Abstract
Background: Elastography is a noninvasive method to assess liver fibrosis by measuring liver stiffness. Studies have compared elas-tography to percutaneous biopsy. Laparoscopic biopsy is associated with decreased sampling error compared to percutaneous biopsy, as laparoscopic biopsies are obtained from both liver lobes and gross nodu-larity can be visualized. Methods: Patients undergoing laparoscopic liver biopsy were enrolled. Gross liver appearance was assessed, and biopsy specimens were blindly evaluated by a pathologist. Elastography (FibroScan) was used to measure liver stiffness. Results: 101 patients were examined. Fibrosis was related to elasticity (Spearman correlation r=0.63; P<.0001). Elasticity was strongly associated with advanced stages of fibrosis (stages 3 and 4; Spearman correlation r2=0.44; P<.001). Significant fibrosis was associated with an irregular liver surface, nodularity, and thickened edge (multiple regression r2=0.41; P<.001). Increased elasticity was associated with a fatty-appearing liver, irregular surface, firmness, and nodularity (multiple regression r2=0.46; P<.001). Receiver operating characteristic curve for elasticity for identifying patients with a liver fibrosis stage of at least 3 or of 4 had an area under the curve (AUC) of 0.85 or 0.86, respectively. AUC was 0.857 when gross nodularity was used as the gold standard for cirrhosis and 0.875 when nodularity/histology were used. Elasticity of at least 7 kPa, at least 9.5 kPa, and at least 11.8 kPa had the highest accuracy for identifying patients with a fibrosis stage of at least 2, at least 3, and 4, respectively. In hepatitis C patients, AUC was 0.921, 0.882, and 0.925 when histology, gross nodularity, and nodularity/histology, respectively, were used as the gold standard for cirrhosis. Conclusion: FibroScan could be useful for detecting advanced stages of fibrosis when validated against laparoscopic liver biopsy.
Keywords: Liver elasticity, laparoscopic liver biopsy, fibrosis, cirrhosis, hepatitis C, liver nodularity
Percutaneous liver biopsy is considered the gold standard for the assessment of liver fibrosis. Unfortunately, this procedure has several limitations. It is an invasive procedure that carries possible complications such as pain, bleeding, and inadvertent biopsy of other organs.1,2 In addition, percutaneous liver biopsies only sample 1/50,000th of the liver, which may not be representative ofthe entire organ.3 Various groups have demonstrated that there is a considerable degree of sampling error and variability associated with percutaneous liver biopsy.4–7 Due to the limitations of sampling, percutaneous liver biopsy should be considered, at best, a flawed gold standard.
With laparoscopic liver biopsy, more representative samples of the liver are obtained with a reduction in sampling error and variability by taking samples from both the right and left lobes.5 In addition, the gross appearance of the liver (eg, nodularity) can be assessed in order to help with the diagnosis of cirrhosis.8 Diagnostic laparoscopy with liver biopsy is a safe and valuable procedure in the evaluation of chronic liver disease and remains an important diagnostic tool at our institu-tion.9 Due to the reduction in sampling error and added benefit of gross assessment, diagnostic laparoscopic liver biopsy should be considered the true gold standard in liver fibrosis assessment.
FibroScan is a novel ultrasound-based instrument that measures transient elasticity and stiffness of the liver expressed in kilopascals (kPa).10 Various studies have compared FibroScan measurements to fibrosis determined by percutaneous liver biopsies and have demonstrated that liver stiffness increases with fibrosis stage. In a study conducted by Sandrin and associates,10 the median hepatic elasticity was 4.2 kPa for a F0 fibrosis score, 4.5–6.25 kPa for a F1 fibrosis score, 5.5–7.8 kPa for a F2 fibrosis score, 8.0–13.7 kPa for a F3 fibrosis score, and 21–34 kPa for a F4 fibrosis score. Various other groups have published similar results for a range of liver diseases.11–25 Recommended cutoff values for cirrhosis and the various stages of fibrosis differ among published studies. Two specific studies that looked at a variety of hepatic diseases reported recommended thresholds of 17.6 kPa and 14.6 kPa for the diagnosis of cirrhosis, with sensitivities of 77% and 79%, specificities of 97% and 95%, and positive predictive values of 91% and 74%, respectively.14,15 A recent meta-analysis of 9 published studies demonstrated pooled estimates of 87% for sensitivity and 91% for specificity for stage 4 fibrosis (cirrhosis). Investigations examining patients with stage 2–4 fibrosis noted pooled estimates of 70% for sensitivity and 84% for specificity.26
All recently published studies assessing the accuracy of FibroScan measurements to predict hepatic fibrosis use percutaneous liver biopsies as the gold standard. The aim of our study was to compare FibroScan measurements to diagnostic laparoscopy with liver biopsy.
Materials and Methods
Our research protocol was approved by our institutional human research review committee. Laparoscopic liver biopsy is routinely used at our institution for diagnostic purposes and is used interchangeably with percutaneous liver biopsy. Patients who required a liver biopsy for diagnostic purposes were consecutively enrolled in the study. Collected data included patient age, gender, and indication for liver biopsy.
Diagnostic Laparoscopy
All patients underwent diagnostic laparoscopy with liver biopsy by one experienced hepatologist, as we have previously described.8,9 Biopsy specimens were obtained from both the right and left hepatic lobes, using an automatic 16-gauge Tru-Cut needle (biopsy gun). In addition, the physician performing the procedure subjectively assessed the gross appearance of the liver by examining liver edge thickness, presence of nodularity, liver surface irregularity, firmness or heaviness when probed, fatty appearance, and hyperemia. The gross appearance of nodularity with firmness to probing was considered diagnostic of cirrhosis.
Histopathology
All obtained specimens were of adequate size according to accepted criteria for satisfactory liver biopsies (at least 25 mm).7,27,28 Biopsy specimens were formalin-fixed and paraffin-embedded. Sections were stained with hema-toxylin and eosin and Masson trichrome. The slides were blindly evaluated by an expert hepatopathologist. Histo-logic findings were assessed according to the grading and staging method based upon the Scheuer system29 modified by Batts and Ludwig.30,31 Inflammatory activity was graded as the following: grade 0=none or minimal; grade 1=portal inflammation; grade 2=mild interface hepatitis; grade 3=moderate interface hepatitis; and grade 4=severe interface hepatitis. Fibrosis was staged as the following: stage 0=normal connective tissue; stage 1=fibrous portal expansion; stage 2=periportal fibrosis or thin rare portal-portal septae; stage 3=fibrous septa with architectural distortion; and stage 4=cirrhosis. The pathologist was blinded to the FibroScan result.
Liver Elastography
FibroScan, or transient elastography, is an instrument that measures transient elasticity of the liver.10 In our study, FibroScan was used to measure liver stiffness within 2 months prior to or following the laparoscopic liver biopsy. The technical details of the instrument and the description of the procedure have been previously described.10 In summary, the measurement is obtained by placing a probe equipped with an ultrasonic transducer mounted on the axis of a vibrator into an intercostal space over the right lobe of the liver. A vibration of mild amplitude and low frequency is transmitted from the vibrator to the liver tissue. This vibration induces an elastic shear wave, which propagates through the tissue. Simultaneously, time pulse-echo ultrasound acquisitions of the propagating shear wave are performed and mea surement of the velocity of the wave is obtained. This measurement is directly related to tissue stiffness. Liver elasticity is measured in kPa.
A technician trained in FibroScan use obtained a minimum of 15 valid measurements per patient and was blinded to the biopsy result.
Statistical Analysis
Spearman correlation analysis was utilized to assess the association between liver elasticity and histopathologic grade and stage. It was also used to evaluate the association between grade and stage.
Multiple regression analysis was used to evaluate the association between liver elasticity with the various histopathologic grades and stages and gross laparoscopic hepatic features. This analysis was also used to evaluate the association of grade and stage with gross laparoscopic hepatic features. A T test was used to compare liver elasticity between adjacent fibrosis stages. A P value of less than .05 was considered statistically significant.
Receiver operating characteristic (ROC) curves were used to evaluate the accuracy of liver elasticity to predict hepatic stage and gross cirrhosis. The index of accuracy was assessed by the area under the ROC curve (AUC), with a value close to 1.0 indicating high diagnostic accuracy.
Results
Patient Characteristics
A total of 105 patients were enrolled in the study. Four patients were excluded (2 patients underwent percutaneous liver biopsy instead of laparoscopic liver biopsy, 1 patient did not undergo liver biopsy, and 1 patient did not have an adequate number of valid FibroScan readings). A total of 101 patients were analyzed. The mean age of the patients was 50.7 years ± 9.2 years, and 50.5% of the patients were men and 49.5% were women. The indications for laparoscopic liver biopsy are listed in Table 1. During the time of liver biopsy or FibroScan measurements, patients with hepatitis C were not on antiviral therapy and patients with alcoholic liver disease were abstinent.
Table 1.
Indications for Laparoscopic Liver Biopsy
| Indication | N (%) |
|---|---|
| Hepatitis C | 65 (64) |
| Elevated liver enzymes | 11 (10.9) |
| Nonalcoholic fatty liver disease | 8 (7.9) |
| Hepatitis B | 8 (7.9) |
| Autoimmune hepatitis | 4 (4) |
| Drug toxicity | 2 (2) |
| Primary biliary cirrhosis | 1 (1) |
| Primary sclerosing cholangitis | 1 (1) |
Liver Biopsy
During laparoscopy, biopsies were obtained from both liver lobes (with a mean of 2.9 biopsies per patient and a majority of biopsies greater than 2.5 cm). The histo-pathologic grade and stage of the patients are listed in Table 2. In patients with different degrees of grade or stage between biopsies, the highest value was used for the analysis. The same stage of fibrosis was found in both liver lobes in 75 patients. The remaining patients had different stages of fibrosis (ie, a difference of at least 1 stage) between the left lobe and the right lobe. Of these patients, 14 had a higher stage of fibrosis in the left lobe and 12 had a higher stage of fibrosis in the right lobe. Twenty (19.8%) patients had cirrhosis based upon histology.
Table 2.
Histopathologic Characteristics, Including Activity Grade, Fibrosis Stage, and Differences in Fibrosis Stage Between Hepatic Lobes
| Activity | N (%) | Fibrosis | N (%) | Fibrosis difference | N (%) |
|---|---|---|---|---|---|
| Grade 0 | 3 (3) | Stage 0 | 14 (13.9) | Right=left | 75 (74.3) |
| Grade 1 | 13 (12.9) | Stage 1 | 10 (9.9) | Right>left | 12 (11.9) |
| Grade 2 | 24 (23.8) | Stage 2 | 32 (31.7) | Right<left | 14 (13.8) |
| Grade 3 | 36 (35.6) | Stage 3 | 25 (24.8) | ||
| Grade 4 | 25 (24.8) | Stage 4 | 20 (19.8) | ||
Grade and stage as assessed by an expert hepatopathologist. When differences in grade or stage were present between biopsies, the highest value was used for analysis. When present, the difference in fibrosis between lobes was by at least 1 stage.
According to the correlation analysis, liver activity (grade) was related to fibrosis (Spearman correlation r=0.74; P<.0001, 95% confidence interval [CI], 0.64–0.82).
Gross Appearance and Liver Histology
The subjectively assessed gross characteristics of the liver on laparoscopic examination are listed in Table 3. Of the 20 patients with biopsy-confirmed cirrhosis, 12 had gross nodularity consistent with frank cirrhosis. The remaining 8 patients had an irregular liver surface (no gross nodular-ity) with a heavy and thickened liver edge. Seven of the 8 patients had a firm liver when probed, and 1 patient had a liver that was soft to probing.
Table 3.
Gross Appearance of Liver on Laparoscopic Examination
| Characteristic | N (%) |
|---|---|
| Thickened liver edge | 62 (61.4) |
| Firm to probing | 25 (24.7) |
| Heavy when lifted with probe | 26 (25.7) |
| Gross nodularity (cirrhosis) | 17 (16.8) |
| Irregular liver surface | 31 (30.7) |
| Hyperemia | 17 (16.8) |
| Fatty appearance | 9 (8.9) |
Characteristics were subjectively assessed by the operator. Liver characteristics were not mutually exclusive.
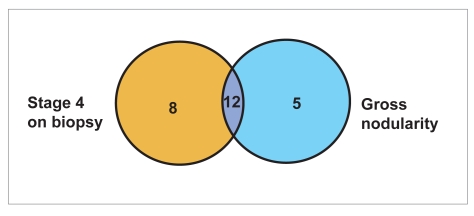
In addition, 5 patients had the gross appearance of cirrhosis (nodularity) but had only stage 3 fibrosis on biopsy. Table 4 and Figure 1 list the number of patients with the diagnosis of cirrhosis based upon histology alone, gross nodularity alone, and histology and/or gross appearance.
Table 4.
Diagnosis of Cirrhosis Based Upon Histology and Gross Appearance
| Diagnostic criteria | N (%) |
|---|---|
| Histology (stage 4) | 20 (19.8) |
| Gross nodularity on laparoscopy | 17 (16.8) |
| Histology and/or gross nodularity | 25 (24.8) |
See Figure 1 for a Venn diagram of patients with cirrhosis based upon histopathology and gross nodularity.
Figure 1.
Venn diagram of patients with cirrhosis based upon histopathology and gross nodularity. (See Table 4.)
Liver Elasticity Measurements
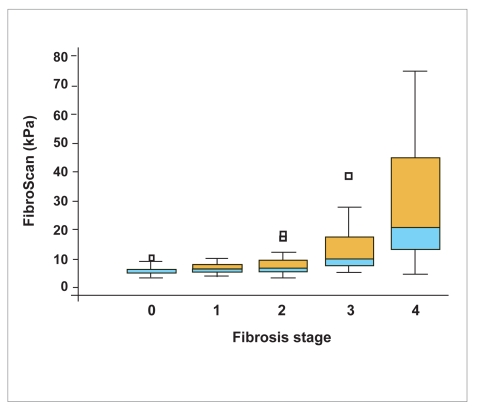
Liver elasticity measurements had a large dispersion (non-normal distribution) with a median of 8.4 kPa and a range of 3.1–75 kPa. Median liver elasticity values (range) for the various fibrosis stages were the following: 5.3 kPa (3.1–10.2 kPa) for stage 0, 6.5 kPa (4.1–10.1 kPa) for stage 1, 7 kPa (3.3–18.4 kPa) for stage 2, 10.1 kPa (5.1–38.5 kPa) for stage 3, and 20.9 kPa (4.5–75 kPa) for stage 4 (Figure 2).
Figure 2.
Box-plot of FibroScan values for different fibrosis stages.
Due to the non-normal distribution of liver elasticity, the measurements of liver elasticity were normalized using a decimal logarithmic transformation (LogE) for analysis. Kawamoto and colleagues32 have previously demonstrated that there is a nearly linear relationship between area of fibrosis and liver elasticity using a logarithmic scale.
In the correlation analysis, liver fibrosis (stage) was related to liver elasticity measurements (Spearman correlation r=0.63; P<.0001; 95% CI, 0.50–0.74), as was activity (Spearman correlation r=0.42; P<.0001; 95% CI, 0.24–0.57).
In multiple regression analysis of liver elasticity with grade and stage, liver elasticity was strongly associated with advanced stages of fibrosis (stage 3, P=.017 and stage 4, P<.0001; multiple regression r2=0.44; P<.001). There was no strong association with grade or lower stages of fibrosis.
The ability of liver elasticity to distinguish adjacent fibrosis stages was evaluated. There was no significant difference in liver elasticity for distinguishing stages F0 versus F1 (P=.38) and F1 versus F2 (P=.25). Liver elasticity was significantly different for stages F2 versus F3 (P=.0004) and F3 versus F4 (P=.003).
In addition, significant liver fibrosis was associated with the presence of an irregular liver surface (P<.0001), nodularity (P=.0001), and thickened liver edge (P=.027) in our patients (multiple regression r2=0.41; P<.001). Increased liver elasticity was also associated with the presence of a fatty-appearing liver (P=.0335), irregular surface (P=.0005), firm liver (P=.0445), and nodularity (P<.0001) in our patients (multiple regression r2=0.46; P<.001).
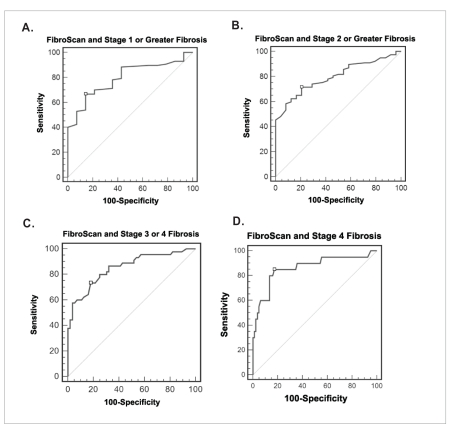
According to the ROC curve for liver elasticity for identifying patients with the various stages of liver fib-rosis, stages of at least 1 had an AUC of 0.8 (95% CI, 0.704–0.869); stages of at least 2 had an AUC of 0.8 (95% CI, 0.706–0.871); stages of at least 3 had an AUC of 0.85 (95% CI, 0.767–0.914); and stage 4 had an AUC of 0.86 (95% CI, 0.779–0.923; Figure 3). FibroScan values of at least 11.8 kPa, at least 9.5 kPa, and at least 7 kPa had the highest accuracy for predicting stages F4, at least F3, and at least F2, respectively. Table 5 lists the sensitivity, specificity, likelihood ratios, and positive and negative predictive values for various FibroScan measurements and stages of fibrosis in our study population.
Figure 3.
Receiver operating characteristic curve for FibroScan for identifying all patients (N=101) with fibrosis using biopsy as a gold standard. A: stage 1 or greater, area under the curve (AUC) of 0.8 (95% confidence interval [CI], 0.7–0.87); B: stage 2 or greater, AUC of 0.8 (95% CI, 0.71–0.87); C: stage 3 or greater, AUC of 0.85 (95% CI, 0.77–0.91); and D: stage 4, AUC of 0.86 (95% CI, 0.78–0.92).
The FibroScan value with the highest accuracy is marked by □ (see Table 5).
Table 5.
Sensitivity, Specificity, Likelihood Ratios, and Predictive Values of FibroScan Measurements for Predicting Fibrosis in All Etiologies of Liver Disease
| Fibrosis | FibroScan (kPa) | Sensitivity (%) | Specificity (%) | LR+ | LR− | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Gold standard: Histology only | |||||||
| Stage 4 | 11.8* | 85 | 82.7 | 4.92 | 0.18 | 55 | 95.7 |
| 18.4 | 60 | 94 | 9.72 | 0.43 | 70.6 | 90.5 | |
| 20.9 | 50 | 96.3 | 13.5 | 0.52 | 76.9 | 88.6 | |
| Stage ≥3 | 7.6 | 86.7 | 67.9 | 2.7 | 0.2 | 68.4 | 86.4 |
| 9.5* | 73.3 | 82.1 | 4.11 | 0.32 | 76.7 | 79.3 | |
| 11.9 | 57.8 | 94.6 | 10.79 | 0.45 | 89.7 | 73.6 | |
| Stage ≥2 | 7* | 71.4 | 79 | 3.43 | 0.36 | 92 | 46.3 |
| 8.7 | 58.4 | 91.7 | 7.01 | 0.45 | 95.7 | 40.7 | |
| Stage ≥1 | 5.3 | 88.5 | 57 | 2.07 | 0.2 | 93 | 44.4 |
| 7 | 66.7 | 85.7 | 4.67 | 0.39 | 96.7 | 29.3 | |
| Gold standard: Gross nodularity on laparoscopy only | |||||||
| Cirrhosis | 11.8 | 76.5 | 78.6 | 3.57 | 0.3 | 41.9 | 94.3 |
| 14 | 70.6 | 85.7 | 4.94 | 0.34 | 50 | 93.5 | |
| 20.4* | 64.7 | 95.2 | 13.59 | 0.37 | 73.3 | 93 | |
| Gold standard: Histology and/or gross nodularity | |||||||
| Cirrhosis | 11.8* | 80 | 85.5 | 5.53 | 0.23 | 64.5 | 92.9 |
| 14.3 | 60 | 89.5 | 5.7 | 0.45 | 65.2 | 87.2 | |
| 18.4 | 56 | 96 | 14.2 | 0.46 | 82.4 | 87 | |
Sensitivity, specificity, likelihood ratios, and predictive values of FibroScan measurements for all patients (N=101) based upon various gold standards (histology and/or gross nodularity).
Highest accuracy.
- LR+
positive likelihood ratio
- LR–
negative likelihood ratio
- NPV
negative predictive value
- PPV
positive predictive value.
For prevalence of fibrosis and cirrhosis, see Tables 2 and 4.
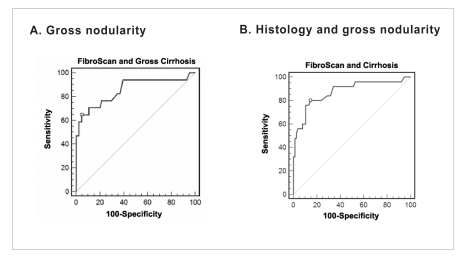
A total of 17 patients had the gross appearance of cirrhosis, though 5 of these patients had only stage 3 fibrosis on biopsy. When gross appearance of cirrhosis was used as the gold standard for the diagnosis of cirrhosis and not histopathology (n=17), the ROC curve had an AUC of 0.857 (95% CI, 0.773–0.918). The highest accuracy for predicting cirrhosis was with the FibroScan measurement of 20.4 kPa, which had a sensitivity of 64.7% and a specificity of 95.2% (Figure 4 and Table 5).
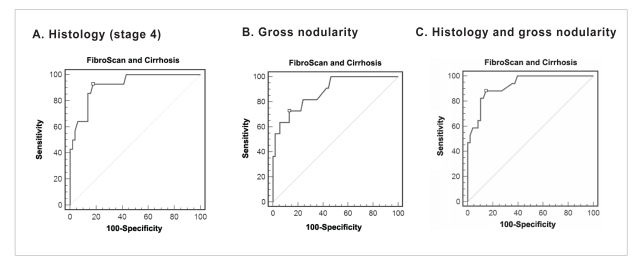
Figure 4.
Receiver operating characteristic curve for FibroScan for identifying all patients (N=101) with fibrosis using gross nodularity and biopsy. A: gross nodularity as the gold standard, area under the curve (AUC) of 0.857 (95% confidence interval [CI], 0.77–0.92); and B: histology and/or gross nodularity as the gold standard, AUC of 0.875 (95% CI, 0.79–0.93).
The FibroScan value with the highest accuracy is marked by □ (see Table 5).
When histology and/or gross appearance were used as diagnostic criteria, 25 patients were found to have cirrhosis. With these criteria, the ROC curve had an AUC of 0.875 (95% CI, 0.794–0.932). The highest accuracy for predicting cirrhosis was with the FibroScan measurement of 11.8 kPa, which had a sensitivity of 80% and a specificity of 85.5% (Figure 4 and Table 5).
A subgroup analysis of hepatitis C patients (n=65) was also performed. The ROC curve for stage 4 had an AUC of 0.921 (95% CI, 0.827–0.973). The highest accuracy was with the FibroScan measurement of 11.8 kPa, which had a sensitivity of 92.9% and a specificity of 82.4% (Table 6 and Figure 5). When gross appearance of cirrhosis was used as the gold standard, the ROC curve had an AUC of 0.882 (95% CI, 0.78–0.95). The highest accuracy for predicting cirrhosis was with the FibroScan measurement of 14.5 kPa, which had a sensitivity of 72.7% and a specificity of 87% (Table 6 and Figure 5). When histology and/or gross appearance were used as diagnostic criteria, the ROC curve had an AUC of 0.925 (95% CI, 0.83–0.98). The highest accuracy for predicting cirrhosis was with the FibroScan measurement of 11.8 kPa, which had a sensitivity of 88.2% and a specificity of 85.4%.
Table 6.
Sensitivity, Specificity, Likelihood Ratios, and Predictive Values of FibroScan Measurements for Predicting Cirrhosis in Patients With Hepatitis C
| Fibrosis | FibroScan (kPa) | Sensitivity (%) | Specificity (%) | LR+ | LR− | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Gold standard: Histology only | |||||||
| Stage 4 | 11.8* | 92.9 | 82.4 | 5.26 | 0.09 | 59.1 | 97.7 |
| 12.8 | 78.6 | 86.3 | 5.72 | 0.25 | 61.1 | 93.6 | |
| 18.4 | 64.3 | 94.1 | 10.93 | 0.38 | 75 | 90.6 | |
| Gold standard: Gross nodularity on laparoscopy only | |||||||
| Cirrhosis | 11.8 | 81.8 | 75.9 | 3.4 | 0.24 | 40.9 | 95.3 |
| 14.5* | 72.7 | 87 | 5.61 | 0.31 | 53.3 | 94 | |
| 18.4 | 63.6 | 90.7 | 6.87 | 0.4 | 58.3 | 92.5 | |
| Gold standard: Histology and/or gross nodularity | |||||||
| Cirrhosis | 11.8* | 88.2 | 85.4 | 6.05 | 0.14 | 68.2 | 95.3 |
| 14.5 | 64.7 | 91.7 | 7.76 | 0.39 | 73.3 | 88 | |
| 18.4 | 58.8 | 95.8 | 14.12 | 0.43 | 83.3 | 86.8 | |
Sensitivity, specificity, likelihood ratios, and predictive values of FibroScan measurements for patients with hepatitis C (n=65) based upon various gold standards (histology and/or gross nodularity).
Highest accuracy.
- LR+
positive likelihood ratio
- LR–
negative likelihood ratio
- NPV
negative predictive value
- PPV
positive predictive value.
Figure 5.
Receiver operating characteristic curve for FibroScan for identifying hepatitis C patients (n=65) with cirrhosis. A: histology (stage 4) as the gold standard, area under the curve (AUC) of 0.92 (95% confidence interval [CI], 0.83–0.97); B: gross nodularity as the gold standard, AUC of 0.88 (95% CI, 0.78–0.95); and C: histology and/or gross nodularity as the gold standard, AUC of 0.925 (95% CI, 0.83–0.98).
The FibroScan value with the highest accuracy is marked by □ (see Table 6).
Discussion
Liver elastography measurement is a promising new mod-ality for assessing liver fibrosis in a safe and noninvasive manner. Recently published studies have used percutaneous liver biopsy as the gold standard in the validation of liver elastography. Unfortunately, percutaneous liver biopsy obtains samples of only the right lobe of the liver and only represents approximately 1/50,000th of the liver. Various groups have demonstrated that there are sampling errors with percutaneous liver biopsies and that the diagnosis of cirrhosis may be missed in 1–67% of cases.33–42 In a previous study from our group, 23.5% of patients with various liver diseases undergoing laparoscopic biopsy were found to have significant histologic differences between their right and left hepatic lobes.43 We have also previously demonstrated in a population of patients with hepatitis C virus infection that laparoscopic liver biopsy samples obtained from the right and left hepatic lobes differed in histologic staging by 33.1%. In that study, sampling error may have led to underdiagnosis of cirrhosis in 14.5% of the patients.5 These findings are consistent with the finding in this current study that 25.7% of patients had a difference of at least 1 stage between the left and right hepatic lobes.
In addition to reducing sampling error, laparoscopy has the advantage of assessing gross cirrhosis. We have previously shown that only 68% of patients have histo-logic confirmation of cirrhosis when nodules on the liver surface and firmness to probing were used as the gold standard for the diagnosis of cirrhosis. This figure represents a 32% sampling error. In this previous study, which used laparoscopy as a gold standard, the sensitivity of liver biopsy was 68% and the specificity was 99%.34 Indeed, 5 patients in our current study had gross characteristics of cirrhosis but only stage 3 fibrosis on biopsy. Laparoscopic liver biopsy is a superior gold standard to percutaneous liver biopsy for the diagnosis of cirrhosis. It was thus used as a gold standard for comparison to FibroScan in this current study.
Liver elasticity was related to both fibrosis and liver activity. On multiple regression analysis, liver elasticity was most strongly associated with stages 3 and 4 fibrosis. Liver elasticity was unable to distinguish among less advanced stages of liver fibrosis (F0 vs F1 and F1 vs F2). This finding may be due to the small sample size within these groups (F0, F1, and F2). Liver fibrosis and grade were indeed related to each other, likely explained by the fact that as liver disease progresses, liver fibrosis and inflammation often increase in tandem. This relationship between fibrosis and activity likely explains the relationship seen between liver elasticity and activity. Further studies are necessary to evaluate whether liver activity is independently associated with liver elasticity measurements.
As expected, significant liver fibrosis was associated with the presence of an irregular liver surface, nodular-ity, and thickened liver edge. Increased liver elasticity was also associated with the presence of a fatty-appearing liver, irregular surface, firm liver, and nodularity. Unfortunately, data on histopathologic steatosis were not collected, and the effect of steatosis on elasticity was not assessed.
ROC curves had an AUC of 0.8 for stages of at least 2, 0.85 for stages of at least 3, and 0.86 for stage 4, with FibroScan values of at least 7 kPa, at least 9.5 kPa, and at least 11.8 kPa (with highest accuracy), respectively. When gross appearance of cirrhosis alone was used as the gold standard, the ROC curve had an AUC of 0.857, with the highest accuracy for predicting cirrhosis with a FibroScan measurement of at least 20.4 kPa. When histology and/or gross appearance were used as diagnostic criteria, the ROC curve had an improved AUC of 0.875. Once again, the highest accuracy for predicting cirrhosis was with the FibroScan measurement of at least 11.8 kPa.
In the subgroup analysis of hepatitis C virus patients, the ROC curve had an AUC of 0.921 for stage 4, with the highest accuracy occurring with the FibroScan measurement of at least 11.8 kPa. When histology and/or gross appearance were used as diagnostic criteria, the AUC improved to 0.925.
FibroScan is a promising new modality for the noninvasive assessment of liver fibrosis. Current studies on FibroScan should be interpreted with some caution due to the limitations of percutaneous liver biopsy, which has been historically used as the gold standard. Unfortunately, few centers in North America use lap-aroscopic liver biopsy, though it is more widely used in Europe. Access to laparoscopic liver biopsy is limited because these procedures are generally performed by surgeons. As percutaneous liver biopsies are an acceptable standard and easily performed by hepatologists or radiologists, it is difficult to justify asking our surgical colleagues to perform a laparoscopic liver biopsy. At our institution, laparoscopic liver biopsies are performedby a hepatologist. As well, all hepatology fellows at our institution are trained in the technique. In the future, further studies using laparoscopic liver biopsy as a gold standard should be considered for diseases other than hepatitis C virus.
Footnotes
Dr. Nudo was the recipient of a 2006 AASLD Advanced Hepatology Fellowship during the time of this research.
References
- 1.van Leeuwen DJ, Wilson L, Crowe DR. Liver biopsy in the mid-1990s: questions and answers. Semin Liver Dis. 1995;15:340–359. doi: 10.1055/s-2007-1007286. [DOI] [PubMed] [Google Scholar]
- 2.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 3.Guido M, Rugge M. Liver biopsy sampling in chronic viral hepatitis. Semin Liver Dis. 2004;24:89–97. doi: 10.1055/s-2004-823103. [DOI] [PubMed] [Google Scholar]
- 4.Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003;38:427–432. doi: 10.1080/00365520310000825. [DOI] [PubMed] [Google Scholar]
- 5.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 6.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Campbell MD, Jeffers LJ, Reddy KR. Liver biopsy and laparoscopy. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff's Diseases of the Liver. 10th ed. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2007. pp. 61–81. [Google Scholar]
- 9.Vargas C, Jeffers LJ, Bernstein D, Reddy KR, Munnangi S, et al. Diagnostic laparoscopy: a 5-year experience in a hepatology training program. Am J Gastroenterol. 1995;90:1258–1262. [PubMed] [Google Scholar]
- 10.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 12.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 13.de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 14.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–1517. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 16.Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411–412. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Kazemi F, Kettaneh A, N'kontchou G, Pinto E, Ganne-Carrie N, et al. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230–235. doi: 10.1016/j.jhep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Melin P, Dacon A, Gauchet A, Schoeny M, Diebold MD. Dépistage non invasif de la fibrose hépatique. Intérê t du FibroScan en consultation d'alcoologie. Alcoologie et Addictologie. 2005;27:191–196. [Google Scholar]
- 19.Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 20.Pares A, Caballeria L, Lazaro E, Garcia-Criado A, Navasa M, et al. Transient elastography: a new and useful non-invasive method for assessing liver damage progression in primary biliary cirrhosis [abstract] Hepatology. 2005;42(suppl 1):A464. [Google Scholar]
- 21.Servin-Abad L, Jeffers L, Bejarano P, Casanova-Romero P, de Medina M, et al. Correlation between liver histology, gross appearance and elasticity measured with the shear elasticity probe. Hepatology. 2006;44(suppl 1):578A. [Google Scholar]
- 22.Saito H, Tada S, Nakamoto N, Kitamura K, Horikawa H, et al. Efficacy of non-invasive elastometry on staging of hepatic fibrosis. Hepatol Res. 2004;29:97–103. doi: 10.1016/j.hepres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, et al. Value of two non-invasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838–845. doi: 10.1002/hep.20814. [DOI] [PubMed] [Google Scholar]
- 24.Masaki N, Imamura M, Kikuchi Y, Oka S. Usefulness of elastometry in evaluating the extents of liver fibrosis in hemophiliacs coinfected with hepatitis C virus and human immunodeficiency virus. Hepatol Res. 2006;35:135–139. doi: 10.1016/j.hepres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, et al. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD) Gut. 2007;56:1330–1331. doi: 10.1136/gut.2007.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Bravo AA, Seth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 28.Sherlock S, Dooly J. Biopsy of the liver. In: Sherlock S, Dooly J, editors. Diseases of the Liver and Biliary System. 10th ed. London, England: Blackwell Science; 1997. pp. 33–42. [Google Scholar]
- 29.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 30.Batts K P, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig J. The nomenclature of chronic active hepatitis: an obituary. Gastro-enterology. 1993;105:274–278. doi: 10.1016/0016-5085(93)90037-d. [DOI] [PubMed] [Google Scholar]
- 32.Kawamoto M, Mizuguchi T, Katsuramaki T, Nagayama M, Oshima H, et al. Assessment of liver fibrosis by a noninvasive method of transient elastography and biochemical markers. World J Gastroenterol. 2006;12:4325–4330. doi: 10.3748/wjg.v12.i27.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdi W, Millan JC, Mezey E. Sampling variability on percutaneous liver biopsy. Arch Intern Med. 1979;139:667–669. [PubMed] [Google Scholar]
- 34.Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, et al. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568–571. doi: 10.1016/s0016-5107(96)70192-x. [DOI] [PubMed] [Google Scholar]
- 35.Nord HJ. Biopsy diagnosis of cirrhosis: blind percutaneous versus guided direct vision techniques—a review. Gastrointest Endosc. 1982;28:102–104. doi: 10.1016/s0016-5107(82)73015-9. [DOI] [PubMed] [Google Scholar]
- 36.Soloway RD, Baggenstoss AH, Schoenfield LJ, Summerskill WH. Observer error and sampling variability tested in evaluation of hepatitis and cirrhosis by liver biopsy. Am J Dig Dis. 1971;16:1082–1086. doi: 10.1007/BF02235164. [DOI] [PubMed] [Google Scholar]
- 37.Bruguera M, Bordas JM, Mas P, Rodes J. A comparison of the accuracy of peritoneoscopy and liver biopsy in the diagnosis of cirrhosis. Gut. 1974;15:799–800. doi: 10.1136/gut.15.10.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrera JL, Brewer TG, Peura DA. Diagnostic laparoscopy: a prospective review of 100 cases. Am J Gastroenterol. 1989;84:1051–1054. [PubMed] [Google Scholar]
- 39.Pagliaro L, Rinaldi F, Craxì A, Di Piazza S, Filippazzo G, et al. Percutaneous blind biopsy versus laparoscopy with guided biopsy in diagnosis of cirrhosis. A prospective, randomized trial. Dig Dis Sci. 1983;28:39–43. doi: 10.1007/BF01393359. [DOI] [PubMed] [Google Scholar]
- 40.Baggenstoss AH. Morphologic and etiologic diagnoses from hepatic biopsies without clinical data. Medicine (Baltimore) 1966;45:435–443. doi: 10.1097/00005792-196645060-00005. [DOI] [PubMed] [Google Scholar]
- 41.Heit HA, Johnson LF, Rabin L. Liver surface characteristics as observed during laparoscopy correlate with biopsy findings. Gastrointest Endosc. 1978;24:288–290. doi: 10.1016/s0016-5107(78)73545-5. [DOI] [PubMed] [Google Scholar]
- 42.Idrovo V, Dailey PJ, Jeffers LJ, Coelho-Little E, Bernstein D, et al. Hepatitis C virus RNA quantification in right and left lobes of the liver in patients with chronic hepatitis C. J Viral Hepat. 1996;3:239–246. doi: 10.1111/j.1365-2893.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 43.Jeffers LJ, Findor A, Tung SN, Reddy R, Silva M, Schiff ER. Minimizing sampling error with laparoscopic guided liver biopsy of right and left lobes. Gastrointest Endosc. 1991;37:A266. [Google Scholar]