Abstract
Neural crest cells (NCCs) form at the dorsal margin of the neural tube and migrate along distinct pathways throughout the vertebrate embryo to generate multiple cell types. A subpopulation of vagal NCCs invades the foregut and colonises the entire gastrointestinal tract to form the enteric nervous system (ENS). The colonisation of embryonic gut by NCCs has been studied extensively in chick embryos, and genetic studies in mice have identified genes crucial for ENS development, including Ret. Here, we have combined mouse embryo and organotypic gut culture to monitor and experimentally manipulate the progenitors of the ENS. Using this system, we demonstrate that lineally marked intestinal ENS progenitors from E11.5 mouse embryos grafted into the early vagal NCC pathway of E8.5 embryos colonise the entire length of the gastrointestinal tract. By contrast, similar progenitors transplanted into Ret-deficient host embryos are restricted to the proximal foregut. Our findings establish an experimental system that can be used to explore the interactions of NCCs with their cellular environment and reveal a previously unrecognised non-cell-autonomous effect of Ret deletion on ENS development.
Keywords: Enteric nervous system, Neural crest, Ret
INTRODUCTION
The enteric nervous system (ENS) of vertebrates is composed of a network of interconnected ganglia that are embedded within the gut wall and control its peristaltic activity, secretions and blood flow (Furness, 2006). ENS progenitors are derived from a subpopulation of vagal neural crest cells that emigrate from the post-otic hindbrain in mouse embryos at embryonic day (E) 8.5–8.75 and assemble transiently at the dorsal aorta. At E9.0–9.5, these pre-enteric neural crest cells (NCCs) invade the foregut to become enteric NCCs, which migrate in a rostrocaudal direction to colonise the entire gastrointestinal tract (Burns, 2005; Durbec et al., 1996; Le Douarin and Teillet, 1973). Many of the studies that defined the origin, migratory pathways and fate of pre-enteric and enteric NCCs have been carried out in avian embryos, owing to the ease of experimental manipulations such as cell-lineage tracing and transplantation (Le Douarin and Kalcheim, 1999; Yntema and Hammond, 1954). Insight into molecular pathways has come from genetic studies in mice, which have identified several genes required for the development of the ENS (Gershon, 1997; Heanue and Pachnis, 2007). One limitation of the mouse studies is that they rely mostly on end-point phenotypic analysis because of the difficulty in effectively monitoring or manipulating ENS progenitors in the dynamic stages of their formation, migration and differentiation during embryogenesis.
One of the genes that is crucial for ENS development is Ret, which encodes a receptor tyrosine kinase (RTK) (Takahashi et al., 1988). During mouse embryogenesis, Ret is first expressed in pre-enteric NCCs assembling at the dorsal aorta and its expression is maintained throughout the colonisation of the gut (Durbec et al., 1996). Mice homozygous for Ret deletion (Ret−/−) have complete intestinal aganglionosis (Schuchardt et al., 1994), whereas partial loss-of-function mutations in this gene lead to colonic aganglionosis (Asai et al., 2006; de Graaff et al., 2001; Uesaka et al., 2008). Interestingly, hypomorphic mutations of RET are responsible for ~50% of familial cases of Hirschsprung’s disease (HSCR), a congenital condition characterised by the absence of enteric ganglia from the distal colon (Amiel et al., 2008). By grafting wild-type ENS progenitors into aganglionic Ret mutant intestine in culture, we have shown that the effect of Ret deletion on enteric NCCs is cell-autonomous and that Ret controls the response of individual cells to their microenvironment (Natarajan et al., 1999).
To further explore the plasticity of enteric NCCs and the role of Ret in ENS development, we have followed the fate of genetically marked cells in cultured post-implantation mouse embryos and organotypic cultures of their gut. We demonstrate that like their endogenous counterparts, exogenous ENS progenitors grafted into the vagal NCC pathway of cultured embryos invade the foregut and colonise the entire intestine. Moreover, by transplanting wild-type ENS progenitors into the vagal NCC pathway of Ret-deficient embryos, we demonstrate that the earlier stages of gut colonisation by NCCs are controlled by Ret in a non-cell-autonomous manner. Our studies reveal novel complexities in the mechanisms of Ret function and provide an experimental paradigm for analysing the effects of mouse mutations on NCCs in general, and on the ENS in particular.
MATERIALS AND METHODS
Animals
Rosa26βgeo, Rosa26R-EYFP and Ret−/− mice have been described previously (Schuchardt et al., 1994; Srinivas et al., 2001; Zambrowicz et al., 1997). Wild-type embryos were obtained from timed matings of CBA/ca females with C57BL/10 males. E8.5 embryos were cultured as described previously (Sturm and Tam, 1993).
Isolation of Ret+ cells from foetal mouse gut and grafting into cultured mouse embryos
Ret+ cells were isolated as described (Lo and Anderson, 1995; Natarajan et al., 1999). Approximately 50 cells were grafted into cultured embryos using a stereomicroscope and a micromanipulator. Cells were loaded individually into a 5 μm diameter needle (borosilicate glass tube, LASER) and delivered at both sides of the embryo to the region between the neural tube and somites 2–4.
Analysis of the ENS of grafted embryos
Gut organ culture was performed as described (Natarajan et al., 1999). Immunostaining, X-Gal staining and in situ hybridisation on whole-mount preparations and cryosections were performed as described (Natarajan et al., 1999; Wong et al., 2005).
RESULTS AND DISCUSSION
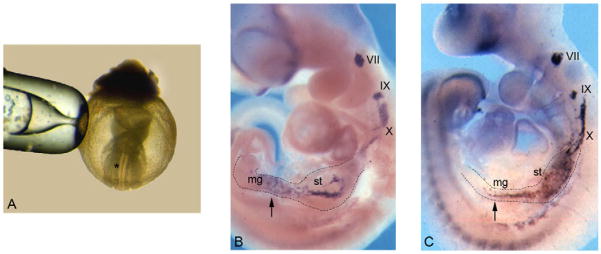
To explore the plasticity of ENS progenitors in vivo, we adopted an ex utero mouse embryo culture system that allows access to, and experimental manipulation of, early NCCs. In this experimental paradigm, post-implantation mouse embryos can be cultured for a short period with growth rate and morphogenesis comparable to those of embryos developing in utero (Sturm and Tam, 1993). For the present experiments, E8.5 mouse embryos (Fig. 1A) were cultured in rolling vials for 3 days, in increasing concentrations of rat serum and oxygen (Trainor et al., 1994). At the end of the culture period (designated hereafter E8.5+3), the majority of embryos (≥90%) reached a stage comparable to E10.5 (Fig. 1B). We have previously demonstrated that cranial NCCs delaminate normally from the dorsal neural tube of cultured embryos and follow appropriate migratory routes to reach their destination (Trainor and Tam, 1995). However, the migration of vagal NCCs, Ret expression and the early stages of enteric neurogenesis have not been examined in this experimental paradigm. Therefore, we carried out whole-mount in situ hybridisation analysis of E8.5+3 embryos using a Ret-specific riboprobe. At E10.5, Ret is expressed in defined subpopulations of NCCs and their derivatives, including the migrating enteric NCCs and the condensing autonomic and sensory ganglia (Enomoto et al., 2001; Pachnis et al., 1993). Ret-expressing cells were observed in cranial sensory ganglia VII, IX and X of E8.5+3 embryos in a pattern and distribution equivalent to those of freshly dissected E10.5 embryos (Fig. 1C). Moreover, a large number of Ret+ cells were observed in the foregut and midgut of cultured embryos. Consistent with the requirement of Ret for ENS formation (Schuchardt et al., 1994), ENS progenitors were absent from the gut of E8.5+3 Ret−/− embryos (data not shown). These experiments suggest that the processes controlling gut colonisation by ENS progenitors in cultured mouse embryos are similar to those for embryos developing in utero. Hence, mouse embryo culture can be used to monitor and experimentally manipulate vagal NCCs at the early stages of enteric neurogenesis ex utero.
Fig. 1. Normal development of the peripheral nervous system in cultured embryos.
E8.5 mouse embryos (A) were cultured for 3 days and processed for whole-mount in situ hybridisation with a Ret-specific riboprobe (B). In situ hybridisation of a freshly dissected E10.5 embryo (C). In both cases, Ret expression is observed in the VII, IX and X cranial sensory ganglia and in the stomach (st) and midgut (mg). The asterisk in A marks the approximate site of cell grafting (see text, and legend to Fig. 2). Arrows indicate enteric neural crest cells (NCCs) within the gut of cultured (B) and freshly dissected (C) embryos.
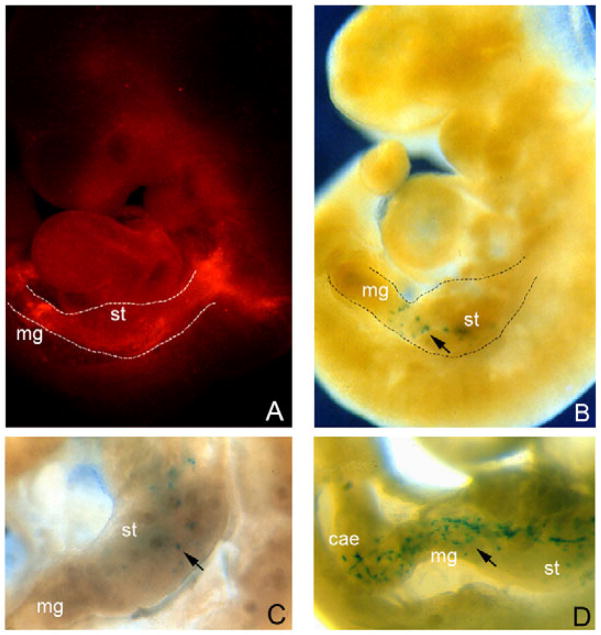
Previous studies have shown that ENS progenitors from quail bowel transplanted into the trunk NCC pathway of younger chick embryos can contribute to the formation of peripheral nerves, sympathetic ganglia and adrenals (Rothman et al., 1990). Moreover, we have previously shown that enteric NCCs isolated from E11.5 mouse embryos and transplanted isochronically into embryonic gut in organotypic culture, colonised all segments of the organ and differentiated into enteric neurons and glia (Natarajan et al., 1999). We aimed to extend these studies to earlier stages of development and explore the capacity of intestinal ENS progenitors to re-enact the migratory behaviour and developmental programme of pre-enteric vagal NCCs upon in vivo transplantation. For this, Ret+ cells were isolated from the gut of E11.5 embryos (using Ret-specific antibodies and FACS analysis) (Natarajan et al., 1999) and grafted heterotopically and heterochronically into the migratory pathway of pre-enteric vagal NCCs. Pilot DiI-labelling experiments indicated that NCCs emerging from the neural tube at the level of somites 2–4 efficiently colonise the gut of cultured embryos (Fig. 2A). Therefore, Ret+ cells were generally grafted at this level between the somitic mesenchyme and the neural tube (Fig. 1A). In order to trace the fate of grafted cells, Ret+ cells were isolated from embryos carrying the Rosa26βgeo or Rosa26R-EYFP alleles, which ubiquitously express the β-geo reporter or the fluorescent protein YFP, respectively. Similar results were obtained with the two reporter lines. Following the graft, 72% (31/43) of transplanted embryos contained β-geo+ cells at the end of the culture period. Interestingly, in the majority of positive embryos, donor cells were restricted to the gastrointestinal tract (Fig. 2B). Occasionally, however, a small number of β-geo+ cells were also detected near the pharyngeal pouch of the third branchial arch (data not shown). Two groups of embryos could be distinguished based on the extent of colonisation of the gastrointestinal tract by the grafted cells. In 66% (20/31) of positive embryos, clusters of β-geo+ cells were found exclusively in the foregut (oesophagus and stomach) (Fig. 2C). However, in 33% (11/31) of embryos, grafted cells were found in both the foregut and midgut (Fig. 2D). In most embryos of this latter group, β-geo+ cells formed a relatively dense network, similar to that formed by intrinsic NCCs at E10.5. In addition, the number of β-geo+ cells in this group clearly exceeded that of the grafted population, suggesting that they had undergone extensive proliferation. Together, these data show that intestinal ENS progenitors retain the capacity to re-enter the foregut and migrate rostrocaudally to colonise more-distal gut segments.
Fig. 2. Colonisation of the gut of E8.5+3 embryos transplanted with Ret+ ENS progenitors.
(A) DiI labelling of NCCs at the level of somites 2–4 results in efficient colonisation of the gut by fluorescent cells. (B) E8.5+3 wild-type mouse embryos transplanted with Ret+ cells isolated from the intestine of E11.5 Rosa26bgeo embryos. At the end of the culture period, β-geo+ cells (arrow) are restricted to the gastrointestinal tract. (C,D) Examples of the two classes of embryos classified according to the extent of gut colonisation by grafted cells. st, stomach; mg, midgut; cae, caecum.
In mouse embryos, Ret is not expressed in the early vagal NCCs, but is specifically induced in the subpopulation that reaches the dorsal aorta and invades the foregut (Durbec et al., 1996; Lo et al., 1997). Induction of Ret expression appears to control the response of prospective enteric NCCs to the chemoattractive activity of Gdnf, which at this stage is expressed by the stomach anlage. Consistent with this view, Ret is necessary for the chemoattraction of enteric NCCs by Gdnf in vitro (Natarajan et al., 2002; Young et al., 2001). Furthermore, in mouse embryos homozygous for a hypomorphic mutation of Ret, vagal NCCs delay their invasion of the foregut and accumulate transiently at the dorsal aorta (D.N. and V.P., unpublished). Our data suggest a model in which Ret plays a key role in ‘sorting’ vagal NCC populations, as expression of this RTK is sufficient to guide them into the gut. Interestingly, exogenous Ret+ cells have occasionally been detected in the third pharyngeal pouch (our unpublished observations), a region that expresses relatively high levels of Gdnf. Although at present we cannot exclude the possibility that apoptotic cell death eliminates Ret+ cells that happen to migrate into Gdnf-free embryonic regions, our findings support the hypothesis that growth factors and their cognate tyrosine kinase receptors serve as guidance systems for the colonisation of specific embryonic sites by distinct subpopulations of NCCs (Wehrle-Haller and Weston, 1997).
The 3-day culture period allows us to follow the colonisation of the foregut and midgut by NCCs, but is not suitable for analysing the colonisation of distal gut segments, which in utero is normally completed by E14.5. We have previously demonstrated that endogenous enteric NCCs present in the foregut of E10.0–10.5 embryos colonise the rest of the gut in organ culture and differentiate into enteric neurons and glia (Natarajan et al., 1999). We reasoned, therefore, that a combination of embryo culture followed by organotypic culture of the gut would allow us to explore the further migration of grafted enteric Ret+ cells and assess their differentiation capacity by extending the period of analysis. For this, the gut of E8.5+3 embryos grafted with genetically marked Ret+ cells was dissected and placed in organ culture for a further 7 days. At the end of this period (designated E8.5+3+7), the grafted cells and their progeny had colonised the entire intestine (Fig. 3A,B) and were arranged radially along with the intrinsic NCC derivatives (Fig. 3C). Therefore, a relatively small number of Ret+ enteric NCCs isolated from the gut of E11.5 mouse embryos and heterochronically transplanted into the vagal region of E8.5 embryos, are able to invade the foregut and migrate in a rostrocaudal direction to colonise its entire length.
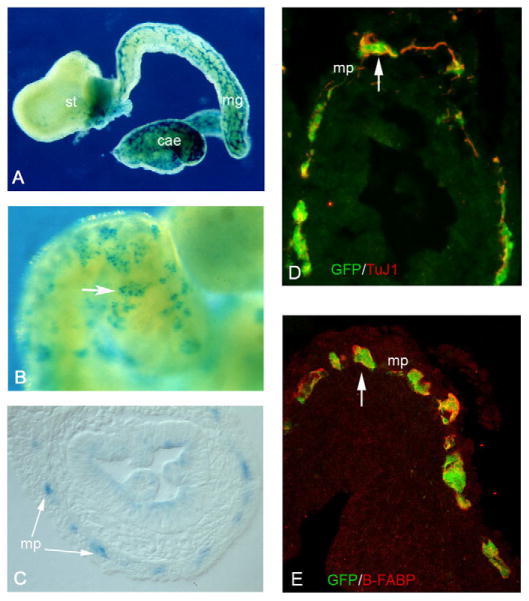
Fig. 3. Ret+ cells grafted to the vagal NCC pathway of E8.5 embryos are capable of colonising the entire length of the gastrointestinal tract.
(A) Whole-mount X-Gal staining of gut from E8.5+3 mouse embryos transplanted with Ret+ cells from the intestine of E11.5 Rosa26bgeo embryos. Note the efficient colonisation of the entire gut by β-geo+ cells. (B) Higher magnification of the gut shown in A. Arrow points to a cluster of β-geo+ cells. (C) Cryosections of E8.5+3+7 gut show that the progeny of grafted cells are localised within the myenteric plexus (mp, arrows). (D,E) Similar cryosections from embryos transplanted with YFP-expressing Ret+ cells were double immunostained for GFP and TuJ1 (D) or for GFP and B-FABP (E). st, stomach; mg, midgut; cae, caecum.
To examine the developmental potential of genetically marked Ret+ cells (isolated from the intestine of E11.5 Rosa26R-EYFP embryos) in our experimental paradigm, cryosections of E8.5+3+7 gut were immunostained for YFP (to identify the grafted cells) and either TuJ1 (Tubb3 – Mouse Genome Informatics) (to identify neurons) or B-FABP (Fabp7) (to identify glia) (Kurtz et al., 1994). We observed significant co-localisation of YFP with these markers, suggesting that grafted Ret+ cells were capable of differentiating into both neurons and glial cells (Fig. 3D,E). The close association of YFP+ cells with the endogenous Tuj1+ and B-FABP+ cells suggests that the transplanted cells and their descendants are capable of homing to the appropriate gut layer, raising the possibility that they can integrate into the intrinsic network of enteric ganglia and establish functional contacts.
The ability of Ret+ intestinal cells grafted into the wall of E11.5 Ret-deficient gut to colonise the entire organ (Natarajan et al., 1999) argues that the effect of the Ret-null mutation on ENS progenitors is cell-autonomous and suggests that the intestine of mutant embryos can support the formation of enteric ganglia by wild-type NCCs. However, these experiments did not address the interaction of wild-type Ret+ cells with the environment of Ret mutant embryos at earlier stages of ENS development, particularly during the invasion of the foregut by nascent Ret+ vagal NCCs emerging at the dorsal aorta. To address this, Ret+ cells from the intestine of E11.5 Rosa26βgeo embryos were grafted into the vagal pathway of E8.5 embryos resulting from intercrosses of Ret+/− mice. At the end of the E8.5+3+7 period, cultured guts were analysed for the presence of β-geo+ cells. In 85% (17/20) of Ret+/− guts, we observed a significant number of β-geo+ cells that had colonised the stomach and the entire intestine (Fig. 4A). Although exogenous β-geo+ cells were found in the foregut of both Ret+/− and Ret−/− embryos at the end of the 3-day culture period (Fig. 4C,D), in all cases of Ret-deficient E8.5+3+7 cultures (5/5), grafted cells were restricted to the proximal foregut while the distal stomach and the entire intestine were devoid of both intrinsic and extrinsic NCC derivatives (Fig. 4B). These findings suggest that although Ret+ cells can invade the foregut of wild-type and Ret-deficient host embryos, in the case of the mutant embryos they are unable to progress further to colonise the remainder of the gastrointestinal tract.
Fig. 4. Non-cell-autonomous effect of the Ret− mutation on the colonisation of E8.5 by Ret+ ENS progenitors.
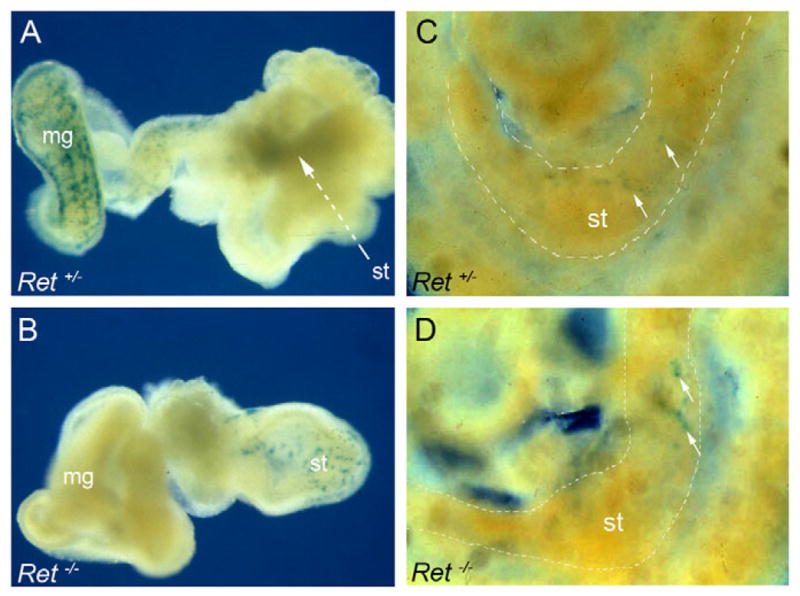
Whole-mount X-Gal staining of E8.5+3+7 guts from Ret+/− (A) and Ret−/− (B) mouse embryos grafted with Ret+ cells from the intestine of E11.5 Rosa26bgeo embryos. Contrary to control gut (A), no β-gal+ cells were detected beyond the anterior foregut of Ret-deficient gut (B). Representative images of Ret+/− (C) and Ret−/− (D) E8.5+3 embryos grafted at E8.5 with Ret+ cells isolated from the intestine of E11.5 embryos. Grafted embryos (three homozygous mutant and 12 heterozygous or wild-type) were cultured for 3 days and then processed for β-gal histochemistry. Arrows in C and D point to β-geo+ cells in the foregut (stomach and duodenum anlage). st, stomach; mg, midgut.
These experiments indicate that the capacity of enteric Ret+ cells to colonise the gut of Ret mutant embryos depends on the site of transplantation; grafting directly into the gut wall allows efficient colonisation of the entire gut (Natarajan et al., 1999), whereas transplantation into the vagal pathway restricts the extrinsic cells to the proximal foregut (this study). Our findings have revealed a previously unrecognised non-cell-autonomous effect of the Ret− mutation on the invasion of the foregut by NCCs. The mechanistic basis for the differential fates in the two experimental paradigms has not been determined. Ret might, directly or indirectly, mediate specific intercellular interactions among vagal NCCs that are necessary for foregut invasion. Such interactions might require a minimal number of Ret+ cells and therefore would not materialise in Ret mutant embryos in which apoptotic cell death eliminates the majority of early ENS progenitors (Taraviras et al., 1999). The requirement of a minimal number of NCCs for the efficient colonisation of the gut has also been suggested by recent studies on avian embryos, which showed that when pre-migratory NCCs are reduced below a critical threshold, they are restricted to the foregut (Barlow et al., 2008; Simpson et al., 2007). Our present findings could also be explained by a model whereby Ret activity within vagal NCCs induces specific changes to the foregut mesenchyme, which in turn facilitates the invasion and rostrocaudal migration of enteric NCCs. Consistent with this idea, it has been demonstrated previously that the absence of enteric neurons and glia from the gut of Ret mutant embryos induces characteristic changes in gene expression in the non-NCC-derived mesenchyme and epithelial cells of the gut (Heanue and Pachnis, 2006; Vohra et al., 2006). Irrespective of the mechanisms implicated, our studies reveal previously unappreciated complexities in the means by which Ret signalling controls the colonisation of mammalian gut by NCCs, and argue that the site of transplantation and the number of wild-type ENS progenitors are critical factors in our efforts to rescue the aganglionic phenotype of animal models of congenital megacolon (HSCR).
In summary, the experimental system described here allows us to monitor the ENS of wild-type embryos ex utero, from the earliest stages of vagal NCC formation to the complete colonisation of the gut. Furthermore, this system allows the generation of chimaeric embryos in which host and grafted cells are of the desired genotype. Such chimaeric embryos will enable us to explore further the interaction between NCCs and the gut microenvironment.
Acknowledgments
This work was supported by the Medical Research Council (MRC, UK) and by a Telethon pre-doctoral fellowship to S.B. (438/b).
References
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, Costantini F. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development. 2006;133:4507–4516. doi: 10.1242/dev.02616. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Burns AJ. Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol. 2005;49:143–150. doi: 10.1387/ijdb.041935ab. [DOI] [PubMed] [Google Scholar]
- de Graaff E, Srinivas S, Kilkenny C, D’Agati V, Mankoo BS, Costantini F, Pachnis V. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15:2433–2444. doi: 10.1101/gad.205001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Furness J. The Enteric Nervous System. Oxford, UK: Blackwell Publishing; 2006. [Google Scholar]
- Gershon MD. Genes and lineages in the formation of the enteric nervous system. Curr Opin Neurobiol. 1997;7:101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Expression profiling the developing mammalian enteric nervous system identifies marker and candidate Hirschsprung disease genes. Proc Natl Acad Sci USA. 2006;103:6919–6924. doi: 10.1073/pnas.0602152103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The Neural Crest. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Lo L, Anderson DJ. Postmigratory neural crest cells expressing c-RET display restricted developmental and proliferative capacities. Neuron. 1995;15:527–539. doi: 10.1016/0896-6273(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Lo L, Sommer L, Anderson DJ. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr Biol. 1997;7:440–450. doi: 10.1016/s0960-9822(06)00191-6. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126:157–168. doi: 10.1242/dev.126.1.157. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Marcos-Gutierrez C, Pachnis V, De Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Le Douarin NM, Fontaine-Perus JC, Gershon MD. Developmental potential of neural crest-derived cells migrating from segments of developing quail bowel back-grafted into younger chick host embryos. Development. 1990;109:411–423. doi: 10.1242/dev.109.2.411. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol. 2007;302:553–568. doi: 10.1016/j.ydbio.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm K, Tam PP. Isolation and culture of whole postimplantation embryos and germ layer derivatives. Methods Enzymol. 1993;225:164–190. doi: 10.1016/0076-6879(93)25013-r. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Buma Y, Iwamoto T, Inaguma Y, Ikeda H, Hiai H. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene. 1988;3:571–578. [PubMed] [Google Scholar]
- Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126:2785–2797. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Tam PP. Cranial paraxial mesoderm and neural crest cells of the mouse embryo: co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development. 1995;121:2569–2582. doi: 10.1242/dev.121.8.2569. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Tan SS, Tam PP. Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos. Development. 1994;120:2397–2408. doi: 10.1242/dev.120.9.2397. [DOI] [PubMed] [Google Scholar]
- Uesaka T, Nagashimada M, Yonemura S, Enomoto H. Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. J Clin Invest. 2008;118:1890–1898. doi: 10.1172/JCI34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra BP, Tsuji K, Nagashimada M, Uesaka T, Wind D, Fu M, Armon J, Enomoto H, Heuckeroth RO. Differential gene expression and functional analysis implicate novel mechanisms in enteric nervous system precursor migration and neuritogenesis. Dev Biol. 2006;298:259–271. doi: 10.1016/j.ydbio.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle-Haller B, Weston JA. Receptor tyrosine kinase-dependent neural crest migration in response to differentially localized growth factors. BioEssays. 1997;19:337–345. doi: 10.1002/bies.950190411. [DOI] [PubMed] [Google Scholar]
- Wong A, Bogni S, Kotka P, de Graaff E, D’Agati V, Costantini F, Pachnis V. Phosphotyrosine 1062 is critical for the in vivo activity of the ret9 receptor tyrosine kinase isoform. Mol Cell Biol. 2005;25:9661–9673. doi: 10.1128/MCB.25.21.9661-9673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–542. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]