Abstract
Background and Purpose
Bone marrow stromal cells (BMSC) decrease neurological deficits in rodents after stroke and concomitantly induce extensive neurite remodeling in the brain, which highly correlates with the improvement of neurological function. We investigated the effects of endogenous tissue plasminogen activator (tPA) on neurite remodeling after BMSC treatment.
Methods
Adult C57BL/6 wild-type (WT) mice and tPA knockout (tPA−/−) mice were subjected to middle cerebral artery occlusion, followed by an injection of 1×106 BMSC (n=18) or phosphate-buffered saline (n=18) into the tail vein 24 hours later. Behavioral tests were performed at 3, 7, and 14 days after middle cerebral artery occlusion. Animals were euthanized at 14 days after stroke.
Results
The effects of BMSC on functional recovery depended on presence or absence of tPA, even after adjusting for imbalanced stroke severity. BMSC significantly improve functional recovery in WT mice compared to WT controls but show no beneficial effect in the tPA−/− mice compared to tPA−/− controls. Axonal density and synaptophysin-positive areas along the ischemic boundary zone of the cortex and striatum in WT mice are significantly higher than in the tPA−/− mice. BMSC treatment significantly increases tPA protein level and activity only in WT mice.
Conclusions
Our results suggest that endogenous tPA promotes BMSC-induced neurite outgrowth and may contribute to functional recovery after stroke.
Keywords: bone marrow stromal cells, functional recovery, neurite remodeling, stroke, tissue plasminogen activator
Tissue plasminogen activator (tPA), a member of the fibrinolytic system, is a serine protease that converts the zymogen plasminogen into the active protease plasmin1 and thus cleaves fibrin and dissolves newly formed clots.2 In addition to its role in the circulation, tPA is expressed in the parenchyma of the rodent central nervous system, where it shapes the function of both neurons and glia.3–5 tPA also participates in tissue remodeling through enhancement of cell migration and differentiation, facilitates axonal growth and path-finding,6 and enhances the late phase of long-term potentiation.7,8 Moreover, various studies have also documented the involvement of tPA in peripheral nerve regeneration9,10: tPA activity is localized to neuron growth cones in culture11; tPA mRNA and enzymatic activities are induced in murine embryonic dorsal root ganglia during the period of axonal outgrowth toward their peripheral targets12 and after peripheral nerve injury10; mice lacking tPA show delayed functional recovery after sciatic nerve crush.13 These experimental findings highlight a role for tPA during neural network reconstitution and synaptic plasticity in the nervous system.
Bone marrow stromal cells (BMSC) are a heterogeneous subpopulation of bone marrow cells that include mesenchymal stem and progenitor cells. BMSC transplantation decreases the thickness of glial scar wall,14,15 accelerates axonal sprouting and regeneration,16 and enhances intercortical and intracortical axonal connections,17 which are directly correlated to the recovery of neurological function.18 How BMSC exert these effects, however, is still an open question. Recent in vitro and in vivo data support a critical role of tPA in facilitating neurite outgrowth.5 BMSC modulate endogenous tPA level and activity in the ischemic boundary zone (IBZ).5 Collectively, these experimental data suggest that endogenous tPA is a mediator of BMSC-induced neurite outgrowth. In the present study, C57B6 wild-type (WT) and tPA knockout (tPA−/−) mice were subjected to focal brain ischemia, BMSC were transplanted, and neurological function and neurite status were evaluated to dissect the role of tPA as well as the interaction between endogenous tPA and BMSC in neurite remodeling after stroke in mice.
Materials and Methods
Animal Middle Cerebral Artery Occlusion Model and Cell Transplantation
Adult male WT C57BL/6J mice (n= 18, Charles River, Wilmington, MA) and tPA−/−mice with C57BL/6J background (n=18, Jackson Laboratory, Bar Harbor, ME) weighing 22 to 25 grams were used in this study. All experiments were conducted in accordance with the standards of the Institutional Animal Care and Use Committee of Henry Ford Hospital. Mice were subjected to permanent monofilament middle cerebral artery occlusion (MCAO).19 At 24 hours after ischemia, randomly selected mice received BMSC (derived from C57BL/6J mice) or vehicle administration. Approximately 1×106 BMSC in 0.5 mL phosphate-buffered saline or phosphate-buffered saline alone was slowly injected via the tail vein over a 5-minute period into each mouse. Immunosuppressant was not used in any animal in this study. Animal mortality for the WT and tPA−/− animals was similar, ≈40%. All mice were euthanized at 14 days after MCAO, among which 12 were used for protein extraction (n=3/group), and the remaining 24 brains (n=6/group) were embedded in paraffin.
Behavioral Tests
A modified neurological severity score (mNSS) and Foot-fault tests were performed by a blinded investigator before MCAO and at 1, 3, 7, and 14 days after MCAO, as previously described.19
Histological and Immunohistochemical Assessment
Mouse brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde, and then brain blocks were embedded in paraffin. One coronal paraffin slide (6-µm-thick) from each of the 7 brain blocks (1-mm-thick) of each mouse was stained with hematoxylin and eosin. The 7 coronal brain sections were traced using a Global Laboratory Image analysis system (Data Translation) for lesion volume evaluation. The infarct volume is presented as a percentage of total contralateral hemisphere volume.20 A standard paraffin-embedded block (within the center of the lesion of MCAO) corresponding to coronal coordinates bregma 0 to 1.0 mm was obtained, from which a series of 6-µm-thick sections were analyzed using light and fluorescent microscopy (Olympus BH-2).21 After deparaffinizing, brain sections were processed for either Bielshowsky silver-Luxol fast blue staining22 or standard immunostaining. Primary antibodies against tPA (polyclonal antibody, 1:50; Santa Cruz Biotech) or synaptophysin (monoclonal antibody, 1:1000; Chemicon) were used.
Direct Casein Zymography Measuring tPA Activity in the Brain5
Brain tissues from mice (n=3/group) along the IBZ ipsilateral to the injury were extracted and homogenized. Protein was isolated with Trizol (Invitrogen) following a standard protocol. tPA activity was measured as previously described.5
Quantification
Immunoreactive signals were analyzed with National Institutes of Health Image software (Image J) based on evaluation of an average of 3 histology slides (6-µm-thick, 54-µm interval, every 10 slides) from the standard block of each animal. For measurements of synaptophysin and tPA immunostaining areas, 8 fields of view were digitized under a ×40 objective and the MCID computer imaging analysis system. The positive area was measured in each field of view. For measurements of axon density, 5 fields of view along the IBZ within the ipsilateral striatum were digitized and evaluated.
Statistical Analysis
The behavioral scores were evaluated for normality and data transformation was considered if data were not normal. As a result, ranked data were used for the analysis because modified neurological severity score data were not normal. Two-way ANOVA with factors of BMSC and tPA was used to test factor effects on stroke severity at baseline and functional recovery after treatment. The baseline functional score was included in the analysis if there was imbalanced stroke severity among groups. Analysis using PROC MIXED in SAS23 started testing for BMSC and tPA interaction, followed by a subgroup analysis, if the P value of the F-test with degree of freedom {1, (N1 – 1)*(N2 – 1)} was significant at the level of 0.05, where N1 and N2 are numbers of mice in WT and tPA−/− groups, respectively. The subgroup analysis was conducted through CONSTRCT statement under PROC MIXED with F-test with degree of freedom (1, N1 /(N2). All histological and immunohistochemical measurements were performed by observers blinded to the treatments and analyzed using the similar analysis approach, as described.
Results
Neurological Outcome and Lesion Volume
Stroke severity measured by Foot-fault and modified neurological severity score was significantly reduced in tPA−/− mice, compared to WT mice, but stroke severity was balanced between the assigned treatment groups for each mouse type. BMSC by tPA interactions were observed (P<0.05) on days 7 and 14 in modified neurological severity score test, and on days 3, 7, and 14 in Foot-fault test adjusting for unbalanced stroke severity at the baseline, which indicated that effects of BMSC on the functional recovery depended on tPA presence/absence. In WT mice, BMSC significantly improved functional recovery at days 7 and 14 (P<0.05), respectively. In contrast, in the tPA−/− mice, no BMSC effect was observed on functional recovery at any time points (Table). In addition, after treatment with BMSC, the more severely injured WT mice showed significantly superior recovery in percentage of left Foot-fault, compared to the less injured tPA−/− mice (P<0.05), after adjusting for baseline unbalanced stroke severity. Therefore, tPA is required for the functional benefit of BMSC.
Table.
Bone Marrow Stromal Cell Effects in Wild-Type and Tissue Plasminogen Activator Knockout Mice*
| WT |
tPA−/− |
|||||||
|---|---|---|---|---|---|---|---|---|
| PBS |
BMSC |
PBS |
BMSC |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Ranked mNSS at day 1 (baseline)‡ | 25.4 | 13.39 | 25.3 | 12.37 | 14.6 | 10.22 | 13.4 | 14.81 |
| Ranked mNSS at day 3‡ | 25.8 | 13.31 | 26.0 | 11.87 | 13.2 | 9.45 | 13.8 | 16.27 |
| Ranked mNSS at day 7†‡ | 30.9 | 14.58 | 21.0§ | 10.65 | 13.7 | 11.96 | 14.1 | 14.36 |
| Ranked mNSS at day 14†‡ | 28.0 | 11.75 | 15.9§ | 6.08 | 13.8 | 13.06 | 18.2 | 18.77 |
| % Foot-fault test at day 1 (baseline) | 25.4 | 4.79 | 25.9 | 5.41 | 21.8 | 3.87 | 22.3 | 5.29 |
| % Foot-fault test at day 3† | 21.2 | 3.43 | 18.3§ | 3.38 | 17.3 | 3.11 | 19.1 | 5.40 |
| % Foot-fault test at day 7† | 18.0 | 4.08 | 12.0§ | 2.89 | 13.8 | 3.77 | 15.3 | 3.84 |
| % Foot-fault test at day 14† | 14.6 | 3.37 | 8.5§ | 2.73 | 10.7 | 4.11 | 11.3 | 4.03 |
BMSC indicates bone marrow stromal cells; mNSS, modified Neurological Severity Score; PBS, phosphate-buffered saline; tPA, tissue plasminogen activator; WT, wild-type.
The stroke severity was significantly different between WT and tPA−/− at baseline (before the BMSC treatment) but was balanced between assigned treated and control groups within each mouse type. Therefore, all the analyses of BMSC effects were adjusted for the baseline stroke severity.
P<0.05 for testing tPA by BMSC interactions.
Ranked within each time interval.
P<0.05 compared to PBS within each mouse type.
Ischemic lesion volumes 14 days after the onset of stroke for the WT mice were 17.7%±3.45% (MCAO alone) and 15.9% ±2.78% (MCAO+BMSC); for the tPA−/− mice, volumes were 11.5%±1.33% (MCAO alone) and 10.9%±2.08% (MCAO+BMSC). No BMSC and tPA interaction was observed, and there was no BMSC effect in either WT or tPA−/− mice in terms of lesion volume.
Neurite Remodeling in the IBZ
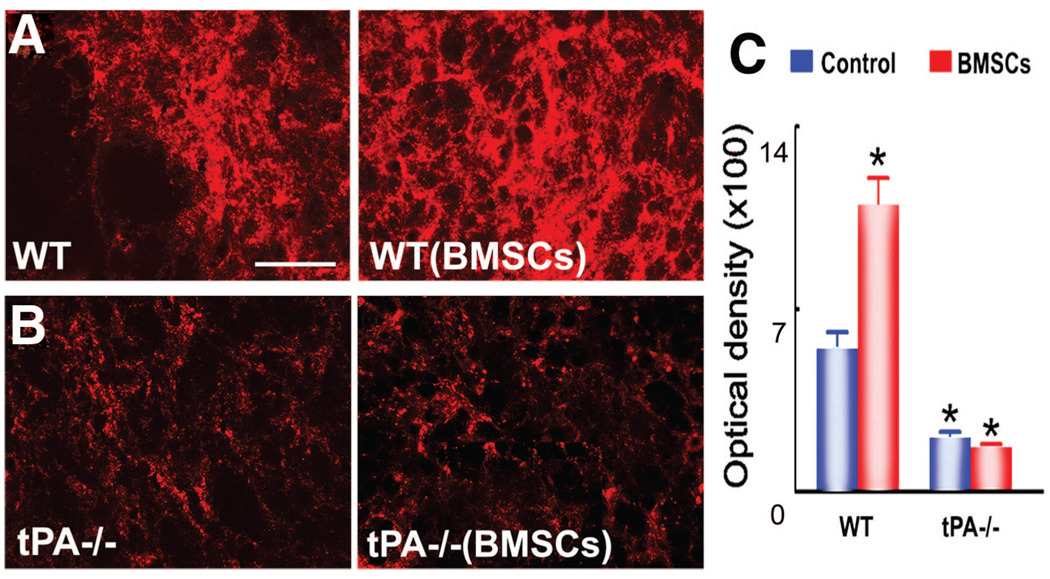
Synaptophysin, a presynaptic vesicle protein, is an indicator of synaptic plasticity and synaptogenesis.24 As shown in Figure 1, WT mice (Figure 1A) showed higher synaptophysin expression in the striatum of the IBZ compared with tPA−/− mice (Figure 1B). BMSC treatment significantly increased the synaptophysin immunopositive area in WT mice (P<0.05), but not in tPA−/− mice (Figure 1C).
Figure 1.
Synaptophysin staining shows axonal remodeling is increased after bone marrow stromal cells (BMSC) treatment in wild-type (WT) mice, but not in tissue plasminogen knockout (tPA−/−) mice. A–C, Compared to WT control animals, WT mice with BMSC treatment show significantly higher expression of synaptophysin immunopositive signals, whereas tPA−/− mice with and without BMSC transplantation have significantly lower signals in the ischemic boundary zone. *P<0.05 vs WT control. Scale bars: 25 µm.
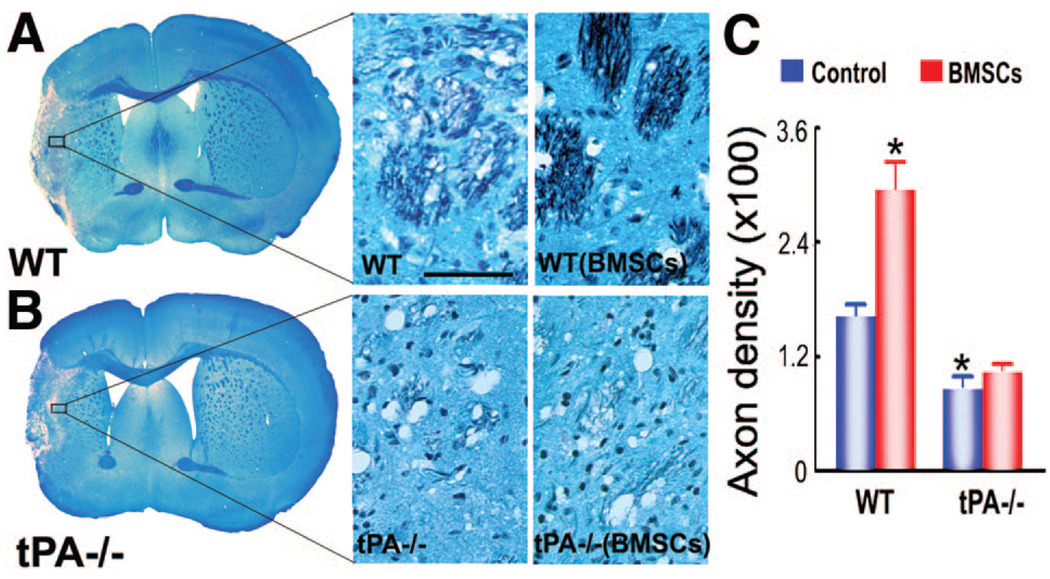
Double-staining for Bielshowsky silver and Luxol fast blue identifies axons and myelin, respectively, in the white matter in the brain. The ischemic attack destroys white matter in the core lesion area and leaves axon-myelin bundles in a partially damaged and disorganized state in the IBZ (Figure 2). Axonal density along the IBZ in the striatum was significantly increased in WT mice (Figure 2A) receiving BMSC transplantation compared with WT controls and tPA−/− controls (Figure 2B) (P<0.05) at 14 days after ischemic attack (Figure 2C).
Figure 2.
Bielshowsky silver and Luxol fast blue double-staining shows axonal remodeling is increased after bone marrow stromal cell (BMSC) treatment in wild-type (WT) mice, but not in tissue plasminogen knockout (tPA−/−) mice. A and B, Axonal density in the ischemic boundary zone (IBZ) in WT mice is significantly higher than that of the tPA−/− mice; corresponding higher magnification pictures in the IBZ are also shown. C, Quantitative data of axon density demonstrate that BMSC treatment significantly increased axonal density in WT mice, whereas no effect shows in tPA−/− animals in the IBZ. *P<0.05 vs WT control. Scale bars: 50 µm.
tPA Level and Activity in the IBZ
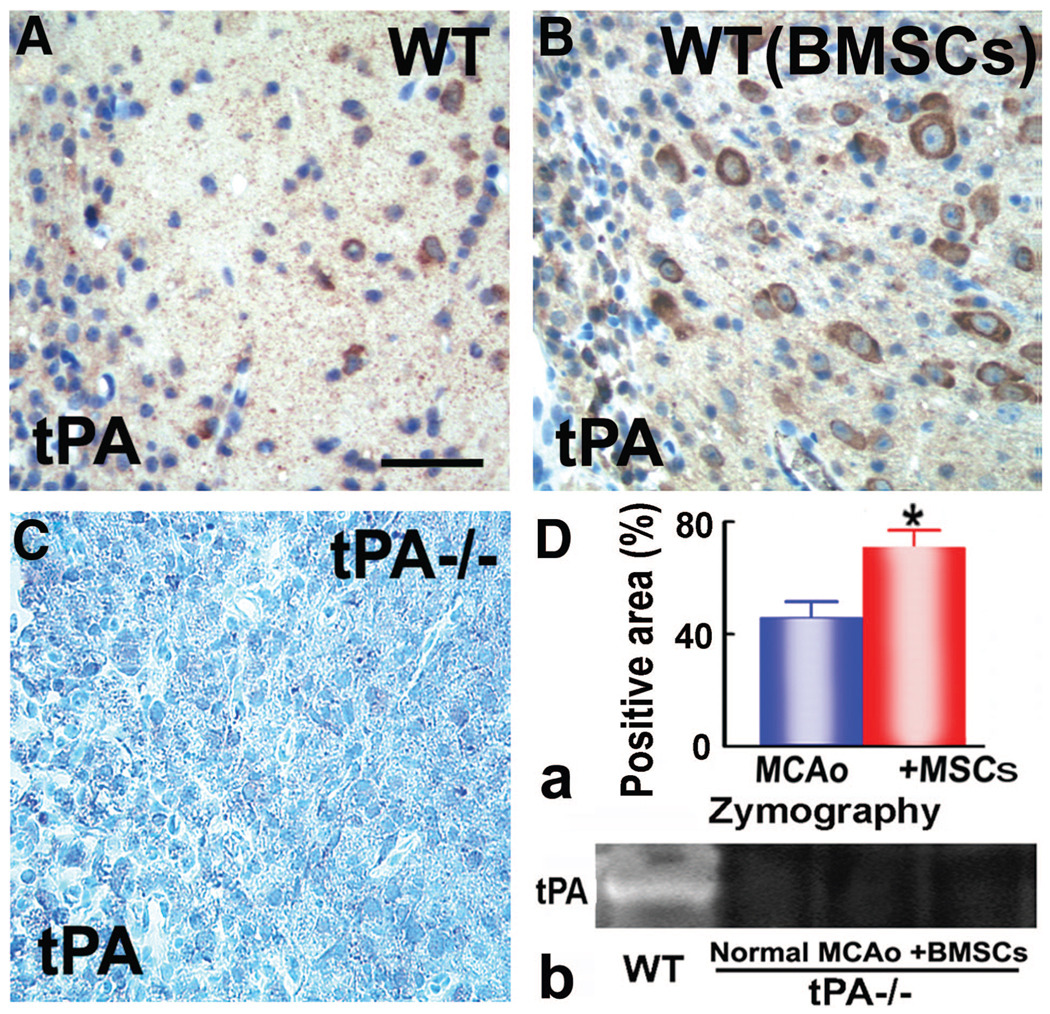
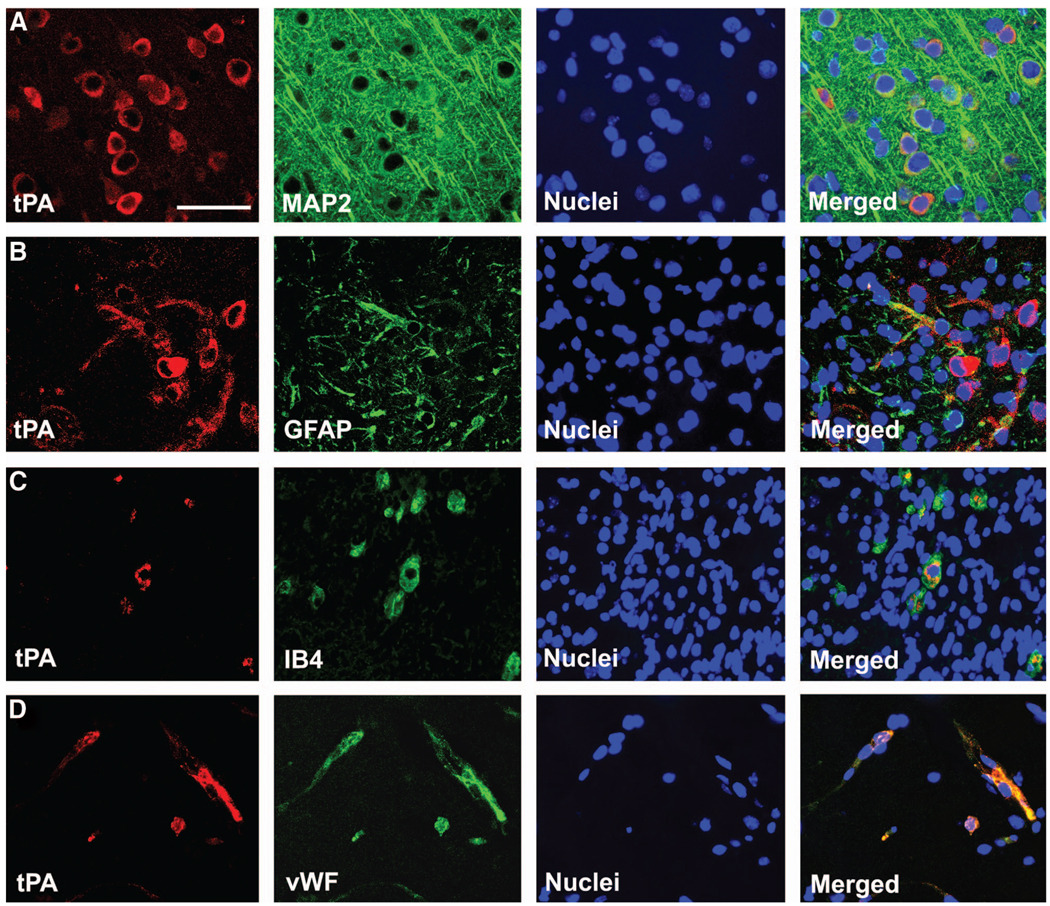
tPA is the primary source of plasminogen activator in the brain.25 Immunostaining with tPA antibody showed positive signals in WT mice (Figure 3A, B, D–a) that were absent in tPA−/− mice (Figure 3C). This indicates the lack of tPA protein in knockout mice. To identify the specific cell types that express tPA in the IBZ, double-staining immunohistochemistry of brain sections were performed. tPA labeling was colocalized with neuronal marker MAP2 (Figure 4A), astrocytic marker glial fibrillary acidic protein (Figure 4B), microglial marker isolectin IB4 (Figure 4C), or endothelial marker von Willebrand factor (Figure 4D). Consistent with these findings, direct casein zymography detected a lytic tPA band in WT mice only (Figure 3D–b). No tPA activity is detectable in tPA−/− mice with or without BMSC treatment.
Figure 3.
tPA protein level in the IBZ in mice subjected to MCAO. A, B; C: there is no tPA signal in tPA−/− mice; D-a: tPA immunostaining shows that tPA protein is significantly increased in WT mice treated with BMSC; D-b: Direct casein zymography demonstrates tPA activity in WT mice, and 0 tPA activity in tPA−/− mice. *P<0.05 versus MCAO alone. Scale bars: 50 µm.
Figure 4.
Double-staining immunohistochemistry of brain sections identifies the cell types that express tissue plasminogen activator (tPA) in the ischemic boundary zone. tPA was colocalized with neuronal marker MAP2 (A), astrocytic marker glial fibrillary acidic protein (B), microglial marker IB4 (C), and endothelial cell marker von Willebrand factor (D). *P<0.05 vs wild-type control. Scale bars: 25 µm.
Discussion
Our data demonstrate that BMSC infusion at 24 hours after MCAO significantly improves neurological recovery in WT mice, consistent with previous results.26,27 Concurrent with the amelioration of functional deficits, WT mice receiving BMSC treatment have significantly higher synaptophysin level, higher axonal density, and more robust increase in tPA protein and activity in the IBZ compared to WT controls. In tPA−/− mice, however, BMSC treatment induces no functional improvement and no increase in synaptophysin expression and axonal density. In addition, compared with WT control mice, tPA−/− mice with and without BMSC treatment show significantly less neurite remodeling in the IBZ. Collectively, these data strongly suggest that endogenous tPA is required for BMSC to stimulate brain plasticity and to improve neurological deficits in mice after focal brain ischemia.
In response to massive neuronal death and denervation after focal ischemia, neurons undergo axonal sprouting and establish new synaptic connections, which may underlie the partial recovery of neurological function over time.28 Cultured medium from BMSC increases neurite outgrowth in primary cultured neurons,29 and BMSC treatment facilitates axonal remodeling in rodents.15,16 Although the exact mechanisms are still not clear, we have demonstrated that endogenous tPA is a key mediator of BMSC-induced neurite outgrowth.5 Therefore, we used loss of function tPA−/− mice in the present study to elucidate BMSC–endogenous tPA interaction and its impact on neurite remodeling after MCAO. In agreement with our hypothesis, BMSC facilitate neurite remodeling and improve neurological deficits after focal brain ischemia in WT mice but show no effects in tPA−/− animals.
Being the major serine protease in the central nervous system, tPA can be produced in the rodent brain by neurons, astrocytes, microglia, and endothelial cells.4,5,30,31 Regardless of its cellular source, tPA is secreted and functions in the extracellular space.3 Endogenous tPA level is tightly regulated both intracellularly and extracellularly within the cells. tPA transcript is modulated in an immediate-early manner and, once in the extracellular space, tPA activity is controlled by inhibitors, including neuroserpin and plasminogen activator inhibitor-1.25,32 This regulation assures the role of tPA in modulating learning,33 synaptic plasticity,34 cell death,4 and the permeability35 as well as coupling of the neurovascular unit36 under physiological conditions. After ischemic brain injury, animal studies show an increase in endogenous tPA activity within the ischemic tissue,37 and genetic deficiency of tPA is associated with decrease in the volume of ischemic lesion and preservation of the function of the blood–brain barrier.35,38 Local tPA level is also significantly increased after peripheral nerve injury, and tPA is crucial for axonal regeneration.9,10 tPA−/− mice show delayed functional recovery after sciatic nerve crush.13 Taken together, this experimental evidence outlines a complicated and controversial role for tPA after injury. Our data are consistent with previous studies showing that tPA−/− mice have smaller lesion volume but less robust axonal remodeling after permanent MCAO compared with WT animals. The major message of this study, however, is that endogenous tPA mediates the beneficial effects of BMSC treatment of brain ischemia. We therefore propose that BMSC injected at 24 hours after MCAO regulates the endogenous tPA/plasminogen activator inhibitor-1 system in such a way that guarantees a consistently increased tPA level and activity in the IBZ in the subacute phase after ischemic attack, which facilitates neurite remodeling and thus may lead to the recovery of neurological deficits.
Conclusion
tPA has pleiotropic effects that can affect brain remodeling, including neurogenesis and angiogenesis. tPA stimulates the expression of MMP,39–41 particularly MMP-9, which enhances progenitor cell migration and contributes to neurogen-esis42–45 and angiogenesis.46 Likewise, tPA can cleave protrophic factors, such as nerve growth factor and brain-derived neurotrophic factor, into active factors that contribute to many aspects of brain plasticity.47–52 Regarding how local tPA facilitates axonal remodeling, there are many possibilities. Previous studies show that tPA indirectly through plasmin3 or directly activates the N-methyl-d-aspartate receptor,36 which can then induce nitric oxide release and foster neuritogenesis.36,53 Given the multifaceted effects of tPA on brain tissue, we cannot exclude other possibilities like plasmin-dependent degradation of extracellular matrix and54 fibrin clearance9 and plasmin-independent proteolytic cleavage of precursor forms of neurotrophins, such as pro-brain-derived neurotrophic factor to the mature neurotrophin brain-derived neurotrophic factor.51,55 These actions can either clear the way for sprouting axons or promote the innate capacity of neurite outgrowth, thus facilitating axonal remodeling after massive neuronal death caused by ischemic attack. Further studies are warranted to clarify the mechanisms by which tPA accelerates axonal regeneration.
Acknowledgments
The authors thank Theradigm for providing primary cultures of BMSC, and Cindi Roberts and Qing-e Lu for technical assistance. tPA−/− mice were generously provided by Dr Costantino Iadecola.
Source of Funding
This work was supported by NINDS grants P01 NS42345, and R01 NS66041.
Footnotes
Disclosure
None.
References
- 1.Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huber K. Plasminogen activator inhibitor type-1 (part one): basic mechanisms, regulation, and role for thromboembolic disease. J Thromb Thrombolysis. 2001;11:183–193. doi: 10.1023/a:1011955018052. [DOI] [PubMed] [Google Scholar]
- 3.Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multi-faceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xin H, Li Y, Shen LH, Liu X, Wang X, Zhang J, Pourabdollah-Nejad DS, Zhang C, Zhang L, Jiang H, Zhang ZG, Chopp M. Increasing tpa activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One. 5:e9027. doi: 10.1371/journal.pone.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci U S A. 1999;96:14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centonze D, Napolitano M, Saulle E, Gubellini P, Picconi B, Martorana A, Pisani A, Gulino A, Bernardi G, Calabresi P. Tissue plasminogen activator is required for corticostriatal long-term potentiation. Eur J Neurosci. 2002;16:713–721. doi: 10.1046/j.1460-9568.2002.02106.x. [DOI] [PubMed] [Google Scholar]
- 8.Mataga N, Nagai N, Hensch TK. Permissive proteolytic activity for visual cortical plasticity. Proc Natl Acad Sci U S A. 2002;99:7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasmin-ogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siconolfi LB, Seeds NW. Induction of the plasminogen activator system accompanies peripheral nerve regeneration after sciatic nerve crush. J Neurosci. 2001;21:4336–4347. doi: 10.1523/JNEUROSCI.21-12-04336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krystosek A, Seeds NW. Peripheral neurons and Schwann cells secrete plasminogen activator. J Cell Biol. 1984;98:773–776. doi: 10.1083/jcb.98.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumi Y, Dent MA, Owen DE, Seeley PJ, Morris RJ. The expression of tissue and urokinase-type plasminogen activators in neural development suggests different modes of proteolytic involvement in neuronal growth. Development. 1992;116:625–637. doi: 10.1242/dev.116.3.625. [DOI] [PubMed] [Google Scholar]
- 13.Siconolfi LB, Seeds NW. Mice lacking tPA, uPA, or plasminogen genes showed delayed functional recovery after sciatic nerve crush. J Neurosci. 2001;21:4348–4355. doi: 10.1523/JNEUROSCI.21-12-04348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Li Y, Qu R, Shen L, Gao Q, Zhang X, Lu M, Savant-Bhonsale S, Borneman J, Chopp M. Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats. Brain Res. 2007;1149:172–180. doi: 10.1016/j.brainres.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 21.Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–2785. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehan D, Hrapchak B. Theory and practice of histotechnology. St. Louis, MO: CV Mosby Co; 1980. [Google Scholar]
- 23.SAS Institute SAS/STAT 9.1. User’s Guide. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 24.Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensiti-zation to psychostimulants. Ann N Y Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence DA, Strandberg L, Ericson J, Ny T. Structure-function studies of the SERPIN plasminogen activator inhibitor type 1. Analysis of chimeric strained loop mutants. J Biol Chem. 1990;265:20293–20301. [PubMed] [Google Scholar]
- 26.Dezawa M, Hoshino M, Nabeshima Y, Ide C. Marrow stromal cells: implications in health and disease in the nervous system. Curr Mol Med. 2005;5:723–732. doi: 10.2174/156652405774641070. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 29.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 30.Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26:209–217. doi: 10.1038/sj.jcbfm.9600181. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber SS, Tan Z, Sun N, Wang L, Zlokovic BV. Immunohisto-chemical localization of tissue plasminogen activator in vascular endo-thelium of stroke-prone regions of the rat brain. Neurosurgery. 1998;43:909–913. doi: 10.1097/00006123-199810000-00107. [DOI] [PubMed] [Google Scholar]
- 32.Yepes M, Lawrence DA. Neuroserpin: a selective inhibitor of tissue-type plasminogen activator in the central nervous system. Thromb Haemost. 2004;91:457–464. doi: 10.1160/TH03-12-0766. [DOI] [PubMed] [Google Scholar]
- 33.Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci. 2003;23:7368–7375. doi: 10.1523/JNEUROSCI.23-19-07368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- 35.Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, Strickland S, Iadecola C. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci U S A. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao G, Reynolds JN, Flavin MP. Temporal profile of tissue plasminogen activator (tPA) and inhibitor expression after transient focal cerebral ischemia. Neuroreport. 2003;14:1689–1692. doi: 10.1097/00001756-200309150-00006. [DOI] [PubMed] [Google Scholar]
- 38.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 39.Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, Kelly PJ. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 41.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 42.Kang SS, Kook JH, Hwang S, Park SH, Nam SC, Kim JK. Inhibition of matrix metalloproteinase-9 attenuated neural progenitor cell migration after photothrombotic ischemia. Brain Res. 2008;1228:20–26. doi: 10.1016/j.brainres.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu L, Tonchev AB, Kaplamadzhiev DB, Boneva NB, Mori Y, Sahara S, Ma D, Nakaya MA, Kikuchi M, Yamashima T. Expression of matrix metalloproteinases in the neurogenic niche of the adult monkey hippocampus after ischemia. Hippocampus. 2008;18:1074–1084. doi: 10.1002/hipo.20466. [DOI] [PubMed] [Google Scholar]
- 45.Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DH, Lilliehook C, Roides B, Chen Z, Chang M, Mobashery S, Goldman SA. Testosterone-induced matrix metalloproteinase activation is a checkpoint for neuronal addition to the adult songbird brain. J Neurosci. 2008;28:208–216. doi: 10.1523/JNEUROSCI.3674-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutiérrez-Fernández A, Gingles NA, Bai H, Castellino FJ, Parmer RJ, Miles LA. Plasminogen enhances neuritogenesis on laminin-1. J Neurosci. 2009;29:12393–12400. doi: 10.1523/JNEUROSCI.3553-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci U S A. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mou X, Peterson CB, Prosser RA. Tissue-type plasminogen activator-plasmin-bdnf modulate glutamate-induced phase-shifts of the mouse suprachiasmatic circadian clock in vitro. Eur J Neurosci. 2009;30:1451–1460. doi: 10.1111/j.1460-9568.2009.06946.x. [DOI] [PubMed] [Google Scholar]
- 50.Gray K, Ellis V. Activation of pro-BDNF by the pericellular serine protease plasmin. FEBS Lett. 2008;582:907–910. doi: 10.1016/j.febslet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 51.Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 52.Nagappan G, Zaitsev E, Senatorov VV, Jr, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of proBDNF by high frequency neuronal activity. Proc Natl Acad Sci U S A. 2009;106:1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamazaki M, Chiba K, Mohri T. Fundamental role of nitric oxide in neuritogenesis of PC12h cells. Br J Pharmacol. 2005;146:662–669. doi: 10.1038/sj.bjp.0706370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kucharewicz I, Kowal K, Buczko W, Bodzenta-Łukaszyk A. The plasmin system in airway remodeling. Thromb Res. 2003;112:1–7. doi: 10.1016/j.thromres.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Barker PA. Whither proBDNF? Nat Neurosci. 2009;12:105–106. doi: 10.1038/nn0209-105. [DOI] [PubMed] [Google Scholar]