Abstract
Background
Plasma interleukin-6 (IL-6) levels increase during liver resection. The source of this IL-6 is hitherto unclear. It has been demonstrated that the hepatosplanchnic area takes up IL-6 but the role of the gut and liver is unknown. The aim of the present study was to investigate the role of the gut and liver in IL-6 homeostasis during liver surgery.
Methods
Before and after partial hepatectomy, IL-6 was measured in blood sampled from the radial artery, and the hepatic and portal vein. Blood flow was measured to assess IL-6 fluxes (flow times AV-differences) across the gut, liver and hepatosplanchnic area.
Results
In 22 patients undergoing liver resection, IL-6 release from the gut after transection was 90.9 (30.1) ng/min (P < 0.001), whereas net IL-6 uptake by the liver equalled 83.4 (41.7) ng/min (P < 0.01). Overall hepatosplanchnic flux was 7.3 (43.5) ng/min after transection and did not differ significantly from zero. Overall hepatosplanchnic flux was 87.8 (41.5) ng/min in the major resection group and −59.8 (67.5) ng/min in the minor resection group (P < 0.05).
Discussion
The gut releases IL-6 and the liver takes up IL-6 before and after liver resection. The loss of IL-6 uptake as a result of a small functional remnant liver could lead to higher IL-6 levels after surgery.
Keywords: liver surgery, interleukin-6, metabolism
Introduction
Major abdominal surgery such as liver resection is followed by an inflammatory response, evidenced by increased systemic levels of pro- and anti-inflammatory cytokines.1–4 Interleukin-6 (IL-6) is one of the archetypical pro-inflammatory cytokines, which contributes to the acute phase response to injury and infection.5–7
Plasma IL-6 levels after liver resection are generally regarded to reflect the magnitude of the systemic inflammatory response and in fact elevated IL-6 levels during and after liver resection have been found repeatedly.1,8–12 Potential triggers for high IL-6 levels are ischaemia–reperfusion injury, hepatocyte injury, intestinal manipulation and demand for liver regeneration.8,9,11,13 Prolonged and excessive release of IL-6 and other circulating cytokines after surgery is associated with systemic inflammatory response syndrome (SIRS) or sepsis.9,10 However, experimental animal studies have shown that the production of IL-6 is particularly important after partial hepatectomy and partial portal vein ligation, as it appears to be crucial in the onset of liver regeneration.13–15 This shows that IL-6 has beneficial effects such as the onset of liver regeneration, but on the other hand high levels of IL-6 are also associated with postoperative sequelae such as SIRS or sepsis.9,10 Therefore a tight regulation of IL-6 levels postoperatively is probably needed.
Two studies performed in man showed that the hepatosplanchnic organs (comprising mainly the portal drained viscera and the liver) are responsible for the uptake of endogenously produced IL-6.16,17 However, the individual role of the gut and liver in determining systemic IL-6 levels was not analysed in these previous studies. It has been hypothesized that IL-6 clearance could be a specific liver function.16 Therefore, liver resection would affect the ability of the liver to take up IL-6 from the blood. Knowledge about the organs involved in IL-6 production and breakdown becomes pivotal not only for understanding the IL-6 response to liver surgery but also in the relation to the role of IL-6 in liver regeneration. The aim of the present study was to investigate which of the hepatosplanchnic organs are responsible for release and uptake of IL-6. In addition, the present study also aimed to investigate IL-6 handling after major vs. minor liver resection.
Methods
Patients
Patients scheduled for a hepatectomy for primary or secondary malignant tumours in otherwise healthy livers at Maastricht University Medical Centre were eligible for inclusion. The study was approved by the medical ethical committee of the Maastricht University Medical Centre+ and conducted in accordance with the revised version of the Declaration of Helsinki (October 2008, Seoul). All patients gave written informed consent. Patients were admitted to the hospital 1 day preoperatively and routine blood tests were performed. Preoperatively, all patients had radial artery and central venous catheters inserted to monitor arterial and central venous blood pressure as part of the standard anaesthetic care.
Surgical procedures
Liver resection was performed as detailed elsewhere.18 A laparotomy was performed by bilateral subcostal incision, followed by intra-operative ultrasonographic assessment of the liver. Once resectability had been confirmed, mobilization of the liver was performed to prepare for hepatic parenchymal transection, which was undertaken using a Cavitron Ultrasonic Surgical Aspirator (Valleylab, Tyco healthcare, Boulder, CO, USA). Argon beam coagulation (Force GSU System; Valleylab, Boulder, CO, USA), clips and sutures were used for haemostasis. If necessary hepatic inflow occlusion was applied by tightening a rubber tube around the entire hepatoduodenal ligament (total Pringle maneuver) or selectively around the left or right portal pedicle using an extra Glissonian approach as considered appropriate for the intended resection.19 Cycles of 15- to 30-min occlusion alternated with 5-min reperfusion were applied. The choice of whether a Pringle manoeuvre was used or not as well as the type of inflow occlusion was made according to the surgeon's preference. Immediately after liver resection weights of resection specimens were recorded in the operating theatre.
Blood sampling and processing
Baseline samples were obtained from the arterial line pre-operatively after induction of anaesthesia. Then, samples were obtained from the arterial line at predefined time points: after mobilization of the liver, before and after transection of the liver and on the first postoperative day (POD). Finally, on the second and third postoperative day blood was drawn by venepuncture (the arterial line was routinely removed at day one). Blood was also drawn from the portal and hepatic vein (from the side of the liver with no tumour) before and after liver transection, to calculate arterio-venous (AV) differences of IL-6 across the gut, liver and hepatosplanchnic area (gut + liver). Blood samples were collected in pre-chilled ethylenediaminetetraacetic acid (EDTA)-containing vacuum tubes (BD vacutainer, Becton Dickinson Diagnostics, Aalst, Belgium) and kept on ice. Haematocrit was determined using microcapillaries. Blood was centrifuged in a pre-chilled centrifuge at 4°C at 2750 g for 15 min. Plasma was immediately stored at −80°C until analysis. IL-6 levels were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) (Hycult Biotechnology, Uden, the Netherlands). The lower limit of detection for IL-6 was 16 ng/l.
Assessment of IL-6 flux across the gut and liver
Net organ fluxes (flow × arterial – venous concentration difference) were calculated across the gut, the liver and the hepatosplanchnic area. In order to calculate organ fluxes, plasma flows were calculated by correcting blood flow for haematocrit [plasma flow = blood flow × (1– haematocrit)]. Mean plasma flow in the portal vein and hepatic artery was obtained in a separate similar series of patients by our group in the recent past.20 These patients were comparable with patients in the present group with respect to baseline characteristics and surgical procedures.
Fluxes were calculated as Fgut= portal plasma flow × ([PV]-[A]), Fsplanchnic= splanchnic plasma flow × ([HV]–[A]), Fliver= Fsplanchnic−Fgut. Hepatosplanchnic plasma flow was calculated as portal flow plus hepatic artery flow. In these equations [PV], [A] and [HV] indicate portal venous, radial artery and hepatic venous plasma concentrations, respectively, and Fsplanchnic, Fliver, Fgut denote splanchnic flux, liver flux and gut flux, respectively. Positive fluxes indicate release, whereas negative fluxes indicate uptake.
Relation between remnant liver volume and systemic IL-6 level
Total liver volume and tumour volume were determined by CT-volumetry according to a previously described method.21 In order to calculate the volume of the resection specimen the actual, measured resection weight was multiplied by a factor of 1.22.22 This conversion factor accounts for liver density (g/ml) and compensates the unperfused state of the resection specimen. The functional remnant liver volume (RLV) percentage was calculated with the following formula:
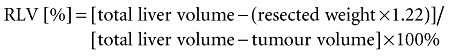 |
Statistics
All data are expressed as mean ± (SEM). Fluxes were tested versus a theoretical mean of zero using Wilcoxon's signed Rank test. The following outcome variables were calculated to analyse serial measurements and compare minor and major resections with respect to IL-6 handling:23 areas under the curve were calculated from baseline to IL-6 peak level and from IL-6 peak level to postoperative day 3, according to the trapezoid method. The slope of the rise in IL-6 levels was calculated by regressing the IL-6 levels at pre-surgery and the peak response (indicated as Sloperise). The slope of the decline in IL-6 levels was calculated by regressing IL-6 levels from peak levels to postoperative day 3 (indicated as Slopedecline).
To compare different subgroups the non-parametric Mann–Whitney U-test was applied. Correlation was calculated with Spearman's test for non-parametric correlations. A multivariable regression analysis was conducted to explore whether there was an independent association of the use of the Pringle manoeuvre with peak IL-6 concentrations. A P-value < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using Prism 4.0 for Windows (Graphpad software, Inc., San Diego, CA, USA).
Results
Patients
Twenty-two patients scheduled for a hepatectomy for primary (n = 2) or secondary malignant liver tumours (n = 20) were included. The median age of the patients was 65 years (30–81). Routine liver tests were within the normal range (Table 1). Patients underwent liver resection because of colorectal liver metastases except for two patients who underwent liver resection for hepatocellular carcinoma and carcinoid tumour, respectively. Ten patients underwent major liver resections (≥3 segments) and 12 a minor liver resection (<3 segments). Time required for mobilisation of the liver was 60 (6) min. Time needed for transection of the liver was 114 (10) min. Plasma flows in the portal vein and hepatic artery were 320 (42) ml/min and 110 (23) ml/min, respectively. Splanchnic plasma flow was calculated to be 430 (47) ml/min.20 In nine patients the liver resection was performed under a total intermittent Pringle manoeuver and in five patients the liver resection was performed under a selective intermittent Pringle manoeuver. The mean period of ischaemia was 18 (2) min and the period of reperfusion was 5 min. In eight patients no Pringle manoeuver was used.
Table 1.
Patient characteristics
| Median (range) | |
|---|---|
| Age (years) | 65 (41–81) |
| Gender (male/female) | M 12 F 10 |
| Height (m) | 1.74 (1.60–1.95) |
| Weight (kg) | 80 (65–106) |
| Body mass index | 26 (23–33) |
| Colorectal carcinoma | n = 20 |
| Hepatocellular carcinoma (HCC) | n = 1 |
| Carcinoid | n = 1 |
| Right hepatectomy | 9 |
| Central hepatectomy | 2 |
| Segmentectomy | 11 |
| AST (IU/L) | 24 (13–46) |
| ALT (IU/L) | 25 (12–55) |
| LDH (IU/L) | 376 (294–529) |
| Gamma-glutamyl transpeptidase (IU/L) | 44 (29–116) |
| Alkaline phosphatase (IU/L) | 91 (59–168) |
| Bilirubin (µM) | 13 (7–37) |
Systemic IL-6 level
Arterial IL-6 levels were below the lower limit of detection pre-operatively. Therefore the cut-off value of the ELISA (16.0 ng/l) was used. Arterial plasma IL-6 increased markedly during and after liver surgery. Before the start of the liver transection arterial IL-6 levels were 73.3 (44.0) ng/l and increased progressively throughout transection to 528.3 (110.3) ng/l directly after transection. Eight hours after the start of surgery, arterial IL-6 had increased further to 1125 (257.0) ng/l (Fig. 1). On postoperative day 1, IL-6 levels had decreased to 529.4 (114.8) ng/l and a further decrease to 236.9 (40.4) ng/l and 66.4 (12.7) ng/l was observed on postoperative days 2 and 3, respectively (Fig. 1).
Figure 1.
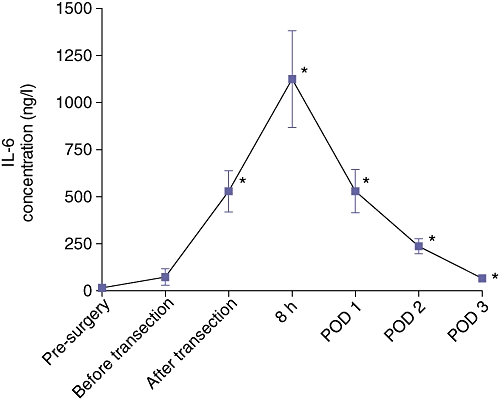
Interleukin-6 (IL-6) levels in patients (n = 22) undergoing liver resection for primary or secondary tumours in an otherwise healthy liver, preoperatively, before and after transection, 8 h after the start surgery and on postoperative day 1, 2 and 3. Data are means ± SEM. *A aignificant difference (P < 0.05) from the baseline sample. POD, postoperative day
IL-6 organ fluxes
IL-6 release from the gut (Fgut) before transection was 10.5 (7.8) ng/min (P < 0.01), whereas net IL-6 uptake by the liver (Fliver) equalled 12.0 (8.4) ng/min (P < 0.01). Overall hepatosplanchnic flux (Fsplanchnic) was −1.6 (1.2) ng/min before transection and did not differ significantly from zero.
IL-6 release from the gut (Fgut) after transection was 90.9 (30.1) ng/min (P < 0.001), whereas net IL-6 uptake by the liver (Fliver) equalled 83.4 (41.7) ng/min (P < 0.01). Overall hepatosplanchnic flux (Fsplanchnic) was 7.3 (43.5) ng/min after transection and did not differ significantly from zero (Fig. 2a–b).
Figure 2.
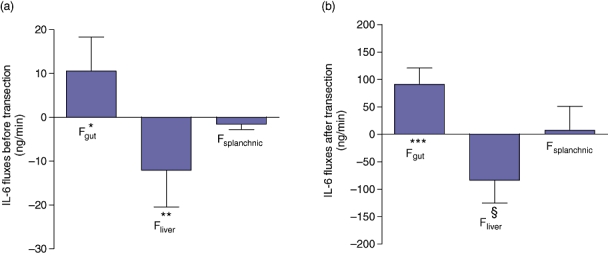
Organ fluxes of interleukin-6 (IL-6) before (a) and after (b) transection (n = 22). The gut releases IL-6 and the liver takes up IL-6. The hepatosplanchnic area flux is close to zero. Data are means ± SEM (*P < 0.01, **P < 0.01, ***P < 0.001, §P < 0.01)
Effects of major versus minor liver resection on IL-6 handling
Patients who underwent major liver resection had significantly greater peak systemic IL-6 levels after resection than those undergoing minor liver resection. The area under the curve for the IL-6 response from baseline to IL-6 peak level and from IL-6 peak level to postoperative day 3 was significantly greater in the major than in the minor liver resection group. Sloperise was also significantly higher in the major than in the minor liver resection group, whereas Slopedecline did not differ significantly (Table 2, Figs 3 and 4a–c).
Table 2.
Interleukin-6 (IL-6) plasma differences between major and minor resection
| Minor liver resection | Major liver resection | P between group | |||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| IL-6 Peakng/l | 707.7 | 146.9 | 1665.0 | 434.1 | 0.04 |
| IL-6 AUC presurgery to peak ng/l*hour | 3.2 *103 | 1.1*103 | 7.1*103 | 1.7*103 | 0.04 |
| IL-6 AUC peak to POD 3ng/l*hour | 15.4 *103 | 2.7*103 | 32.4*103 | 8.0*103 | 0.03 |
| IL-6 Slope riseng/l/hour | 62.5 | 12.8 | 203.4 | 59.0 | 0.02 |
| IL-6 Slope declineng/l/hour | −10.6 | 3.0 | −19.2 | 6.6 | ns |
Figure 3.
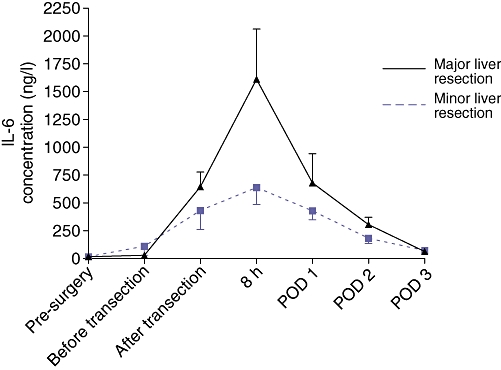
Interleukin-6 (IL-6) levels for minor (n = 10) vs. major (n = 12) liver resections. POD, postoperative day
Figure 4.
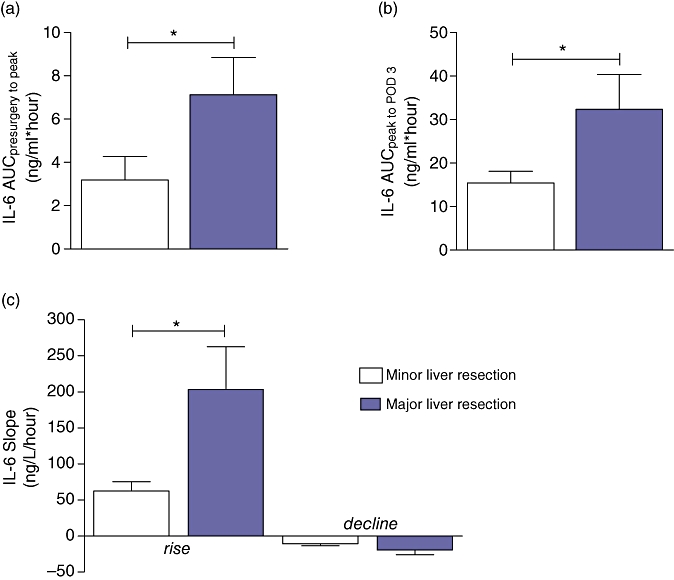
(a) Area under curvepresurgery to peak, (b) area under curvepeak to POD3 and (c) sloperise in patients who underwent major vs. minor liver resection. Data are means ± SEM. *Significant difference (P < 0.05)
On multivariable analysis, the use of the Pringle manoeuver was also an independent predictor of high peak IL-6 levels (P = 0.04). The extent of the operation (minor versus major resection), when corrected for the Pringle manoeuver, was also independently and significantly related (major versus minor, odds ratio = 1020, confidence interval 233–1808, P = 0.01) with peak IL-6.
IL-6 release from the gut (Fgut) after transection [151.2 (62.0) ng/min] was higher after major compared with minor liver resection [40.7 (9.4) ng/min] (P = 0.006). IL-6 uptake by the liver (Fliver) was 63.4 (30.9) ng/min in the major resection group and 100.5 (73.7) ng/min in the minor liver resection group, which was not significantly different (P = 0.87). Overall hepatosplanchnic flux (Fsplanchnic) was 87.8 (41.5) ng/min in the major resection group and −59.8 (67.5) ng/min in the minor resection group (P < 0.05) (Fig. 5). This indicates net IL-6 release from the splanchnic area after major resection versus uptake in the minor resection group.
Figure 5.
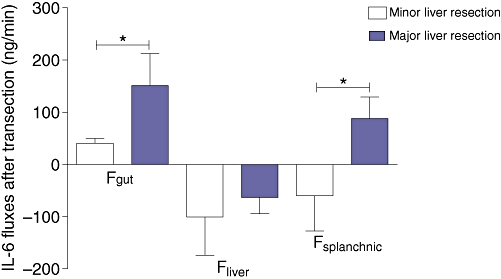
After transection interleukin-6 (IL-6) release from the gut is significantly higher in the major liver resection group. There is a net IL-6 release from the hepatosplanchnic area in the major liver resection group compared with IL-6 uptake in the minor resection group. Data are means ± SEM. *Significant difference (P < 0.05)
Relation between remnant liver volume and systemic IL-6 level
Mean total liver volume in the whole group (n = 22) was 1837 ± 101 ml and mean tumour volume was 23 ± 10 ml. Total functional liver volume (total liver volume – tumour volume) was 1814 ± 104 ml. Resection weight was 522 ± 69 g. After correction using the previously mentioned conversion factor the volume of the resection specimen weight was 637 ± 84 ml. The remnant liver volume was 1199 ± 133 ml. Mean functional remnant liver volume percentage was 65 ± 5%. There was a significant correlation between the IL-6 AUC peak to POD3 and the remnant liver volume (P < 0.05, r = −0.46) (Fig. 6).
Figure 6.
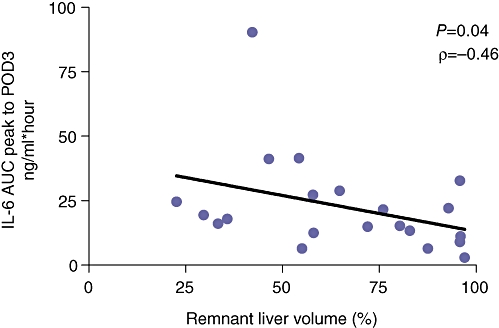
Spearman's correlation analysis of remnant liver volume with interleukin-6 (IL-6) AUCpeak tot POD3
Discussion
The present study investigated the contribution of hepatosplanchnic organs to the release and uptake of IL-6 in patients undergoing liver resection. To the best of our knowledge the present study is the first in humans to demonstrate that the gut releases IL-6 and the liver takes up IL-6 before and after liver transection. The IL-6 flux across the hepatosplanchnic area was not significantly different from zero in the whole group of minor and major liver resections before and after liver resection. This suggests that this region does not in general contribute to systemic elevation of IL-6 levels. In the group undergoing major liver resection a net release of IL-6 from the splanchnic area was observed after liver resection.
Febbraio et al. used a human physical exercise model showing that in the physiological situation the hepatosplanchnic organs took up IL-6 while systemic levels were elevated.16 The authors assumed that the intact human liver was probably responsible for this negative IL-6 flux across the hepatosplanchnic area. However, the previous study did not specify the individual role of the gut and liver in determining systemic IL-6 levels. The present study involved patients undergoing liver surgery allowing direct access to the portal and hepatic vein. Therefore, the role of the liver and gut in IL-6 metabolism could be studied. IL-6 release from the gut increased after liver resection. Conversely, the liver took up IL-6 to the same extent after liver resection and consequently the IL-6 inter-organ balance over the hepatosplanchnic area was unchanged and remained at zero in the whole group.
Comparison of the major and minor liver resection group revealed that the gut released significantly more IL-6 in the major liver resection group after resection. Because liver uptake of IL-6 did not entirely match gut IL-6 release, this led to a net IL-6 release from the hepatosplanchnic area after major hepatectomy. Eventually this caused significantly higher systemic IL-6 concentrations in the major liver resection group. Although these data show that the gut is responsible for significant IL-6 production, it must be assumed, also based on the present data that extra-splanchnic production of IL-6 occurs as well. The difference in IL-6 handling between major and minor liver resections could most likely be a consequence of a smaller functional remnant liver volume diminishing IL-6 uptake capacity in the major liver resection group. In the present study, a significant correlation between the remnant liver volume and area under the curve of IL-6 levels confirmed this association. However, this is only circumstantial evidence for the hypothesis that the IL-6 uptake capacity of the liver is related to the size of the remnant liver after liver resection.
The condition of a liver containing (metastatic) tumours can be different from a normal liver in a physiological condition. Blood was sampled from the non-tumorous side of the liver and the (metastatic) liver tumour was probably not producing IL-6 because IL-6 baseline samples were not above the cutoff value of the ELISA (16 ng/l) in the present patients. In addition, the data in the current paper regarding the splanchnic fluxes of IL-6 were comparable to the data given by Febbraio et al.,16 measured in healthy volunteers, providing circumstantial evidence for the validity of the present data.
On postoperative days 1, 2 and 3, IL-6 levels were higher in the major liver resection group. The area under the curve for this period was significantly different between the two groups. However, the Slope decline was not different between the two groups. This may have been because of the relatively small group size (type II error), but there are two other potential explanations. First, reduced IL-6 levels result from rapidly decreased production from the gut postoperatively. Second, it is known that in humans there is a very rapid increase in liver mass and function during the first 7 days after partial hepatectomy. Rapid restoration of liver mass may compensate any differences in diminished liver function between the major and minor liver resection group. It is highly likely that this will not be the case when postoperative liver failure occurs. The Pringle manoeuvre was also an independent predictor of high peak IL-6 levels. This can probably be explained by the fact that the Pringle manoeuvre leads to a temporary total or partial abrogation of IL-6 liver uptake ultimately leading to higher peak IL-6 levels in the Pringle group compared with the non-Pringle group. In accordance with the results in the present study, Castell et al. showed that intravenous injection of radio-actively labelled IL-6 into rats caused IL-6 to disappear from plasma. This radiolabelled IL-6 was mostly localized on the surface of the parenchymal liver cells,24 indicating liver uptake. Although the nature of this process requires further clarification, an explanation for this process may be that IL-6 is necessary for liver regeneration.14,25 Cressman et al. showed that IL-6 plays a crucial role in the onset of liver regeneration and in promoting hepatocyte proliferation in mice after partial hepatectomy.14 Most likely, specific binding of IL-6 to a membrane bound IL-6 receptor in the hepatocyte or via a soluble IL-6 receptor may play an important role in inducing liver regeneration.26
Although there appears to be an obvious relation between IL-6 and liver regeneration, the absence of IL-6 merely delays restoration of liver mass and IL-6 does not appear to be the only inducer of liver regeneration.14 Blindenbacher et al. concluded that the major role of IL-6 after partial hepatectomy in mice is the induction of an adaptive response ensuring body homeostasis and survival.25 In line with this, administration of IL-6 can actually improve the regenerative response of the liver after acute injury in mice.27 In contrast, hyper-IL-6 (agonistic protein consisting of IL-6 linked the soluble IL-6 receptor) or chronically elevated IL-6 levels are associated with inhibition of liver regeneration after injury.27,28 Studies have shown that IL-6 acts as a pro-survival factor of apoptosis-mediated liver injury and that it is mainly the onset of liver regeneration that plays a role in the inhibition of hepatocyte apoptosis.29 Based on these observations and also based on the data of the present study it could be assumed that it is likely that the liver is not simply clearing IL-6 during liver resection. On the contrary, IL-6 might play an important role intracellularly in hepatocytes, not only to activate signal pathways that induce liver regeneration but also to act as a pro-survival factor of apoptosis-mediated liver injury.
In the present study it was observed that the gut is responsible for IL-6 release during liver surgery. In the major liver resection group the gut released significantly more IL-6 after resection than in the minor resection group. These observations suggest that the gut plays a significant role in the inflammatory response after liver surgery. Little is known about the role of IL-6 in the intestinal response to liver surgery or other gut injury. The gut may release IL-6 as a result of major liver resection compromising the functional integrity of the gut barrier. Wang et al. showed in rats that bacterial translocation from the gut is caused by intestinal injury and increased gut capillary permeability after major hepatectomy.30 On the other hand apoptosis in gastrointestinal epithelium has been associated with liver surgery as well. Jin et al. showed in mice that IL-6 plays a crucial regulatory role in in vivo enterocyte homeostasis.31 IL-6 appears to affect enterocyte apoptotic pathways, resulting in increased enterocyte resistance to apoptosis and oxidative injuries. The exact biological significance of IL-6 as a pro-survival factor in intestinal apoptosis and apoptosis-mediated liver injury after liver resection in humans remains unclear.
In conclusion, in the present study it was observed that the gut releases IL-6 and that the liver takes up IL-6 in humans before and after liver resection. The loss of IL-6 uptake as a result of small remnant functional liver volume could lead to higher IL-6 levels during and after surgery. However, the exact consequences of high IL-6 levels circulating after liver resection are unknown. The liver might take up IL-6 in order to regulate IL-6 levels and prevent excessively elevated systemic IL-6 levels. Equally this uptake may play a role in liver regeneration.
Acknowledgments
S. dello was supported by a Kootstra Talent Fellowship from Maastricht University.
Conflicts of interest
None declared.
References
- 1.Jerin A, Pozar-Lukanovic N, Sojar V, Stanisavljevic D, Paver-Erzen V, Osredkar J. Balance of pro- and anti-inflammatory cytokines in liver surgery. Clin Chem Lab Med. 2003;41:899–903. doi: 10.1515/CCLM.2003.136. [DOI] [PubMed] [Google Scholar]
- 2.Kanaoka Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Inagaki M, et al. Analysis of host response to hepatectomy by simultaneous measurement of cytokines in the portal vein, caval vein and radial artery. J Int Med Res. 2002;30:496–505. doi: 10.1177/147323000203000505. [DOI] [PubMed] [Google Scholar]
- 3.Ueda T, Sakabe T, Oka M, Maeda Y, Nishida M, Murakami F, et al. Levels of interleukin (IL)-6, IL-8, and IL-1 receptor antagonist in the hepatic vein following liver surgery. Hepatogastroenterology. 2000;47:1048–1051. [PubMed] [Google Scholar]
- 4.de Jong KP, von Geusau BA, Rottier CA, Bijzet J, Limburg PC, de Vries EG, et al. Serum response of hepatocyte growth factor, insulin-like growth factor-I, interleukin-6, and acute phase proteins in patients with colorectal liver metastases treated with partial hepatectomy or cryosurgery. J Hepatol. 2001;34:422–427. doi: 10.1016/s0168-8278(00)00030-1. [DOI] [PubMed] [Google Scholar]
- 5.Geiger T, Andus T, Klapproth J, Hirano T, Kishimoto T, Heinrich PC. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988;18:717–721. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- 6.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Marinkovic S, Jahreis GP, Wong GG, Baumann H. IL-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. J Immunol. 1989;142:808–812. [PubMed] [Google Scholar]
- 8.Guidi L, Tricerri A, Costanzo M, Adducci E, Ciarniello M, Errani AR, et al. Interleukin-6 release in the hepatic blood outflow during normothermic liver ischaemia in humans. Dig Liver Dis. 2003;35:409–415. doi: 10.1016/s1590-8658(03)00156-7. [DOI] [PubMed] [Google Scholar]
- 9.Lan AK, Luk HN, Goto S, Chen SM, Eng HL, Chen YS, et al. Stress response to hepatectomy in patients with a healthy or a diseased liver. World J Surg. 2003;27:761–764. doi: 10.1007/s00268-003-6955-2. [DOI] [PubMed] [Google Scholar]
- 10.Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94:767–773. doi: 10.1093/bja/aei143. [DOI] [PubMed] [Google Scholar]
- 11.Badia JM, Ayton LC, Evans TJ, Carpenter AJ, Nawfal G, Kinderman H, et al. Systemic cytokine response to hepatic resections under total vascular exclusion. Eur J Surg. 1998;164:185–190. doi: 10.1080/110241598750004625. [DOI] [PubMed] [Google Scholar]
- 12.van de Poll MC, Derikx JP, Buurman WA, Peters WH, Roelofs HM, Wigmore SJ, et al. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. 2007;31:2033–2038. doi: 10.1007/s00268-007-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavien PA. IL-6, a key cytokine in liver regeneration. Hepatology. 1997;25:1294–1296. doi: 10.1002/hep.510250544. [DOI] [PubMed] [Google Scholar]
- 14.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science (New York, NY) 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 15.Liska V, Treska V, Skalicky T, Mirka H, Kobr J, Sykora R, et al. Cytokines and liver regeneration after partial portal vein ligation in porcine experimental model. Bratisl Lek Listy. 2009;110:447–453. [PubMed] [Google Scholar]
- 16.Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, et al. Hepatosplanchnic clearance of interleukin-6 in humans during exercise. Am J Physiol Endocrinol Metab. 2003;285:E397–E402. doi: 10.1152/ajpendo.00134.2003. [DOI] [PubMed] [Google Scholar]
- 17.Garibotto G, Sofia A, Balbi M, Procopio V, Villaggio B, Tarroni A, et al. Kidney and splanchnic handling of interleukin-6 in humans. Cytokine. 2007;37:51–54. doi: 10.1016/j.cyto.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Dejong C, Garden O. Neoplasms of the liver. In: Majid AA, Kingsnorth A, editors. Advanced Surgical Practice. London: Greenwich medical Media; 2003. pp. 146–156. [Google Scholar]
- 19.Topal B, Aerts R, Penninckx F. Laparoscopic intrahepatic Glissonian approach for right hepatectomy is safe, simple, and reproducible. Surg Endosc. 2007;21:2111. doi: 10.1007/s00464-007-9303-z. [DOI] [PubMed] [Google Scholar]
- 20.van de Poll MC, Siroen MP, van Leeuwen PA, Soeters PB, Melis GC, Boelens PG, et al. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr. 2007;85:167–172. doi: 10.1093/ajcn/85.1.167. [DOI] [PubMed] [Google Scholar]
- 21.Dello SA, van Dam RM, Slangen JJ, van de Poll MC, Bemelmans MH, Greve JW, et al. Liver volumetry plug and play: do it yourself with image J. World J Surg. 2007;31:2215–2221. doi: 10.1007/s00268-007-9197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang S, Lee SG, Kim KH, Park KM, Ahn CS, Moon DB, et al. Correlation of blood-free graft weight and volumetric graft volume by an analysis of blood content in living donor liver grafts. Transplant Proc. 2002;34:3293–3294. doi: 10.1016/s0041-1345(02)03603-5. [DOI] [PubMed] [Google Scholar]
- 23.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castell JV, Geiger T, Gross V, Andus T, Walter E, Hirano T, et al. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988;177:357–361. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]
- 25.Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674–682. doi: 10.1053/jhep.2003.50378. [DOI] [PubMed] [Google Scholar]
- 26.Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol (Berl) 2006;195:173–183. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 27.Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43:474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- 28.Wustefeld T, Rakemann T, Kubicka S, Manns MP, Trautwein C. Hyperstimulation with interleukin 6 inhibits cell cycle progression after hepatectomy in mice. Hepatology. 2000;32:514–522. doi: 10.1053/jhep.2000.16604. [DOI] [PubMed] [Google Scholar]
- 29.Galun E, Axelrod JH. The role of cytokines in liver failure and regeneration: potential new molecular therapies. Biochim Biophys Acta. 2002;1592:345–358. doi: 10.1016/s0167-4889(02)00326-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Parsson H, Soltesz V, Johansson K, Andersson R. Bacterial translocation and intestinal capillary permeability following major liver resection in the rat. J Surg Res. 1995;58:351–358. doi: 10.1006/jsre.1995.1054. [DOI] [PubMed] [Google Scholar]
- 31.Jin X, Zimmers TA, Zhang Z, Peirce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2009;59:149–151. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]


