Abstract
The male mouse medial amygdala is an important site for integration of main and accessory olfactory information. Exposure to biologically relevant chemical signals from the same species (conspecific) results in a general pattern of immediate early gene (IEG) expression in medial amygdala different from that elicited by chemical signals from other species (heterospecific), of no demonstrable biological relevance. The neuropeptide oxytocin (OT) in the medial amygdala has been shown to be necessary for social recognition. In the present set of experiments, male mice with intracerebroventricular (i.c.v.) cannulae were injected with either PBS (vehicle control) or oxytocin antagonist (OTA) (1ng in 1 μL PBS) and exposed to conspecific (female mouse urine) and heterospecific (steer urine and worn cat collar) chemical stimuli. Similarly to our previous report with intact male mice (Samuelsen and Meredith, 2009a), PBS-injected mice exhibit different immediate early gene (IEG) expression patterns in the medial amygdala according to the biological relevance of the chemical stimuli. However, OTA injection eliminates the increase in IEG expression in the medial amygdala to any of the tested conspecific or heterospecific stimuli. Importantly, OTA injection disrupts avoidance of an unfamiliar predator odor, worn cat collar. Here we suggest that the disruption of social recognition behavior in male mice with altered OT receptor activity results from an inability of the medial amygdala to process relevant conspecific (and heterospecific) chemosensory signals.
Keywords: Oxytocin, Olfaction, Pheromone, Immediate Early Gene (IEG), Predator, Behavior
Introduction
Rodents are especially reliant upon chemical signals to communicate information pertaining to complex behaviors such as reproductive readiness, individual relatedness, social rank and territorial ownership (Desjardins et al., 1973; Hurst and Beynon, 2004; Nakamura et al., 2007). Chemical signals containing pre-programmed information may be necessary for efficient behavioral function (Lin et al., 2007; Samuelsen and Meredith, 2009a). A rodent’s ability to discriminate categories of chemosignals from their own species (conspecific) and that of other species (heterospecific) is necessary for their survival and reproduction (Meredith et al., 2008; Pankevich et al., 2006; Meredith and Westberry, 2004; Murphy, 1980; Johnston and Brenner, 1982). Rodents easily discriminate between conspecific chemosignals (Kavaliers and Colwell, 1995; Mossman and Drickamer, 1996; Pankevich et al., 2004) and respond with characteristic behaviors and neural response to some heterospecific chemosignals (Samuelsen and Meredith, 2009a,b; Gouat et al., 1998; Dielenberg et al., 1999; Dielenberg et al., 2001; Meredith and Westberry, 2004; Fendt, 2006; Kobayakawa et al., 2007; Takahashi et al., 2005).
As the first site of main and accessory olfactory information convergence (Shipley and Adamek, 1984; Coolen and Wood, 1998; Pitkaen et al. 1997; Meredith, 1998;), the medial amygdala has been shown to be important for normal behavioral responses to chemical signal information (Blanchard et al., 2005; Lehman et al., 1980; Petrulis and Johnston, 1999; Dulac and Torello, 2003; Martel and Baum, 2008; Kang et al., 2008). Exposure to different biologically relevant chemosensory stimuli (reproductive, territorial or predator odors) characteristically changes immediate early gene (IEG) expression in the medial amygdala (Meredith and Westberry, 2004; Samuelsen and Meredith, 2009a). This characteristic medial amygdala response is dependent on intact vomeronasal organs (Samuelsen and Meredith, 2009b). Chemical signal information, via the medial amygdala, is thought to be an important contribution to hypothalamic circuits involved in defensive and reproductive behavior (Canteras, 2002; Choi et al., 2005).
The neuropeptide oxytocin (OT) has been implicated in many social behaviors, including maternal care (Takayanagi et al., 2005), maternal aggression (Bosch et al., 2005; Consiglio et al., 2005; Lubin et al., 2007), social attachment (Insel and Hulihan, 1995) and social recognition (Ferguson et al., 2000; Ferguson et al., 2001; Crawley et al., 2007; Choleris et al., 2008). The mouse medial amygdala contains many neurons expressing OT receptor (Insel et al., 1993). Mice deficient in OT appear not to recognize a previously encountered conspecific during subsequent trials (Ferguson et al., 2000; Choleris et al., 2008). Social recognition by oxytocin-knockout (OTKO) male mice is rescued by i.c.v. OT injection only before their exposure to conspecific females (Ferguson et al., 2001). OT during social recognition is important for the initial acquisition or storage of information about the social-stimulus animal, not for memory consolidation or retrieval. It is possible that OT deficient mice do not recognize a familiar conspecific because there is a defect in processing of chemosensory information at the level of the medial amygdala. This social recognition behavior can be rescued with direct injection of OT into the posterior medial amygdala of OTKO mice (Ferguson et al., 2000).
Previously we have shown that the IEG expression pattern in medial amygdala is different after exposure to different categories of biological relevant and non-relevant chemical stimuli (Samuelsen and Meredith, 2009a,b; Meredith and Westberry, 2004). Conspecific and biologically relevant heterospecific predator odors increase IEG expression in both the anterior and posterior medial amygdala. Biologically non-relevant heterospecific chemical signals increase IEG expression only in the anterior medial amygdala, failing to alter IEG expression in posterior medial amygdala. Given the importance of the medial amygdala to categorization of biologically relevant chemical communication-signals and the necessary role of OT in social recognition, we reasoned that OT might be essential for normal processing of chemical signals in medial amygdala. Failure of social recognition may be due to a failure to recognize or to assign appropriate values to chemosensory stimuli. To investigate this hypothesis, we used i.c.v. injection of oxytocin antagonist (OTA) to disrupt OT function, assessed IEG response in the medial amygdala as a measure of categorization of chemosensory communication signals, and monitored behavioral responses to the chemical signals.
Here we show that i.c.v OTA injection blocks significant medial amygdala response to critical chemical signals in sexually inexperienced (naïve) male mice. OTA-injection eliminates all significant medial amygdala IEG expression above control levels to all tested chemical signals, regardless of biological relevance. The tested stimuli include female mouse urine (fMU), known to convey reproductive and agonistic information to male mice (Hurst and Beynon, 2004; Pankevich et al., 2004; Pankevich et al., 2006; Nakamura et al., 2007), steer urine, a heterospecific stimulus, known to elicit avoidance in other conspecific bovines, but presumably irrelevant for mice (Boissy et al., 1998), and pieces of worn cat collar, a potential predator-stimulus which mice and rats normally avoid (Dielenberg et al., 1999; Dielenberg et al., 2001; Takahashi et al., 2005; Takahashi et al., 2007; Samuelsen and Meredith, 2009a,b).
Experimental Procedures
Animals
24 (Exp. 1) and 48 (Exp. 2) sexually naive 3 month old male C57 BL/6 mice (Jackson Laboratory) were single-housed and maintained on a reverse 12/12hr light/dark cycle with food and water ad libitum. Animals had no contact with any heterospecific stimuli before the experimental session, no contact with females since weaning and no prior contact with the female conspecific stimulus donors. Animals were in good health and nutritional status and all animal procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
Stimuli
Female C57 BL/6 mouse urine was collected from 3-4 adult mice placed in a metabolic cage over a 5 day period. Five days of collection were used in order to collect urine from all estrus stages of normally cycling female mice (Champlin and Dorr, 1973). Steer urine was removed via syringe from the bladder of a recently slaughtered male castrate and frozen until dilution. As in our previous experiments (Meredith and Westberry, 2004; Samuelsen and Meredith, 2009a,b), all liquid stimuli were diluted 1:10 by weight with distilled water (purified by reverse osmosis and polishing with activated carbon) and centrifuged for 30 min (Fisher clinical centrifuge at medium speed). The supernatant was decanted and held at -20°C until presentation. Heterospecific stimuli were diluted to avoid any neo-phobia; conspecific stimuli are diluted equally to match. Soft nylon cat-collars (CC) (PETCO Single Ply Nylon Collar) were worn for 2-weeks by a neutered male house cat. The collars were removed, placed in zip-lock plastic bags and held at -20°C until use. Similar sized pieces of nylon fabric cut from clean collars of the same type were used as a substrate for presentation of all the chemical stimuli, in order to provide similar control stimulation for all stimuli.
Intracerebroventricular Cannulation Surgery (i.c.v) and Injections
All male mice were fitted with intracerebroventricular cannulas. Using a mixture of ketamine and xylazine as anesthesia, mice were placed in a stereotaxic apparatus and a 1 cm midline incision was made across the top of the skull. The skin was pulled back, secured and the periosteum was removed. A 1 mm hole was drilled 1100μm lateral to bregma. A 26 gauge guide tube (Plastics One) was implanted 2600μm below the surface of the skull into the lateral ventricle. The guide tube was secured to the skull with dental cement and a dummy cannula was inserted into the guide tube to maintain patency. Mice were allowed 7 days to recover from surgery before beginning their respective experiment. Injections were made in awake animals using a 33 gauge injector cannula, attached to PE-20 tubing and fitted to a 10μL Hamilton syringe. Using a World Precision Instruments syringe pump, injections of either 1 μL sterile 0.1M phosphate buffered saline vehicle (PBS) or 1 μL OTA [(d(CH2)51,Tyr(Me)2,Thr4,Orn8,des-Gly-NH29)-Vasotocin, catalog # H-2908 BACHEM] (1ng in 1 μL sterile PBS) was delivered to awake, restrained mice over 90s. The OTA dose was chosen to replicate the social recognition experiments performed by Ferguson et al. 2000 and 2001 and subsequently used to test conspecific and heterospecific chemical stimuli in the present study. The injector cannula was left in place for a further 120s to allow for diffusion away from the injector cannula. The injector cannula was then removed and the dummy cannula replaced. Mice were allowed a further 10 minutes before the behavioral test.
Testing Procedure and Stimulus Presentation
Social Recognition Experiment
The procedure used by Ferguson et al., 2001 was followed as closely as possible. In order to minimize sexual behavior during the social recognition trials, male mice were exposed several times daily during the post-surgery recovery period to a different ovariectomized (OVX) stimulus female C57 BL/6 mouse. The social recognition trial began after the male mouse had spent 10 minutes in its home cage after i.c.v injection (above). A novel stimulus OVX female mouse was introduced into the home cage of the subject male for a 5 minute interaction. After 5 minutes, the stimulus OVX female was removed. After a 30 minute inter-trial interval, another stimulus OVX female was introduced into the home cage of the test mouse. For the “different female” (DIFF) trials, the stimulus OVX female introduced during the second trial was novel. For the “same female” (SAME) trials, the stimulus OVX female was the same for both encounters. All OVX stimulus females were only exposed to one male mouse per trial. Investigation was defined as direct, active, olfactory exploration of the stimulus OVX female by the test male, consisting of nosing and sniffing the head and anogenital regions, as well as close following and pursuit. Any sexual behaviors, such as mounting, were not included as measures of investigation (Ferguson et al., 2001). Behaviors were recorded using a computer program and numbered key pad with each key corresponding to a different behavior. All animals were tested in the first 6 hours of the dark phase of the light cycle in a room lit by red light.
Biologically-Relevant Chemical Stimuli Exposure for IEG Expression
After the 7 day surgery recovery period, a second group of mice were injected with either PBS (control) or OTA, as above. After the 10 min post-injection time in a clean test cage, mice were exposed to a 2.5cm × 1.27cm piece of clean nylon fabric cut from a clean unworn cat collar (control) or an equivalent piece of nylon collar fabric containing one of the chemical stimuli. The liquid stimuli, fMU or SU, were presented by pipetting ~200μl of the liquid directly onto a piece of clean collar and placing it in the middle of the test cage. The worn cat-collar stimulus (CC), carrying chemosensory stimuli from the cat’s fur was similarly presented in the middle of a clean cage. All stimuli were left in the test cage for the entire 15 minute trial. Behavior was recorded using a computer program and numbered key pad with each key corresponding to a different behavior. The computer records the latency, the number of presses and total elapsed time each key is depressed. Behaviors recorded were: duration of grooming, the number of times the mouse contacted or very closely investigated the stimulus (within 1mm), the cumulative duration of investigation of the stimulus, number of rears, time spent rearing and general investigation of the cage. All animals were tested in the first 6 hours of the dark phase of the light cycle in a room lit by red light.
Cannula Placement Histology and Immunocytochemistry (ICC)
Thirty minutes after the 15 minute behavioral trial, male mice in the social recognition experiment were anesthetized with Nembutal (Ovation Pharmaceuticals, Deerfield IL) and perfused with cold 0.1M PBS followed by 4% paraformaldehyde (PFA). The next morning brains were placed in 30% sucrose for cyroprotection. Using a freezing microtome, brains were sliced into 40μm sections. Free-floating coronal sections were mounted on slides and stained with cresyl violet to allow for easy detection of proper cannula placement. Tissue from male mice in the biologically-relevant chemical stimulus experiment underwent similar ICC processing as previously reported (Samuelsen and Meredith, 2009a,b). Mice were anesthetized using Nembutal forty-five minutes after the initial stimulus exposure and perfused with cold 0.1M PBS followed by 4% PFA. Brains were removed and post-fixed overnight in 4% PFA. The next morning brains were placed in 30% sucrose for cyroprotection. Using a freezing microtome, brains were sliced into 40μm sections. Alternate free-floating coronal sections were washed in 0.1M PBS, blocked in a solution of 5% normal goat serum (30 min) and incubated in rabbit anti-FRAs primary antibody solution (SC253 – detects c-Fos, Fos B, Fra-1 and Fra-2; 1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA) for 20-24 hours at room temperature. The next day, sections were washed in 0.1M PBS and incubated in biotinylated goat anti-rabbit secondary antibody solution (1:400; Vector Laboratories, Burlingame, CA) for 2 hours. Sections were processed in ABC reagent (Vector Laboratories, Burlingame, CA) for 1 hour and stained with diamino benzidine (DAB) (Vector Laboratories, Burlingame, CA). FRAs expression was assessed by averaging numbers of densely labeled cell nuclei within areas of interest on both sides of the brain in three adjacent sections per anatomical area. Areas of interest included: 1) Anterior medial amygdala (MeA), which was divided into ventral anterior medial amygdala (MeAv) and dorsal anterior medial amygdala (MeAd); 2) Posterior medial amygdala (MeP), which was divided into ventral posterior medial amygdala (MePv) and dorsal posterior medial amygdala (MePd); both as indicated in the mouse brain atlas (Paxinos and Franklin, 2003). Image analysis software (ImagePro plus, Media Cybernetics, Inc.) was used to count all densely labeled cell nuclei within the borders of the neuroanatomical nucleus of interest. The numbers are presented as means and standard errors.
Statistics
The effect of drug injection (PBS vs. OTA) or exposure type (SAME vs. DIFF) during the social recognition test was analyzed using a paired t-test with the Bonferroni correction for running multiple tests, comparing the total duration of investigation during the first and second OVX stimulus exposures. The social recognition data are presented as relative duration of investigation (RDI). This was calculated by dividing the duration of trial one by the duration of trial two, as originally reported by Ferguson et al (2001). IEG expression comparisons were analyzed for each experiment by two-way analysis of variance (ANOVA) with factors drug (PBS vs. OTA) and stimulus (CON, fMU, SU and CC). Post-hoc comparisons were made using the Holm-Sidak test. Behavioral responses were analyzed using two-way ANOVAs, with factors: surgery (PBS vs. OTA) and stimulus (CON, fMU, SU and CC), and Holm-Sidak post-hoc comparison tests. Reported behaviors include number and cumulative duration of close investigation or contact with the stimulus or its fabric substrate duration of grooming and number of stretch attend postures.
Results
In our social recognition test, we repeated work by Ferguson et al. (2001) to ensure our animals and test conditions produced similar results: i.e. that i.c.v. injection of OTA eliminates the decrease in investigation to a familiar stimulus female compared to investigation of a novel female. To further understand the role of OT in processing chemosensory information in the medial amygdala, we examined the effect of an identical icv-OTA injection on medial amygdala IEG response to conspecific and heterospecific chemical stimuli varying in biologically relevance.
Social Recognition Experiment
Upon initial exposure to novel OVX stimulus female, male mice injected i.c.v. with either PBS or OTA exhibited vigorous investigation behaviors. After 5 minutes, the female was removed and a novel female placed in the cage 30 minutes later. Both PBS-injected and OTA-injected male mice behaved similarly toward the different (DIFF) female, with no significant difference in investigation time (p>0.05). However, in trials where the same female (SAME) was placed back in the test cage after the first encounter, PBS-injected mice exhibit a significant reduction in investigation time as compared to the first encounter (p<0.005). OTA-injected males did not exhibit a decrease in investigation time during a second exposure of the SAME female (p>0.05) (Figure 1). This replicates previous findings that deficient OT action results in a social recognition deficit in male mice to OVX conspecific females (Ferguson et al., 2000; Ferguson et al., 2001; Choleris et al., 2008).
Figure 1. The relative duration of investigation (RDI) of conspecifics.
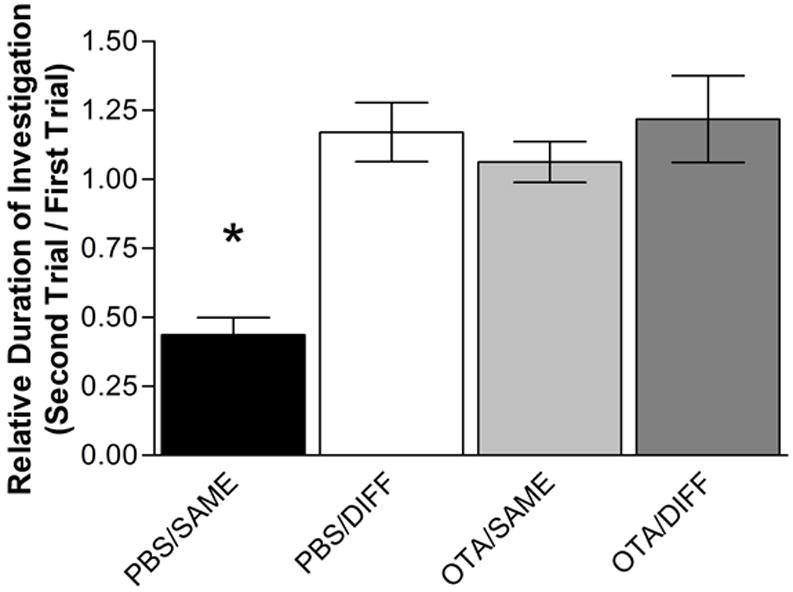
The RDI for male mice injected i.c.v. with PBS or OTA and exposed in trial two to the either a DIFF female or the SAME female from trial one. The data are presented as RDI ± SEM (Ferguson 2001; see Experimental Procedures). Male mice injected with either PBS or OTA and exposed to a DIFF female showed no change in investigation. However, male mice injected i.c.v. with PBS and exposed to the SAME female exhibited a significant decrease in investigation. OTA injection i.c.v. prevents the formation of a social memory for the SAME female and the male mouse investigates the SAME female as much as in the first trial.
Biologically-relevant Chemical Stimulus Exposure
Male mice injected i.c.v with PBS and exposed to fMU had significantly greater FRAs expression in MeA, F(3,47)=27.6, p<0.001 and MeP, F(3,47)=65.4, p<0.001, compared to PBS injected males exposed to clean control fabric (Figure 2). They also had significantly increased FRAs expression compared to control in all four medial amygdala subdivisions (MeAv: F(3,47)=18.5, p<0.001; MeAd: F(3,47)=21.6, p<0.001; MePv: F(3,47)=27.1, p<0.001; MePd: F(3,47)=68.2, p<0.001). PBS injected mice also had significantly greater IEG expression in response to fMU than OTA injected mice, in MeA, F(1,47)=27.5, p<0.001 and MeP, F(1,47)=55.2, p<0.001 and in each subdivision (MeAv: F(1,47)=21.4, p<0.001; MeAd: F(1,47)=19.9, p<0.001; MePv: F(1,47)=10.8, p<0.003; MePd: F(1,47)=71.3, p<0.001). There was no significant difference in FRAs expression between OTA-injected mice exposed to fMU and PBS-injected control mice exposed to control fabric, in any region or subdivision of medial amygdala.
Figure 2. Categorical response to chemical signals in the medial amygdala after PBS or OTA i.c.v injection.
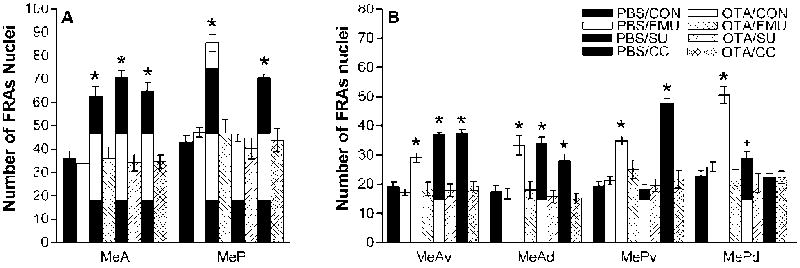
(A) In PBS mice, all stimuli elicited increased FRAs expression (mean number of nuclei ± SEM) overall in MeA, but only the biologically relevant stimuli: the conspecific stimulus, fMU, and the heterospecific predator odor, CC, increased FRAs expression overall in MeP. In OTA mice, no stimuli increased FRAs expression in either MeA or MeP. (B) Among the subdivisions of anterior and posterior medial amygdala, all stimuli increased FRAs expression in MeAv and MeAd in PBS-injected mice. In MePv, both fMU and CC increased FRAs expression, but in MePd only fMU significantly increased FRAs expression. In OTA-injected mice, no stimuli increased FRAs expression in any subdivision of medial amygdala. * indicates a significant difference from both PBS-injected control mice and their stimulus-exposed counterparts; + indicates a significant difference between PBS- and OTA-injected mice exposed to the same stimulus. Refer to the results section for p values.
PBS injected male mice exposed to the heterospecific odor, SU, exhibited significantly higher FRAs expression in MeAv, F(3,47)=57.7, p<0.001, MeAd, F(3,47)=22.8, p<0.001, and overall MeA, F(3,47)= 46.5, p<0.001, as compared to PBS-injected control males exposed to control fabric (Figure 2). Also, PBS-injected mice exposed to SU exhibited a significant increase compared to OTA injected males exposed to SU in total MeA, F(1,47)= 51.7, p<0.001, and in subdivisions MeAv, F(1,47)= 65.6, p<0.001 and MeAd F(1,47)= 27.6, p<0.001. There was no significant FRAs expression in the measured areas of MeP or its subdivisions in either PBS-injected mice or OTA-injected mice, after exposure to SU. There was no significant difference in FRAs expression between OTA injected mice and PBS control mice exposed to clean control fabric in any region or subdivision of medial amygdala.
Male mice injected with PBS exposed to a fabric-piece from a cat-collar (CC) worn for 2 weeks, exhibited significantly greater FRAs expression in MeA, F(3,47)= 33.1, p<0.001, and MeP, F(3,47)= 27.1, p<0.001 compared to PBS-injected mice exposed to a piece of clean control collar (the same clean nylon fabric substrate as for other tests). Also, FRAs expression was increased compared to control, in the subdivisions MeAv, F(3,47)= 62.3, p<0.001, MeAd, F(3,47)= 9.5, p<0.005, and MePv, (F(3,47)= 89.1, p<0.001. There was no significant difference in the MePd subdivision with CC exposure. PBS-injected mice also had greater FRAs expression than OTA-injected mice in response to CC in MeA, F(1,47)= 36.9, p<0.001, and MeP, F(1,47)= 25.7, p<0.001, and in each subdivision except MePd (MeAv: F(1,47)= 61.0, p<0.001; MeAd: F(1,47)= 13.9, p<0.001; MePv: F(1,47)= 75.1, p<0.001). There was no significant difference in FRAs expression between CC-exposed OTA injected mice and PBS injected control mice exposed to clean-fabric control in any region or subdivision of medial amygdala. Representative brain sections of the FRAs expression in both MeA and MeP to CC exposure in PBS and OTA animals are shown in Figure 3.
Figure 3. Representative coronal sections (40 μM) showing outlines within which FRAs-immunoreactive nuclei were counted.
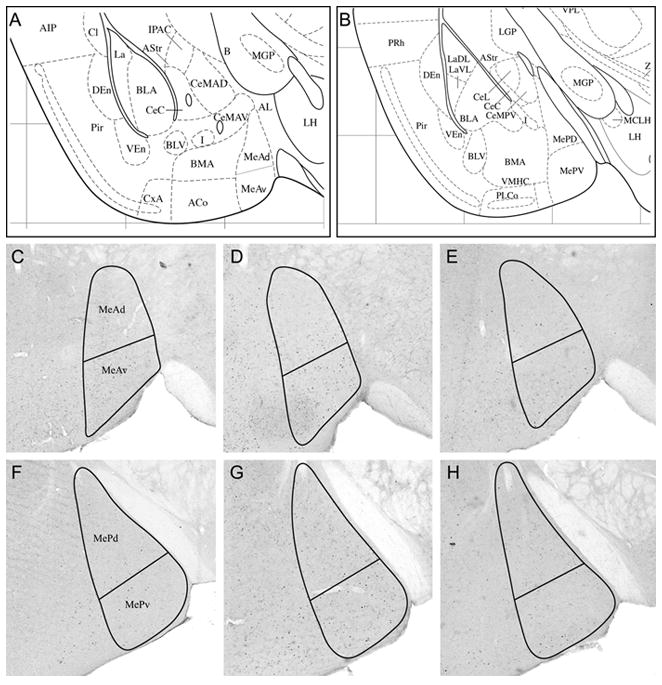
(A,B) Ventrolateral forebrain structures of the coronal sections used to measure IEG response in medial amygdala (from the Paxinos and Franklin (2003) mouse brain atlas). (A) At the level of anterior medial amygdala (MeA); Panels C, D, E are approximately at this level (1.06 mm posterior to bregma); (B) Posterior medial amygdala (MeP); Panels F, G, H are approximately at this level (1.58 mm posterior to bregma). Representative sections showing FRAs-labeled nuclei in anterior medial amygdala (C, D) and posterior medial amygdala (F,G) in PBS-injected mice exposed to (C, F) clean control collar or to (D,G) worn cat collar (CC). E and H show the reduced FRAs expression in MeA and MeP of an OTA-injected mouse exposed to CC.
Behavioral Response to Social Odor /Chemical-Stimulus Exposure
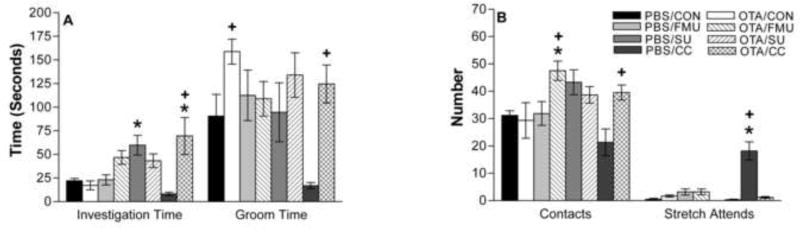
Investigation times for the stimulus-scented fabric pieces had a significant main effect of drug (p<0.02), a significant effect of stimulus exposure (p<0.02) and a significant interaction (p<0.001). PBS-injected mice spent significantly more time investigating SU than clean control fabric pieces (F(1,47) = 8.7, p<0.01). OTA-injected mice spent significantly more time investigating worn CC compared either to OTA-injected mice exposed to clean control fabric pieces (F(1,47) = 8.7, p<0.005) or to PBS-injected mice exposed to CC (F(1,47) = 22.9, p<0.001).
The number of contacts/close investigations of the stimuli had a main effect of drug (p<0.025), a significant effect of stimulus exposure (p<0.015) and a significant interaction (p<0.002). OTA-injected mice contacted fMU-scented fabric pieces significantly more times than PBS-injected mice (F(1,47) = 7.1, p<0.015); also more than PBS-injected mice exposed to clean fabric pieces (F(1,47) = 9.6, p<0.005). OTA-injected mice contacted the CC stimulus significantly more times than PBS-injected mice exposed to CC (F(1,47) = 9.6, p<0.005).
Time spent grooming had only a main effect of drug (p<0.001), with OTA-injected mice showing more grooming. There was no main effect of stimulus (p=0.08) and no significant interaction (p=0.085). OTA-injected mice spent significantly more time grooming than PBS-injected mice in the presence of a clean control piece of fabric (F(1,47) = 5.0, p<0.03). OTA-injected mice also spent significantly more time grooming in the presence a CC stimulus than did PBS-injected mice (F(1,47) = 12.5, p<0.001). Grooming in PBS-injected mice in the presence of scented CC was low compared to control PBS-injected males exposed to clean collar pieces, but not significantly different in the 2-Way ANOVA. Restricting the analysis to PBS-injected animals in a One-Way ANOVA did reveal a significant suppression of grooming in the presence of CC pieces, and a significant suppression of investigation time (Figure 4).
Figure 4. Behavioral response of PBS- and OTA-injected mice to conspecific and heterospecific chemical signals.
(A) PBS mice exposed to SU spent significantly more time (mean seconds ± SEM) than control investigating the stimulus. OTA-injected mice exposed to CC spent significantly more time investigating the stimulus than both control and PBS-injected mice investigating CC. OTA-injected mice groomed significantly more in the presence of a control collar than PBS-injected mice exposed to control. Also, OTA-injected mice exposed to CC spent significantly more time grooming than PBS-injected mice in the presence of CC. (B) OTA-injected mice exposed to fMU contact the stimulus significantly more than both control exposed PBS-injected mice and PBS-injected mice exposed to fMU. Only PBS-injected mice exposed to CC showed significantly more stretch-attend behavior to the stimulus than control or their OTA-injected counterparts. * indicates significant difference from control; + indicates significant difference between PBS- and OTA-injected mice exposed to same stimulus. Refer to the results section for p values.
The stretch-attend posture has been proposed as a measure of mouse defensive behavior (Yang et al., 2004). Stretch-attend behavior showed a main effect of drug (p<0.001), and of stimulus (p<0.001) and a significant interaction (p<0.001). PBS-injected mice exhibited significantly greater numbers of stretch attend movements than OTA-injected mice in response to CC (F(1,47) =, p<0.0001) and significantly more than PBS-injected mice exposed to clean control collar-pieces (F(1,47) = 84.2, p<0.0001). There was no difference in any of the other measured behaviors.
Discussion
OT has been repeatedly shown to be essential for normal social recognition behavior in mice (Ferguson et al., 2000; Ferguson et al., 2001; Choleris et al., 2003; Kavaliers et al., 2006; Choleris et al., 2008) In this report, we provide evidence that male mice made deficient in OT activity, by i.c.v. OTA injection, not only fail to recognize a familiar conspecific female, but also have significantly depressed IEG responses in the medial amygdala to all tested biological chemosignals, regardless of apparent biological relevance. We have previously reported that the pattern of IEG expression in medial amygdala to biologically relevant chemical signals (such as conspecific chemosignals or predator odors) is categorically different from the pattern for other heterospecific stimuli. Heterospecific chemosensory stimuli, with no obvious biological relevance, do elicit a response in anterior medial amygdala, but have little or no effect on behavior. In particular, biologically relevant stimuli increased IEG expression in both MeA and MeP, but stimuli with no apparent relevance increased expression only in MeA. This is the case for both mice (Samuelsen and Meredith, 2009a) and hamsters (Meredith and Westberry, 2004). These categorical medial amygdala responses are also eliminated by removing the vomeronasal organ in mice (Samuelsen and Meredith, 2009b) and hamsters (Fewell and Meredith 2002, Westberry and Meredith, unpublished). OT in the medial amygdala seems necessary for normal social recognition behavior in male mice. Oxytocin-knockout (OTKO) mice and mice injected with OTA fail to recognize previously encountered conspecifics (this report, Ferguson et al., 2000) and OT injected into medial amygdale restores social-recognition behavior (Ferguson et al., 2001). The fact that mice deficient in OT have little to no IEG expression in MeP in response to a conspecific female (Ferguson 2000), suggested to us that blocking OT action might disrupt the IEG expression pattern in MeP to other biologically relevant communication signals. Although we tested only a small sample of stimuli, our results suggest that OT in the medial amygdala is necessary for normal categorization of chemical-communication signals by the medial amygdala, and possibly necessary for normal amygdala response to all biological stimuli.
An obvious caveat when using i.c.v. injections is losing area specificity, as injections will spread to the entire brain. For the present study, direct injections into the medial amygdala were not an option as this would eliminate the ability to examine IEG patterns in this region. Therefore, the possibility that OTA activity in other areas, particularly the olfactory bulbs (OB), may be responsible for the defect observed in conspecific and heterospecific behavioral chemical IEG expression patterns. However, previous work with OTKO mice showed no effect on Fos expression in the OB after exposure to a conspecific and injections of OT into the OB showed no effect on the disrupted social recognition behavior, while direct injections into medial amygdala recovered this behavior (Ferguson et al., 2001). Previous experiments in hamsters (Meredith and Westberry, 2004) and unpublished preliminary data in mice show no difference in OB FRAs expression patterns after exposure to conspecific and heterospecific odors. Therefore the medial amygdala was the primary focus of these experiments.
In our previous studies, the chemical stimuli, other than CC, were presented on polyester tip swabs. In order to limit the number of animals in this study, we decided to present all stimuli on pieces of clean nylon collar fabric, eliminating the unnecessary swab-control groups. The change of presentation material did not affect the behavioral responses of PBS-injected mice to stimuli on the new substrate. They behaved similarly to the previously reported investigative behavior of intact male mice (Samuelsen and Meredith, 2009a). In our previous experiments, using swabs as presentation materials, mice would shred the control swabs leading to a high level of investigation time for clean-swab controls. The mice would not shred any swab that contained a chemical stimulus. This led to most chemosensory stimuli having a significantly lower investigation time, unusual compared to most of the published literature. The only chemical stimulus that was not significantly lower than control was steer urine (SU; which we chose as a stimulus likely to have little biological relevance for mice; Samuelsen and Meredith, 2009a). In the present study, PBS-injected mice spent significantly more time investigating SU as compared to control collar-fabric pieces, and mice did not shred the clean collar-fabric controls, resulting in a low control investigation time. This low investigation time for the control condition allowed the significant effect of OTA injection on investigation of SU to be clearly seen. Investigation of SU by OTA-injected mice did not differ from investigation of clean (control) fabric pieces by OTA-injected animals, suggesting that the quality of the SU that increased investigation time was not recognized by mice that had undergone i.c.v OTA-injection.
Upon exposure to predator-derived chemical stimuli, prey species exhibit a wide variety of behavioral changes including suppression of overall activity, avoidance of the area containing the predator chemical stimuli, and inhibition of non-defensive behaviors such as grooming (Blanchard and Blanchard, 1989; McGregor et al., 2002; Apfelbach et al., 2005). In this experiment, we report that the behavioral response to a predator odor is disrupted by i.c.v OTA injection. OTA mice spent significantly more time investigating a piece of worn CC as compared to both OTA-injected mice exposed to a clean collar-fabric control and PBS-injected mice exposed to worn CC. In a previous experiment (Samuelsen and Meredith, 2009a), intact males, as PBS-injected (sham-treated) males here, appeared to avoid worn CC pieces. This apparent avoidance of CC was also eliminated by vomeronasal organ lesions (Samuelsen and Meredith, 2009b).
The “stretch-attend” posture, noted by Grant and Mackintosh (1963), and defined as a posture in which the body is stretched forward with the animal either motionless or slowing moving toward the stimulus, is used as a behavioral measurement of an animal’s risk assessment (Yang et al., 2004). Only PBS-injected mice exposed to CC showed a significant difference from control in the number of stretch attend movements performed. This group also showed significantly more stretch-attend behavior than OTA-injected mice exposed to CC. Grooming behavior, a non-defensive behavior, was also significantly reduced in PBS-injected mice exposed to CC, but showed no difference from control in OTA-injected mice. Taken together, these data suggest that normal OT function may be necessary for appropriate behavioral responses to biologically relevant predator stimuli.
In female mice, social recognition is dependent upon estrogen and OT (Choleris et al., 2003; Choleris et al., 2006; Agmo et al., 2008) The impairment in the social recognition paradigm for female OTKO mice is similar to the impairment in estrogen receptor-alpha knock out (ERαKO) and estrogen receptor-beta knock out (ERβKO) female mice (Choleris et al., 2003). When presented simultaneously with a choice between a familiar and novel male both OTKO and ERαKO female mice show a significant impairment of social recognition. OT receptor is not upregulated in the medial amygdala of ERKO mice (Young et al., 1998), suggesting that the social recognition deficit in ERKO may be due in part to disruption of normal estrogen function in altering OT receptor expression in medial amygdala. ERβKO female mice perform significantly better than chance; however the performance is still significantly less than wild- type female mice (Choleris et al., 2006). These results support the theory that social recognition by female mice is dependent upon OT and ERα, while ERβ also facilitates social recognition (Choleris et al., 2009). ERαKO male mice exhibit a similar failure in social recognition (Imwalle et al., 2002). Interestingly, ERαKO and ERβKO male mice exhibit no behavioral deficits in the detection of predator chemical signals (Kavaliers et al., 2008). While, OTKO, ERαKO and ERβKO males have no olfactory impairments in simple tests, they fail to recognize and avoid odors of parasitized conspecifics (Kavaliers et al., 2005). It seems that OT, possibly modulated by estrogen, may affect the behavioral response of male mice both to conspecific mice in different physiological states and degrees of familiarity, as well as, to some conspecific and heterospecific chemical stimuli (shown here). All of these effects could result from a failure to correctly categorize biologically relevant stimuli; a capacity we tentatively assign to the medial amygdala.
Here we provide evidence that regardless of the biologically relevance of the chemical-communicative signal, normal OT function is required for both the behavioral response and categorical IEG expression pattern in the medial amygdala. We propose that categorization by the medial amygdala of conspecific and heterospecific chemical stimuli reflect their biological relevance in the animal’s environment and are dependent upon OT action. Our behavioral data suggest that the categorization we see in IEG expression within the medial amygdala predicts the animal’s evaluation of important stimuli.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC 005813 and T32 DC00044 (M.M.) and fellowship F31 DC08062 (C.L.S.).
Abbreviations
- CC
cat collar
- CON
control
- fMU
female mouse urine
- ERαKO
estrogen receptor-alpha knockout
- ERβKO
estrogen receptor-beta knockout
- FRAs
Fos-related antigens
- i.c.v.
intracerebroventricular
- IEG
immediate early gene
- MeAv/d
anterior medial amygdala, ventral/dorsal divisions
- MePv/d
posterior medial amygdala, ventral/dorsal divisions
- PFA
paraformaldehyde
- OB
olfactory bulb
- OT
oxytocin
- OTA
oxytocin antagonist
- OTKO
oxytocin knockout
- OVX
ovariectomized
- RDI
relative duration of investigation
- SU
steer urine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Agmo A, Choleris E, Kavaliers M, Pfaff DW, Ogawa S. Social and sexual incentive properties of estrogen receptor alpha, estrogen receptor beta, or oxytocin knockout mice. Genes Brain Behav. 2008;7(1):70–7. doi: 10.1111/j.1601-183X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29(8):1123–44. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: Effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29(8):1243–53. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.05.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci. 2003;100(10):6192–7. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci USA. 2008;104:4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5(7):528–39. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav. 2005;85:354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41(3):145–63. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182(115):939–41. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Arnold JC, McGregor IS. Low-dose midazolam attenuates predatory odor avoidance in rats. Pharmacol Biochem Behav. 1999;62:197–201. doi: 10.1016/s0091-3057(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4(7):551–62. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Grant EW, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21(1963):246–259. [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941(1-2):91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakes EM, Rissman EF. Estrogen receptor alpha influences socially motivated behaviors. Horm Behav. 2002;42(4):484–91. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young L, Witt DM, Crews D. Gonadal steroids have paradoxical effects on brain oxytocin receptors. J Neuroendocrinol. 1993;5:619–628. doi: 10.1111/j.1365-2826.1993.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109(4):782–9. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Johnston R, Brenner D. Species specificity of scent marking in hamsters. Behav Neural Biol. 1982;35:46–55. [Google Scholar]
- Kavaliers M, Choleris E, Agmo A, Braun WJ, Colwell DD, Muglia LJ, Ogawa S, Pfaff DW. Inadvertent social information and the avoidance of parasitized male mice: a role for oxytocin. Proc Natl Acad Sci. 2006;103(11):4293–8. doi: 10.1073/pnas.0600410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Pfaff DW. Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci Biobehav Rev. 2005;29(8):1347–59. doi: 10.1016/j.neubiorev.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Devidze N, Choleris E, Fudge M, Gustafsson JA, Korach KS, Pfaff DW, Ogawa S. Estrogen receptors alpha and beta mediate different aspects of the facilitatory effects of female cues on male risk taking. Psychoneuroendocrinology. 2008;33(5):634–42. doi: 10.1016/j.psyneuen.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210(4469):557–60. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur J Neurosci. 2008;29(2):368–76. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odors’ are equal: cat odor but not 2,4,5 trimethylthiazoline (TMT; fox odor) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann N Y Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Meredith M, Samuelsen CL, Blake C, Westberry J. Selective responses of medial amygdala subregions to reproductive and defensive chemosignals from conspecific and heterospecific species. In: Hurst JL, editor. Chemical signals in Vertebrates: 11. Springer; 2008. pp. 367–378. [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kikusui T, Takeuchi Y, Mori Y. The critical role of familiar urine odor in diminishing territorial aggression toward a castrated intruder in mice. Physiol Behav. 2007;90:512–517. doi: 10.1016/j.physbeh.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci. 2006;120:925–936. doi: 10.1037/0735-7044.120.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates: Compact Second Edition. Pub. Elsevier Science & Technology Books; 2003. [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;13(2):345–57. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Research. 2009a;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals. Review Neuroscience. 2009b doi: 10.1016/j.neuroscience.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav Neurosci. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102(44):16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Social memory in the male labrotory rat. J Comp Physiol Psycol. 1982;96:1000–1006. [Google Scholar]
- Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D, Wall PM, Blanchard RJ, Blanchard DC. The rat exposure test: a model of mouse defensive behaviors. Physiol Behav. 2004;81(3):465–73. doi: 10.1016/j.physbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor α is essential for induction of oxytocin receptor by estrogen. NeuroReport. 1998;9(5):933–6. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]