Wilson disease (WD), an inborn copper metabolism defect, is traditionally diagnosed on the basis of clinical features, positive family history, biochemical parameters, the presence of Kayser-Fleischer rings on slit lamp eye examination, and neurologic abnormalities.1,2 Occasionally, however, patients may only have hepatic manifestations that mimic nearly any other hepatopathy. Some of these patients may have normal laboratory results such as normal serum ceruloplasmin levels and urine copper excretion, or they may display normal neurologic function. In this subset of patients, the diagnosis of WD becomes complicated and subsequent management difficult. Another interesting facet of WD is the predisposition to hepatocellular carcinoma (HCC), which is a rare but fatal complication in the absence of early diagnosis and initiation of treatment.3 To date, 23 cases of HCC associated with WD have been reported in the literature.3–13 This case study briefly reviews the pertinent literature1–13 and adds another atypical case of WD, which was initially recognized as HCC on fineneedle aspiration cytology and subsequently confirmed by strong family history, the presence of cirrhosis, elevated serum ceruloplasmin levels, increased urinary copper, and significant copper deposition in the liver parenchyma.
Case Report
A 59-year-old white man complained of gradually progressive pedal edema, increasing abdominal girth, and a 15-pound weight gain over a 3-week period. His past medical history included hypertension, diabetes mellitus type II, hyperlipidemia, osteoarthritis, osteomyelitis of the left foot with status post-below-the-knee amputation 15 years ago and cervical myelopathy with status postdiscectomy of the C5 through C6 vertebrae 5 years ago. The patient's medications were aspirin 325 mg QD, atenolol 50 mg QD, ibuprofen 600 mg TID, NPH 35 U subcutaneous BID, lisinopril 20 mg QD, metformin 850 mg BID, niacin 500 mg QHS, psyllium 1 tsp QD, and beclomethasone (Beconase AQ, GlaxoSmithKline) 1 spray in each nostril QD. He did not have any known drug allergies. The patient lived alone, worked as a contractor, was a social drinker, had a prior history of smoking 10–15 packs a year, and had no history of illicit drug use. His mother died at the age of 90 from a stroke, and his father, a nondrinker, died in his forties from liver cirrhosis.
Abnormal physical findings included deep tan, mild scleral icterus, palpable liver edge 4 cm below the right costal margin, two-plus edema to the level of the knees, and left below-the-knee amputation stump. There was no evidence of ataxia, asterixis, or neurologic abnormalities.
Pertinent laboratory data were normal hemograms and differential counts, electrolytes within reference limits, prothrombin time of 39.4 sec (ref, 22.8–39.8), aspartate aminotransaminase of 203 IU/L (ref, 1–50), alanine aminotransaminase of 177 IU/L (ref, 1–53), alkaline phosphatase of 631 IU/L (ref, 30–110), total bilirubin of 3.8 mg/dL (ref, 0.1–1.2), direct bilirubin of 3.4 mg/dL (ref, 0–0.8), and albumin of 2.1 g/dL (ref, 3.5–5).
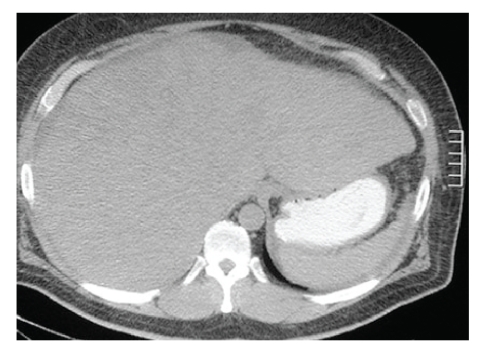
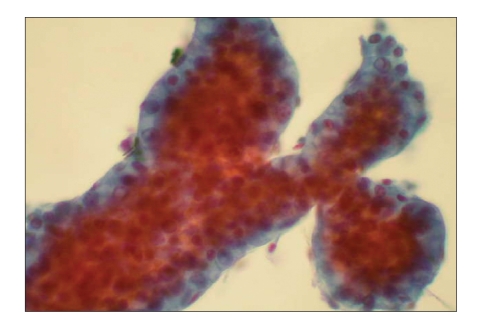
Hepatitis panel testing was negative, and other miscellaneous measurements included iron of 112 pg/dL (ref, 35–150), total iron-binding capacity of 331 pg/dL (ref, 250–450), iron saturation of 33.8% (ref, 10–50%), ferritin of 166 ng/mL (ref, 22–322), ceruloplasmin of 223 mg/dL (ref, 20–60), and hemochromatosis genotyping that tested negative for the 282Y and H63D mutations. Autoimmune studies (antinuclear antibody, antimitochondrial, antismooth muscle, and alfa-1-antitrypsin) were also negative. Other findings included polyclonal gammopathy serum on protein electrophoresis, consistent with acute inflammatory pattern, carcinoembryonic antigen of 1.8 ng/mL (ref, 0.5–5), beta-human chorionic gonadotrophin of less than 2.0 mIU/mL (ref, 0–10), alfa-fetoprotein of 3.8 ng/mL (ref, 1.3–8.1), and carbohydrate antigen 19-9 of less than 3 IU/mL (ref, 0–36). Computed tomography (CT) scan of the abdomen revealed a diffusely enlarged liver and probable cirrhosis (Figure 1). Subsequent CT scan-guided fine-needle aspiration cytology revealed a well-differentiated trabecular HCC in micronodular cirrhosis (Figure 2).
Figure 1.
Computed tomography scan reveals a diffusely enlarged liver of probable cirrhosis without distinct mass lesions.
Figure 2.
Fine-needle aspiration cytology displays characteristic trabecular growth pattern of malignant hepatocytes associated with occasional benign surface endothelial cells (Papanicolaou stain, 400x).
Further discussion with the patient's family revealed that a niece, at the age of 20, was transplanted for fulminant hepatic failure secondary to WD. Slit lamp examination was negative for Kayser-Fleischer rings, and serum copper levels measured 0.25 pg/mL (ref, 0.75–1.45), whereas urinary copper measured 117 Hg/24 hours (ref, 15–60).
The patient was immediately treated with symptomatic regimens. Two hospital readmissions within 2-week intervals were highlighted by watery diarrhea, pruritus, right upper quadrant abdominal pain, increasing jaundice, alcoholic stools, and dark urine. Ascitic fluid analysis was transudative and negative for malignancy. The hospital stay was also characterized by gradual deterioration. The patient became terminal and developed hypotension, shock, acute renal failure, hyperkalemia, and metabolic acidosis. Intravenous hydration, pressors, and antibiotics had no discernable effects.
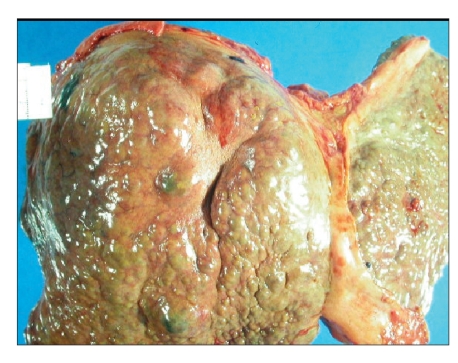
Autopsy confirmed the diagnosis of a well-differentiated HCC in WD-related liver cirrhosis with metastases in both lungs (Figure 3). The liver copper stain was positive, and the liver copper load was greater than 1,000 pg/g (ref, 10–35). Brain imaging revealed hepatic encephalopathy with negative copper stain/deposition and no lenticular nucleus degeneration.
Figure 3.
Postmortem evaluation confirmed the diagnosis of well-differentiated hepatocellular carcinoma arising in Wilson disease-related cirrhosis.
Discussion
WD is an autosomal recessive metabolic disorder with an incidence of 1:40,000. The WD gene is located on chromosome 13 and codes for a copper-transporting P-type ATPase-ATP7B. Mutations of the WD gene cause impaired biliary copper excretion, resulting in copper accumulation in many vital areas, including the liver, central nervous system, and cornea. Patients may therefore develop cirrhosis, neurologic manifestations, and KayserFleischer rings.1–13
Although hepatitis and cirrhosis are well-recognized complications of WD, HCC is extremely rare as a complication. The first case of HCC associated with WD was reported in 1959. To date, there have been 23 such cases reported in the literature.3–13 The patients were almost exclusively male and ranged from 12 to 66 years of age (mean age, 39 years). The average disease duration was 18 years, and the majority of cases received treatment with D-penicillamine chelation. The diagnosis of HCC was confirmed in 12 patients at autopsy, in 3 patients during liver transplantation, and, in 1 case each, via liver core needle biopsy, exploratory surgery, surgical lung biopsy for metastasis, and liver fine-needle aspiration cytology. In 4 cases, the diagnosis remained undisclosed.
The diagnosis of WD in our patient was based upon positive family history, presence of cirrhosis, increased serum ceruloplasmin levels, increased urinary copper, and abundant copper deposition in the liver. Etiologies that are more common for HCC and liver cirrhosis, such as hepatitis B virus, hepatitis C virus, alcohol abuse, hemochromatosis, and chronic Aspergillus flavus exposure, were excluded clinically, biochemically, and genetically. Among the 23 prior cases in the literature, our index case is one of the oldest patients with HCC and WD and the second to be initially recognized as HCC.3–13 This atypical presentation of WD occurs in 10–14% of patients and is largely attributed to the diversity of WD gene mutations and phenotypes.3
Carcinogenesis in WD is thought to be the result of accumulated copper in the liver and underlying cirrhosis. Although copper accumulation was originally thought to have a potential protective effect against hepatocarcinogenesis, recent evidence suggests otherwise. Copper deposition in the liver is actually a risk factor for the development of HCC. Some researchers have also observed that decreased copper in patients following D-penicillamine and other chelator treatments may enhance the risk of developing HCC. A consensus on this topic, however, has yet to be developed.1–12
The treatment of WD-related HCC does not differ from HCCs of other etiologies and consists of either surgery or chemoembolization, with variable results. Several cases managed with chemoembolization have resulted in early death because of fulminant liver failure. This unfavorable outcome can be explained on the basis of overwhelming hepatocytic degeneration induced by excessive copper deposits and the effect of anticancer treatment. Liver transplantation is a promising alternative and has also been advocated for patients with end-stage WD. Liver transplantation may not only restore liver function but may also alleviate other clinical symptoms and neurologic dysfunctions of WD.3
As a preventive measure in WD-related HCC, it is important to make an early diagnosis of WD to arrest further progression of hepatitis and cirrhosis. All patients with unresolving abnormal liver function tests should be screened for the possibility of HCC.3
Summary
A 59-year-old white man with atypical presentations of WD was initially diagnosed with HCC. Subsequent follow-up evaluations supported the diagnosis of WD with a strong family history, elevated serum ceruloplasmin levels, increased urinary copper, and significant copper deposition in the liver. The development of HCC in WD is most likely secondary to excessive copper deposition in the liver and concurrent cirrhosis.
References
- 1.Das SK, Ray K. Wilson's disease: an update. Nat Clin Pract Neurol. 2006;2:482–493. doi: 10.1038/ncpneuro0291. [DOI] [PubMed] [Google Scholar]
- 2.Loudianos G, Gitlin JD. Wilson's disease. Sem Liver Dis. 2000;29:353–364. doi: 10.1055/s-2000-9389. [DOI] [PubMed] [Google Scholar]
- 3.Xu R, Bu-Ghanim M, Fiel MI, Schiano T, Cohen E, Thung SN. Hepatocellular carcinoma associated with an atypical presentation of Wilson's Disease. Semin Liver Dis. 2007;27:122–127. doi: 10.1055/s-2007-967203. [DOI] [PubMed] [Google Scholar]
- 4.Savas N, Canan O, Ozcay F, Bilezikci B, Karakayali H, et al. Hepatocellular carcinoma in Wilson's disease: a rare association in childhood. Pediatr Transplant. 2006;10:639–643. doi: 10.1111/j.1399-3046.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 5.Ozçay F, Canan O, Bilezikçi B, Torgay A, Karakayali H, Haberal M. Effects of living donor liver transplantation on outcome of children with inherited liver disease and hepatocellular carcinoma. Clin Transplant. 2006;20:776–782. doi: 10.1111/j.1399-0012.2006.00571.x. [DOI] [PubMed] [Google Scholar]
- 6.Avdinli M, Harmanci O, Ersoy O, Iskit AT, Ozcebe O, et al. Two unusual cases with Wilson's disease: hepatoma and fulminant hepatitis treated with plasma exchange. J Natl Med Assoc. 2006;98:1989–1991. [PMC free article] [PubMed] [Google Scholar]
- 7.Kumagi T, Horiike N, Abe M, Kurose K, Iuchi H, et al. Small hepatocellular carcinoma associated with Wilson's disease. Intern Med. 2005;44:439–443. doi: 10.2169/internalmedicine.44.439. [DOI] [PubMed] [Google Scholar]
- 8.Iwadate H, Ohira H, Suzuki T, Abe K, Yokokawa J, et al. Hepatocellular carcinoma associated with Wilson's Disease. Intern Med. 2004;43:1042–1045. doi: 10.2169/internalmedicine.43.1042. [DOI] [PubMed] [Google Scholar]
- 9.Harada M. Wilson's disease and hepatocellular carcinoma [editorial] Intern Med. 2004;43:1021–1013. doi: 10.2169/internalmedicine.43.1012. [DOI] [PubMed] [Google Scholar]
- 10.Agret F, Vallet-Pichard A, Landau A, Carnot F, Pol S. Late presentation of Wilson's disease as cirrhosis complicating hepatocellular carcinoma [in French] Gastroenterol Clin Biol. 2003;27:130–131. [PubMed] [Google Scholar]
- 11.Walshe JM, Waldenstrom E, Sams V, Nordlinder H, Westermark K. Abdominal malignancies in patients with Wilson's disease. QJM. 2003;96:657–662. doi: 10.1093/qjmed/hcg114. [DOI] [PubMed] [Google Scholar]
- 12.Polio J, Enriquez RE, Chow A, Wood WM, Atterbury CE. Hepatocellular carcinoma in Wilson's disease: case report and review of the literature. J Clin Gastroenterol. 1989;11:220–224. doi: 10.1097/00004836-198904000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Lygren T. Hepatolenticular degeneration (Wilson's disease) and juvenile cirrhosis in the same family. Lancet. 1959;1:275–276. doi: 10.1016/s0140-6736(59)90202-8. [DOI] [PubMed] [Google Scholar]