Abstract
Background
Patients with familial adenomatous polyposis (FAP) develop duodenal and ampullary polyps that may progress to malignancy via the adenoma–carcinoma sequence.
Objective
The aim of this study was to review a large series of FAP patients undergoing pancreaticoduodenectomy for advanced duodenal and ampullary polyposis.
Methods
A retrospective case notes review of all FAP patients undergoing pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis was performed.
Results
Between October 1993 and January 2010, 38 FAP patients underwent pancreaticoduodenectomy for advanced duodenal and ampullary polyps. Complications occurred in 29 patients and perioperative mortality in two. Postoperative histology revealed five patients to have preoperatively undetected cancer (R = 0.518, P < 0.001).
Conclusions
Pancreaticoduodenectomy in FAP is associated with significant morbidity, but low mortality. All patients under consideration for operative intervention require careful preoperative counselling and optimization.
Keywords: familial adenomatous polyposis, adenomatosis, pancreaticduodenectomy
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominant, generalized disorder of tissue growth regulation secondary to mutations of the adenomatous polyposis coli (APC) gene on chromosome 5.1,2 The defining phenotypic feature is the development of multiple colorectal adenomas in the early adolescent period, which usually undergo malignant change by the third or fourth decades of life. However, mortality caused by colorectal cancer in FAP has significantly decreased following the widespread introduction of prophylactic colectomy, and duodenal cancer and desmoid disease are now the most significant causes of death in patients with FAP.3
Familial adenomatous polyposis patients develop multiple adenomatous polyps in the small as well as the large bowel and these can similarly become symptomatic or undergo malignant change.4–7 Small bowel polyps occur almost exclusively within the duodenum and ampulla, although they also occur on ileostomies and within ileoanal pouches.8 The cumulative incidence of duodenal polyps in FAP increases with age to reach 65% at 38 years and 90–95% by 70 years.9,10
Duodenal adenomas can undergo dysplastic change, most often in the second and third parts of the duodenum,11 where prolonged contact with bile acids may lead to mutational changes in the surrounding mucosa.12–14 However, only approximately 5% of all duodenal polyps will undergo malignant change,10 via the adenoma–carcinoma sequence,4–7 and the cumulative incidence of duodenal cancer in FAP is 4.5% at 57 years.9
The Spigelman scoring system takes into account the polyp number, size, histology and degree of dysplasia15 (Table 1) and is applicable to duodenal and periampullary regions.16 Higher scores indicate greater risk for neoplastic transformation: the risk for developing duodenal cancer over 10 years is 2% in Spigelman stages II and III, and 36% in stage IV.17 However, the identification of patients at risk for developing neoplastic disease remains problematic because, although the cumulative incidence of Spigelman stage IV is 52% at the age of 70 years,9 the rate of progression between stages is highly variable and the disease can take 4–11 years to progress from one stage to another.18
Table 1.
Spigelman scoring for duodenal and periampullary polyps in familial adenomatous polyposis15
| Polyps, n | 1–4 | 5–20 | >20 |
| Polyps, size | 1–4 mm | 5–10 mm | >10 mm |
| Histology | Tubular adenoma | Tubulovillous adenoma | Villous adenoma |
| Dysplasia | Mild | Moderate | Severe |
| Points | 1 | 2 | 3 |
Ampullary adenomatosis has been shown to correlate strongly with the degree of duodenal polyp burden and is estimated to occur in approximately 28% of FAP patients.16 Further, the risk for malignant transformation may be higher in patients with a larger periampullary than duodenal polyp burden and the cumulative risk for developing stage IV disease and adenocarcinoma in the periampullary region by 60 years of age has been estimated as 20% and 10%, respectively.19
Early identification of malignant duodenal and ampullary invasion may be insufficient to decrease mortality and many centres therefore offer operative intervention following the diagnosis of benign but advanced disease (Spigelman stage III or IV).17 St Mark's Hospital in London introduced endoscopic surveillance for duodenal and ampullary polyps in 1988 (Table 2); outcomes of the practice of utilizing pancreaticoduodenectomy for the management of advanced duodenal and ampullary adenomatosis are analysed and presented here.
Table 2.
Current follow-up and management protocol of familial adenomatous polyposis patients with duodenal and ampullary polyposis, according to Spigelman stage
| Spigelman stage | Points | Action |
|---|---|---|
| 0–I | 0–4 | Repeat endoscopy at 5 years |
| II | 5–6 | Repeat endoscopy at 3 years |
| III | 7–8 | Repeat endoscopy at 6 months |
| Consider endoscopic therapy or chemoprevention | ||
| IV | 9–12 | Repeat endoscopy at 6 months |
| Computed tomography and endoscopic ultrasound staging | ||
| Consider surgery or endoscopic therapy + chemoprevention | ||
| Cancer | N/A | Surgery |
This protocol was developed by the Polyposis Registry at St Mark's Hospital, in combination with the Department of Hepatopancreaticobiliary Surgery at University College London Hospital
N/A, not applicable
Materials and methods
Endoscopic surveillance
Since the initiation of a surveillance programme in 1988, all FAP patients undergoing follow-up at the Polyposis Registry (St Mark's Hospital, London) are subject to endoscopic surveillance for duodenal and ampullary polyps. Surveillance commences at the age of 25 years (no small bowel polyps requiring treatment at younger ages have been observed) via a side-viewing duodenoscope to optimize views of the ampullary and periampullary region. Duodenal polyp size is measured by comparison with open biopsy forceps and the number of polyps defined as the number of discrete areas of mucosal abnormality. Multiple biopsies are taken for assessment of histology and dysplasia, and completion of Spigelman staging.
Patients are followed according to their Spigelman stage (Table 2) and referred for consideration of surgery when they are diagnosed with Spigelman stage IV disease or with suspected or histologically proven malignancy. Patients with diffuse duodenal or ampullary adenomatosis (>20 polyps; score of 3 for polyp number on Spigelman staging) are also considered for surgery at Spigelman stage III.
Surgical management
All FAP patients in the Polyposis Registry with advanced duodenal or ampullary adenomatosis are referred to a single centre (University College London Hospital [UCLH]) for consideration of pancreaticoduodenectomy (unless a patient has a particular preference for referral elsewhere). All patients undergo preoperative staging via computed tomography (CT) and endoscopic ultrasound (EUS), and further examinations and assessments (including cardiopulmonary exercise [CPEX] testing) are arranged as necessary. All patients undergo pancreaticoduodenectomy with reconstruction via pancreaticogastrostomy (PG) formed by the burial of the pancreatic remnant in the posterior wall of the stomach utilizing two layers of 3–0 polydioxanone sutures (PDS) (Ethicon, Inc., Somerville, NJ, USA), and the insertion of a common bile duct (CBD) stent.
Postoperative complications
Anastomotic leaks were diagnosed via CT or contrast study and postoperative haemorrhage via CT angiogram or clinically. Clinical diagnosis was also used to identify both pancreatic exocrine insufficiency (based on a clinical diagnosis of steatorrhoea, supplemented by faecal elastase tests where necessary) and delayed gastric emptying (based upon symptoms of nausea, vomiting and decreased oral intake, some of which may be attributable to bile reflux); however, such gastrointestinal (GI) diagnoses can be difficult to assess in FAP patients who have undergone prior colectomy.
Patient identification
Familial adenomatous polyposis patients undergoing pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis were retrospectively identified from surgical databases at UCLH, data held at the Polyposis Registry and via contact with general practitioners and alternative operating centres. Following patient identification, a review of computerized and written hospital notes, pathology results and operative notes was undertaken and all data entered into a relational database using Microsoft Office Access 2007 (Microsoft, Redmond, WA, USA).
Statistical analysis
Differences among proportions of categorical variables (i.e. postoperative morbidity and mortality) were compared using Fisher's exact test. Pearson's correlation coefficient model was used to indicate the strength and direction of the relationship between preoperative endoscopic biopsy histology and surgical resection histology. Outcome measures of survival and all-cause mortality rates were analysed using the Kaplan–Meier log-rank test. Two-tailed P-values of <0.05 were considered to indicate statistical significance. Statistical analysis was carried out using spss Version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Demographics
Between October 1993 and January 2010, 46 patients were referred from the Polyposis Registry at St Mark's Hospital for consideration of surgical intervention. Of these, 41 patients were referred to a single centre (UCLH) and five were referred to alternate UK centres and have been excluded from analysis. Of the 41 patients, one underwent polypectomy and cholecystectomy and two were found to be inoperable at laparotomy; of the latter two patients, one had widespread, intra-abdominal, malignant disease and one had extensive intra-abdominal desmoid disease.
Thus, 38 FAP patients underwent pancreaticoduodenectomy for advanced duodenal and ampullary polyps at a single centre, representing approximately 5.0% of all patients undergoing primary colonic surgery for FAP at St Mark's Hospital. The study cohort included 17 male and 21 female FAP patients with a median age of 49 years (range: 32–67 years).
Preoperative staging
Four of the 38 patients were referred with Spigelman stage III disease; all four underwent pancreaticoduodenectomy for diffuse polyps involving the duodenal or ampullary regions. Of the remaining patients, 27 had Spigelman stage IV disease and four had a preoperative diagnosis of malignancy on endoscopic surveillance (one duodenal, three ampullary). In three patients, the preoperative Spigelman stage was not given.
Surgical management
All 38 patients were operated upon at a single centre, undergoing pancreaticoduodenectomy by one of two consultant surgeons. The number of patients undergoing pancreaticoduodenectomy increased over time: 14 of the 38 patients underwent resection during 1993–2001 and 24 underwent resection during 2002–2010 (P = 0.003, one-sample t-test). The median length of stay was 38 days (interquartile range [IQR]: 15–51 days) and the median follow-up was 42 months (IQR: 13–99 months).
Postoperative histology
Postoperative histology revealed five patients to have preoperatively undetected cancer (four duodenal, one ampullary). Two further patients were also stage-upgraded on surgical histology (from Spigelman stage III to IV), whereas four patients were downgraded (from Spigelman stage IV to III). Therefore, postoperative histology revealed nine cancers in total, of which five were duodenal and four were ampullary. Correlation between preoperative endoscopic and postoperative surgical staging (Fig. 1) revealed a Pearson's kappa correlation coefficient of 0.518 (P < 0.001).
Figure 1.
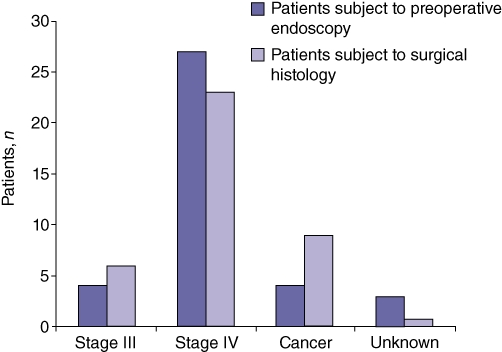
Analysis of preoperative and operative staging showed five patients to have preoperatively undetected cancer (four duodenal and one ampullary; Pearson's kappa correlation coefficient: 0.518 [P < 0.001])
All-cause and cancer-related five-year survival (Fig. 2) was lower in patients with postoperative histology illustrating frank malignancy (n = 9) than in patients with Spigelman stage III or IV disease (n = 29) (62.5% vs. 81.6% [P = 0.325], 62.5% vs. 88.5% [P = 0.116], respectively), although these differences did not reach statistical significance.
Figure 2.
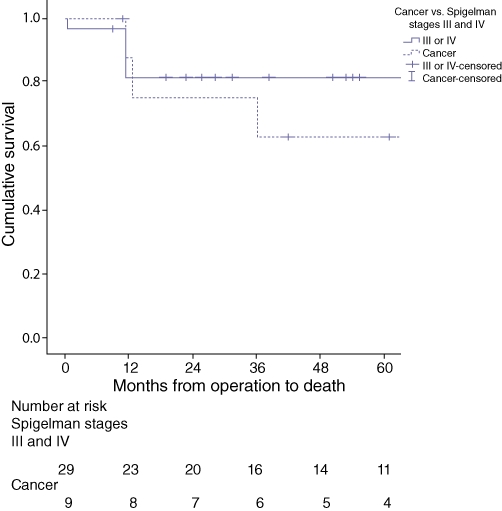
Longterm follow-up of 38 familial adenomatous polyposis patients undergoing resection for advanced duodenal and ampullary adenomatosis illustrates a shorter, overall 5-year survival in patients with postoperative histology demonstrating frank malignancy compared with those with Spigelman stage III or IV disease (62.5% vs. 81.6%; P = 0.325)
Outcome
Two patients died while in hospital, both from multi-organ failure after re-operation for intra-abdominal haemorrhage resulting from a pseudoaneurysm which developed following a diagnosed anastomotic leak. Another six patients died following discharge, four from metastatic disease (one at 11 months, one at 13 months and two at 3 years after surgery), one from a glioblastoma (at 10 months after surgery) and one from unknown reasons (Fig. 3).
Figure 3.
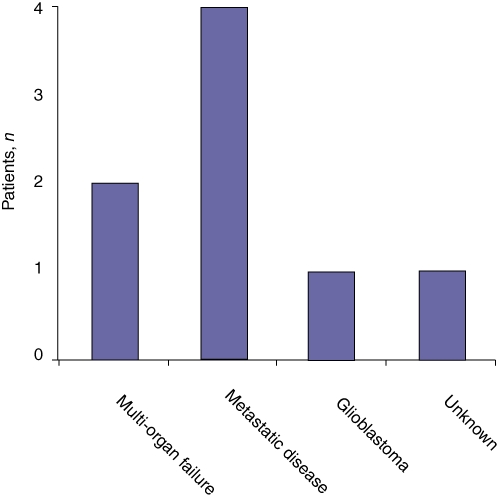
All-cause mortality in 38 familial adenomatous polyposis patients undergoing resection for advanced duodenal and ampullary adenomatosis
Postoperative complications were diagnosed in 29 patients (Fig. 4). The most common complication was anastomotic leak, which occurred in 16 patients, who were treated via radiological drain insertion, although one required laparotomy for biliary peritonitis and three developed enterocutaneous fistulae. Significant postoperative haemorrhage occurred in seven patients (five with confirmed anastomotic leaks), necessitating three re-laparotomies for the cessation of haemorrhage (two patients died) and two angiographic embolizations of a gastroduodenal artery and a jejunal vessel. Exocrine pancreatic insufficiency occurred in 13 patients and delayed gastric emptying in four.
Figure 4.
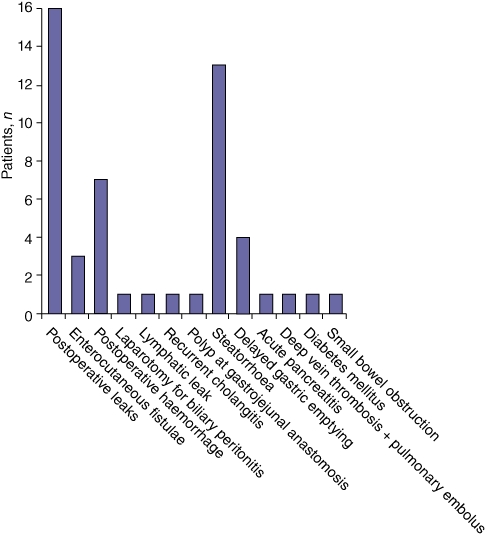
Postoperative complications in 38 familial adenomatous polyposis patients undergoing pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis
Patients were subsequently grouped according to their highest-grade complication on the revised Clavien–Dindo classification20 (Table 3, Fig. 5), which revealed that 19 patients had a complication of grade III or above. However, there was no significant difference in complication rate between patients with Spigelman stage III/IV (n = 23) and patients with malignancy (n = 6) (P = 0.830).
Table 3.
Clavien–Dindo classification of surgical complications (adapted from Dindo et al. 200420)
| Grade | Definition |
|---|---|
| Grade I | Any deviation from the normal postoperative course, without the need for medication (excluding anti-emetics, laxatives, analgesics, anti-pyrexials, diuretics, electrolytes) or intervention (excluding physiotherapy) |
| Grade II | Pharmacological treatment including blood transfusion and parenteral nutrition (excluding medications allowed for Grade I) |
| Grade III | Surgical, endoscopic or radiological intervention |
| Grade IIIa | Intervention not under general anaesthesia |
| Grade IIIb | Intervention under general anaesthesia |
| Grade IV | Life-threatening complication requiring high dependency unit/intensive therapy unit management |
| Grade IVa | Single-organ dysfunction |
| Grade IVb | Multi-organ dysfunction |
| Grade V | Patient death |
| Suffix ‘d’ | Complication present at the time of discharge |
Figure 5.
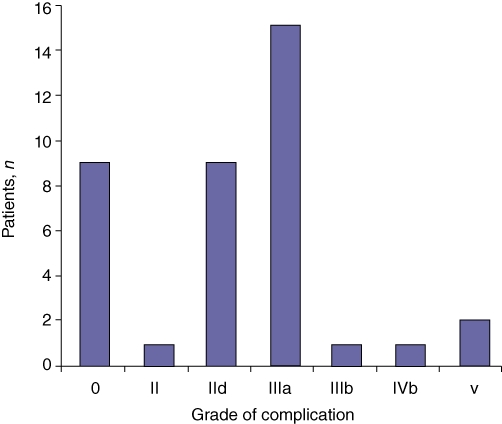
Analysis of highest-grade complication per patient, as defined by Clavien–Dindo grade, in 38 familial adenomatous polyposis patients undergoing resection for advanced duodenal and ampullary adenomatosis
Discussion
This report represents the largest published series of pancreaticoduodenectomies for advanced duodenal and ampullary adenomatosis in FAP. The results suggest that pancreaticoduodenectomy for advanced duodenal and ampullary polyps in FAP is associated with significant perioperative morbidity. However, a mortality rate of 5% is comparable to figures derived from series investigating outcomes of pancreaticoduodenectomy for non-FAP malignant lesions over the same time period, which demonstrate mortality rates of 3–25%.21–29
The use of pancreaticoduodenectomy in this FAP cohort is associated with higher morbidity rates than those found in most pancreaticoduodenectomy series for non-FAP lesions (approximately 34–57%21,22,29), probably because surgical intervention is significantly more technically demanding in FAP patients. All the patients in this study had previously undergone colonic resection and therefore had significant intra-abdominal adhesions and desmoplastic change, rendering mobilization technically more difficult. Further, the non-diseased, normal-texture pancreas, non-dilated ducts and increased tendency towards postoperative ileus or small bowel obstruction30 in FAP patients may challenge anastomotic integrity and contribute to a greater tendency for anastomotic leak.
Pancreaticogastrostomy reconstruction was originally chosen over pancreaticojejunostomy (PJ) for the FAP patients in this series because PG has been associated with a decreased incidence of postoperative leak, particularly in patients with normal pancreatic texture,31–33 which possibly occurs in PJ patients secondary to jejunal limb oedema, obstruction and distension, which may lead to anastomotic tension. The close proximity of the stomach and pancreatic remnant, in combination with the easy placement of nasogastric tubes for decompression, may decrease anastomotic tension in PG patients.34 Further, lack of enterokinase in the gastric remnant in PG patients may prevent pancreatic autodigestion35 and the stomach's excellent blood supply facilitates the formation of a sound anastomosis.34
Despite this, a significant incidence of anastomotic leak was discovered in this series and, in addition, some recent randomized studies and meta-analyses have demonstrated no benefit of one method of reconstruction over another.36,37 Further, PG formation has also been associated with pancreatic enzyme inactivation secondary to the reflux of gastric juices,38–40 which may help to explain the high incidence of pancreatic insufficiency seen in this FAP cohort.
The number of patients undergoing pancreaticoduodenectomy increased over the time course of the series, probably in response to improvements in the management of colonic disease which enable more patients to survive to an age at which duodenal disease becomes significant. These include the generalized introduction of upper GI surveillance and the identification of more patients with advanced, benign disease, as well as enhanced disease awareness, which results in increased referrals to the Polyposis Registry and similar centres.
Endoscopic ultrasound was not available during the first decade of this series (1993–2003) and thus was not utilized in all patients. There remains a paucity of published data regarding the use of EUS in the assessment of upper GI polyps in FAP, partly because the small numbers of cases make it impossible to perform a randomized controlled trial for the direct comparison of CT with CT and EUS. Experiences to date indicate that duodenoscopy and EUS (performed with one oblique viewing electronic echoendoscope) are complementary to CT and provide useful information about polyp size, number and heterogeneity, depth of invasion (if present), nodal status and evidence of biliary and pancreatic obstruction, and EUS is now performed routinely as part of disease staging. In particular, EUS may be useful for the identification of positive nodal status and/or early pancreatic/biliary obstruction, thus expediting the decision to refer for surgery rather than to continue endoscopic surveillance. However, further data on the utility of EUS in this context are required.
Despite the high morbidity rates associated with the use of pancreaticoduodenectomy in this study, it remains the most definitive treatment for advanced duodenal and ampullary adenomatosis in FAP. Pharmacological and endoscopic therapies can be helpful in delaying or preventing the need for surgery, but, once advanced or diffuse adenomatosis is present, surgical strategies are frequently required. Non-steroidal anti-inflammatory drugs, in particular, have been shown to be of some benefit. The use of celecoxib resulted in a significant (14–31%) reduction in duodenal involved areas,41 although its effect upon cancer prevention remains unknown. The use of sulindac led to non-significant duodenal polyp regression in one study42 and had no effect upon periampullary polyps in another.43 However, treatment with ranitidine44 and calciferol has been shown to have no effect.45 Endoscopic therapy with argon plasma coagulation and Nd-YAG laser has been attempted, with varying results.46
Endoscopic mucosal resection may represent a less invasive method than surgery for managing some stage III and IV patients, possibly in combination with drug therapies. However, open polypectomy via duodenotomy for the removal of large polyps has proved largely unsuccessful because not only does this procedure incorporate the risks of laparotomy and the triggering of desmoid disease, but any benefit is short-lived as polyps recur after 6–36 months18,47,48 and progression to further Spigelman stages occurs after 53 months.47 Similarly, advanced ampullary lesions can be locally removed by endoscopic49 or open ampullectomy. However, endoscopic ampullectomy carries a risk of incomplete resection margins, repeat procedures and complications (20%).50 To date, surgical ampullectomy has been shown to carry a low risk of both morbidity and recurrence in patients without invasive disease51,52 or with invasive lesions measuring <2 cm.53
Pylorus-preserving pancreaticoduodenectomy, although considered a less aggressive procedure than definitive pancreaticoduodenectomy, still carries significant complication and mortality rates (40% and 4.5%, respectively)54 and the inherent nature of the procedure means that de novo lesions are capable of arising in residual small bowel.55 Pancreas-sparing duodenectomy was developed as a similarly less aggressive procedure for the treatment of duodenal disease because it leaves the entire pancreas in situ and decreases the number of anastomoses required. However, it has been shown to have short-term morbidity (62% vs. 57%; P > 0.05) and mortality (4% vs. 3%; P > 0.05) rates comparable to those of standard pancreaticoduodenectomy for ampullary adenocarcinoma.29 Further, operative techniques that spare the pancreas leave areas of ampullary mucosa that may still undergo malignant change56 and may also result in increased anastomotic tension secondary to delayed gastric emptying, as well as adversely affecting the function of the patient's ileoanal pouch.8
Wide disease variability exists among FAP patients and only 36% of patients with stage IV disease will develop cancer.9,57,58 Therefore, given the high postoperative morbidity and mortality rates, some have previously advocated a watch-and-wait policy in patients with advanced but benign disease. However, once malignant duodenal or ampullary disease is established, prognosis is poor.3,59 Further, a discrepancy between endoscopic biopsy and surgical resection histology has been noted in this study and others54,60,61 and some patients may therefore have preoperatively undetected malignancy, further mandating the use of definitive resection in advanced but benign disease.
For patients in whom complete resection is possible, the longterm prognosis can be good. However, although distal small bowel polyps are less likely to develop62 and less likely to undergo dysplastic and malignant change than duodenal polyps,63 further malignant lesions may still be diagnosed in residual small bowel if FAP patients are followed for sufficient periods of time.64
Currently, the selection of FAP patients requiring surgical intervention for high risk of duodenal and ampullary malignant transformation remains challenging; improved methods of identifying patients at risk are required. Thus, FAP patients with advanced duodenal and ampullary adenomatosis require careful counselling and preoperative optimization as surgical intervention still carries significant rates of morbidity and mortality.
Acknowledgments
JRAS is supported by the Jason Boas Fellowship awarded by the No Surrender Charitable Trust.
Conflicts of interest
None declared.
References
- 1.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 2.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 3.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149–1151. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- 4.Spigelman AD, Talbot IC, Penna C, Nugent KP, Phillips RK, Costello C, et al. Evidence for adenoma–carcinoma sequence in the duodenum of patients with familial adenomatous polyposis. The Leeds Castle Polyposis Group (Upper Gastrointestinal Committee) J Clin Pathol. 1994;47:709–710. doi: 10.1136/jcp.47.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakatsubo N, Kashiwagi H, Okumura M, Kamoshida T, Takahashi A, Spigelman AD. Malignant change in a duodenal adenoma in familial adenomatous polyposis: report of a case. Am J Gastroenterol. 1998;93:1566–1568. doi: 10.1111/j.1572-0241.1998.00485.x. [DOI] [PubMed] [Google Scholar]
- 6.Sellner F. Investigations on the significance of the adenoma–carcinoma sequence in the small bowel. Cancer. 1990;66:702–715. doi: 10.1002/1097-0142(19900815)66:4<702::aid-cncr2820660419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Stolte M, Pscherer C. Adenoma–carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376–382. doi: 10.3109/00365529609006414. [DOI] [PubMed] [Google Scholar]
- 8.Morpurgo E, Vitale GC, Galandiuk S, Kimberling J, Ziegler C, Polk HC. Clinical characteristics of familial adenomatous polyposis and management of duodenal adenomas. J Gastrointest Surg. 2004;8:559–564. doi: 10.1016/j.gassur.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard F, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–386. doi: 10.1136/gut.2003.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 1993;36:1059–1062. doi: 10.1007/BF02047300. [DOI] [PubMed] [Google Scholar]
- 11.Domizio P, Talbot IC, Spigelman AD, Williams CB, Phillips RK. Upper gastrointestinal pathology in familial adenomatous polyposis: results from a prospective study of 102 patients. J Clin Pathol. 1990;43:738–743. doi: 10.1136/jcp.43.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scates DK, Spigelman AD, Nugent KP, Phillips RK, Venitt S. DNA adducts, detected by 32P-postlabelling, in DNA treated in vitro with bile from patients with familial adenomatous polyposis and from unaffected controls. Carcinogenesis. 1993;14:1107–1110. doi: 10.1093/carcin/14.6.1107. [DOI] [PubMed] [Google Scholar]
- 13.Spigelman AD, Crofton-Sleigh C, Venitt S, Phillips RK. Mutagenicity of bile and duodenal adenomas in familial adenomatous polyposis. Br J Surg. 1990;77:878–881. doi: 10.1002/bjs.1800770811. [DOI] [PubMed] [Google Scholar]
- 14.Spigelman AD, Owen RW, Hill MJ, Phillips RK. Biliary bile acid profiles in familial adenomatous polyposis. Br J Surg. 1991;78:321–325. doi: 10.1002/bjs.1800780318. [DOI] [PubMed] [Google Scholar]
- 15.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 16.Bertoni G, Sassatelli R, Nigrisoli E, Pennazio M, Tansini P, Arrigoni A, et al. High prevalence of adenomas and microadenomas of the duodenal papilla and periampullary region in patients with familial adenomatous polyposis. Eur J Gastroenterol Hepatol. 1996;8:1201–1206. doi: 10.1097/00042737-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10-year prospective study. Gut. 2002;50:636–641. doi: 10.1136/gut.50.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heiskanen I, Kellokumpu I, Jarvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;31:412–416. doi: 10.1055/s-1999-41. [DOI] [PubMed] [Google Scholar]
- 19.Björk J, Akerbrant H, Iselius L, Bergman A, Engwall Y, Wahlström J, et al. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127–1135. doi: 10.1053/gast.2001.28707. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. doi: 10.1097/00000658-200209000-00012. discussion 366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, et al. Standard vs. extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicentre, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120–124. doi: 10.1016/0002-9610(91)90371-j. discussion 124–125. [DOI] [PubMed] [Google Scholar]
- 25.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Longterm survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996;83:625–631. doi: 10.1002/bjs.1800830512. [DOI] [PubMed] [Google Scholar]
- 27.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. discussion 72–73. [DOI] [PubMed] [Google Scholar]
- 28.Trede M. The surgical treatment of pancreatic carcinoma. Surgery. 1985;97:28–35. [PubMed] [Google Scholar]
- 29.de Castro SM, van Eijck CH, Rutten JP, Dejong CH, van Goor H, Busch OR, et al. Pancreas-preserving total duodenectomy vs. standard pancreatoduodenectomy for patients with familial adenomatous polyposis and polyps in the duodenum. Br J Surg. 2008;95:1380–1386. doi: 10.1002/bjs.6308. [DOI] [PubMed] [Google Scholar]
- 30.Nyam DC, Brillant PT, Dozois RR, Kelly KA, Pemberton JH, Wolff BG. Ileal pouch–anal canal anastomosis for familial adenomatous polyposis: early and late results. Ann Surg. 1997;226:514–519. doi: 10.1097/00000658-199710000-00012. discussion 519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takano S, Ito Y, Watanabe Y, Yokoyama T, Kubota N, Iwai S. Pancreaticojejunostomy vs. pancreaticogastrostomy in reconstruction following pancreaticoduodenectomy. Br J Surg. 2000;87:423–427. doi: 10.1046/j.1365-2168.2000.01395.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. doi: 10.3748/wjg.v11.i16.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J, et al. Meta-analysis of pancreaticojejunostomy vs. pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. Br J Surg. 2006;93:929–936. doi: 10.1002/bjs.5407. [DOI] [PubMed] [Google Scholar]
- 34.Aranha GV, Hodul P, Golts E, Oh D, Pickleman J, Creech S. A comparison of pancreaticogastrostomy and pancreaticojejunostomy following pancreaticoduodenectomy. J Gastrointest Surg. 2003;7:672–682. doi: 10.1016/s1091-255x(02)00432-8. [DOI] [PubMed] [Google Scholar]
- 35.Pikarsky AJ, Muggia-Sullam M, Eid A, Lyass S, Bloom AI, Durst AL, et al. Pancreaticogastrostomy after pancreatoduodenectomy. A retrospective study of 28 patients. Arch Surg. 1997;132:296–299. doi: 10.1001/archsurg.1997.01430270082016. [DOI] [PubMed] [Google Scholar]
- 36.Lai EC, Lau SH, Lau WY. Measures to prevent pancreatic fistula after pancreatoduodenectomy: a comprehensive review. Arch Surg. 2009;144:1074–1080. doi: 10.1001/archsurg.2009.193. [DOI] [PubMed] [Google Scholar]
- 37.Adams DB. The pancreatic anastomosis: the danger of a leak, which anastomotic technique is better? J Gastrointest Surg. 2009;13:1182–1183. doi: 10.1007/s11605-009-0865-z. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura H, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, et al. Predictive factors for exocrine pancreatic insufficiency after pancreatoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2009;13:1321–1327. doi: 10.1007/s11605-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 39.Rault A, SaCunha A, Klopfenstein D, Larroudé D, Epoy FN, Collet D, et al. Pancreaticojejunal anastomosis is preferable to pancreaticogastrostomy after pancreaticoduodenectomy for longterm outcomes of pancreatic exocrine function. J Am Coll Surg. 2005;201:239–244. doi: 10.1016/j.jamcollsurg.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Fish JC, Smith LB, Williams RD. Digestive function after radical pancreaticoduodenectomy. Am J Surg. 1969;117:40–45. doi: 10.1016/0002-9610(69)90283-9. [DOI] [PubMed] [Google Scholar]
- 41.Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, et al. A randomized, double blind, placebo-controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857–860. doi: 10.1136/gut.50.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 43.Richard CS, Berk T, Bapat BV, Haber G, Cohen Z, Gallinger S. Sulindac for periampullary polyps in FAP patients. Int J Colorectal Dis. 1997;12:14–18. doi: 10.1007/s003840050071. [DOI] [PubMed] [Google Scholar]
- 44.Wallace MH, Forbes A, Beveridge IG, Spigelman AD, Hewer A, Venitt S, et al. Randomized, placebo-controlled trial of gastric acid-lowering therapy on duodenal polyposis and relative adduct labelling in familial adenomatous polyposis. Dis Colon Rectum. 2001;44:1585–1589. doi: 10.1007/BF02234376. [DOI] [PubMed] [Google Scholar]
- 45.Seow-Choen F, Vijayan V, Keng V. Prospective randomized study of sulindac vs. calcium and calciferol for upper gastrointestinal polyps in familial adenomatous polyposis. Br J Surg. 1996;83:1763–1766. doi: 10.1002/bjs.1800831232. [DOI] [PubMed] [Google Scholar]
- 46.Kashiwagi H, Spigelman AD. Gastroduodenal lesions in familial adenomatous polyposis. Surg Today. 2000;30:675–682. doi: 10.1007/s005950070077. [DOI] [PubMed] [Google Scholar]
- 47.Penna C, Phillips RK, Tiret E, Spigelman AD. Surgical polypectomy of duodenal adenomas in familial adenomatous polyposis: experience of two European centres. Br J Surg. 1993;80:1027–1029. doi: 10.1002/bjs.1800800833. [DOI] [PubMed] [Google Scholar]
- 48.Dixon E, Vollmer CM, Sahajpal A, Cattral MS, Grant DR, Taylor BR, et al. Transduodenal resection of periampullary lesions. World J Surg. 2005;29:649–652. doi: 10.1007/s00268-005-7578-6. [DOI] [PubMed] [Google Scholar]
- 49.Alarcon FJ, Burke CA, Church JM, van Stolk RU. Familial adenomatous polyposis: efficacy of endoscopic and surgical treatment for advanced duodenal adenomas. Dis Colon Rectum. 1999;42:1533–1536. doi: 10.1007/BF02236201. [DOI] [PubMed] [Google Scholar]
- 50.Dittrick GW, Mallat DB, Lamont JP. Management of ampullary lesions. Curr Treat Options Gastroenterol. 2006;9:371–376. doi: 10.1007/BF02738525. [DOI] [PubMed] [Google Scholar]
- 51.Ouaïssi M, Panis Y, Sielezneff I, Alves A, Pirrò N, Robitail S, et al. Longterm outcome after ampullectomy for ampullary lesions associated with familial adenomatous polyposis. Dis Colon Rectum. 2005;48:2192–2196. doi: 10.1007/s10350-005-0187-5. [DOI] [PubMed] [Google Scholar]
- 52.Ouaïssi M, Sielezneff I, Alves A, Pirro N, Heyries L, Robitail S, et al. [Longterm outcome following 26 surgical ampullectomies.] Ann Chir. 2006;131:322–327. doi: 10.1016/j.anchir.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Demetriades H, Zacharakis E, Kirou I, Pramateftakis MG, Sapidis N, Kanellos I, et al. Local excision as a treatment for tumours of ampulla of Vater. World J Surg Oncol. 2006;4:14. doi: 10.1186/1477-7819-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallagher MC, Shankar A, Groves CJ, Russell RC, Phillips RK. Pylorus-preserving pancreaticoduodenectomy for advanced duodenal disease in familial adenomatous polyposis. Br J Surg. 2004;91:1157–1164. doi: 10.1002/bjs.4527. [DOI] [PubMed] [Google Scholar]
- 55.Murakami Y, Uemura K, Sasaki M, Morifuji M, Hayashidani Y, Sudo T, et al. Duodenal cancer arising from the remaining duodenum after pylorus-preserving pancreatoduodenectomy for ampullary cancer in familial adenomatous polyposis. J Gastrointest Surg. 2005;9:389–392. doi: 10.1016/j.gassur.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Sarmiento JM, Thompson GB, Nagorney DM, Donohue JH, Farnell MB. Pancreas-sparing duodenectomy for duodenal polyposis. Arch Surg. 2002;137:557–562. doi: 10.1001/archsurg.137.5.557. discussion 562–563. [DOI] [PubMed] [Google Scholar]
- 57.Bülow S, Alm T, Fausa O, Hultcrantz R, Järvinen H, Vasen H. Duodenal adenomatosis in familial adenomatous polyposis. DAF Project Group. Int J Colorectal Dis. 1995;10:43–46. doi: 10.1007/BF00337586. [DOI] [PubMed] [Google Scholar]
- 58.Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance programme. Gastrointest Endosc. 1999;49:358–364. doi: 10.1016/s0016-5107(99)70013-1. [DOI] [PubMed] [Google Scholar]
- 59.Beckwith PS, van Heerden JA, Dozois RR. Prognosis of symptomatic duodenal adenomas in familial adenomatous polyposis. Arch Surg. 1991;126:825–827. doi: 10.1001/archsurg.1991.01410310035004. discussion 827–828. [DOI] [PubMed] [Google Scholar]
- 60.Kashiwagi H, Spigelman AD, Talbot IC, Phillips RK. Overexpression of p53 in duodenal tumours in patients with familial adenomatous polyposis. Br J Surg. 1996;83:225–228. [PubMed] [Google Scholar]
- 61.Cahen DL, Fockens P, de Wit LT, Offerhaus GJ, Obertop H, Gouma DJ. Local resection or pancreaticoduodenectomy for villous adenoma of the ampulla of Vater diagnosed before operation. Br J Surg. 1997;84:948–951. doi: 10.1002/bjs.1800840711. [DOI] [PubMed] [Google Scholar]
- 62.Groves CJ, Beveridge G, Swain DJ, Saunders BP, Talbot IC, Nicholls RJ, et al. Prevalence and morphology of pouch and ileal adenomas in familial adenomatous polyposis. Dis Colon Rectum. 2005;48:816–823. doi: 10.1007/s10350-004-0835-1. [DOI] [PubMed] [Google Scholar]
- 63.Zuidema MF, Dekker W. A patient with a metastasizing jejunal carcinoma 17 years after colectomy for familial polyposis coli. Neth J Med. 1989;34:317–321. [PubMed] [Google Scholar]
- 64.Ruo L, Coit DG, Brennan MF, Guillem JG. Longterm follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg. 2002;6:671–675. doi: 10.1016/s1091-255x(02)00045-8. [DOI] [PubMed] [Google Scholar]


