Abstract
Acetylcholinesterase (AChE) is the main enzyme for the breakdown of acetylcholine. Nowadays, usage of the inhibitors of this enzyme is one of the most important types of treatment of mild to moderate neurodegenerative diseases such as Alzheimer’s disease. Herbal medicines can be a new source of inhibitors of this enzyme. In this study we examined around 100 different plants to evaluate their inhibitory properties for AChE enzyme. Plants were scientifically identified and their extracts were prepared by methanol percolation. Acetylcholinesterase activity was measured using a colorimetric method in the presence or absence of the extracts. Eserine was used as a positive control. Methanol extracts of the Levisticum officinale, Bergeris integrima and Rheum ribes showed more than 50% AChE inhibitory activity. The inhibition kinetics were studied in the presence of the most effective extracts. L. officinale and B. integrima inhibited AChE activity in a non-competitive manner, while R. ribes competitively inhibitied the enzyme as revealed by double-reciprocal Linweaver-Burk plot analysis. Under controlled condition, Km and Vmax values of the enzyme were found to be 9.4 mM and 0.238 mM/min, respectively. However, in the presence of L. officinale, B. integrima, and R. ribes extracts, Vmax values were 0.192, 0.074 and 0.238 mM/min, respectively. Due to the competitive inhibition of the enzyme by R. ribes extract, the Km value of 21.2 mM was obtained. The concentration required for 50% enzyme inhibition (IC50 value) was 0.5, 0.9, and 0.95 mg/ml for the L. officinale, B. integrima and R. ribes extracts, respectively. The IC50 of the eserine was determined to be 0.8 mg/ml.
Keywords: Acetylcholinesterase, Inhibitor, Levisticum officinale, Bergeris integrima, Rheum ribes
INTRODUCTION
The main role of acethylcholinestrase (AChE) is to rapidly hydrolyze acetylcholine at the cholinergic synapses, ending the transmission of nerve impulses (1). The use of AChE inhibitors in order to enhance cholinergic function in the brain is the main strategy in treatment of Alzheimer’s disease (AD) which is characterized by loss or decline in memory and cognitive impairment (2,3). AD is the most common cause of dementia in the elderly and is responsible for 60 to 80 percent of the cases (2). Degeneration and loss of basal forebrain cholinergic innervation is accepted as a major cause of cognitive impairment and memory loss for the disease (4–6).
The pathological hallmarks of AD are the senile plaques and neurofibrillary tangles. The accumulation of plaques and tangles, and the progression of other pathological processes, leads to a massive neuronal loss, which is usually preceded by synapse loss (1). Several AChE inhibitors such as tacrine, donepzil, rivastigmine and galanthamine, are available for the treatment of mild to moderate AD (7). Although the use of these drugs are beneficial in the treatment of AD symptoms, they may also cause some adverse side effects (8). The most common side effects of these drugs include: anorexia, diarrhea, fatigue, nausea, muscle cramps as well as gastrointestinal, cardiorespiratory, genitourinary and sleep disturbances (6,8). Therefore, cheaper and safer AChE inhibitors with higher efficacy, bioavailability and fewer side effects, particularly from natural sources, have been extensively investigated and research should be continued.
MATERIALS AND METHODS
Plant material
Different parts of all plants such as flowers, fruits, seeds, aerial parts and roots were collected from various provinces of Iran or obtained from the medical herbal markets in Kerman city. Scientific names of the collected plants were authenticated. A voucher specimen of each plant was retained in the herbarium in the Herbal Medicine Research Center, Faculty of the Pharmacy, Kerman University of Medical Sciences, Kerman, Iran.
Extraction of plant material
Each plant was air dried in the dark, and grounded into fine powder. The powdered materials (20 g) were extracted with 200 ml of absolute methanol for 24 h at room temperature. The suspensions were then filtered and air-dried. The extracts were stored at -20 °C until being used (9).
Chemicals
Acetylthiocholine iodide (ATCI), Electric eel acetylcholinesterase and 5-5’-thiobis-2-nitrobenzoic acid (DTNB) were purchased from Sigma (USA). Eserine was obtained from Merck (Germany). All other reagents were of analytical grade.
Acetylcholinesterase activity assay
The AChE activity was measured according to the method developed by Ellman et al. (10). This method employs ATCI as a synthetic substrate for AChE. In this procedure 10 μl of methanol extract containing 50 μg of crude extract was added to the reaction mixture containing 20 μl of enzyme solution (0.1 U/ml) and 950 μl sodium phosphate buffer (pH 8, 0.1 M). Reaction mixture was incubated for 15 min at 25 °C. Then, 10 μl of DTNB (10 mM) was added and the reaction initiated by the addition of substrate (10 μl of ATCI 14 mM solution). Based on this procedure, ATCI is broken down to thiocholine and acetate by the enzyme and thiocholine is reacted with DTNB to produce yellow color. The intensity of yellow color was measured at 410 nm after 10 min. Eserine (20 μl of 0.1 mM solution in phosphate buffer) was used as positive control. The percentage of enzyme inhibition was calculated using the following formula(11).
where, At is the absorbance of the tested extract and Ac is the absorbance of the standard control.
Kinetic study
In order to elucidate the type of inhibition of the effective extracts, the enzyme activity was measured in the presence of an increasing concentrations of ACTI (2-20 mM), and in the presence or absence of two concentrations of each extract (4 and 8 mg/ml). Inhibition mode was determined by double-reciprocal Lineweaver-Burk plot analysis of the data resulted from enzyme assays containing various concentrations of ACTI and the extracts.
RESULTS
Plants with acetylcholinesterase inhibitory effect
Among all plants examined, B. integrima, L. officinale and R. ribes showed the strongest inhibition on the enzyme activity (80.2, 97.6 and 72.4%, respectively). Alhagi camelorum, Marrubium anisodon, Vaccinium arcto-staphilus, Peganum harmala, Rosa damascene and Valeriana hispida, Myrtus communis, Nepta saccharata and Quercus infectoria showed inhibitory effect between 20-50%. The other plants showed inhibitory effect less than 20% or had no effect on enzyme activity. Acetylcholinesterase inhibitory activity of all plants is shown in Table 1.
Table 1.
Acetylcholinesterase inhibitory activity of plants.
| Plants name | Family | Used part | Inhibition % |
|---|---|---|---|
| Achillea eriophora | Asteraceae | Aerial parts | N.E |
| Acantholepis orientalis | Asteraceae | Aerial parts | N.E |
| Achillea phillea | Composiatae | Aerial parts | 9 |
| Achillea wilhelmsii | Asteraceae | Aerial parts | 0.1 |
| Acroptilon repens | Asteraceae | Aerial parts | N.E |
| Alhagi camelorum | Fabaceae | Aerial parts | 29.7 |
| Anacardium occidentale | Anacardiaceae | Rhizomes | 4.6 |
| Alpinia officinarum | Zingiberaceae | Rhizomes | 0.4 |
| Althaea officinalis | Malvaceae | Flowers | 1.7 |
| Apium graveolens | Umbelliferae | Leaves | 4.7 |
| Arctium lappa | Asteraceae | Roots | N.E |
| Artemisia santolina | Asteraceae | Aerial parts | 4.9 |
| Biebersteinia multifida | Berberdaceae | Aerial parts% fruits | 2 |
| Berberis integrima | Berberdaceae | Roots | 80.2 |
| Bunium persicum | Apiaceae | Seeds | 16.8 |
| Camellia sinensis | Theaceae | Leaves | N.E |
| Cannabis sativa | Cannabaceae | Seeds | N.E |
| Cardaria draba | Brassicaceae | Aerial parts% flowers | N.E |
| Carthamus oxyacantha | Asteraceae | aerial parts | N.E |
| Chaerophyllum khorassanicum | Apiaceae | Aerial parts | N.E |
| Cichorium intybus | Asteraceae | Roots | 12.7 |
| Cinnamomum zeylanicum | Lauraceae | Derm | 0.5 |
| Citrus aurantium | Rutaceae | Flowers | 7.4 |
| Citrus sinensis | Rutaceae | Fruits hull | 1.2 |
| Clematis officinalis | Ranunculaceae | Aerial parts | 18 |
| Convolvulus pilosellaefolius | Concolvulaceae | Aerial parts | 10.4 |
| Cordia mixa | Boraginaceae | Fruits | 9 |
| Crocus sativa | Iridaceae | Leaves | N.E |
| Cuminum cyminum | Apiaceae | Seeds | 9.9 |
| Eremostachys laciniata | Lmiaceae | Whole the plant | 2.2 |
| Eremurus persicus | Liliaceae | Aerial parts | 7 |
| Eremurus persicus | Liliaceae | Flowers | N.E |
| Eremurus persicus | Liliaceae | Fruits | N.E |
| Eucaliptus galbie | Myrtaceae | Leaves | N.E |
| Euphorbia hebecarpa | Euphorbiaceae | Aerial parts% flowers | N.E |
| Falcaria vulgaris | Umbelliferaceae | Aerial parts | 6.6 |
| Ferula assafoetida | Apiaceae | Aerial parts% flowers | N.E |
| Ferula oopoda | Apiaceae | Aerial parts | N.E |
| Ferulago angulata | Apiaceae | Aerial parts | 5.3 |
| Ficus carica | Moraceae | Leaves | 3.7 |
| Foeniculum vulgare | Apiaceae | Fruits | N.E |
| Francoeuria undulata | Asteraceae | Aerial parts | 3.1 |
| Fumaria parviflora | Fumariaceae | Aerial parts | 15.5 |
| Glycyrrhiza glabra | Fabaceae | Aerial parts | N.E |
| Gundelia tournefortii | Asteraceae | Aerial parts | N.E |
| Heracleum persicum | Apiaceae | Fruits | 6.5 |
| Hibiscus gossypifolius | Malvaceae | Flowers | 0.5 |
| Hyoscyamus senecionis | Solanaceae | Aerial parts% flowers | 3.5 |
| Laurus nobilis | Lauraceae | Leaves | 6.5 |
| Lawsonia inermis | Lythraceae | Leaves | 8.6 |
| Levisticum officinale | Apiaceae | Roots | 97.6 |
| Linum usitatissimum | Liliaceae | Seeds | N.E |
| Malva sylvestris | Malvaceae | Flowers | 1.5 |
| Marrubium anisodon | Lamiaceae | Aerial parts | 27.7 |
| Matricaria aurea | Asteraceae | Flowers | N.E |
| Mentha longifolia | Lamiaceae | Aerial parts | N.E |
| Mentha piperita | Lamiaceae | Leaves | 4.2 |
| Myrtus communis | Myrtaceae | Leaves | 20.4 |
| Nepeta crispa | Lamiaceae | Aerial parts | 6 |
| Nepeta saccharata | Lamiaceae | Whole the plant | 21.5 |
| Nigella sativa | Ranunculaceae | Seeds | N.E |
| Origanum majorana | Lamiaceae | Whole the plant | 7.9 |
| Otostegia persica | Lamiaceae | Aerial parts | 0.06 |
| Outreya carduiformis | Asteraceae | Aerial parts | 12.3 |
| Peganum harmala | Nitrariaceae | Aerial parts | 29.8 |
| Piper nigrum | Pipereaceae | Fruit | 3.7 |
| Pistacia vera | Anacardiaceae | Fruits hull | 5.5 |
| Punica granatum | Lythraceae | Fruits hull | 11.5 |
| Quercus infectoria | Fagaceae | Galls | 21.4 |
| Rheum ribes | Polygonaceae | Rhizomes | 72.4 |
| Rosa damascene | Rosaceae | Floret | 27.9 |
| Rosmarinus officinalis | Lamiaceae | Aerial parts | N.E |
| Rubia tinctorium | Rubiaceae | Roots | 8.8 |
| Salix alba | Salicaceae | Aerial parts | 3.5 |
| Salvadora persica | Salvadoraceae | Wood | 19 |
| Salvia rhytidea | Lamiaceae | Whole the plant | N.E |
| Sanguisorba minor | Rosaceae | Aerial parts | 18 |
| Scorphularia frigid | Scorophulariaceae | Aerial parts | 2.9 |
| Sizigium aromaticus | Caryophyllaceae | Floret | N.E |
| Solanum dulcamara | Solanaceae | Fruits | 4.8 |
| Sonchus asper | Asteraceae | Aerial parts | N.E |
| Sophora alopecuroides | Fabaceae | Aerial parts | 3 |
| Stachys inflate | Lmiaceae | Aerial parts | 5.2 |
| Stachys lavandulifolia | Lamiaceae | Aerial parts | 7.4 |
| Terminalia chebulla | Combretaceae | Fruits | N.E |
| Teucrium polium | Lamiaceae | Aerial parts | 10 |
| Teucrium scordium | Lamiaceae | Aerial parts | N.E |
| Thymus serpyllum | Lamiaceae | Aerial parts | N.E |
| Tragopogon carcifolius | Compositae | Areial parts | 4.7 |
| Trigonella foenum graecum | Fabaceae | Seeds | 1.8 |
| Urtica dioica | Urticacea | Aerial parts | 2.9 |
| Verbascum kermanensis | Scrophulariaceae | Leaves | 2.7 |
| Verbascum songaricum | Scrophulariaceae | Aerial parts | N.E |
| Zataria multiflora | Lamiaceae | Aerial parts | 8.2 |
| Zhumeria majdae | Lamiaceae | Leaves | 8.5 |
| Zingiber officinale | Zingiberaceae | Rhizomes | 0.6 |
| Ziziphus spinachristi | Rhamnaceae | Leaves | 10.9 |
N.E: No Effect
Kinetic analysis
The inhibition modes of the three most active plant extracts were analyzed by doublereciprocal Lineweaver-Burk plot. B. integrima and L. officinale inhibited the enzyme activity in a non-competitive manner (Fig. 1 and 2), whereas R. ribes showed competitive inhibition (Fig. 3). The Km value of the substrate, ATCI, for the Electric eel acetylcholinesterase was 9.4 mM and the Vmax was 0.238 mM/min. When 8 mg/ml of each extract was added to the enzyme mixtures, the kinetics demonstrated competitive inhibition on enzyme activity by R. ribes with a Vmax of 0.238 mM/min and a Km value of 21.2 mM. IC50 for L. officinale, B. integrima and R.ribes were 0.5, 0.9, and 0.95 mg/ml, respectively (Table 2). The Ki values of 1.6, 5.5 and 6.37 mg/ml were found for L. officinale, B. integrima and R. ribes, respectively.
Fig. 1.
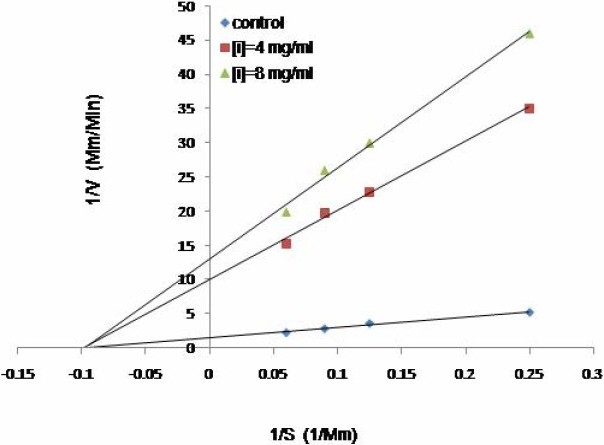
The Lineweaver-Burk plot of kinetic analysis of acetylcholinestrase at two different concentrations of L. officinale (4 and 8 mg/ml) in the presence of four different ATCI concentrations.
Fig. 2.
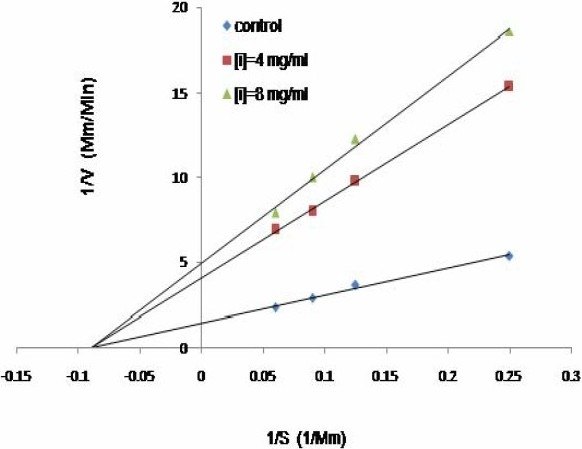
The Lineweaver-Burk plot of kinetic analysis of acetylcholinestrase at two different concentrations of B. integrima (4 and 8 mg/ml) in the presence of four different triolein concentrations.
Fig. 3.
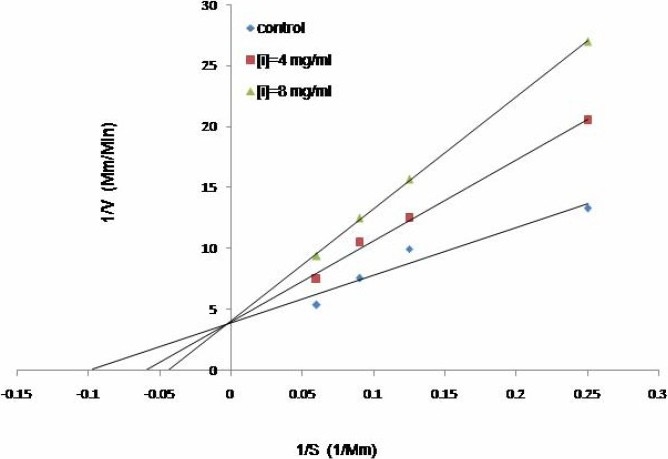
The Lineweaver-Burk plot of kinetic analysis of acetylcholinestrase at two different concentrations of R. ribes (4 and 8 mg/ml) in the presence of four different ATCI concentrations.
Table 2.
The IC50 values of the methanol extracts compared to eserine as a positive control.
| Plants name | Family | IC50 value (mg/ml) |
|---|---|---|
| Levisticum officinale | Apiaceae | 0.5 |
| Rheum ribes | Polygonaceae | 0.95 |
| Berberis integrimma | Berberidacea | 0.9 |
| Eserine | --- | 0.8 |
DISCUSSION
Acetylcholinesterase inhibitors are used for the treatment of Alzheimer’s disease. These inhibitors may interact with the central cholinergic system function to improve memory and cognitive disorders in the patients by decreasing the breakdown of acetylcholine in brain synapses (12). Nature is an unlimited resource for providing chemicals and biological compounds which are unique and complex insofar as their chemical synthesis seems impossible. The anti-cholinesterase activity of some plants in the world has been approved (12). In this study we concluded that roots of L. officinale and B. integrima and rhizomes of R. ribes possess a strong anticholinesterase activity. IC50 values (concentration required to inhibit 50% of enzyme activity) were calculated from the regression equation obtained from various concentrations of the test compounds (Table 2). Previously, it has been demonstrated (13) that the methanol extracts of punica granatum and Rosa damascene have more than 50% inhibitory effect on alpha manosidase activity, but these extracts did not show strong inhibitory effects on acetylcholinesterase. Extract of L. officinale exhibited strong inhibitory effects on the alpha glucosidase and the pancreatic lipase but exhibited weak inhibition on alpha manosidase (13,14). The plant extract demonstrated apoptotic activity on humans leukaemia cell line (15), and had an anti-mycobacterial activity as well (16). B. integrima produced no effect on the alpha glucosidase and the pancreatic lipase activity (14,17). 1-methylmalate, one of the B. integrima fruit components, increased the anti-microbial activity against Staphylococcus aureus (18). Isoquinoline alkaloids attained from the root of Turkish berberis species showed an anti-inflamatory and anti-nociceptive effects (19), whereas in our study this plant exhibited the anti-cholinesterase activity. R. ribes has shown to have hypo-glycemic effects in the alloxan induced diabetic rats but did not reveal hypoglycemic activity in healthy mice (20). It was also demonstrated that the herb possesses some anti-depressive activity (21). Some drugs such as rasagiline, used in the treatment of Alzheimer’s disease, retain the neuroprotective properties with their anti-cholinesterase and monoamine oxidase inhibitory effects and, has shown anti-depressant activity in animals (22). Therefore, the anti-depressant properties of R. ribes could be due to the anti-cholinesterase activity shown in this study. Other findings indicated that some components of R. ribes extract demonstrated selective cytotoxic activity on cancerous cell lines (23). The stem and root of R. ribes exhibited an antioxidant activity (24), but this plant did not have any inhibitory effect on the alpha glucosidase or the pancreatic lipase activity (14,17). The methanol extracts of Rosa damascene, Quercus infectoria, Eucalyptus galbie, Myrtus communis, Terminalia chebulla, Punica granatum, Camellia sinensis, and Cinnamomum zeylanicum showed more than 50 percent inhibitory activity on the pancreatic lipase or alpha glucosidase (14,17), while they revealed no or little effect on cholinesterase activity. One of the most important anti-cholinesterase drugs, tacrine, proved to have both competitive and non-competitive inhibitory activities on acetylcholinesterase (25). Tolserin, the novel experimental AD therapeutic agent, inhibits the acetylcholinesterase in a non-competitive manner (26). R. ribes showed some similarities in kinetic properties to tacrine. L. officinale and B. integrima were non-competitive inhibitors of the enzyme, as acting similar to tolserin. A competitive-inhibitor binds to the active site of the enzyme and affects Km of the reaction. Components of R. ribes extract with competitive inhibition, may bind to the enzyme and block its activity. When an inhibitor binds to the enzyme and/or enzyme-substrate complex, it is considered as non-competitive inhibition where the inhibitor affects only the Vmax of the reaction but has no effect on complex formation between the enzyme and the substrate. Therefore, two extracts that showed non-competitive inhibition on activity, probably have components that bind to enzyme or enzyme-substrate complex (27).
CONCLUSION
According to our results, it is possible to assume that R. ribes may contain some components that are functionally or structurally similar to tacrine. The same might be true for L. officinale and B. integrima regarding the kinetic properties of the tolserin. Results of this study indicated that these plants may offer great potentials for the treatment of different diseases including AD, and their anti-acetylcholinesterase properties introduce them as promising candidates for more detailed in vitro and in vivo studies. Besides, these plants can be examined in order to isolate and identify the active ingredients, and this may serve as a foundation to find safer and more effective agent (s) for therapeutic use.
Acknowledgments
This study was financially supported by the research funds provided by Vice Chancellor for Research and the Kerman Physiology Research Center, Kerman Univercity of Medical Sciences, Kerman, Iran.
REFERENCES
- 1.Alzheimer’s Association. 2008 Alzheimer’s disease facts and figures. Alzheimers Dement. 2008;4:110. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam M. The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 3.Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB. Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth. 2006;97:445–52. doi: 10.1093/bja/ael233. [DOI] [PubMed] [Google Scholar]
- 4.Munoz DG, Feldman H. Causes of Alzheimer’s disease. Can Med Assoc J. 2000;162:65–72. [PMC free article] [PubMed] [Google Scholar]
- 5.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005;105:145–158. [PubMed] [Google Scholar]
- 7.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 8.Chattipakorn S, Pongpanparadorn A, Pratchayasakul W, Pongchaidacha A, Ingkaninan K, Chattipakorn N. Tabernaemontana divaricata extract inhibits neuronal acetylcholinesterase activity in rats. J Ethnopharmacol. 2007;110:61–68. doi: 10.1016/j.jep.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Sharma N, K, Sharma V, Seo SY. Screening of some medicinal plants for anti-lipase activity. J Ethnopharmacol. 2005;97:453, 456. doi: 10.1016/j.jep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed T, Gilani AH. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol Biochem Behav. 2009;91:554. doi: 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Gholamhoseinian A, Fallah H, Sharifi-Far F, Mirtajaddini M. Alpha mannosidase inhibitory effect of some Iranian plant extracts. Int J Pharmacol. 2008;4:460–465. [Google Scholar]
- 14.Gholamhoseinian A, Fallah H, Sharifi-Far F, Mirtajaddini M. The inhibitory effect of some Iranian plants extracts on the alpha glucosidase. Iran J Basic Med Sci. 2008;11:1–9. [Google Scholar]
- 15.Bogucka-Kocka A, Smolarz HD, Kocki J. Apoptotic activities of ethanol extracts from some Apiaceae on human leukaemia cell lines. Fitoterapia. 2008;79:487–497. doi: 10.1016/j.fitote.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Schinkovitz A, Stavri M, Gibbons S, Bucar F. Antimycobacterial polyacetylenes from Levisticum officinale. Phytother Res. 2008;22:681–684. doi: 10.1002/ptr.2408. [DOI] [PubMed] [Google Scholar]
- 17.Gholamhoseinian A, Shahouzehi B, Sharifi-far F. Inhibitory Effect of Some Plant Extracts on Pancreatic Lipase. Int J Pharmacol. 2010;6:18–24. [Google Scholar]
- 18.Alimirzaee P, Gohari AR, Hajiaghaee R, Mirzaee S, Jamalifar H, Monsef-Esfahani HR, et al. 1-methyl malate from Berberis integerrima fruits enhances the antibacterial activity of ampicillin against Staphylococcus aureus. Phytother Res. 2009;23:797. doi: 10.1002/ptr.2641. [DOI] [PubMed] [Google Scholar]
- 19.Kupeli E, Kosar M, Yesilada E, Husnu K, Baser C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72:645–657. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 20.Ozbek H, Ceylan E, Kara M, Ozgkçe F, Koyuncu M. Hypoglycemic effect of Rheum ribes roots in alloxan induced diabetic and normal mice. Medicine. 2001;3:138. [Google Scholar]
- 21.Sayyah M, Boostani H, Pakseresht S. Efficacy of hydroalcoholic extract of Rheum ribes L. in treatment of major depressive disorder. Iran Red Crescent Med J. 2009;3:573–575. [Google Scholar]
- 22.Youdim MBH, Weinstock M. Novel neuroprotective anti-Alzheimer drugs with anti-depressant activity derived from the anti-Parkinson drug, rasagiline. Mech Ageing Dev. 2002;123:1081–1086. doi: 10.1016/s0047-6374(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 23.Sardari S, Shokrgozar MA, Ghavami G. Cheminformatics based selection and cytotoxic effects of herbal extracts. Toxicol In Vitro. 2009;23:1412–421. doi: 10.1016/j.tiv.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Oztürk M, Aydogmus-Oztürk F, Duru ME, Topçu GI. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): an edible medicinal plant. Food Chem. 2007;103:623–630. [Google Scholar]
- 25.Alhomida AS, Al-Rajhi AA, Kamal MA, Al-Jafari AA. Kinetic analysis of the toxicological effect of tacrine (Cognex®) on human retinal acetylcholin-esterase activity. Toxicology. 2000;147:33–39. doi: 10.1016/s0300-483x(00)00177-3. [DOI] [PubMed] [Google Scholar]
- 26.Kamal MA, Greig NH, Alhomida AS, Al-Jafari AA. Kinetics of human acetylcholinesterase inhibition by the novel experimental Alzheimer therapeutic agent, tolserine. Biochem Pharmacol. 2000;60:561–570. doi: 10.1016/s0006-2952(00)00330-0. [DOI] [PubMed] [Google Scholar]
- 27.Nelson DL, Michel MC. Enzymes. Enzyme kinetics as an approach to understanding mechanism. 4th ed. New York: Freeman company; 2005. Principles of biochemistry. [Google Scholar]


