Abstract
Ethanol is a potent teratogen for the developing central nervous system (CNS), and fetal alcohol syndrome (FAS) is the most common nonhereditary cause of mental retardation. Ethanol disrupts neuronal differentiation and maturation. It is important to identify agents that provide neuroprotection against ethanol neurotoxicity. Using an in vitro neuronal model, mouse Neuro2a (N2a) neuroblastoma cells, we demonstrated that ethanol inhibited neurite outgrowth and the expression of neurofilament (NF) proteins. Glycogen synthase kinase 3β (GSK3β), a multi-functional serine/threonine kinase negatively regulated neurite outgrowth of N2a cells; inhibiting GSK3β activity by retinoic acid (RA) and lithium induced neurite outgrowth, while over-expression of a constitutively active S9A GSK3β mutant prevented neurite outgrowth. Ethanol inhibited neurite outgrowth by activating GSK3β through the dephosphorylation of GSK3β at serine 9. Cyanidin-3-glucoside (C3G), a member of the anthocyanin family rich in many edible berries and other pigmented fruits, enhanced neurite outgrowth by promoting p-GSK3β(Ser9). More importantly, C3G reversed ethanol-mediated activation of GSK3β and inhibition of neurite outgrowth as well as the expression of NF proteins. C3G also blocked ethanol-induced intracellular accumulation of reactive oxygen species (ROS). However, the antioxidant effect of C3G appeared minimally involved in its protection. Our study provides a potential avenue for preventing or ameliorating ethanol-induced damage to the developing CNS.
Keywords: Alcohol, Development, Differentiation, Fetal alcohol syndrome, Neuroprotection
Introduction
Fetal alcohol spectrum disorders (FASD) are caused by maternal alcohol consumption during pregnancy (Riley and McGee 2005), and fetal alcohol syndrome (FAS), the most severe form of FASD, is associated with increased fetal demise, intrauterine growth restriction, central nervous system (CNS) malformations, mental retardation, and craniofacial and skeletal defects. Epidemiologic data indicate that in the US, FAS occurs in 0.2–1.5 per 1,000 live births, whereas alcohol-related birth defects and brain developmental disorders occur in approximately 9 per 1,000 live births (Stromland and Pinazo-Duran 2002). FAS is the most common nonhereditary cause of mental retardation (Stratton et al. 1996; May and Gossage 2001). Moderate or even low levels of prenatal alcohol abuse can also be harmful and impair cognitive behavior and motor functions (Abel 1984; Mattson et al. 2001; O’Malley and Nanson 2002; O’Callaghan et al. 2003, 2007; Riley and McGee 2005). Prenatal exposure to alcohol disrupts many events of neuronal development, including neuronal survival, migration, neuromorphogenesis and synapse formation (Diamond and Gordon 1997; Luo and Miller 1998; Goodlett and Horn 2001; Olney 2004). These alcohol-induced alterations may underlie many of the behavioral deficits observed in FAS. Despite attempts to increase public awareness of the risks involved, increasing numbers of women are drinking during pregnancy (Ebrahim et al. 1999). Therefore, it is important to develop strategies that prevent or ameliorate alcohol-induced damages to the brain.
Anthocyanins are a group of natural phenolic compounds responsible for the coloring of many plants and fruits. Evidence suggests that anthocyanins may serve as a natural antioxidant (Prior 2003; Bagchi et al. 2004). Anthocyanins are reported to have many beneficial effects on human health, including reducing the risk of cardiovascular diseases, anti-inflammatory and anti-carcinogenic functions, involvement in diabetes prevention and vision improvement (Hou 2003; Bagchi et al. 2004; Ghosh and Konishi 2007; Zafra-Stone et al. 2007). Berry anthocyanins are beneficial in reducing age-associated oxidative stress, as well as in improving neuronal and cognitive brain function (Hou 2003; Bagchi et al. 2004). Cyanidin is the most common anthocyanin; it is present in 90% of fruits (Prior 2003). Cyanidin glucosides tend to have higher antioxidant capacity than peonidin or malvidin glucosides (Prior 2003). We purified cyanidin-3-glucoside (C3G) from blackberries and demonstrated that it has potent antioxidant and anti-tumor capacity (Ding et al. 2006). C3G inhibits neoplastic transformation and cancer cell metastasis (Ding et al. 2006). The present study is designed to determine whether C3G is beneficial in protecting ethanol neurotoxicity. We examined the effect of ethanol and C3G on the differentiation of mouse neuroblastoma Neuro2a (N2a) cells, a widely used in vitro model of neuronal differentiation. In response to serum starvation or treatment with retinoic acid (RA) or dibutyryl cAMP, N2a cells undergo neuronal differentiation characterized by cell cycle arrest, neurite outgrowth and up-regulation of neurofilament (NF) proteins (De Girolamo et al. 2000; Dasgupta and Milbrandt 2007).
We demonstrate here that ethanol inhibits neurite outgrowth and the expression of NFs in N2a cells. Treatment of C3G antagonizes the inhibitory effect of ethanol. Glycogen synthase kinase 3β (GSK3β), a multifunctional serine/threonine kinase, appears to play an important role in neuronal differentiation and mediates the action of C3G.
Materials and Methods
Materials
The antibodies directed against GSK3β and phospho-GSK3β (Ser9 and Tyr216) were purchased from Cell Signaling Technology, Inc. (Beverly, MA). The antibodies directed against phospho-Tau (Ser396) and actin were purchased from Sigma Chemical Co. (St. Louis, MO). The antibody directed against light-chain NF was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antioxidants N-acetyl-L-cysteine (NAC) and glutathione monoethyl ester (GSH-MEE) were obtained from Calbiochem (La Jolla, CA). C3G was purified from blackberries using high performance liquid chromatography as previously described (Ding et al. 2006). The purity of C3G isolated by this method was 99%.
Cell Culture and Ethanol Exposure Protocol
Neuro2a cells obtained from ATCC were grown in Eagle’s MEM containing 10% fetal bovine serum (FBS), 2 mM L-glutamine and 25 μg/ml gentamicin, 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C with 5% CO2. A method utilizing sealed containers was used to maintain ethanol concentrations in the culture medium. With this method, ethanol concentrations in the culture medium can be accurately maintained (Luo et al. 2001). A pharmacologically relevant concentration of 400 mg/dl was used in this study. In general, the concentration for in vitro studies is higher than that required to produce a similar effect in vivo (Luo et al. 2001).
Measurement of Neurite Outgrowth
Cells were viewed by phase-contrast microscopy using an inverted microscope. Neurite length was measured as the distance between the center of the cell soma and the tip of its longest neurite. The neurite had to meet the following requirements: it must emerge from an isolated cell (not a clump of cells), it must not contact other cells or neurites, and it must be longer than the diameter of the cell body. For each experiment, 30 cells per well were randomly measured with MetaMorph software (Molecular Devices, Sunnyvale, CA). The measurements were performed in triplicates (3 wells).
Cell Transfection
V5-tagged GSK3β S9A construct (substitution of serine 9 to alanine) carried by vector pcDNA3 was previously described (Ma et al. 2007). Cell transfection was carried out with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions.
Immunoblotting
Cells were washed with phosphate-buffered saline (PBS, pH 7.4) and lysed with RIPA buffer [150 mM NaCl, 50 mM Tris (pH 8.0), 1% Nonidet P-40 (NP-40), 0.1% sodium dodecylsulfate (SDS), 0.5% deoxycholic acid sodium, 0.1 mg/ml phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and 3% aprotinin] on ice for 10 min, solubilized cells were centrifuged, and the supernatant was collected.
The procedures for immunoblotting and quantitative analysis of proteins imaged in the blots have been previously described (Chen et al. 2004). Briefly, after the protein concentrations were determined, aliquots of the protein samples (20–40 μg) were loaded into the lanes of a SDS-polyacrylamide gel. The protein samples were separated by electrophoresis, and the separated proteins were transferred to nitrocellulose membranes. The membranes were blocked with either 5% BSA or 5% nonfat milk in 0.010 M PBS (pH 7.4) and 0.05% Tween-20 (TPBS) at room temperature for 1 h. Subsequently, the membranes were probed with primary antibodies directed against target proteins for 2 h at room temperature or overnight at 4°C. After three quick washes in TPBS, the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (Amersham, Arlington Hts. IL) diluted at 1:2,000 in TPBS for 1 h. The immune complexes were detected by the enhanced chemiluminescence method (Amersham). In some cases, the blots were stripped and re-probed with an anti-actin antibody. The density of immunoblotting was quantified with the software Quantity One (Bio-Rad Laboratories, Hercules, CA). The expression of target proteins was normalized to the levels of actin.
GSK3β Small Interfering RNA (siRNA)
GSK3β siRNA (Catalog # 6301; Cell Signaling Tech., Inc) was transfected into SK-N-MC cells with oligofectamine reagent (Invitrogen) according to the manufacturer’s protocol. At 48 h after transfection, the cells were subjected to immunoblotting analysis for GSK3β expression.
Detection of Intracellular Reactive Oxygen Species
Intracellular reactive oxygen species (ROS) levels were measured using the fluorescent dye carboxy-H2DCFDA staining method as previously described (Chen et al. 2008). Once incorporated into cells, H2DCFDA is converted into a nonfluorescent polar derivative (H2DCF) by cellular esterases. H2DCF is rapidly oxidized to the highly fluorescent 2,7-dichlorofluorescein (DCF) by intracellular ROS, mainly H2O2 (LeBel et al. 1992). Therefore, the intensity of the DCF signal reflects the quantity of intracellular ROS. N2a cells were treated with ethanol in the presence or absence of C3G or other antioxidants for the indicated times. After treatment, cells were transferred to luminometer cuvettes containing 10 μM carboxy-H2DCFDA (C400) (Molecular Probes, Inc., Eugene, OR) in pre-warmed PBS buffer for 30 min. Intracellular ROS levels (DCF signals) were measured with a flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA) at an emission wavelength of 525 nm.
Statistical Analysis
Differences among treatment groups were tested using analysis of variance (ANOVA). Differences in which P was less than 0.05 were considered statistically significant. In cases where significant differences were detected, specific post-hoc comparisons between treatment groups were examined with Student–Newman–Keuls tests.
Results
GSK3β Mediates Neurite Outgrowth of N2a Cells
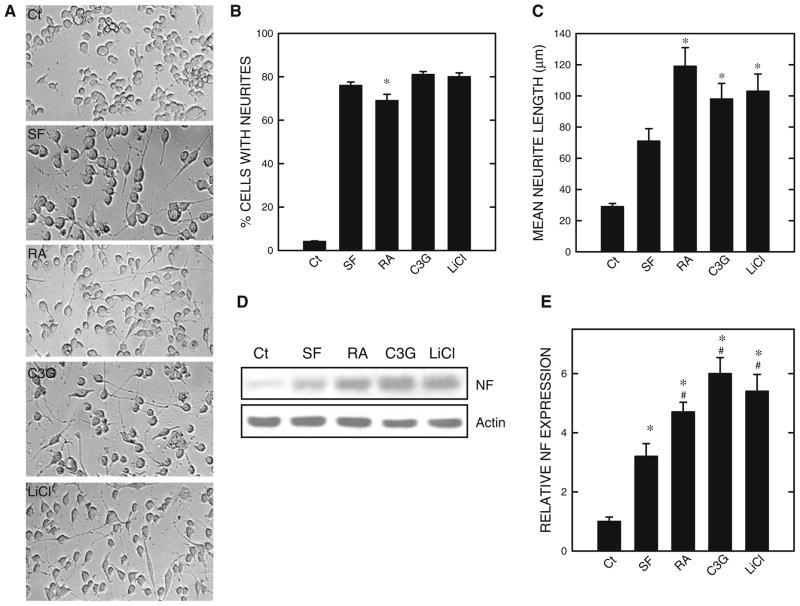
Neuronal differentiation of N2a cells was induced by serum starvation or treatment of RA or C3G in the absence of serum (Fig. 1). The percentage of cells extending neurites under these treatment conditions was similar, approximately 70–80% (Fig. 1b). RA or C3G further increased the average length of neurites (Fig. 1c), compared to serum starvation only. The intermediate filaments of neuronal cells NFs are important components of the cytoskeletal network in neurons. High levels of NFs are detected along axons of neurons. NFs consist of three subunits, NF-H, NF-M, and NF-L, corresponding to heavy, medium, and light chains, respectively. Consistent with morphological differentiation, light-chain neurofilament (NF-L, 68 kDa) was upregulated by serum starvation or treatment with RA or C3G (Fig. 1d and e). It was noted that RA- or C3G-mediated upregulation of NF-L expression was more than that induced by serum starvation.
Fig. 1.
Regulation of neurite outgrowth of N2a cells. N2a cells were maintained in a culture medium containing 10% serum. To induce differentiation, the culture medium was removed and replaced with a new medium containing 0% serum (SF) supplemented with/without retinoic acid (RA, 5 μM), cyanidin-3-glucoside (C3G, 5 μM), or LiCl (10 mM). a. At 48 h after serum starvation (SF) and treatment of RA, C3G, or LiCl, cell morphology was examined under an inverted light microscope. b. The percentage of cells baring neurites in 100 cells was calculated as described under the section “Materials and Methods”. c. The average length of neurites was determined. For each experiment, the result was the mean of 100 cells. * denotes significant difference from control or SF-treated cultures. The experiment was replicated three times. d. Cell lysates were collected and the expression of light-chain neurofilament (NF) was determined by immunoblotting. The experiment was replicated three times. e. The relative amounts of NF were measured by densitometry and normalized to the expression of actin. * denotes significant difference from SF-treated cultures
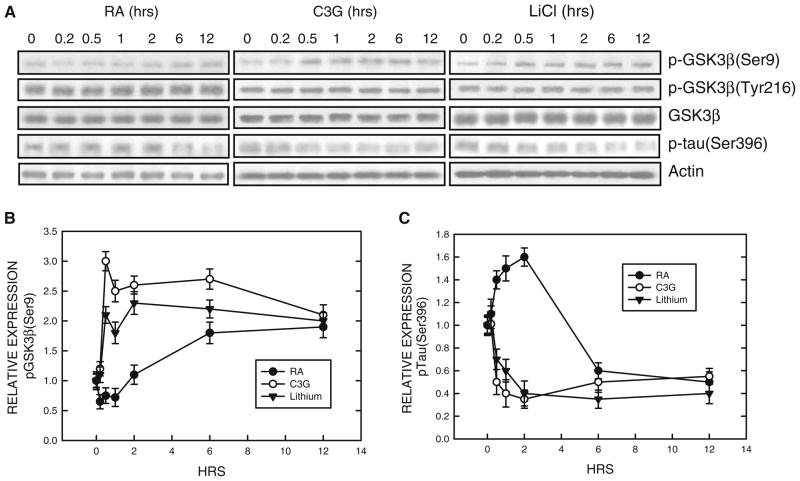
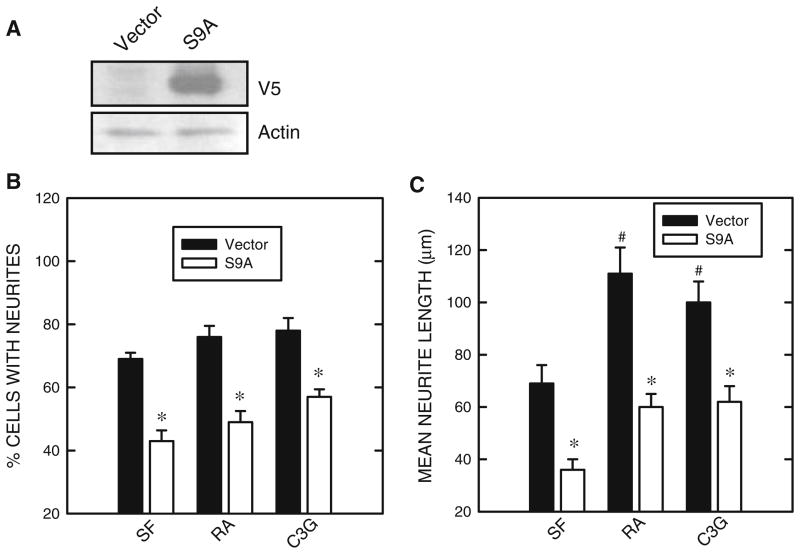
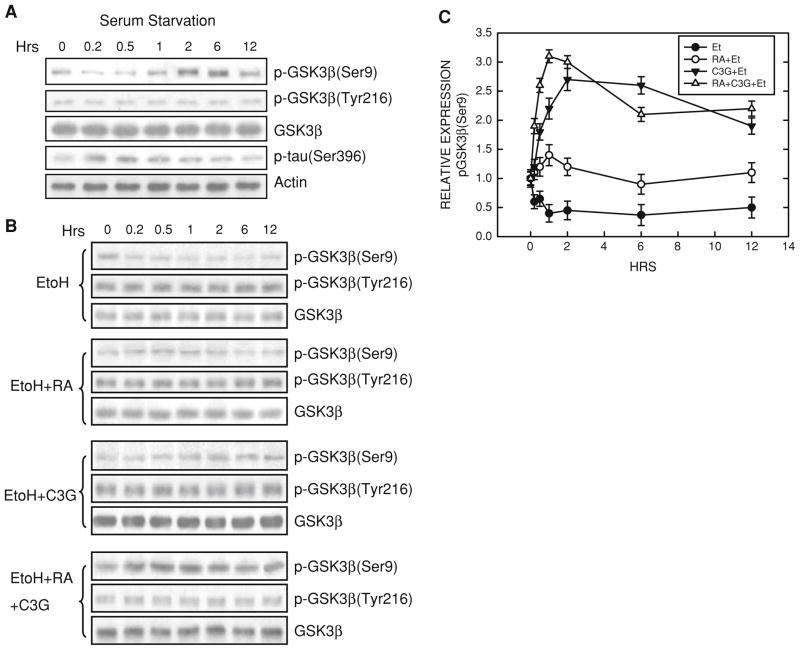
Previous studies suggested that GSK3β may negatively regulate the differentiation of N2a cells (García-Pérez et al. 1999; Orme et al. 2003). We confirmed this and showed an inhibitor of GSK3β, lithium, promoted neurite outgrowth and expression of NF-L (Fig. 1). We sought to determine whether treatment of RA or C3G inhibited GSK3β activity. The activity of GSK3β is regulated by its phosphorylation status; phosphorylation at serine 9 inhibits GSK3β activity while phosphorylation at tyrosine 216 (Tyr216) activates GSK3β. Both RA and C3G induced phosphorylation of GSK3β at Ser9 [p-GSK3β(Ser9)] without affecting p-GSK3β(Tyr216) (Fig. 2). However, the time sequences of p-GSK3β(Ser9) induced by RA and C3G treatment were different. RA-induced p-GSK3β(Ser9) expression was not evident until 6 h of exposure. C3G produced a much more rapid and stronger increase in p-GSK3β(Ser9) expression; C3G-induced p-GSK3β(Ser9) occurred at 0.5 h after exposure. Inhibition of GSK3β activity by these treatments was confirmed by a dephosphorylation of its substrate Tau at serine 396 [p-Tau(Ser396)] (Fig. 2). Generally, the expression levels of p-GSK3β(Ser9) inversely correlated to p-Tau(Ser396). LiCl directly inhibits GSK3β activity and also stimulates GSK3β phosphorylation at serine 9 (Zhang et al. 2003); its inhibition on GSK3β activity in N2a cells was verified (Fig. 2). To further establish the role of GSK3β and the effect of p-GSK3β(Ser9) on neurite outgrowth, we transfected N2a cells with a constitutively active S9A GSK3β mutant construct. This S9A construct functions as a dominant active protein (Ma et al. 2007). As shown in Fig. 3, over-expression of the S9A GSK3β mutant inhibited neurite outgrowth induced by all treatments (serum starvation, RA or C3G) (Fig. 3). Together, these results indicated that inhibition of GSK3β by stimulating p-GSK3β(Ser9) promoted N2a cell differentiation.
Fig. 2.
Regulation of GSK3β phosphorylation. a. N2a cells grown in a medium containing 0% serum were treated with retinoic acid (RA, 5 μM), cyanidin-3-glucoside (C3G, 5 μM), or LiCl (10 mM) for specified times. Cell lysates were collected and the expression of phosphorylated GSK3β [p-GSK3β(Ser9) and p-GSK3β(Tyr216)], p-Tau (Ser396) and total GSK3β was examined with immunoblotting. The blots were stripped and re-probed with an anti-actin antibody. The experiment was replicated three times. b. The relative amounts of phosphorylated GSK3β(Ser9) were measured by densitometry and normalized to the expression of total GSK3β. Each data point (mean ± SEM) is the mean of three independent experiments. c. The relative amounts of p-Tau(Ser396) were measured as described above
Fig. 3.
Effect of GSK3β mutant (S9A) on neurite outgrowth. a. N2a cells were transfected with either a construct of GSK3β mutant (S9A) carrying a V5 tag or an empty vector. Forty-eight hours after transfection, cell lysates were collected and the expression of S9A GSK3β was determined by immunoblotting using an anti-V5 antibody. b. N2a cells were transfected with either a GSK3β mutant S9A construct or an empty vector. Twenty-four hours after transfection, cells were induced to differentiate as described under Fig. 1. The percentage of cells baring neurites was calculated. c. The average length of neurites was determined. For each experiment, the result was the mean of 100 cells. The experiment was replicated three times. * denotes significant difference from vector-transfected cultures. # denotes significant difference from vector-transfected and SF-treated cultures
C3G Protects N2a Cells Against Ethanol Inhibition of Neurite Outgrowth by Modulating GSK3β Activity
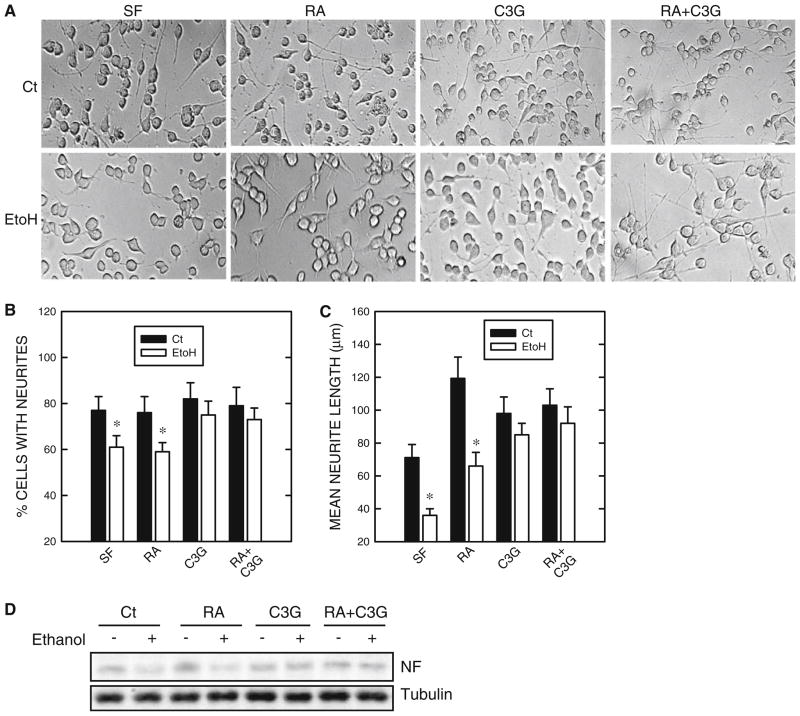
Ethanol inhibited serum starvation and RA-induced neurite outgrowth as well as the expression of NF-L (Fig. 4). Ethanol decreased both the percentage of cells baring neurites and the average length of neurites. Consistent with its inhibition of neurite outgrowth, ethanol also reduced the expression of NF-L (Fig. 4d). However, when C3G was included, ethanol-mediated inhibition of neuronal differentiation was reversed. C3G not only completely blocked the ethanol-induced decrease in the percentage of cells baring neurites (Fig. 4b), but it significantly inhibited ethanol-mediated reduction in neurite length (Fig. 4c). Consistent with the results on neurite outgrowth, C3G blocked ethanol-mediated down-regulation of NF-L expression (Fig. 4d).
Fig. 4.
Effect of ethanol on neurite outgrowth. N2a cells grown in a medium containing 0% serum were treated with retinoic acid (RA, 5 μM), cyanidin-3-glucoside (C3G, 5 μM), or both (5 μM of each) in the absence or presence of ethanol (400 mg/dl) for 48 h. a Cell morphology was examined under an inverted light microscope. b The percentage of cells baring neurites was calculated. c The average length of neurites was determined. * denotes significant difference from non-ethanol-treated controls. d Cell lysates were collected and the expression of light-chain neurofilament (NF) was determined by immunoblotting. The experiment was replicated three times
Serum starvation caused an initial and brief dephosphorylation of p-GSK3β(Ser9), followed by an increase in p-GSK3β(Ser9) at 2 h and 6 h (Fig. 5a). As expected, the levels of p-Tau(Ser396) inversely correlated to that of p-GSK3β(Ser9). Serum starvation did not affect p-GSK3β(Tyr216) and the expression of GSK3β. Ethanol exposure antagonized serum starvation- and RA-induced up-regulation of p-GSK3β(Ser9) (Fig. 5b). However, C3G-treated cells were resistant to ethanol-caused dephosphorylation of p-GSK3β(Ser9). Although ethanol antagonized RA-induced up-regulation of p-GSK3β(Ser9), inclusion of C3G completely reversed ethanol antagonism.
Fig. 5.
Effect of ethanol on GSK3β phosphorylation. a. Serum starvation was initiated. At specified times after serum starvation, cell lysates were collected and the expression of phosphorylated GSK3β [p-GSK3β(Ser9) and p-GSK3β(Tyr216)], p-Tau (Ser396) and total GSK3β was examined with immunoblotting. b. N2a cells grown in a medium containing 0% serum were treated with retinoic acid (RA, 5 μM), cyanidin-3-glucoside (C3G, 5 μM), or both in the absence or presence of ethanol (400 mg/dl) for specified times. Cell lysates were collected and the expression of phosphorylated GSK3β [p-GSK3β(Ser9) and p-GSK3β(Tyr216)] and total GSK3β was examined with immunoblotting. The experiment was replicated three times. c. The relative amounts of p-GSK3β(Ser9) were measured by densitometry and normalized to the expression of total GSK3β. Each data point (mean ± SEM) is the mean of three independent experiments
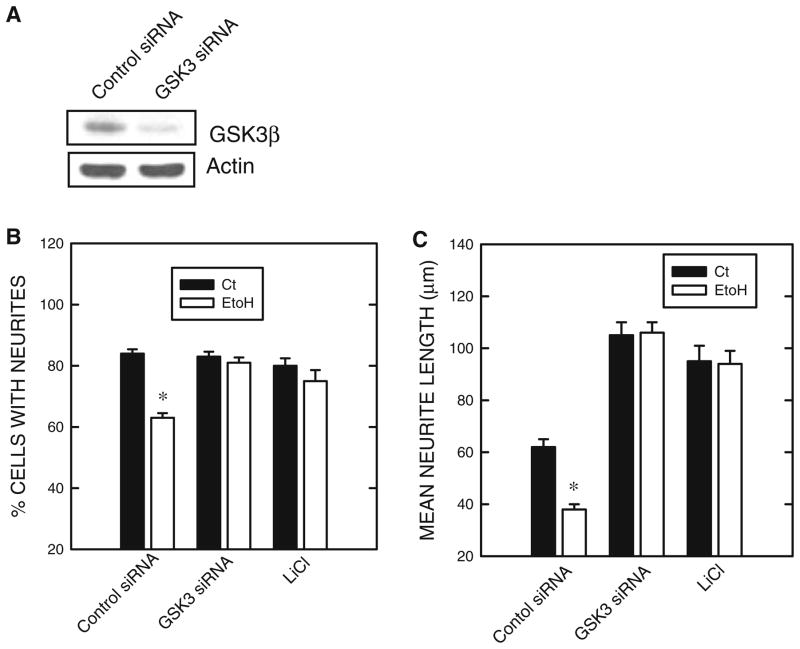
We further investigated the role of GSK3β in ethanol-induced repression of neurite outgrowth using a GSK3β siRNA and lithium. As shown in Fig. 6a, GSK3β siRNA effectively knocked down the expression of GSK3β. More importantly, both GSK3β siRNA and lithium significantly ameliorated ethanol-mediated inhibition of neurite outgrowth in N2a cells (Fig. 6b).
Fig. 6.
Effect of GSK3β siRNA and lithium on ethanol-induced inhibition of neurite outgrowth. a N2a cells were treated with a GSK3β siRNA or a scrambled siRNA (control siRNA) for 48 h as described under the section “Materials and Methods”. The expression of GSK3β and actin was determined with immunoblotting b. N2a cells were treated with a GSK3β siRNA or a scrambled siRNA for 24 h, and serum starvation was initiated in the presence of ethanol (0 or 400 mg/dl). LiCl treatment (0 or 10 mM) began 30 min prior to serum starvation. Forty hours later, the percentage of cells baring neurites B and the average length of neurites (c) were determined as described in Fig. 1. The experiment was replicated three times. * denotes significant difference from non-ethanol controls
The Antioxidant Property of C3G is Minimally Involved in Its Neuroprotective Effect
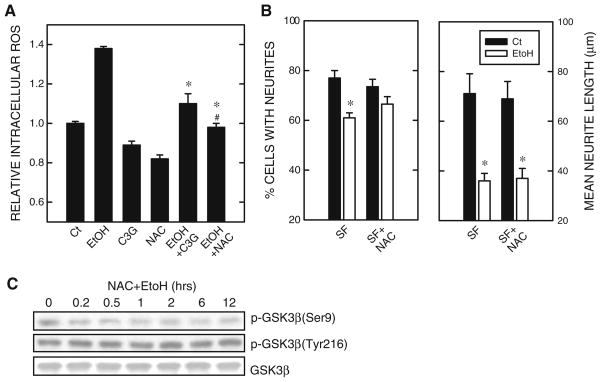
We have previously shown that ethanol causes intracellular accumulation of ROS and induces oxidative stress (Chen et al. 2008). Since C3G is a potent antioxidant (Ding et al. 2006), we sought to determine whether the neuroprotective effect of C3G was mediated by blocking ethanol-induced ROS production. As shown in Fig. 7a, both C3G and antioxidant NAC effectively inhibited ethanol-induced intracellular ROS accumulation. Unlike C3G, however, NAC did not promote neurite outgrowth (data not shown). NAC only modestly mitigated ethanol-induced reduction in a percentage of cells baring neurites, but did not alter ethanol’s inhibition on the growth of neurites (average neurite length) (Fig. 7b). Furthermore, NAC had little effect on ethanol-induced dephosphorylation of p-GSK3β(Ser9) (Fig. 7c). These results indicated that the neuroprotective effect of C3G was minimally mediated by its antioxidant property.
Fig. 7.
Effect of antioxidants on ethanol-induced inhibition of neurite outgrowth. a N2a cells were pretreated with C3G (5 μM) or antioxidant NAC (10 mM) for 30 min, then exposed to ethanol (0 or 400 mg/dl) for 6 h. Intracellular reactive oxygen species (ROS) were measured as described under the section “Materials and Methods”. ROS levels were expressed relative to untreated controls. Each data point represents the mean of three independent experiments. * denotes significant difference from ethanol-treated cultures. # denotes significant difference from ethanol + C3G group. b N2a cells were pretreated with antioxidant NAC (10 mM) for 30 min, then induced to differentiate by serum starvation (SF) in the presence or absence of ethanol (400 mg/dl) for 48 h. The percentage of cells baring neurites (panel on the left) and the average length of neurites (panel on the right) were determined as described under Fig. 1. The experiment was replicated three times. * denotes significant difference from non-ethanol-treated controls. c N2a cells were pretreated with antioxidant NAC (10 mM) for 30 min, then subject to serum starvation (SF) and ethanol exposure (400 mg/dl) for specified times. Cell lysates were collected and the expression of phosphorylated GSK3β [p-GSK3β(Ser9) and p-GSK3β(Tyr216)] and total GSK3β was examined with immunoblotting. The experiment was replicated three times
Discussion
We demonstrate that GSK3β is a negative regulator of N2a cell differentiation. GSK3β activity is inhibited during serum starvation- or RA-induced differentiation. GSK3β, a multifunctional serine/threonine kinase, regulates diverse biological processes, including glycogen metabolism, insulin signaling, cytoskeleton modification, transcription activation, and cell cycle/survival regulation. The activity of GSK3β is regulated by site-specific phosphorylation. Full activity of GSK3β generally requires phosphorylation at Tyr216, and conversely, phosphorylation at serine 9 (Ser9) inhibits GSK3β activity. Ser9 phosphorylation is probably the most important regulatory mechanism of GSK3β activation. Our results indicate that serum starvation or RA treatment inhibits GSK3β activity by promoting p-GSK3β(Ser9). Inhibition of GSK3β activity by lithium also induces neurite outgrowth in N2a cells. Lithium-mediated promotion of neurite outgrowth in N2a cells has been previously reported (García-Pérez et al. 1999; Orme et al. 2003). Contrarily, activation of GSK3β by over-expressing a constitutively active GSK3β mutant (S9A) significantly inhibits serum starvation- and RA-induced neurite outgrowth (Fig. 3). Therefore, GSK3β negatively regulates neurite outgrowth of N2a cells. Studies using primary neurons also indicate the involvement of GSK3β in the regulation of neurite outgrowth and neuronal differentiation. For example, lithium is shown to enhance neurite outgrowth of cultured cerebellar granule neurons, while expression of constitutively active GSK3β blocks neurite outgrowth (Muñoz-Montaño et al. 1999). Inhibition of GSK3β by selective inhibitors, a dominant-negative form of GSK3β or genetic knockdown promotes dendrite initiation and growth in cultured cortical neurons and hippocampal neurons in slice cultures (Naska et al. 2006). However, one report suggests that GSK3β may be a positive regulator of neuronal differentiation under certain circumstances because lithium is shown to inhibit neurite outgrowth in cultured hippocampal neurons generated from 18-day rat embryos (Takahashi et al. 1999).
We are the first to demonstrate that GSK3β mediates the effect of ethanol on neurite outgrowth. Ethanol exposure not only decreases the percentage of cells baring neurites, but also reduces the length of those neurites. Ethanol-induced inhibition of neurite outgrowth is likely mediated by its modulation of GSK3β activity. Ethanol dephosphorylates GSK3β at serine 9 without affecting p-GSK3β(Tyr216). Although it remains unclear whether GSK3β is a direct target of ethanol action, evidence from both in vitro and in vivo studies suggest that it may mediate ethanol neurotoxicity. Ethanol exposure during development activates GSK3β in cerebellar neurons probably by impairing insulin signaling (de la Monte and Wands 2002). Furthermore, lithium is shown to reduce ethanol-induced caspase-3 activation in the developing brain and apoptosis in cultured neurons (Zhong et al. 2006; Chakraborty et al. 2008).
We revealed that C3G reverses ethanol-induced inhibition of neurite outgrowth. C3G treatment is sufficient to promote N2a cell differentiation; this is consistent with its inhibition of GSK3β activity. C3G drastically up-regulates p-GSK3β(Ser9), and over-expressing a constitutively active GSK3β mutant (S9A) significantly inhibits C3G-induced neurite outgrowth (Fig. 3). These results indicate that C3G-induced promotion of neurite outgrowth is also mediated by its inhibition on GSK3β. More importantly, C3G-treated cells are more resistant to ethanol-induced inhibition of neurite outgrowth as well as NF expression (Fig. 4). Ethanol blocks RA-induced up-regulation of p-GSK3β(Ser9) but fails to inhibit C3G-mediated promotion of p-GSK3β(Ser9). Thus, the resistance is likely due to C3G-mediated p-GSK3β(Ser9) up-regulation being unaffected by ethanol (Fig. 5). It is unclear why ethanol inhibits p-GSK3β(Ser9) regulated by RA but not that regulated by C3G. Several up-stream kinases are capable of regulating p-GSK3β(Ser9), including p70 S6 kinase, extracellular signal-regulated kinases (ERKs), p90Rsk (also called MAPKAP kinase-1), protein kinase B (also called Akt), certain isoforms of protein kinase C (PKC), and cyclic AMP-dependent protein kinase (protein kinase A) (Grimes and Jope 2001). In addition, p-GSK3β(Ser9) is subject to regulation by protein phosphatases, such as PP2A and PP2B (Lee et al. 2005). It is likely that the mechanism underlying C3G regulation of p-GSK3β(Ser9) is different from that by RA or serum starvation. This possibility is currently under investigation.
It has been demonstrated that ethanol produces ROS (Chen et al. 2008), and C3G is a potent antioxidant (Ding et al. 2006). Like other commonly used antioxidants, such as NAC or GSH-MEE, C3G is effective at scavenging ethanol-induced ROS production (Fig. 6). NAC or GSH-MEE, however, fail to promote p-GSK3β(Ser9) and induce neurite outgrowth of N2a cells (data not shown). Unlike C3G, these antioxidants do not override ethanol-induced dephosphorylation of GSK3β at serine 9 (Fig. 6). As a result, these antioxidants do not offer protection against ethanol-induced inhibition of neurite outgrowth (Fig. 6). These results suggest that antioxidant properties of C3G are minimally involved in its modulation of neurite outgrowth.
Research suggests anthocyanins may exert their beneficial effects either through their ability to lower oxidative stress and inflammation or by directly altering the signaling involved in neuronal communication, calcium buffering ability, neuroprotective stress shock proteins, plasticity, and stress signaling pathways (Shukitt-Hale et al. 2008). Regardless of the mechanisms of C3G’s action, our study supports that C3G is beneficial in ameliorating alcohol neurotoxicity in vitro. Anthocyanins found in berry fruits have been implicated in improving aged-related neuronal and behavioral deficits in animal models (Galli et al. 2002; Andres-Lacueva et al. 2005; Shukitt-Hale et al. 2007, 2008). Anthocyanins are also shown to protect neurons against focal cerebral ischemic injury in rat brains (Sweeney et al. 2002; Shin et al. 2006).
Anthocyanins are able to cross the blood–brain barrier and localize in various brain regions important for motor, learning and memory functions. In a study where rats are fed a blueberry supplementation or a control diet for 8–10 weeks, several anthocyanins (cyanidin-3-O-beta-galactoside, cyanidin-3-O-beta-glucoside, cyanidin-3-O-beta-arabinose, malvidin-3-O-beta-galactoside, malvidin-3-O-beta-glucoside, malvidin-3-O-beta-arabinose, peonidin-3-O-beta-arabinose, and delphinidin-3-O-beta-galactoside) are found in the cerebellum, cortex, hippocampus or striatum of rats, but not in the controls (Andres-Lacueva et al. 2005). In another study where rats are fed a blackberry anthocyanin-enriched diet for 15 days, C3G is the predominant form of anthocyanins detected in the brain homogenate, and C3G content is higher in the brain than in the plasma (Talavéra et al. 2005). Peripheral C3G is able to cross the blood–brain barrier and distribute in brain regions that are affected by ethanol. It is therefore a feasible candidate with therapeutic potential for the treatment of alcohol neurotoxicity.
Acknowledgments
This research was supported by grants from the National Institutes of Health (AA015407), and the National Natural Science Foundation of China (30470544, 30471452, and 30570580). Dr. Z-J. Ke was also supported by the One Hundred Talents Program of the Chinese Academy of Sciences.
Abbreviations
- C3G
Cyanidin-3-glucoside
- FASD
Fetal alcohol spectrum disorders
- FAS
Fetal alcohol syndrome
- GSK3β
Glycogen synthase kinase 3β
- ROS
Reactive oxygen species
Contributor Information
Gang Chen, Department of Internal Medicine, University of Kentucky College of Medicine, 124C Combs Research Building, 800 Rose Street, Lexington, KY 40536, USA.
Kimberly A. Bower, Department of Internal Medicine, University of Kentucky College of Medicine, 124C Combs Research Building, 800 Rose Street, Lexington, KY 40536, USA
Mei Xu, Department of Internal Medicine, University of Kentucky College of Medicine, 124C Combs Research Building, 800 Rose Street, Lexington, KY 40536, USA.
Min Ding, National Institute for Occupational Safety and Health, Morgantown, WV 26505, USA.
Xianglin Shi, Graduate Center for Toxicology, University of Kentucky, 232 Bosomworth, Lexington, KY 40536, USA.
Zun-Ji Ke, Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200031, People’s Republic of China.
Jia Luo, Email: jialuo888@uky.edu, Department of Internal Medicine, University of Kentucky College of Medicine, 124C Combs Research Building, 800 Rose Street, Lexington, KY 40536, USA.
References
- Abel EL. Prenatal effects of alcohol. Drug Alcohol Depend. 1984;14:1–10. doi: 10.1016/0376-8716(84)90012-7. [DOI] [PubMed] [Google Scholar]
- Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Sen CK, Bagchi M, Atalay M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry (Mosc) 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Saito M, Mao RF, Wang R, Vadasz C, Saito M. Lithium blocks ethanol-induced modulation of protein kinases in the developing brain. Biochem Biophys Res Commun. 2008;367:597–602. doi: 10.1016/j.bbrc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- Chen G, Ma C, Bower KA, Shi X, Ke Z, Luo J. Ethanol promotes endoplasmic reticulum stress-induced neuronal death: involvement of oxidative stress. J Neurosci Res. 2008;86:937–946. doi: 10.1002/jnr.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Girolamo LA, Billett EE, Hargreaves AJ. Effects of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine on differentiating mouse N2a neuroblastoma cells. J Neurochem. 2000;75:133–140. doi: 10.1046/j.1471-4159.2000.0750133.x. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. Cell Mol Life Sci. 2002;59:882–893. doi: 10.1007/s00018-002-8475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol Rev. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- Ding M, Feng R, Wang SY, Bowman L, Lu Y, Qian Y, Castranova V, Jiang BH, Shi X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemo-therapeutic activity. J Biol Chem. 2006;281:17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- Ebrahim SH, Diekman ST, Floyd RL, Decoufle P. Comparison of binge drinking among pregnant and nonpregnant women, United States, 1991–1995. Am J Obstet Gynecol. 1999;180:1–7. doi: 10.1016/s0002-9378(99)70139-0. [DOI] [PubMed] [Google Scholar]
- Galli RL, Shukitt-Hale B, Youdim KA, Joseph JA. Fruit polyphenolics and brain aging: nutritional interventions targeting age-related neuronal and behavioral deficits. Ann N Y Acad Sci. 2002;959:128–132. doi: 10.1111/j.1749-6632.2002.tb02089.x. [DOI] [PubMed] [Google Scholar]
- García-Pérez J, Avila J, Díaz-Nido J. Lithium induces morphological differentiation of mouse neuroblastoma cells. J Neurosci Res. 1999;57:261–270. doi: 10.1002/(SICI)1097-4547(19990715)57:2<261::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;16:200–208. [PubMed] [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25:175–184. [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Hou DX. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med. 2003;3:149–159. doi: 10.2174/1566524033361555. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Lee YI, Seo M, Kim Y, Kim SY, Kang UG, Kim YS, Juhnn YS. Membrane depolarization induces the undulating phosphorylation/dephosphorylation of glycogen synthase kinase 3beta, and this dephosphorylation involves protein phosphatases 2A and 2B in SH-SY5Y human neuroblastoma cells. J Biol Chem. 2005;280:22044–22052. doi: 10.1074/jbc.M413987200. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–167. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Luo J, Lindström CL, Donahue A, Miller MW. Differential effects of ethanol on the expression of cyclo-oxygenase in cultured cortical astrocytes and neurons. J Neurochem. 2001;76:1354–1363. doi: 10.1046/j.1471-4159.2001.00129.x. [DOI] [PubMed] [Google Scholar]
- Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower KA, Odetallah M, Ding M, Ke Z, Luo J. The role of glycogen synthase kinase 3beta in the transformation of epidermal cells. Cancer Res. 2007;67:7756–7764. doi: 10.1158/0008-5472.CAN-06-4665. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Montaño JR, Lim F, Moreno FJ, Avila J, Díaz-Nido J. Glycogen synthase kinase-3 modulates neurite outgrowth in cultured neurons: possible implications for neurite pathology in Alzheimer’s Disease. J Alzheimers Dis. 1999;1:361–378. doi: 10.3233/jad-1999-1602. [DOI] [PubMed] [Google Scholar]
- Naska S, Park KJ, Hannigan GE, Dedhar S, Miller FD, Kaplan DR. An essential role for the integrin-linked kinase-glycogen synthase kinase-3 beta pathway during dendrite initiation and growth. J Neurosci. 2006;26:13344–13356. doi: 10.1523/JNEUROSCI.4462-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W. Maternal alcohol consumption during pregnancy and physical outcomes up to 5 years of age: a longitudinal study. Early Hum Dev. 2003;71:137–148. doi: 10.1016/s0378-3782(03)00003-3. [DOI] [PubMed] [Google Scholar]
- O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W. Prenatal alcohol exposure and attention, learning and intellectual ability at 14 years: a prospective longitudinal study. Early Hum Dev. 2007;83:115–123. doi: 10.1016/j.earlhumdev.2006.05.011. [DOI] [PubMed] [Google Scholar]
- O’Malley KD, Nanson J. Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Can J Psychiatry. 2002;47:349–354. doi: 10.1177/070674370204700405. [DOI] [PubMed] [Google Scholar]
- Olney JW. Fetal alcohol syndrome at the cellular level. Addict Biol. 2004;9:137–149. doi: 10.1080/13556210410001717006. [DOI] [PubMed] [Google Scholar]
- Orme MH, Giannini AL, Vivanco MD, Kypta RM. Glycogen synthase kinase-3 and Axin function in a beta-catenin-independent pathway that regulates neurite outgrowth in neuroblastoma cells. Mol Cell Neurosci. 2003;24:673–686. doi: 10.1016/s1044-7431(03)00229-x. [DOI] [PubMed] [Google Scholar]
- Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. 2003;78:570S–578S. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Shin WH, Park SJ, Kim EJ. Protective effect of anthocyanins in middle cerebral artery occlusion and reperfusion model of cerebral ischemia in rats. Life Sci. 2006;79:130–137. doi: 10.1016/j.lfs.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey AN, Jenkins D, Rabin BM, Joseph JA. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol Aging. 2007;28:1187–1194. doi: 10.1016/j.neurobiolaging.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Lau FC, Joseph JA. Berry fruit supplementation and the aging brain. J Agric Food Chem. 2008;56:636–641. doi: 10.1021/jf072505f. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battagila F, editors. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. National Academy Press; Washington DC: 1996. [Google Scholar]
- Stromland K, Pinazo-Duran MD. Ophthalmic involvement in the fetal alcohol syndrome: clinical and animal model studies. Alcohol Alcohol. 2002;37:2–8. doi: 10.1093/alcalc/37.1.2. [DOI] [PubMed] [Google Scholar]
- Sweeney MI, Kalt W, MacKinnon SL, Ashby J, Gottschall-Pass KT. Feeding rats diets enriched in lowbush blueberries for six weeks decreases ischemia-induced brain damage. Nutr Neurosci. 2002;5:427–431. doi: 10.1080/1028415021000055970. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yasutake K, Tomizawa K. Lithium inhibits neurite growth and tau protein kinase I/glycogen synthase kinase-3beta-dependent phosphorylation of juvenile tau in cultured hippocampal neurons. J Neurochem. 1999;73:2073–2083. [PubMed] [Google Scholar]
- Talavéra S, Felgines C, Texier O, Besson C, Gil-Izquierdo A, Lamaison JL, Rémésy C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J Agric Food Chem. 2005;53:3902–3908. doi: 10.1021/jf050145v. [DOI] [PubMed] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- Zhong J, Yang X, Yao W, Lee W. Lithium protects ethanol-induced neuronal apoptosis. Biochem Biophys Res Commun. 2006;350:905–910. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]