Abstract
Background
Laparoscopic liver resection (LLR) is now considered a feasible alternative to open liver resection (OLR) in selected patients. Nevertheless studies comparing LLR and OLR are few and concerns remain about long-term oncological equivalence. The present study compares outcomes with LLR vs. OLR using meta-analytical methods.
Methods
Electronic literature searches were conducted to identify studies comparing LLR and OLR. Short-term outcomes evaluated included operating time, blood loss, length of hospital stay, peri-operative morbidity and resection margin status. Longer-term outcomes included local and distant recurrence, and overall (OS) and disease-free survival (DFS). Meta-analyses were performed using the Mantel–Haenszel method and Cohen's d method, with results expressed as odds ratio (OR) or standardized mean difference (SMD), respectively, with 95% confidence intervals (CI).
Results
Twenty-six studies met the inclusion criteria with a population of 1678 patients. LLR resulted in longer operating time, but reduced blood loss, portal clamp time, overall and liver-specific complications, ileus and length of stay. No difference was found between LLR and OLR for oncological outcomes.
Discussion
LLR has short-term advantages and seemingly equivalent long-term outcomes and can be considered a feasible alternative to open surgery in experienced hands.
Keywords: colorectal metastases, non-colorectal metastases, hepatocellular carcinoma, cholangiocarcinoma, adenoma
Introduction
Over the past 30 years surgery of the gastro-intestinal (GI) tract has been revolutionized by minimally invasive techniques. Laparoscopic surgery is now considered the approach of choice for a variety of procedures, including cholecystectomy,1 appendicectomy,2 splenectomy,3 anti-reflux surgery4 and more recently colorectal resection.5 Proven benefits of laparoscopic surgery include reduced post-operative pain, shortened duration of hospitalization and improved cosmetic outcome, and have been reported in almost all surgical sub-specialties. The successful application of laparoscopy to solid-organ surgery, such as hepatic resection, represents a potential hurdle and liver surgeons have, until relatively recently, been wary of embracing the laparoscopic movement. The first laparoscopic liver resection (LLR) for benign disease was reported in 1992 by Gagner,6 and the first report of LLR for malignancy was published soon after that in 1994.7 These early reports demonstrated the feasibility of LLR, and since then an estimated 3000 cases of LLR have been performed worldwide for varying indications including hepatocellular carcinoma (HCC), colorectal cancer metastases (CRCM) and various benign pathologies.8
Nevertheless, there remain a number of concerns regarding LLR. Liver mobilization and parenchymal transection can be difficult laparoscopically, and achieving control of haemorrhage can also present a significant challenge.9,10 Furthermore, there is the theoretical risk of gas embolism during division of the hepatic veins under pneumoperitoneum.11 The arduous learning curve and concerns regarding the ease with which the necessary skills can be disseminated amongst liver surgeons constitute further reservations. These technical considerations aside, there remains uncertainty regarding the adequacy of oncological resection with LLR when compared with conventional open liver resection (OLR) for cancer. To date, no randomized-controlled trials (RCT) have addressed this question, and these issues combined have meant that widespread adoption of LLR has been slow to gain momentum.
A recent Consensus Conference (November 2008, Louisville, Kentucky, USA) aimed to address some of these concerns and attempted to define more clearly the role of LLR in surgical practice.12 The Consensus Statement suggested that an RCT would be valuable, however, there was recognition of the probable difficulties with the required study population size and lengthy study duration. Until a prospective trial is undertaken, outcome comparison between LLR and OLR is entirely contingent upon data from available case–control series. A previously published meta-analysis evaluated short-term outcomes in a small number of studies comparing LLR and OLR,13 but since that time many more studies have been published, meaning that comparison can now also be drawn with regard to long-term outcomes, including cancer-specific recurrence rate and survival. The present study is a meta-analysis updating peri-operative outcomes for benign and malignant conditions, and for the first time, reporting on survival and recurrence rates for malignant pathology with LLR vs. conventional OLR.
Methods
Study selection
Two authors (R.M and A.H.M) independently carried out electronic literature searches using MEDLINE (1950 to August 2009), EMBASE (1980 to August 2009), CINAHL (1982 to August 2009) and the Cochrane library to identify studies comparing LLR and OLR. The following medical subject heading (MeSH) terms and key words were used: ‘laparoscopic liver resection’, ‘laparoscopic hepatic resection’, ‘Laparoscopic hepatectomy’ and ‘laparoscopic vs. open’. The ‘related articles’ function was used to broaden the search and all abstracts, studies and citations retrieved were scanned. Reference lists of all relevant publications were then hand searched for additional studies missed by the search strategy, and this method of cross-referencing was continued until no further relevant publications were identified. The latest date on which a literature search was undertaken was 20th December 2009 (Fig. 1).
Figure 1.
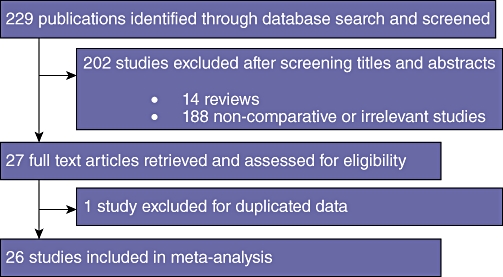
Modified PRISMA flow diagram showing study methodology
Study inclusion criteria and data extraction
A study methodology was carried out in accordance with the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) recommendations for improving the standard of meta-analyses.14 In order to be included in the meta-analysis, studies had to report on peri- and post-operative outcomes when comparing LLR and OLR. Only studies published in the English language were included. Where multiple studies describing the same patient population were identified, the most recent publication was used. In cases of doubt, the authors were contacted for further information to ensure accuracy. Where there was disagreement over eligibility of a study, an additional reviewer (K.C.) assessed the article until a consensus was reached. Two reviewers (R.M. and A.M.) independently extracted the following data from all eligible studies according to a pre-specified protocol: first author, year of publication, study population characteristics, study design, number of subjects operated on, number of laparoscopic cases, number of open cases, matching criteria between laparoscopic and open patient groups, indication for surgery, type of liver resection performed and use of adjuvant chemotherapy. The following outcome parameters were extracted for comparison by meta-analytical assessment: operation duration, estimated blood loss, need for portal triad clamping, portal triad clamping time, length of hospital stay, time to resumption of oral diet, 30-day mortality rate, resection margin status, rate of resection margin <1 cm, rate of positive resection margin, overall and liver-specific complication rate(s), hepatic tumour recurrence (stating where available whether hepatic recurrence was at a site of previous resection, or remote), extra-hepatic tumour recurrence, overall disease-free survival (DFS) and cost.
Assessment of methodological quality and validity
Having met the inclusion criteria, studies were subjected to an assessment of methodological quality and validity by two reviewers (R.M. and A.H.M.) and graded on strength of evidence using the revised grading system of the Scottish Intercollegiate Guidelines Network (SIGN).15 In the event of a discrepancy in assigned grade, studies were re-evaluated until consensus was reached.
Statistical analysis
Statistical analysis was performed using MedCalc for Windows, version 11.3 (MedCalc software, Mariakerke, Belgium). For categorical data, the odds ratio (OR) was calculated using the Mantel–Haenszel method,16 and for continuous data the standardized mean difference (SMD) was calculated with Cohen's d method.17 The results are provided with corresponding 95% confidence intervals (CI) and P-values; results were deemed significant at P < 0.05. Additionally the finding of a SMD >0.5 or <−0.5 was considered indicative of a medium effect size (Cohen's rule of thumb17).
Heterogeneity was examined using the Q statistic18 and quantified with I2. Statistical significance was fixed at 0.10 rather than the conventional level of 0.05 for heterogeneity because of the low power of this test.19 Heterogeneity was rejected if P was greater than 0.10. When there was significant heterogeneity, the DerSimonian–Laird random effect model (REM)20 was used to calculate summary effect size while the fixed effect model (FEM) was used when there was no heterogeneity.
Publication bias was identified visually looking for asymmetry in Begg's funnel plots, that is, plots of effect size against their precision (inverse of standard error) for continuous data and effect size against sample size for categorical data.21 The degree of asymmetry in funnel plots was assessed using Egger's regression analysis.22 Funnel plots were considered symmetrical if the 95% CI of the intercept (α) of Egger's regression crossed zero.
Results
Literature search and description of studies
The predefined search strategy identified 229 potentially relevant publications. After screening titles and abstracts, 202 publications were excluded, comprising 14 review articles and 188 publications that did not meet inclusion criteria or were found to be non-comparative. Full text articles for the remaining 27 studies were retrieved and reviewed in detail. Examination of references from these 27 studies did not reveal any additional relevant publications. Duplicated data were identified in one publication and this was excluded from analysis.23 In total, 26 studies, published between 1998 and 2009 met the a priori determined inclusion criteria and were entered into the meta-analysis. Study characteristics are summarized in Tables 1 and 2.
Table 1.
Summary table of characteristics of studies comparing outcomes after laparoscopic vs. open liver resection (all studies – evidence grade 3)
| Author | Year | Location | Study type | No. | Lap/ Open | Matching criteria | Indication for surgery | Evidence grade |
|---|---|---|---|---|---|---|---|---|
| Rau et al.24 | 1998 | Germany | CM; L(P) O(R) | 34 | 17/17 | a, b, h, k, l | B (L15 O15); M (L2 O2)a | 3 |
| Shimada et al.25 | 2001 | Japan | CM; L(P) O(R) | 55 | 17/38 | a, b, e, f, h(i), k-n, p | M (L17 O38)b | 3 |
| Farges et al.26 | 2002 | France | CM; L(P) O(R) | 42 | 21/21 | a, b, d, i, k, l, n | B (L21 O21) | 3 |
| Mala et al.27 | 2002 | Norway | CM; L(R) O(R) | 27 | 13/14 | a-c, h(ii), j-m | M (L13 O14)a | 3 |
| Laurent et al.28 | 2003 | France | CM; L(P) O(R) | 27 | 13/14 | a, b, e-g, h(i), k, l, n | M (L13 O14)b | 3 |
| Lesurtel et al.29 | 2003 | France | CM; L(P) O(R) | 38 | 18/20 | a-c, e, h, i, l, n | B (L12 O13); M (L6 O7)§ | 3 |
| Morino et al.30 | 2003 | Italy | CM; L(R) O(R) | 60 | 30/30 | a-c, e, k, l, n | B (L16 O5); M (L14 O25)c | 3 |
| Buell et al.31 | 2004 | USA | CM; L(P) O(R) | 117 | 17/100 | l, m | B (L12 O n.a); M (L5 O n.a)d | 3 |
| Kaneko et al.32 | 2005 | Japan | CM; L(R) O(R) | 58 | 30/28 | a, b, f, g, h(i), k, l, n | M (L30 O28)b | 3 |
| Aldrighetti et al.33 | 2008 | Italy | CM; L(P) O(R) | 40 | 20/20 | a, b, e, f, l, n, p | B (L5 O5); M (L15 O15)e | 3 |
| Polignano et al.34 | 2008 | UK | CM; L(P) O(R) | 50 | 25/25 | a-c, e, k, l-n | B (L4 O2); M (L21 O23)f | 3 |
| Troisi et al.35 | 2008 | Belgium | CM; L(R) O(R) | 40 | 20/20 | a-d, i, k, l, n | B (L20 O20) | 3 |
| Lee et al.36 | 2008 | Hong Kong | CM; L(P) O(R) | 50 | 25/25 | a-c, e, g, j-n, p | B (L6 O2); M (L19 O23)g | 3 |
| Abu Hilal et al.37 | 2008 | UK | CM; L(R) O(R) | 44 | 24/20 | a, b, h(i, ii, iii), i, n | B (L7 O5); M (L17 O15)h | 3 |
| Topal et al.38 | 2008 | Belgium | CM; L(P) O(P) | 152 | 76/76 | a, c, e, h(i, ii), i, k, l, n, o | n.a | 3 |
| Cai et al.39 | 2009 | China | CM; L(P) O(R) | 38 | 19/19 | a, b, f-i, k, l, n | B (L18 O18)i; M (L1 O1)j | 3 |
| Sarpel et al.40 | 2009 | USA | CM; L(R) O(R) | 76 | 20/56 | a, b, e, h(i), l | M (L20 O56)b | 3 |
| Tranchart et al.41 | 2009 | France | CM; L(R) O(R) | 84 | 42/42 | a-c, e-g, h(i), l, n | M (L42 O42)b | 3 |
| Tsinberg et al.42 | 2009 | USA | CM; L(25P 6R) O(R) | 74 | 31/43 | a, b, k, l, n | B (L17 O12); M (L14 O31)k | 3 |
| Rowe et al.43 | 2009 | Canada | CM; L(P) O(R) | 30 | 18/12 | a, b, e, g, h(i,ii, iii), i, n | B (L4 O1); M (L14 O11)l | 3 |
| Ito et al.44 | 2009 | USA | CM; L(R) O(R) | 130 | 65/65 | a, b, d, e, h(i, ii, iii), i, l-n | B (L28 O18); M (L37 O47)m | 3 |
| Endo et al.45 | 2009 | Japan | CM; L(R) O(R) | 21 | 10/11 | b, e, g, h(i), l-n | M (L10 O11)b | 3 |
| Dagher et al.46 | 2009 | France | CM; L(P) O(R) | 72 | 22/50 | a-h(i, ii, iii), i, k, l, n, o | B (L7 O14); M (L15 O36)n | 3 |
| Carswell et al.47 | 2009 | UK | CM; L(R) O(R) | 20 | 10/10 | a-c, h, i, n | B (L4 O6); M (L6 O4)o | 3 |
| Castaing et al.48 | 2009 | France | CM; L(R) O(R) | 120 | 60/60 | a, b, h(ii), k-m, o | M (L60 O60)a | 3 |
| Belli et al.49 | 2009 | Italy | CM; L(R) O(R) | 179 | 54/125 | a-c, e, g, h(i), k, m | M (L54 O125)b | 3 |
CM, comparative matched; L, laparoscopic; P, prospective; O, open; R, retrospective; B, benign disease; n.a, not available; M, malignancy; HCC, hepatocellular carcinoma.
Matching criteria: (a) age (b) gender (c) ASA grade (d) BMI (e) liver cirrhosis (f) pre-operative liver function tests (g) Child-Pugh score (h) malignant disease (i) HCC (ii) colorectal metastases (iii) other (i) benign disease (j) previous liver resection (k) location of lesion (l) size of lesion (m) number of lesions (n) type/extent of resection (o) adjuvant chemotherapy (p) histology.
Matched cases of LLR and OLR for HCC, CRCM and breast metastases.
CRCM in all cases.
HCC in all cases.
CRCM-14 (L5 O9), other metastases-9 (L5 O4); HCC-12 (L3 O9), gallbladder cancer-3 (L0 O3), lymphoma-1 (L1 O0).
Metastases-1, lymphoma-1, HCC-3.
Unspecified metastases-14 (L7 O7); HCC-16 (L8 O8).
CRCM-36 (L16 O20), HCC-6 (L4 O2), gallbladder cancer-2 (L1 O1).
HCC-32 (L16 O16), CRCM-8 (L3 O5), intra-hepatic cholangiocarcinoma-1 (L0 O1), other-1 (L0 O1).
HCC-3 (L2 O1), CRCM-29 (L15 O14).
One patient with presumed benign disease (hepatolithiasis) in laparoscopic group was found intra-operatively to have cholangiocarcinoma (resected).
Liver cancer (unspecified)-2 (L1 O1).
HCC-17 (L4 O13), CRCM-25 (L9 O 16), other-3 (L1 O2).
HCC-13 (L9 O4), unspecified metastases-12 (L5 O7).
HCC-17 (L12 O15), unspecified metastases-57 (L25 O32).
HCC-14 (L4 O10), CRCM-33 (L10 O23), other-4 (L1 O3).
CRCM-8 (L6 O2), HCC-1 (L0 O1), Leiomyosarcoma-1 (L0 O1).
Table 2.
Data on type of resection, laparoscopic conversion rates, mortality and adjuvant therapy from included studies
| Author | Procedures performed | Conversion | 30-day mortality | Adjuvant therapy |
|---|---|---|---|---|
| Rau et al.24 | NAR (L2 O2) S (L7 O4) BiS (L8 O10) TriS (L0 O1) | 1/17 (6%) | NA | NA |
| Shimada et al.25 | NAR (L10 O na) LLS (L7 O na) α | NA | NA | NA |
| Farges et al.26 | NAR (L9 O9) S (L4 O4) BiS (L8 O8) | NA | NA | NA |
| Mala et al.27 | NAR (L11 O10) LLS (L2 O4) | NA | NA | L0 O1 |
| Laurent et al.28 | NAR (L3 O4) S (L7 O7) BiS (L3 O3) | 2/13 (15%) | L (0) O (2) | TACE (L2 O2) |
| Lesurtel et al.29 | LLS (L18 O20) | 2/18 (11%) | NA | NA |
| Morino et al.30 | NAR (L5 O5) S (L12 O12) BiS (L13 O13) | NA | NA | nana |
| Buell et al.31 | S (L4 O n.a) BiS (L5 O n.a) TriS (L4 O na) LLS (L7 O na) RL (L1 O na) | NA | L(1) O (n.a) | Na |
| Kaneko et al.32 | PH (L20 O20) LLS (L10 O8) | 1/30 (3%) | NA | nana |
| Aldrighetti et al.33 | LLS (L20 O20) | NA | NA | nana |
| Polignano et al.34 | NAR (L5 O6) S (L4 O5) BiS (L6 O5) LLS (L10 O9) | 2/25 (8%) | NA | NA |
| Troisi et al.35 | NAR (L7 O6) S (L5 O5) BiS (L6 O4) LLS (L1 O3) LHH (L2 O1) | 2/20 (1%) | NA | nana |
| Lee et al.36 | NAR (L16 O15) LLS (L11 O11) | 2/25 (8%) | NA | NA |
| Abu Hilal et al.37 | LLS (L24 O20) | NA | NA | NA |
| Topal et al.38 | Na | 7/76 (9%) | NA | NA |
| Cai et al.39 | LHH (L19 O19) | 2/19 (11%) | NA | NA |
| Sarpel et al.40 | Na | a | b | NA |
| Tranchart et al.41 | NAR (L10 O10) S (L15 O13) BiS (L3 O7) LLS (L9 O7) LHH (L 2 O2) RHH (L3 O3) | 2/42 (5%) | L(1) O(1) | NA |
| Tsinberg et al.42 | S (L23 O28) BiS (L23 O28) | NA | NA | NA |
| Rowe et al.43 | S (L17 O11) BiS (L0 O1) LHH (L1 O0) | 1/18 (6%) | NA | NA |
| Ito et al.44 | NAR (L26 O28) S (L49 O47) BiS (L16 O18) LLS (L11 O9) RPS (L1 O1) | 13/65 (20%) | NA | NA |
| Endo et al.45 | LLS (L10 O11) | NA | NA | NA |
| Dagher et al.46 | RHH (L22 O50) | 2/22 (9%) | L(0) O(1) | NA |
| Carswell et al.47 | LLS (L10 O10) | 1/10 (10%) | NA | NA |
| Castaing et al.48 | NAR (L18 O8) S (L6 O16) BiS (L5 O9) LHH (L1 O2) RHH (L23 O18) CH (L1 O1) LL (L6 O6) | 6/60 (10%) | L(1) O(1) | L34 O34 |
| Belli et al.49 | ≥3 segments (L3 O39)≤2 segments (L51 O86) | 4/54 (7%) | L(1) O(5) | TACE (L5); SC (L1) |
L, lapropscopic; O, open; NAR, non-anatomical resection; S, segmentectomy; BiS, bisegmentectomy; LLS, left lateral sectionectomy; HCC, hepatocellular carcinoma; TACE, transarterial chemo-embolization; TriS, trisegmentectomy; RL, right lobectomy; LHH, left hemihepatectomy; RHH, right hemihepatecfomy; RPS, right posterior sectionectomy; SC, systemic chemotherapy; CH, central hepatectomy; LL, left lobectomy.
Four cases converted to open but not included in study.
One per-operative death but unclear whether in laparoscopic or open group, NA not available.
The combined population from included studies was 1678 (range 20–179). A laparoscopic resection was performed in 717 patients (43%) and an open resection in 961 (57%). The indication for resection was malignant disease in 445 out of 717 cases of LLR (62%) compared with 628 out of 961 cases of OLR (65%). Of the 445 cases of malignant disease in the LLR group, 267 were for HCC, 142 were for CRCM and 36 were for other malignancies. All studies included in the meta-analysis provided comparative data for LLR vs. OLR for benign and malignant disease. One study involved prospective data collection for both groups (evidence level 3).38 In 12 studies, prospective LLR cases were compared with retrospective matched OLR cases24–26,28,29,31,33,34,36,39,43,46 (evidence level 3). Retrospective data collection and matching for both LLR and OLR was conducted in a further 12 studies27,30,32,35,37,40,41,44,45,47–49 (evidence level 3). In one study the laparoscopic cohort consisted of 25 prospective and 6 retrospective cases compared with a retrospective matched OLR cohort42 (evidence level 3).
The cumulative reported conversion-to-open rate for all laparoscopic procedures was 7% (50 out of 717). Outcome data comparing converted cases with purely laparoscopic or open cases were not described in any study included in this meta-analysis.
Thirty-day mortality occurred in 0.6% of patients undergoing LLR (4 out of 717) compared with 1% of patients undergoing OLR (10 out of 961). The reported incidence of gas embolism with LLR was 0.1% (1 out of 717).46 There were no reported cases of port-site recurrence after LLR for malignant disease.
Selection criteria for LLR
Seven studies did not provide any clearly described selection criteria for LLR.24,27,30–32,38,40 The remaining studies described heterogenous selection methods for LLR. Irrespective of the indication for surgery, the two most frequently reported inclusion criteria for LLR were a tumour ≤5 cm in size25,28,29,36,42 for malignant lesions45,49 and tumours involving the left and/or antero-lateral Couinaud segments (that is 2, 3, 4, 5, or 6).25,28,29,35–37,42,45,49 Other stipulated selection criteria were: only benign tumours,26,35 first-time liver resection,25,47,48 only patients with non-cirrhotic liver or Child's A or B stage cirrhosis,39,41,46,47 a tumour involving ≤2 segments,34,44 a tumour ≤8 cm42 for benign lesions,46 a tumour ≤12 cm,33 American Society of Anesthesiologists (ASA) grade ≤2,35 ASA grade ≤3 and41,46 a tumour not associated with major vascular/biliary involvement.41,44,46 Of note, body mass index (BMI) was not outlined as an independent inclusion/exclusion condition in any of the studies.
Meta-analysis results
Operative parameters for benign and malignant disease
Seventeen studies,24,26,28,29,32–35,39–43,45,46,48,49 comprising 1023 patients, provided data on operation duration (Fig. 2). Median operation times for laparoscopic cases were 220 (range 161–362 min) and 204 min (range 128–366 min) for open surgery. Pooled analysis demonstrated that operation duration was significantly longer in LLR compared with OLR (Table 3; SMD = 0.536; 95% CI = 0.120 to 0.952; P = 0.012), although significant between-study heterogeneity was observed (P < 0.001).
Figure 2.
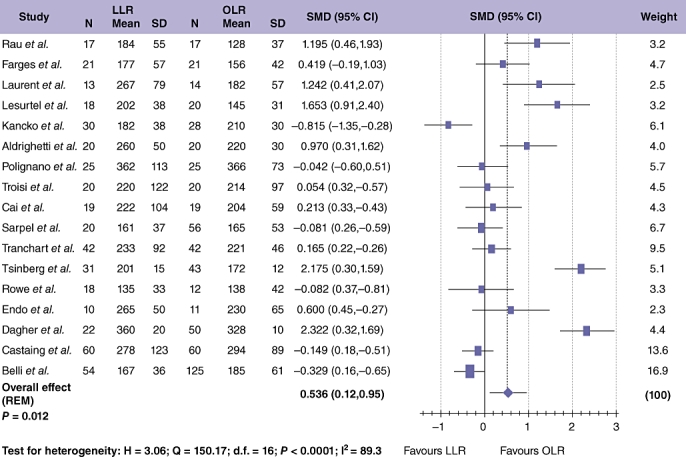
Pooled estimates of procedure duration for laparoscopic liver resection (LLR) vs. open liver resction (OLR). The solid squares denote individual standardized mean differences (SMD) and the horizontal lines represent 95% confidence intervals (CI). The diamonds denote overall pooled SMD
Table 3.
Results of meta-analyses for peri-operative outcome measures after laparoscopic liver resection (LLR) vs. open liver resction (OLR)
| Peri-operative outcomes | Number of studies | Patients | Lap/Open | SMD/OR (95% C.I) | P-value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| I2 | Q | DF | P-value | ||||||
| Operation duration | 17 | 1023 | 440/583 | 0.536 (0.120, 0.952)a | 0.012 | 89.3% | 150.17 | 16 | <0.0001 |
| Estimated blood loss | 15 | 847 | 370/477 | −1.109 (−1.549, −0.669)a | <0.0001 | 90.9% | 153.85 | 14 | <0.0001 |
| Portal triad clamping | 12 | 847 | 372/475 | 0.211 (0.091, 0.491)b | 0.003 | 68.9% | 35.41 | 11 | <0.0001 |
| Portal triad clamping time | 3 | 105 | 51/54 | 0.907 (−0.658, 2.472)a | 0.255 | 92.4% | 26.18 | 2 | <0.0001 |
| Length of stay | 17 | 1009 | 399/610 | −1.109 (−1.549, −0.669)a | <0.0001 | 89.0% | 144.94 | 16 | <0.0001 |
| Time to resumption of diet | 5 | 245 | 112/133 | −2.651 (−4.532, −0.770)a | 0.006 | 96.7% | 122.94 | 4 | <0.0001 |
| Overall complication rate | 25 | 1561 | 700/861 | 0.452 (0.345, 0.590)b | <0.0001 | 13.8% | 27.83 | 24 | 0.267 |
| Liver specific complication rate | 19 | 1060 | 498/562 | 0.636 (0.422, 0.960)b | 0.012 | 9.0% | 19.78 | 18 | 0.345 |
Lap, laparoscopic; SMD, standardized mean difference; OR, odds ratio; I2, index used to measure the extent of study heterogeneity in meta-analysis; Q, statistic used to assess for study heterogeneity in meta-analysis; DF, degrees of freedom.
SMD with 95% CI.
OR with 95% CI.
Data regarding estimated blood loss were available in 15 studies,24,26,28–30,32–34,39,41–43,45,46,49 with a combined population of 847 patients (Fig. 3). The median blood loss was 320 ml for LLR (range 122–620 ml) and 483 ml for OLR (range 214–895 ml). Meta-analytical assessment of the pooled data showed significantly reduced blood loss with LLR compared with OLR (Table 3; SMD =−1.109; 95% CI =−1.549 to −0.669; P < 0.001), although again significant study heterogeneity was noted.
Figure 3.
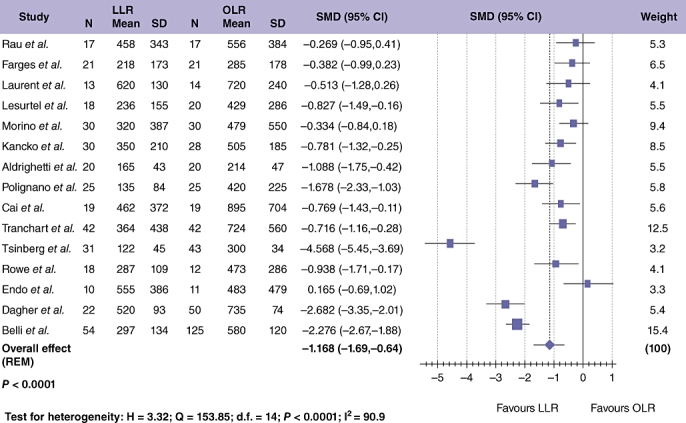
Pooled data of estimated blood loss for laparoscopic liver resection (LLR) vs. open liver resction (OLR)
Twelve studies, including 847 patients, evaluated the need for portal triad clamping.27–30,34,35,41,44,46–49 Meta-analysis demonstrated a significantly lower rate of portal triad clamping in LLR compared with OLR (Table 3; OR = 0.211; 95% CI = 0.091 to 0.491; P = 0.003), and significant heterogeneity was observed between these studies (P < 0.001). Additionally, comparative data on duration of vascular pedicle clamping were available in three of these studies,28,29,35 and meta-analysis found no significant difference in clamping duration (where required) between LLR and OLR (Table 3; SMD = 0.907; 95% CI =−0.658 to 2.472; P = 0.255). Sensitivity analyses were conducted to exclude studies with large effect sizes and wide 95% CI; however, it was not possible to eliminate the observed heterogeneity.
Post-operative parameters for benign and malignant disease
Data regarding the overall complication rate were available in 25 studies24–30,32–49 which included 1561 patients (Fig. 4). Meta-analysis showed significantly fewer overall complications after LLR compared with OLR (Table 3; OR = 0.452; 95% CI = 0.345 to 0.590; P < 0.001). This finding was not associated with significant study heterogeneity. Liver-specific complications (including all cases of biliary leak/collection, bleeding and post-operative abscess formation) were reported in 19 studies,24–30,32–46,48 including 1060 patients. Meta-analysis demonstrated a significant difference in the incidence of liver-specific complications with LLR compared with OLR (Table 3; OR = 0.636; 95% CI = 0.422 to 0.960; P = 0.012) with no significant between study heterogeneity. Length of stay (LOS) was examined, assessing 1009 patients (Fig. 5).24–26,28–33,35,39,41–43,45,46,49 Median LOS for LLR was 8 days (range 3–20 days), and 10 days with OLR (range 6–32 days). Duration of hospital stay was significantly shorter after LLR compared with OLR (Table 3; OR =−1.109; 95% CI =−1.549 to −0.669; P < 0.001). One study assessed the adjusted odds of LOS ≥ 6 days and found this to be significantly lower in patients undergoing LLR.40 None of the studies described the use of a specific enhanced recovery after the surgery programme for either LLR or OLR. Data on time to resumption of diet after LLR and OLR were compared in 5 studies evaluating 245 patients.26,32,35,42,45 Results favoured LLR for earlier return to oral dietary intake (Table 3; OR =−2.651; 95% CI =−4.532 to −0.770; P = 0.006), with significant between study heterogeneity (P < 0.001). Sensitivity analyses eliminated the observed heterogeneity (by exclusion of one outlying study42) and although this reduced the SMD to −0.995 (95% CI =−1.412 to −0.601), statistical significance was retained (P = 0.005).
Figure 4.
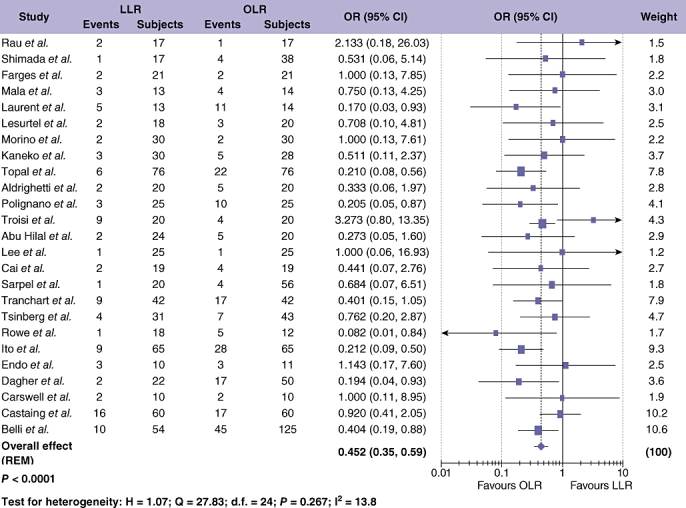
Pooled estimates of overall complication rate for laparoscopic liver resection (LLR) vs. open liver resction (OLR). The solid squares denote individual odds ratio (OR) and the horizontal lines represent 95% confidence intervals (CI). The diamonds denote overall pooled OR
Figure 5.
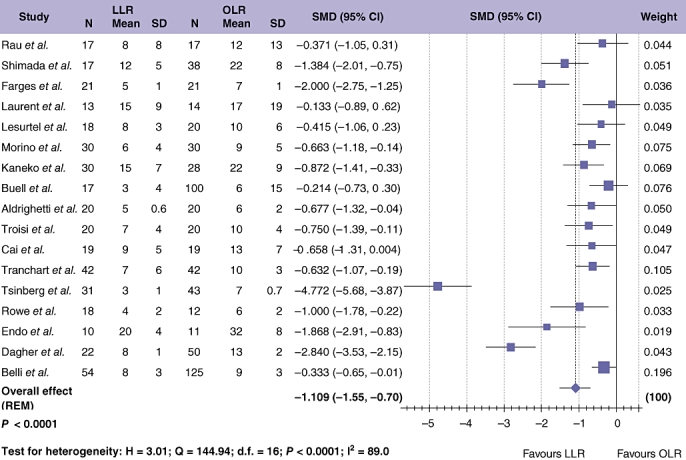
Pooled estimates of length of hospital stay for laparoscopic liver resection (LLR) vs. open liver resction (OLR). The solid squares denote individual standardized mean differences (SMD) and the horizontal lines represent 95% confidence intervals (CI). The diamonds denote overall pooled SMD
Resection margin outcomes for LLR vs. OLR for all cases of malignant disease
Data on the surgical resection margin were available in eight studies, comprising 392 patients.25,28,33,41,42,45,46,48 Meta-analysis of pooled data did not find any significant difference in the size of the resection margin with LLR compared with OLR (Table 4; OR =−0.356; 95% CI =−1.061 to 0.349; P = 0.318). Six studies compared the incidence of a resection margin <1 cm in size with LLR and OLR, evaluating a total of 269 patients.27–31,49 Meta-analysis of this data showed an increased incidence of a resection margin <1 cm in OLR (Table 4; OR = 0.511; 95% CI = 0.293 to 0.893; P = 0.03), with no significant between study heterogeneity. Involvement of the resection margin was evaluated between LLR and OLR in 8 studies on 591 patients.25,27,31,35,41,42,48,49 Resection margin positivity was greater in OLR than LLR with no significant between study heterogeneity (Table 4; OR = 0.524; 95% CI = 0.296 to 0.927; P = 0.026).
Table 4.
Results of meta-analyses for resection margin status, recurrence rate and survival after laparoscopic liver resection (LLR) vs. open liver resction (OLR) for cases of malignant disease
| Pathological and oncological outcomes | Number of studies | Patients | Lap/Open | SMD/OR (95% CI) | P-value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| I2 | Q | DF | P-value | ||||||
| Studies comparing resection margin status for LLR vs. OLR for all types of malignant disease | |||||||||
| Resection margin (mm) | 8 | 392 | 215/177 | −0.356 (−1.061, 0.349)a | 0.318 | 89.9% | 69.41 | 7 | <0.0001 |
| Resection margin <1 cm | 6 | 351 | 138/213 | 0.511 (0.293, 0.893)b | 0.030 | 13.5% | 5.77 | 5 | 0.328 |
| Resection margin positive | 8 | 591 | 250/341 | 0.524 (0.296, 0.927)b | 0.026 | 0.00 | 5.56 | 7 | 0.591 |
| Studies comparing long-term outcome with LLR vs. OLR for HCC only | |||||||||
| Hepatic recurrence rate | 4 | 366 | 129/237 | 0.832 (0.527, 1.314)b | 0.430 | 0.00 | 0.64 | 3 | 0.887 |
| Disease-free survival | 6 | 424 | 166/258 | 0.990 (0.605, 1.621)b | 0.969 | 0.00 | 1.88 | 5 | 0.864 |
| Overall survival | 7 | 500 | 186/314 | 1.500 (1.002, 2.246)b | 0.049 | 0.00 | 3.62 | 6 | 0.728 |
Lap, laparoscopic; SMD, standardized mean difference; OR, odds ratio; I2, index used to measure the extent of study heterogeneity in meta-analysis; Q, statistic used to assess for study heterogeneity in meta-analysis; DF, degrees of freedom.
SMD with 95% CI.
OR with 95% CI.
Recurrence and survival outcomes after LLR vs. OLR for malignant disease
Eleven studies provided oncological data on LLR vs. OLR for CRCM, with a combined number of 212 patients.24,27,29,30,33,35,37,42,46–48 However, these data were not suitable for meta-analytical evaluation as outcomes for CRCM were presented alongside other cancer types with no clear distinction of results based on histology. Only three studies evaluated CRCM only,25,28,47 of which only one provided oncological outcome data.47
For HCC, data on hepatic tumour recurrence were available from four studies comparing LLR and OLR, with a cumulative study population of 366 patients (Fig. 6).28,40,41,49 A distinction between hepatic recurrences that were local to the resection site compared with distant hepatic recurrences was only made in two of these studies. Laurent and co-workers found all hepatic recurrences were remote to the site of surgery.28 Tranchart et al.41 reported tumour recurrence at the site of a previous resection in 2 cases of LLR and one case of OLR out of a total study population of 84. Meta-analysis of pooled data demonstrated no difference in the rate of hepatic tumour recurrence after LLR vs. OLR for HCC (Table 4; OR = 0.832; 95% CI = 0.527 to 1.314; P = 0.430).
Figure 6.
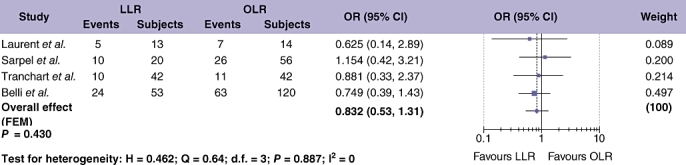
Pooled estimates of hepatic recurrence rate after laparoscopic liver resection (LLR) vs. open liver resction (OLR) for hepatocellular carcinoma (HCC). The solid squares denote individual odds ratios (OR) and the horizontal lines represent 95% confidence intervals (CI). The diamonds denote overall pooled OR
Data on the extra-hepatic recurrence rate in HCC were available from four studies, but were unsuitable for meta-analysis.42,44,48,49 DFS results for HCC were extracted from six studies on 424 patients (Fig. 8),25,28,32,41,45,49 and showed no difference in DFS when comparing LLR and OLR (OR = 0.990; 95% CI = 0.605 to 1.621; P = 0.969), with significant between study heterogeneity (Table 4). Seven studies, assessing 500 patients, provided data on overall survival (OS) after LLR compared with OLR for cases of HCC only (Fig. 7).25,28,32,40,41,45,49 Meta-analysis showed a tendency towards increased OS after LLR compared with OLR (OR = 1.50; 95% CI = 1.002 to 2.246; P = 0.049) with no significant between study heterogeneity (Table 4).
Figure 8.
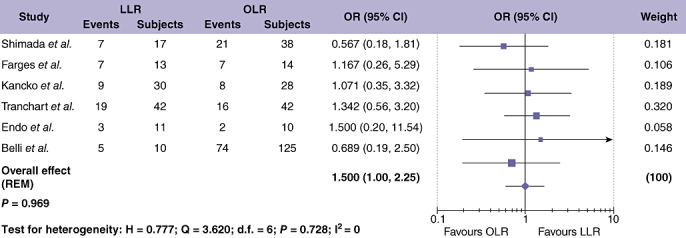
Pooled estimates of the disease-free survival rate after laparoscopic liver resection (LLR) vs. open liver resction (OLR) for hepatocellular carcinoma (HCC). The solid squares denote individual odds ratios (OR) and the horizontal lines represent 95% confidence intervals (CI). The diamonds denote overall pooled OR
Figure 7.
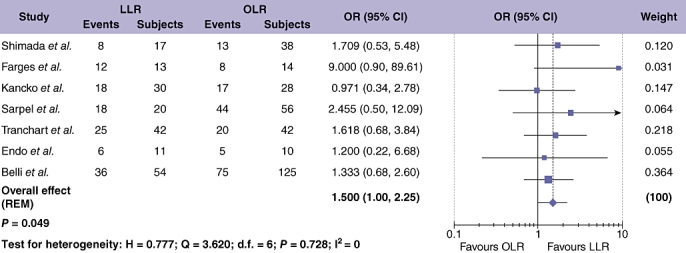
Pooled estimates of overall survival rate after laparoscopic liver resection (LLR) vs. open liver resction (OLR) for hepatocellular carcinoma (HCC). The solid squares denote individual odds ratios (OR) and the horizontal lines represent 95% confidence intervals (CI). The diamonds denote overall pooled OR
Cost outcomes following LLR versus OLR
Data on cost outcomes were limited to two studies and were not suitable for meta-analysis.34,42 Polignano et al. reported increased disposable instrument costs with LLR compared with OLR. However, these expenses were offset by reduced high dependency unit (HDU) and ward stay costs, meaning that total costs were significantly lower with LLR.34 Similarly Tsinberg et al. also reported significantly reduced hospital and overall costs with LLR compared with OLR.42
Discussion
Minimally invasive surgery (MIS) has revolutionized surgical practice over the past 30 years. Advances in both technique and technology have allowed the laparoscopic approach to be applied to increasingly complex surgical procedures. LLR has been slow to gain acceptance, but is now gradually becoming an established part of international practice. Widespread implementation has been hindered by a number of factors. Perceived hazards include gas embolism, port-site tumour recurrence and impaired ability to control bleeding. Furthermore, LLR is technically challenging with a long learning curve, hence dissemination of skills and training has been difficult. Finally, making a strong case for routine LLR for liver tumours has been difficult as long-term data has been lacking, and the adequacy of oncological resection compared with OLR has been questioned.
To date, close to 3000 cases of LLR have been reported in the literature,8 although high-quality evidence is scarce. No randomized trials have been undertaken comparing LLR with OLR, and so the assumed benefits of a laparoscopic approach remain unproven. In 2007, Simillis et al. performed a meta-analysis of case-matched studies up to 2005 examining short-term outcomes with LLR compared with OLR.13 Although limited to only eight studies, this meta-analysis demonstrated favourable short-term outcomes with LLR. No long-term data were evaluated however. Since then, a further 18 studies have been published comparing LLR with OLR, and intermediate and long-term data are now available for analysis. The present study provides an up-to-date summary of the evidence comparing outcomes after LLR and OLR. In particular, the study is the first to provide meta-analytical evaluation of pathological, and in the case of HCC, oncological outcomes achieved with LLR and OLR.
Twenty-six studies met predefined inclusion criteria and were entered into the meta-analytical process, providing a total of 1678 liver resections. In all, 717 (43%) procedures were attempted laparoscopically with a conversion rate of 7%. With respect to peri-operative outcomes, LLR takes significantly longer to perform compared with conventional open surgery. In keeping with trends observed in other sub-specialties, it is likely that as experience with LLR increases, procedure duration will shorten. Indeed it is even conceivable that in the future LLR may take less time than OLR, as the latter involves larger, more time-consuming incisions. It is worth noting that contrary to our findings, Simillis et al. found no increase in operating time with LLR.13 One possible explanation is that more difficult laparoscopic procedures are now being attempted, accounting for increased procedure duration. This suggestion is supported by the fact that the studies evaluated by Simillis et al. included only one laparoscopic right hepatectomy (0.6%), compared with 49 laparoscopic right hepatectomies included in the present analysis (7%). On a cautionary note, the validity of LOS as a comparative outcome measure is frequently questioned, as it can be influenced by factors often entirely disconnected from the surgical intervention. In this respect, the authors acknowledge that data on discharge criteria would be a useful addition to the data presented here, although these data were found to be consistently lacking from the included studies.
The present study also finds superior short-term outcomes including reduced blood loss, fewer overall complications, fewer liver-specific complications, shortened LOS and earlier resumption of oral intake after LLR compared with OLR. This is consistent with data on other laparoscopic abdominal procedures,5 and more recent work conducted by our group adds further weight to this data.50
The issue of haemorrhage warrants particular attention as intra-operative bleeding during liver parenchymal transection is of great concern, with a reported incidence of 7% in the literature.51 Early studies demonstrated that LLR results in reduced blood loss after minor liver resections30 and left lateral sectionectomies.29 However, it has been unclear if this finding would also apply to more complex resections. Dagher et al. recently reported on outcomes in closely matched patients undergoing right hepatectomy. In their study of 72 patients, they found blood loss to be reduced with LLR compared with OLR (519 ± 93 ml and 735 ± 74 ml, respectively; P = 0.038).46 Although few surgeons would counsel against conversion in the face of major haemorrhage, the increasingly sophisticated array of laparoscopic equipment available for the control of bleeding may be supporting more complex surgery, a view reiterated in a recent multi-national consensus conference examining the role of LLR.12
The present study provides the first pooled analysis of oncological outcomes in LLR, and a total of 1073 cancers were identified in the literature. LLR can achieve equivalent resection margins compared with OLR. In fact, LLR was associated with a lower incidence of margin involvement and a margin <1 cm. A possible explanation for this finding is that more challenging oncological resections with concerns regarding margin positivity and proximity of major vascular and/or biliary structures are still performed using an open approach. Consistent with this, selection criteria for LLR, where stated, included smaller and anatomically easier lesions, with no re-operative cases, and although matching criteria were frequently adopted in the studies examined (Table 1), not all reports examined here matched for tumour size and type of resection.
In relation to survival and recurrence rates, CRCM and HCC should be considered separately. The publications included in the present study incorporate 212 resections for CRCM; however, these data were not suitable for meta-analysis. In the currently available literature, data on LLR for CRCM are therefore largely reliant on data from non-comparative studies. Nguyen et al. reported oncological outcomes from a large multi-institutional cohort of patients undergoing LLR for CRCM and found that resection margin status and overall 5-year survival were comparable with data from contemporary open series.8 Well-conducted randomized studies are necessary to specifically address this question.
With respect to HCC, relatively more data are available and we identified seven studies reporting comparative oncological outcome data for LLR and OLR.25,28,32,40,41,45,49 Meta-analysis of these data indicates an equivalent hepatic recurrence rate with laparoscopic and open resection. A significant trend towards improved OS after LLR was noted (P = 0.049), although no significant difference was found for DFS. This parity would seem logical, and yet there are emerging data, to suggest that MIS may be oncologically superior to conventional open surgery; experimental studies have shown that open surgery results in increased systemic levels of circulatory cytokines and vascular endothelial growth factor (VEGF) compared with laparoscopy; these pro-inflammatory biomarkers have been implicated in tumour growth, survival and proliferation.52 In addition, enhanced recovery and reduced post-operative complications after laparoscopic surgery may facilitate early institution of adjuvant therapy, which may also improve cancer outcomes.
Special mention is warranted in the case of cirrhotic patients undergoing OLR for HCC, where post-operative ascites and hepatic decompensation can pose significant difficulties. As the abdominal wall is relatively spared from injury in LLR, this may reduce any loss of collateral venous circulation when compared with open surgical incisions, and potentially limit post-operative rises in portal pressure. In support of this, some studies noted a significant increase in post-operative cirrhotic decompensation with OLR compared with LLR (26.1% vs. 7.1%, respectively; P = 0.03).23,28,41 Similarly, in the present study, we found the rate of overall and liver specific complications to be lower with LLR. Further studies are necessary to evaluate this finding in more detail.
LLR may also be advantageous in cases of HCC complicating established chronic liver disease. Liver transplantation remains the main strategy in these cases; however, this is frequently preceded by liver resection or radiofrequency ablation for disease control, given the shortage of donor livers. Recent reports have suggested that liver transplantation after a previous laparotomy is technically more difficult and associated with significant blood loss.53 Recently, Laurent and colleagues evaluated procedure duration and blood loss with liver transplantation after a previous LLR or OLR.54 Patients who underwent a LLR as the first procedure had shorter operating times and reduced blood loss, suggesting that LLR may facilitate the transplant procedure.
The present study is subject to a number of limitations. The studies evaluated in this meta-analysis are non-randomized comparative studies and not the highest-quality evidence. Nevertheless, it remains unclear if meta-analyses of non-randomized studies systematically over-estimate effect sizes compared with meta-analyses of randomized trials,55 and in light of the scarcity of high-quality evidence in this field, data from the meta-analysis of non-randomized studies can be informative. A further criticism is that most studies included in the present analysis were conducted at large tertiary centres with potentially significant differences in case mix, protocol and surgical expertise. The low rate of conversion to open surgery, and low peri-operative mortality rates in the laparoscopic group also suggest that these cases were performed by experienced surgeons with advanced laparoscopic skills, who have already ascended their learning curves, thereby limiting generalizability. Additionally, the degree of statistical heterogeneity between the meta-analysed publications cautions against over-interpretation of our results. While the a priori decision to utilize a random effects model, combined with the use of sensitivity analyses, helped to diminish the effect of the observed heterogeneity, it is unlikely to have abolished it entirely. The authors acknowledge that this meta-analysis includes a highly diverse collection of procedures, ranging from non-anatomical resection to right hepatectomy, performed for equally varied indications including benign disease, CRCM and HCC. Although this is undoubtedly a limitation, independent data comparing LLR and OLR for specific pathologies or procedures are scarce at present. In the future, it will be possible to eliminate this weakness as more focused studies are conducted. A meta-analysis comparing left lateral sectionectomy (LLS) by laparoscopic and open means would be particularly useful as laparoscopic LLS is increasingly considered the approach of choice by experienced liver surgeons.
In conclusion, the present study indicates that in appropriately selected cases, LLR results in largely superior peri-operative outcomes. Analysis of cancer-related outcomes for HCC also suggests seemingly equivalent oncological results. These findings are in keeping with evidence that has accumulated over the past decade from large-scale multi-centre randomized studies on the use of laparoscopic colorectal surgery for benign and malignant pathology.5,56,57 While the challenge of designing and conducting appropriate clinical trials for laparoscopic liver resection remains, the available evidence as summed in the present report suggests that skilled liver surgeons should not be wary of embracing the laparoscopic approach.
Conflicts of interest
None declared.
References
- 1.Keus F, de Jong JAF, Gooszen HG, van Laarhoven CJHM. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;(18) doi: 10.1002/14651858.CD006231. CD006231. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J, Boddy A, Rhodes M. Choice of approach for appendicectomy: a meta-analysis of open versus laparoscopic appendicectomy. Surg Laparosc Endosc Percutan Tech. 2007;17:245–255. doi: 10.1097/SLE.0b013e318058a117. [DOI] [PubMed] [Google Scholar]
- 3.Winslow ER, Brunt LM. Perioperative outcomes of laparoscopic versus open splenectomy: a meta-analysis with an emphasis on complications. Surgery. 2003;134:647–653. doi: 10.1016/s0039-6060(03)00312-x. discussion 654–655. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson GG, Watson DI, Britten-Jones R, Mitchell PC, Anvari M. Laparoscopic Nissen fundoplication. Ann Surg. 1994;220:137–145. doi: 10.1097/00000658-199408000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, et al. UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 6.Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc. 1992;6:97–98. [Google Scholar]
- 7.Wayand W, Woisetschlager R. Laparoscopic resection of liver metastasis. Chirurg. 1993;64:195–197. [PubMed] [Google Scholar]
- 8.Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 9.Dagher I, O'Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250:856–860. doi: 10.1097/SLA.0b013e3181bcaf46. [DOI] [PubMed] [Google Scholar]
- 10.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 11.Schmandra TC, Mierdl S, Bauer H, Gutt C, Hanisch E. Transoesophageal echocardiography shows high risk of gas embolism during laparoscopic hepatic resection under carbon dioxide pneumoperitoneum. Br J Surg. 2002;89:870–876. doi: 10.1046/j.1365-2168.2002.02123.x. [DOI] [PubMed] [Google Scholar]
- 12.Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 13.Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms – a meta-analysis. Surgery. 2007;141:203–211. doi: 10.1016/j.surg.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334–336. doi: 10.1136/bmj.323.7308.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. UK: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 17.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 18.Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edn. London: BMJ Books; 2001. pp. 40–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psychol Methods. 2001;6:203–217. [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belli G, Fantini C, D'Agostino A, Cioffi L, Langella S, Russolillo N, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc. 2007;21:2004–2011. doi: 10.1007/s00464-007-9503-6. [DOI] [PubMed] [Google Scholar]
- 24.Rau HG, Buttler E, Meyer G, Schardey HM, Schildberg FW. Laparoscopic liver resection compared with conventional partial hepatectomy – a prospective analysis. Hepatogastroenterology. 1998;45:2333–2338. [PubMed] [Google Scholar]
- 25.Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541–544. doi: 10.1007/s004640080099. [DOI] [PubMed] [Google Scholar]
- 26.Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg. 2002;9:242–248. doi: 10.1007/s005340200026. [DOI] [PubMed] [Google Scholar]
- 27.Mala T, Edwin B, Gladhaug I, Fosse E, Soreide O, Bergan A, et al. A comparative study of the short-term outcome following open and laparoscopic liver resection of colorectal metastases. Surg Endosc. 2002;16:1059–1063. doi: 10.1007/s00464-001-9176-5. [DOI] [PubMed] [Google Scholar]
- 28.Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763–769. doi: 10.1001/archsurg.138.7.763. [DOI] [PubMed] [Google Scholar]
- 29.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236–242. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 30.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic versus open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914–1918. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 31.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804–811. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, et al. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–194. doi: 10.1016/j.amjsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Aldrighetti L, Pulitanò C, Catena M, Arru M, Guzzetti E, Casati M, et al. A prospective evaluation of laparoscopic versus open left lateral hepatic sectionectomy. J Gastrointest Surg. 2008;12:457–462. doi: 10.1007/s11605-007-0244-6. [DOI] [PubMed] [Google Scholar]
- 34.Polignano FM, Quyn AJ, de Figueiredo RS, Henderson NA, Kulli C, Tait IS. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc. 2008;22:2564–2570. doi: 10.1007/s00464-008-0110-y. [DOI] [PubMed] [Google Scholar]
- 35.Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, et al. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. 2008;22:38–44. doi: 10.1007/s00464-007-9527-y. [DOI] [PubMed] [Google Scholar]
- 36.Lee KF, Cheung YS, Chong CN, Tsang YY, Ng WW, Ling E, et al. Laparoscopic versus open hepatectomy for liver tumours: a case control study. Hong Kong Med J. 2007;13:442–448. [PubMed] [Google Scholar]
- 37.Abu Hilal M, McPhail MJ, Zeidan B, Zeidan S, Hallam MJ, Armstrong T, et al. Laparoscopic versus open left lateral hepatic sectionectomy: a comparative study. Eur J Surg Oncol. 2008;34:1285–1288. doi: 10.1016/j.ejso.2008.01.018. Epub 2008 Mar 7. [DOI] [PubMed] [Google Scholar]
- 38.Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc. 2008;22:2208–2213. doi: 10.1007/s00464-008-0023-9. [DOI] [PubMed] [Google Scholar]
- 39.Cai XJ, Wang YF, Liang YL, Yu H, Liang X. Laparoscopic left hemihepatectomy: a safety and feasibility study of 19 cases. Surg Endosc. 2009;23:2556–2562. doi: 10.1007/s00464-009-0454-y. [DOI] [PubMed] [Google Scholar]
- 40.Sarpel U, Hefti MM, Wisnievsky JP, Roayaie S, Schwartz ME, Labow DM. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol. 2009;16:1572–1577. doi: 10.1245/s10434-009-0414-8. [DOI] [PubMed] [Google Scholar]
- 41.Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170–1176. doi: 10.1007/s00464-009-0745-3. [DOI] [PubMed] [Google Scholar]
- 42.Tsinberg M, Tellioglu G, Simpfendorfer CH, Walsh RM, Vogt D, Fung J, et al. Comparison of laparoscopic versus open liver tumor resection: a case-controlled study. Surg Endosc. 2009;23:847–853. doi: 10.1007/s00464-008-0262-9. [DOI] [PubMed] [Google Scholar]
- 43.Rowe AJ, Meneghetti AT, Schumacher PA, Buczkowski AK, Scudamore CH, Panton ON, et al. Perioperative analysis of laparoscopic versus open liver resection. Surg Endosc. 2009;23:1198–1203. doi: 10.1007/s00464-009-0372-z. [DOI] [PubMed] [Google Scholar]
- 44.Ito K, Ito H, Are C, Allen PJ, Fong Y, DeMatteo RP, et al. Laparoscopic versus open liver resection: a matched-pair case control study. J Gastrointest Surg. 2009;13:2276–2283. doi: 10.1007/s11605-009-0993-5. [DOI] [PubMed] [Google Scholar]
- 45.Endo Y, Ohta M, Sasaki A, Kai S, Eguchi H, Iwaki K, et al. A comparative study of the long-term outcomes after laparoscopy-assisted and open left lateral hepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2009;19:171–174. doi: 10.1097/SLE.0b013e3181bc4091. [DOI] [PubMed] [Google Scholar]
- 46.Dagher I, Di Giuro G, Dubrez J, Lainas P, Smadja C, Franco D. Laparoscopic versus open right hepatectomy: a comparative study. Am J Surg. 2009;198:173–177. doi: 10.1016/j.amjsurg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Carswell KA, Sagias FG, Murgatroyd B, Rela M, Heaton N, Patel AG. Laparoscopic versus open left lateral segmentectomy. BMC Surg. 2009;9:14. doi: 10.1186/1471-2482-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- 49.Belli G, Limongelli P, Fantini C, D'Agostino A, Cioffi L, Belli A, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041–1048. doi: 10.1002/bjs.6680. [DOI] [PubMed] [Google Scholar]
- 50.Abu Hilal M, Underwood T, Zuccaro M, Primrose J, Pearce N. Short- and medium-term results of totally laparoscopic resection for colorectal liver metastases. Br J Surg. 2010;97:927–933. doi: 10.1002/bjs.7034. [DOI] [PubMed] [Google Scholar]
- 51.Chen HY, Juan CC, Ker CG. Laparoscopic liver surgery for patients with hepatocellular carcinoma. Ann Surg Oncol. 2008;15:800–806. doi: 10.1245/s10434-007-9749-1. [DOI] [PubMed] [Google Scholar]
- 52.Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411–1419. doi: 10.1007/s00464-003-8275-x. [DOI] [PubMed] [Google Scholar]
- 53.Adam R, Azoulay D. Is primary resection and salvage transplantation for hepatocellular carcinoma a reasonable strategy? Ann Surg. 2005;241:671–672. doi: 10.1097/01.sla.0000159077.23253.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laurent A, Tayar C, Andreoletti M, Lauzet JY, Merle JC, Cherqui D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:310–314. doi: 10.1007/s00534-009-0063-0. [DOI] [PubMed] [Google Scholar]
- 55.MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AM. A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health Technol Assess. 2000;4:1–154. [PubMed] [Google Scholar]
- 56.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;13:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 57.Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]


