Abstract
Methanolic extract of 15 Iranian medicinal plants were prepared and tested for their cytotoxic activities against three cancer cell lines (MCF7, HepG2, WEHI164) and one normal cell line (MDBK). Some plants showed cytotoxic activities. The extract of Ferula szowitsiana root, which proved to be the most active, was chosen for further phytochemical studies. The major compounds of the most potent acetone extract were isolated. They were identified as chimgin and chimganin, two known monoterpenoids, by spectroscopic means. Their cytotoxic activity was evaluated in three cell lines. The results show that these compounds are responsible, at least in part, for the cytotoxic activity of this plant.
Keywords: Cytotoxicity, Ferula szowitsiana, Monoterpenoid
INTRODUCTION
Cancer is a general term applied to a series of malignant diseases which may affect many different parts of the body. If the process is not arrested, it may progress until it causes the death of the organism(1). Cancer is one of the major causes of death in developed countries, together with cardiac and cerebrovascular diseases(2). Conventional cancer treatments include surgery, radiation and chemotherapy. The dispersed nature of end-stage disease drives the need for systematic therapy and chemotherapy aims to wipe out all cancerous colonies within the patient’s body, including metastasized cancer cells(3). Currently, much commonly used anticancer therapeutics represent broadly cytotoxic agents. These agents have been frequently discovered using cell-based cytotoxicity assays.
Drug discovery from medicinal plants has played an important role in the treatment of cancer and, indeed, most new clinical applications of plant secondary metabolites and their derivatives over the last half century have been applied toward combating cancer(4,5).
Traditional medicine over the years has proved to be an invaluable guide in drug discovery and Iran has a long history in this field and is a great source of new bioactive compounds.(6,7).
In this study we collected 15 plant species from different parts of Iran. These plants have ethnomedicinal reports or have shown antifungal properties in a previous screening of native Iranian plants for their in vitro antifungal activity against 19 fungal strains(8,9). The methanolic extracts of these plants were prepared and their antiproliferative activities were screened against different cancer and normal cell lines, i.e. MCF7 (human breast carcinoma), HepG2 (hepatocellular carcinoma), WEHI (fibrosarcoma) and MDBK (cow’s normal kidney cell). Extract of F. szowitsiana showed the most potent antiproliferative activity against human tumor cells. This plant is used in folk medicine for the treatment of various diseases such as dermal wounds and asthma(6). Fractionation of the methanolic extract with acetone led to the isolation of two cytotoxic compounds. Both these compounds have previously been isolated from some Ferula species but this is the first report of their isolation from F. szowitsiana (10–12).
MATERIALS AND METHODS
All solvents were purchased from Merck, Germany. NMR spectra were recorded on a Bruker Ultra shild NMR spectrometer (500 MHz for 1Hand 125 MHz for 13CNMR) using CDCl3. Electron Impact mass spectra were obtained using a Finnigan MAT-EI-TSQ at 70 eV. Melting points were determined on a Reichert-jung apparatus and UV spectra were taken on a Shimadzu UV-3100 spectro-photometer.
Plants and extracts
The selected plants (Table 1) were collected at different localities of Iran and were identified at Traditional Medicine & Materia Medica Research Center, Shaheed Beheshti University of Medical Sciences, Tehran, Iran. Voucher specimens of the plants were deposited in the herbarium of the Traditional Medicine and Materia Medica Research Center. The shade dried and ground plant parts were extracted with methanol for 24 h while stirring at room temperature. The solvent was evaporated under reduced pressure at a temperature of 45°C. The resulting crude extracts were stored until assayed.
Table 1.
Plant species investigated in this study
| Plant name | Family | Part used | Traditional uses | Voucher |
|---|---|---|---|---|
| Anchusa azurea Mill. | Boraginaceae | Aerial part | Cold, sedative | TMRC 273 |
| Biebersteinia multifida DC. | Geraniaceae | Root | Dermal wounds | TMRC 486 |
| Buxus hyrcana Pojark. | Buxaceae | Aerial part | Antifungal | TMRC 1161 |
| Caccinia macranthera (Branks & soland.) Brand. | Boraginaceae | Root | Dermal infections, liver disorders, dyspepsia | TMRC 510 |
| Capparis spinosa L. | Capparidaceae | Aerial part | Rheumatism, headache, digestive disorders, hemorrhoid | TMRC 1295 |
| Chenopodium butrys L | Chenopodiaceae | Aerial part | Antifungal | TMRC 1296 |
| Convolvulus commutatus Boiss. | Convolvulaceae | Aerial part | Infected wounds | TMRC 564 |
| Echium italicum L. | Boraginaceae | Aerial part | Dermal wounds | TMRC 1299 |
| Ferula szowitsiana DC. | Apiaceae | Root | Dermal wound, asthma, cough | TMRC 965 |
| Glaucium oxylobum Boiss.& Buhse | Papaveraceae | Aerial part | Antifungal | TMRC 1283 |
| Leontice leontopetalum L. | Berberidaceae | Root | Rheumatism, joint pain and inflammation | TMRC 1287 |
| Parrotia persica (DC.) C. A. Mey | Hammamelidaceae | Bark | Broken bone | TMRC 1281 |
| Perovskia abrotanoides Karel. | Lamiaceae | Root | Leishmaniasis | TMRC 801 |
| Stachys turcomanica Trautv. | Lamiaceae | Aerial part | Influenza, bronchitis, footinflammation, toothache | TMRC 491 |
| Zygophyllum fabago L. | Zygophylaceae | Aerial part | Digestive problems, Diarrhea | TMRC 2189 |
Cytotoxicity assay
The cytotoxicity bioassay was performed against three cancer cell lines (MCF-7, HepG2, WEHI) and one normal cell line (MDBK). Cells were grown in monolayer cultures in Dulbecco’s Modified Eagle’s Medium (DMEM) and RPMI 1640 supplemented with 5% FBS (Gibco), 100 U/ml penicillin, 10 µg/ml streptomycin, and maintained at 37°C in a 5% CO2 incubator. For testing, cells were washed with PBS (phosphate buffer saline), harvested by tripsinization, plated (104 cell/well) in 96-well plates, and incubated for 24 h at 37°C in the incubator. Afterwards, they were exposed to different concentrations of plant extracts and incubated for further 72h followed by MTT [3-(4,5- dimethylthiazol-2yl)-2,5- biphenyl tetrazolium bromide] assay at 570 nm(13). Viability was defined as the ratio (expressed as a percentage) of absorbance of treated cells to untreated cells. The selectivity index (SI) was determined as the ratio of the concentration at which growth was inhibited by 50% (IC50) on normal cells to cancer cells.
Extraction and isolation
The ground roots of F. szowitsiana (40 g) which were collected from the Kalaleh region in Golestan province, at the height of 500-700 m, were extracted with methanol over night. After filtration, the solvent was evaporated to dryness under reduced pressure at 40°C. The residue was extracted by acetone. The dried acetone extract (6 g, semisolid reddish brown gum) was fractioned by column chromatography using Silica gel 60 eluting first with hexane, followed by a gradient of hexane CHCl3 up to 100% CHCl3 and CHCl3 -acetone up to 15% acetone. Eight fractions were obtained. Fraction 3 was separated by Preparative Thin Layer Chromatography (PTLC) over silica gel, using CH2 Cl2 : EtOAc (9.5:0.5) as mobile phase to obtain 175 mg compound 1 (Chimganin). Fraction 5 was crystallized from EtOH twice and afforded 500 mg of compound 2 (Chimgin).
Chimganin (1,7,7-Trimethylbicyclo[2.2.1] heptan-2-yl-4-hydroxy-3-methoxybenzoate) (compound 1)
Colorless crystals, m.p.80-82 °C (lit. 85 °C);1 HNMR (500 MHz, CDCl3) (α=endo, β=exo): δ 7.70 (1H, dd, H-6’), 7.60 (1H, d, H-2’), 6.98 (1H, d, H-5’), 5.12 (1H, dt, H-2β), 3.96 (3H, s, OMe), 2.50 (1H, m, H-3β), 1.16 (1H, m, H-3α), 2.16 (1H, m, H-6β), 1.45 (1H, m, H-6α), 1.84 (1H, m, H-5β), 1.34 (1H, m, H-5α), 1.76 (1H, t, H-4), 1.13 (3H, s, H-9), 0.95 (3H, s, H-8), 0.94 (3H, s, H-10).13 C NMR (125 MHz, CDCl3): 167.13 (C-7’),150.28 (C-4’), 146.59 (C-3’), 124.36 (C-6’), 114.51 (C-5’), 123.40 (C-1’), 112.23 (C-2’), 80.75 (C-2), 56.46 (OMe), 49.50 (C-7), 48.27 (C-1), 45.42 (C-4), 37.34 (C-3), 28.52 (C-5), 27.54 (C-6), 19.92 (C-8), 19.34 (C-9), 14.05 (C-10). EIMS (70 eV) m/z (rel.int.): 304 (M+) (15), 136 (10), 121 (100), 118 (35), 93 (20).
Chimgin (1,7,7- Trimethylbicyclo [2.2.1] heptan-2-phydroxybenzoate) (compound 2)
White needles, m.p. 150-152 °C (lit. 154-155 °C).
1 H NMR (500 MHz, CDCl3) (α=endo, β=exo):δ 7.92 (1H, d, H-6’), 7.92 (1H, d, H-2’), 6.88 (1H, d, H-5’), 6.88 (1H, d, H-3’), 5.05 (1H, dt, H-2β), 2.45 (1H, m, H-3β), 1.09 (1H, dd, H-3α), 2.13 (1H, m, H-6β), 1.40 (1H, m, H-6α), 1.77 (1H, m, H-5β), 1.31 (1H, m, H-5α), 1.71 (1H, t, H-4), 0.95 (3H, s, H-9), 0.90 (3H, s, H-10), 0.89 (3H, s, H-8).13 CNMR (125 MHz, CDCl3): 167.34 (C-7’), 161.99 (C-4’), 132.01 (C-6’), 132.01 (C-2’), 115.73 (C-3’), 115.73 (C-5’), 122.44 (C-1’), 80.38 (C-2), 48.22 (C-1), 49.44 (C-7), 45.41 (C-4), 37.33 (C-3), 28.49 (C-5), 27.79 (C-6), 20.12 (C-8), 19.30 (C-9), 13.10 (C-10). EIMS (70 eV) m/z (rel.int): 274(M+) (15), 153 (10) 136 (37), 121 (100), 93 (50).
Cytotoxicity determination of Chimgin and Chimganin
The cytotoxicity of two isolated compounds was evaluated against MCF-7, HepG2 and MDBK cell lines by the MTT assay.
RESULTS
The cytotoxic activity of 15 different medicinal plants of Iran against MCF-7, HepG2, WEHI and MDBK cell lines was evaluated. The results of this screening are summarized in Table 2. While most of the extracts appeared almost inactive (IC50 values above 100 µg/ml)(14), the methanolic extract of F. szowitsiana (yield 22.4%) showed a promising antiproliferative activity. It showed IC50 values less than 100 µg/ml in all evaluated cell lines and in 3 cell lines out of 4 it showed the highest cytotoxicity. Further-more, Perovskia abrotanoides exhibited moderate cytotoxicity against three cell lines, whereas Buxus hyrcana and Parrotia persica where only active against one cell line. According to the results of the bioassay, F. szowitsiana was selected for further studies in order to identify the active compounds.
Table 2.
In vitro cytotoxicity of methanol extracts of selected medicinal plants. Tamoxifen was used as positive control
| Plant name | IC50 a value |
|||
|---|---|---|---|---|
| MCF-7 | HepG2 | WEHI | MDBK | |
| Anchusa azurea Mill. | >100 | >100 | >100 | >100 |
| Biebersteinia multifida DC. | >100 | >100 | >100 | >100 |
| Buxus hyrcana Pojark. | 44.4 | >100 | >100 | >100 |
| Caccinia macranthera (Branks & soland.) | >100 | >100 | >100 | >100 |
| Capparis spinosa L. | >100 | >100 | >100 | >100 |
| Chenopodium butrys L | >100 | >100 | >100 | >100 |
| Convolvulus commutatus Boiss. | >100 | >100 | >100 | >100 |
| Echium italicum L. | >100 | >100 | >100 | >100 |
| Ferula szowitsiana DC. | 29 | 40.6 | 79 | 38 |
| Glaucium oxylobum Boiss.& Buhse | >100 | >100 | >100 | >100 |
| Leontice leontopetalum L. | >100 | >100 | >100 | >100 |
| Parrotia persica (DC.) C. A. Mey | >100 | >100 | 97.4 | >100 |
| Perovskia abrotanoides Karel. | 93 | >100 | 40.6 | 62.3 |
| Stachys turcomanica Trautv. | >100 | >100 | >100 | >100 |
| Zygophyllum fabago L. | >100 | >100 | >100 | >100 |
| Tamoxifen | 6.5 | 13.8 | 89.6 | 19.1 |
The concentration at which growth was inhibited by 50%, µg/ml
The bioassay-guided fractionation of the methanolic extract led to isolation of two monoterpenoids (Fig. 1). The 1H NMR of compound 1 revealed the occurrence of a 1,2,4- trisubstituted aromatic system. In addition it showed characteristic signals of a bornyl moiety in which H-2 (exo) appears as a doublet of triplets (J = 9.5, 2.9 Hz) due to a “W” coupling to the coplanar H-6 (exo)(15). By comparison of its spectroscopic data with the literature values(16), it was identified as chimganin. Compound 2 showed similar NMR data but was characterized by a p-substituted aromatic system and identified as chimgin(16). The cytotoxicity of compounds 1 and 2 was evaluated against three different cell lines. IC50 was calculated from dose-response curves (Table 3).
Fig. 1.
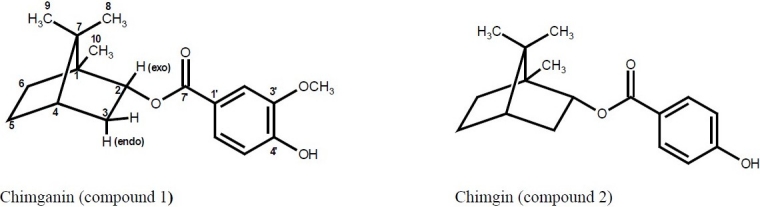
Structures of the isolated monoterpenoids
Table 3.
In vitro cytotoxicity of isolated compounds
| Compounds | IC50 (µM) |
||
|---|---|---|---|
| Cell lines | |||
| MCF-7 | HepG2 | MDBK | |
| Chimgin (compound 2) | 45.2 | 67.1 | 69.7 |
| Chimganin (compound 1) | 28 | 74 | 30.9 |
DISCUSSION
This study aimed to investigate the cytotoxic activity of 15 Iranian traditional medicinal plants against three cancer cell lines and one normal cell line. Some plant’s methanolic extracts showed low or no cytotoxicity against the cell lines, whereas F. szowitsiana showed the most potent cytotoxicity against all of them.
Bioassay-guided fractionation led to the isolation of two monotepenoids from the root extract of F. szowitsiana. Both bornyl esters have been isolated before from different Russian Ferula species(10–12), but are reported here for the first time from F. szowitsiana. Previous investigations revealed the occurrence of prenylated coumarin derivatives in the roots of F. szowitsiana (17). One of these coumarins, umbelliprenin, inhibited the growth of a human melanoma cell line with an IC50 value of 12.3 µM(18).
CONCLUSION
Both compounds, Chimganin and Chimgin, showed significant cytotoxic effects with lower IC50 values compared to the extract and were just slightly less active than tamoxifen which was used as positive control. Thus we conclude that these two compounds are responsible, at least in part, for the observed cytotoxicity of the extract of F. szowitsiana.
Acknowledgments
The authors would like to thank Dr. K. Jenett-Siems, Institut fur Pharmazie, Freie Universitat Berlin, for reading of the manuscript and providing useful suggestions and by Ms. Pirani and Mr. Ghorbani, Traditional Medicine & Materia Medica Research Center, Shaheed Beheshti University of Medical Sciences for plant collection and identification.
REFERENCES
- 1.Evans WC. Trease and Evans pharmacognosy. London: WB Saunders; 2002. [Google Scholar]
- 2.Ueda J, Tezuka Y, Banskota AH, Tran Q, Harimaya y, Saiki I, et al. Antiproliferative activity of vietnamese medicinal plants. Biol Pharm Bull. 2002;25:753–760. doi: 10.1248/bpb.25.753. [DOI] [PubMed] [Google Scholar]
- 3.Kintzios SE, Barberaki MG. Plants that fight cancer. Boca Raton: CPR Press; 2003. [Google Scholar]
- 4.Newman DJ, Cragg GM, Sander KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 5.Butter MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 6.Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran. J Ethnopharmacol. 2005;102:58–68. doi: 10.1016/j.jep.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Naghibi F, Mosaddegh M, Mohammadi Motamed S, Ghorbani A. Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iranian J Pharm Res. 2005;2:63–79. [Google Scholar]
- 8.Amin G, Dehmoobed-sharifabadi A, Salehi Surmaghi MH, Yasa N, Aynechi Y, Emami M, et al. Screening of Iranian plants for antifungal activity. Daru. 2002;9:38. [Google Scholar]
- 9.Amin G, Dehmoobed-sharifabadi A, Salehi Surmaghi MH, Yasa N, Aynechi Y, Emami M, et al. Screening of Iranian plants for antifungal activity. Daru. 2002;10:78. [Google Scholar]
- 10.Kerimov SS, Saidkhodzhaev AI, Malikov VM. Esters of Ferula calcarea. Khim Prir Soedin. 1987;5:765. [Google Scholar]
- 11.Ahmad VU, Noorwala M, Mohammad FV, Qadir MH. Constituents of Ferula baluchistanica. Fitoterapia. 1994;65:183. [Google Scholar]
- 12.Kerimov YuB, Abyshev AZ, Serkerov SV, Isaev DI, Bairamov PB. Phenol derivatives from the roots of Ferula persica. Khim Prir Soedin. 1992;5:579. [Google Scholar]
- 13.Carmichael J, DeGroff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 14.Cordell GA, Kinghorn AD, Pezzuto JM. Separation, structure elucidation, and bioassay of cytotoxic natural products. In: Colegate SM, Molyneux RJ, editors. Bioactive natural products: detection, isolation, and structural determination. Florida: CRC Press; 1993. pp. 198–201. [Google Scholar]
- 15.Duclos R, Lu D, Guo J, Makriyannis A. Synthesis and characterization of 2-substituted bornane pharmacophores for novel cannabinergic ligands. Tetrahedron Lett. 2008;49:5587–5589. doi: 10.1016/j.tetlet.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadyrov A, Nikonov GK. Structure of tschimganin and tschimgi. Khim Prir Soedin. 1972;8:59–63. [Google Scholar]
- 17.Iranshahi M, Afra P, Ramezani M, Jaafari M, Sadeghian H, Bassarello C, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochem. 2007;68:554. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Barthomeuf C, Lim S, Iranshahi M, Chollet P. Umbelliprenin from Ferula szowitsiana inhibits the growth of human M4Beu metastatic pigmented malignant melanoma cells through cell-cycle arrest in G1 and induction of caspase-dependent apoptosis. Phytomedicine. 2008;15:103–111. doi: 10.1016/j.phymed.2007.04.001. [DOI] [PubMed] [Google Scholar]


