Abstract
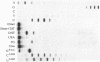
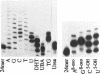
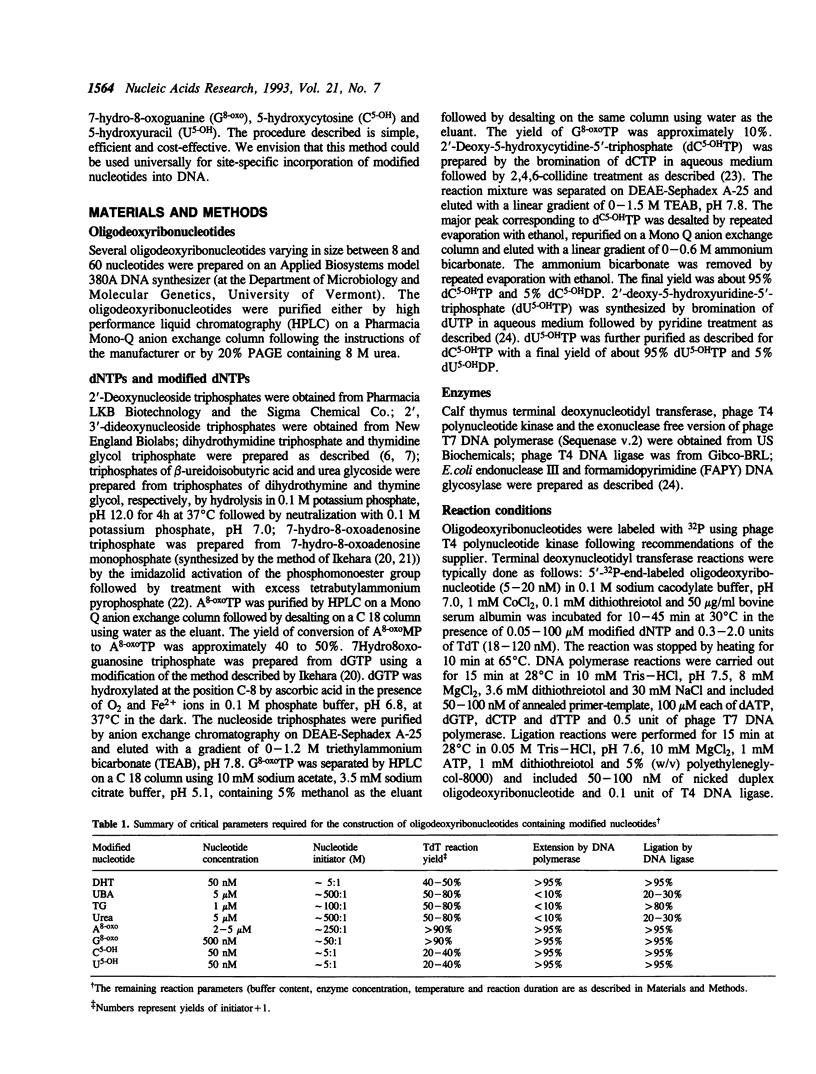
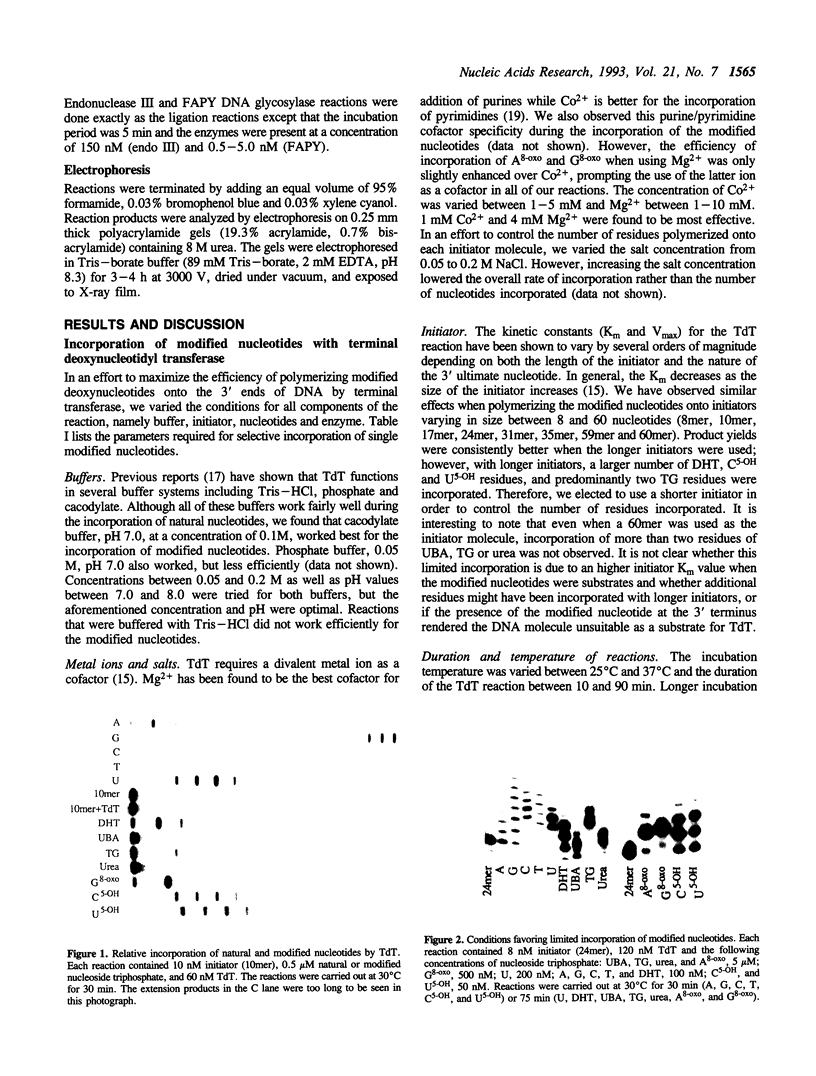
Calf thymus terminal deoxynucleotidyl transferase was used to incorporate several products of oxidative base damage onto the 3' end of oligodeoxyribonucleotides. Under the defined conditions described in this report, single residues of dihydrothymine, beta-ureidoisobutyric acid, thymine glycol, urea, 7-hydro-8-oxoadenine, 7-hydro-8-oxoguanine, 5-hydroxycytosine and 5-hydroxyuracil were incorporated into oligodeoxyribonucleotides of different lengths. The reaction is both efficient and cost effective. The 3' termini of the reaction products were suitable substrates for ligation by phage T4 DNA ligase, facilitating greatly the construction of oligodeoxyribonucleotides containing unique and site specific oxidative DNA lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. M., Bollum F. J. Molecular biology of terminal transferase. CRC Crit Rev Biochem. 1986;21(1):27–52. doi: 10.3109/10409238609113608. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Multiple roles of divalent cation in the terminal deoxynucleotidyltransferase reaction. J Biol Chem. 1990 Oct 15;265(29):17436–17440. [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Functional effects of cis-thymine glycol lesions on DNA synthesis in vitro. Biochemistry. 1987 Aug 25;26(17):5398–5403. doi: 10.1021/bi00391a027. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Pattabiraman N., Jarvis W., Beardsley G. P. Modeling and molecular mechanical studies of the cis-thymine glycol radiation damage lesion in DNA. Biochemistry. 1987 Aug 25;26(17):5404–5409. doi: 10.1021/bi00391a028. [DOI] [PubMed] [Google Scholar]
- Eaton M. A., Hutchinson D. W. Poly(5-hydroxycytidylic acid) . Biochim Biophys Acta. 1973 Sep 7;319(3):281–287. doi: 10.1016/0005-2787(73)90167-6. [DOI] [PubMed] [Google Scholar]
- Guschlbauer W., Duplaa A. M., Guy A., Téoule R., Fazakerley G. V. Structure and in vitro replication of DNA templates containing 7,8-dihydro-8-oxoadenine. Nucleic Acids Res. 1991 Apr 25;19(8):1753–1758. doi: 10.1093/nar/19.8.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Ide H., Kow Y. W., Wallace S. S. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985 Nov 25;13(22):8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide H., Melamede R. J., Wallace S. S. Synthesis of dihydrothymidine and thymidine glycol 5'-triphosphates and their ability to serve as substrates for Escherichia coli DNA polymerase I. Biochemistry. 1987 Feb 10;26(3):964–969. doi: 10.1021/bi00377a042. [DOI] [PubMed] [Google Scholar]
- Ide H., Petrullo L. A., Hatahet Z., Wallace S. S. Processing of DNA base damage by DNA polymerases. Dihydrothymine and beta-ureidoisobutyric acid as models for instructive and noninstructive lesions. J Biol Chem. 1991 Jan 25;266(3):1469–1477. [PubMed] [Google Scholar]
- Ide H., Wallace S. S. Dihydrothymidine and thymidine glycol triphosphates as substrates for DNA polymerases: differential recognition of thymine C5-C6 bond saturation and sequence specificity of incorporation. Nucleic Acids Res. 1988 Dec 9;16(23):11339–11354. doi: 10.1093/nar/16.23.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Tada H., Kaneko M. Studies of nucleosides and nucleotides. XXXV. Purine cyclonucleosides. 5. Synthesis of purine cyclonucleoside having 8,2'-O-anhydro linkage and its cleavage reactions. Tetrahedron. 1968 Apr;24(8):3489–3498. doi: 10.1016/s0040-4020(01)92646-8. [DOI] [PubMed] [Google Scholar]
- Kouchakdjian M., Bodepudi V., Shibutani S., Eisenberg M., Johnson F., Grollman A. P., Patel D. J. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry. 1991 Feb 5;30(5):1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Borden A., Banerjee S. K., LeClerc J. E. Mutation frequency and spectrum resulting from a single abasic site in a single-stranded vector. Nucleic Acids Res. 1990 Apr 25;18(8):2153–2157. doi: 10.1093/nar/18.8.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Rabkin S. D., Strauss B. S. A role for DNA polymerase in the specificity of nucleotide incorporation opposite N-acetyl-2-aminofluorene adducts. J Mol Biol. 1984 Sep 25;178(3):569–594. doi: 10.1016/0022-2836(84)90239-0. [DOI] [PubMed] [Google Scholar]
- Rouet P., Essigmann J. M. Possible role for thymine glycol in the selective inhibition of DNA synthesis on oxidized DNA templates. Cancer Res. 1985 Dec;45(12 Pt 1):6113–6118. [PubMed] [Google Scholar]
- Singer B., Chavez F., Spengler S. J., Kuśmierek J. T., Mendelman L., Goodman M. F. Comparison of polymerase insertion and extension kinetics of a series of O2-alkyldeoxythymidine triphosphates and O4-methyldeoxythymidine triphosphate. Biochemistry. 1989 Feb 21;28(4):1478–1483. doi: 10.1021/bi00430a008. [DOI] [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]