Abstract
The chromatin associated cellular proteins LEDGF/p75 and LEDGF/p52 have been implicated in transcriptional regulation, cell survival and autoimmunity. LEDGF/p75 also appears to act as a chromatin docking factor or receptor for HIV-1 and other lentiviruses and also to play a role in leukemogenesis. For both the viral and cellular roles of this protein, a key feature is its ability to act as a molecular adaptor and tether proteins to the chromatin fiber. This chapter reviews the emerging roles of LEDGF/p75 and LEDGF/p52 in diverse cellular processes and disease states.
1. History and nomenclature: transcriptional coactivator p75, LEDGF/p75, DFS70
Between 1998 and 2003, research in four apparently unrelated fields — transcriptional regulation, cell survival, autoimmunity and virology — independently identified a polypeptide that migrated in denaturing SDS gels with an approximate molecular weight of 75 kDa [15, 34, 65, 82]. The initial discovery came from micro-sequencing of a protein that co-purified with the general transcriptional coactivator positive cofactor 4 (PC4) [34]. Two splice variant cDNAs were identified, one encoding the 75 kDa species (p75) and the other a smaller polypeptide, p52. Like PC4, both proteins enhanced activity of the general transcription machinery in vitro and so were designated transcriptional coactivators p75 and p52. A year later, p75 was isolated from a mouse lens epithelium library and was reported to protect these cells from oxidative damage [82]. These workers named the protein Lens Epithelium-Derived Growth Factor p75 (LEDGF/p75), in part because it is one of seven members of the Hepatoma-derived growth factor (HDGF) family [22]. The latter term has entered common usage. The implied possibilities that LEDGF/p75 might be secreted or act as a signal-transducing growth factor have been discarded on the basis that the protein is constitutively nuclear [15, 49, 53] and is not detectable in culture supernatants or serum (our and others unpublished data). In addition, LEDGF/p75 knockout mice show no defects in lens development [87]. The general view at present is that LEDGF/p75 is a ubiquitously expressed nuclear transcription factor-type protein with a role in transcriptional regulation [27–29, 34, 45, 73, 75, 76, 98]. In 2000, p75 was again identified by screening of a cDNA library with human serum reactive against the nuclear auto-antigen dense fine speckled protein of 70 kDa (DFS70) [65]. This line of work has suggested a role for LEDGF/p75 in autoimmunity [32, 65] and additional studies have suggested roles in cell survival and prevention of apoptosis [33, 76]. More recently, the protein was isolated by Cherepanov et al. as a polypeptide that co-immunoprecipitated with human immunodeficiency virus type 1 (HIV-1) integrase (IN) when the latter was over-expressed outside the viral context [15].
LEDGF/p75 and LEDGF/p52 bind chromatin tightly [50, 64, 89] and interact with other nuclear proteins [5, 6, 34, 35, 55, 98]. Several of these proteins are tethered to the chromatin fiber by LEDGF/p75. These properties underlie roles for these proteins in transcriptional regulation, which in turn appears to influence the processes of cellular transformation, differentiation, and survival. We now also know that lentiviruses, the genus of retroviruses to which HIV-1 belongs, have exploited the chromatin tethering capacity of LEDGF/p75 during their obligate life cycle step of integration into a host chromosome [48, 78, 92]. LEDGF/p75 dependence is found not only in the primate group of lentiviruses, but also in the two other main groups (ungulate and feline lentiviruses) [10, 12, 48, 49, 56]. Here we will discuss evidence for the cellular and the viral cofactor activities of LEDGF/p75, concentrating on recent findings. For additional reviews that focus on the HIV-1 cofactor role comprehensively see [18, 26, 68, 90].
1.1 Gene organization and splice variants
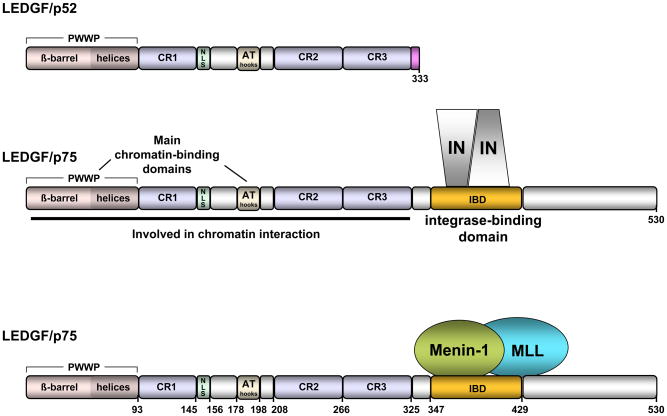
LEDGF/p75 and LEDGF/p75 are illustrated in Figure 1. Both are encoded by the gene PSIP1 (PC4- and SFRS-interacting protein 1) which is 35.7 kb in length and located on human chromosome 9p22.3 [80]. There are 15 exons and 14 introns. Gene to protein relationships are presented in Table 1. The two encoded splice variants share the same N-terminal 325 amino acids (encoded by exons 1–9), but have different C-termini, 8 amino acids in the case of p52 and 205 in the case of p75. This C-terminal portion of LEDGF/p75 contains the IN binding domain (IBD, residues 340–417) which is central to the protein’s virological and cellular significance [14, 93].
Figure 1.
Domain structure and binding partners of LEDGF/p75 and LEDGF/p52.
Table 1.
Gene-to-protein relationship in LEDGF proteins
| Exon | Amino acids | Protein domain | p75 mRNA segment |
|---|---|---|---|
| 1 | 1–23 | PWWP | 1–382 |
| 2 | 24–50 | PWWP | 383–459 |
| 3 | 51–96 | PWWP, CR1 | 460–598 |
| 4 | 97–131 | CR1 | 599–703 |
| 5 | 136–152 | CR1, NLS | 704–766 |
| 6 | 153–184 | NLS, AT | 767–863 |
| 7 | 185–210 | AT, CR2 | 864–939 |
| 8 | 211–286 | CR2, CR3 | 940–1168 |
| 9 | 287–326 | CR3 | 1169–1287 |
| 10 | 327–344 | 1288–1343 | |
| 11 | 345–368 | IBD | 1344–1414 |
| 12 | 369–402 | IBD | 1415–1516 |
| 13 | 403–472 | IBD | 1517–1731 |
| 14 | 473–511 | 1732–1843 | |
| 15 | 512–530 | 1844-poly(A) signal | |
| 9-A | *287–326 | CR3 | |
| 10-A | *327–333 | C-terminal region |
Exons 9-A and 10-A encode CR3 and the C-terminal region of LEDGF/p52. Exon 9 and exon 9-A have identical nucleotide sequences, except different terminal amino acids are encoded (Q for 9, and H for 9-A)
Additional LEDGF/p52 splice variants were identified recently by mRNA analyses in promyelocytic leukemia cells [43]. They elaborate the theme of variant C-terminal domains determining diverse bi-molecular adaptor roles in tandem with the chromatin-binding properties of the N-terminal portion. One, p52b, was highly expressed at the mRNA level. In the putative encoded protein, the last eight amino acids of p52 are substituted by 25 extra amino acids derived also from intron 9 of the gene. The other three LEDGF/p52 variants are expressed at lower levels and they lack exon 6 and exhibit other sequence modifications [43]. However, endogenous protein expression of these p52 splice variants has not been documented.
Northern blots have identified a 1.8 kb species of mRNA corresponding to LEDGF/p52 and two additional species of 3.4 and 2.8 kb that correspond to p75 [34]. These mRNAs and proteins are ubiquitously expressed, with LEDGF/p75 being considerably more abundant in the majority of tissues, although greater relative amounts of p52 mRNA were detected in the brain, testis and thymus [34], suggesting that splicing of PSIP1-derived mRNAs can be regulated in tissue-specific fashion.
2. Chromatin binding
LEDGF/p75 and p52 are nuclear proteins that attach to chromatin avidly throughout the cell cycle, though as determined by studying GFP fusions, p52 may have a more restricted intra-nuclear distribution [64]. The major determinants of sub-cellular distribution are located in the shared N-terminal region (Figure 1) [50, 52, 89, 93]. Nuclear import is determined by a nuclear localization signal (NLS) at residues 148–156 [52, 81, 93]. This NLS belongs to the classical basic (SV40 large T antigen) family and nuclear import requires functional Ran, the adaptor protein importin-alpha, and the nuclear import receptor importin-beta [52, 93]. NLS deletion leads to cytoplasmic localization of newly synthesized LEDGF/p75. However, in dividing cells, the NLS is actually dispensable for nuclear localization. The protein is chromatin-trapped with such efficiency during mitotic mingling of nuclear and cytoplasmic contents that stably expressed NLS-mutants are seen only in tight association with chromosomes [93]. Chromatin binding is mediated in part through the N-terminal PWWP domain (residues 1–93) [50, 77, 89]. Importantly, although there is extensive homology between the PWWP domain of LEDGF/p75 and the same domain in a related IBD-containing HDGF family member, hepatoma derived growth factor-related protein 2 (HRP-2), this latter protein (and any associated IN) dissociates from chromatin in mitosis and does not display the tight Triton-resistant chromatin binding of LEDGF/p75 in any part of the cell cycle [93]. It is thus clear that the chromatin binding of PWWP-containing proteins is greatly influenced by other protein regions.
LEDGF/p75 resists extraction from chromatin when cells are lysed in isotonic buffers containing the detergent Triton X-100 [50]. Triton-resistance is determined primarily by the cooperative interaction of the PWWP domain with two downstream AT hook motifs (residues 178–198, Figure 1). Flanking relatively charged regions (CR1, CR2, and CR3) contribute to a lesser extent [50]. Turlure et al. also implicated the NLS region in chromatin binding [89]. Mutations that disrupt the PWWP domain partially impair Triton-resistant chromatin binding, while simultaneous mutational inactivation of the PWWP and the AT hook domains completely abolishes it [50]. Although such mutants localize to the nuclear compartment during interphase, they are excluded from chromatin during mitosis [50, 77]. Shun et al. have further identified specific amino acid mutations within the domain that disrupt chromatin binding, e.g. W21A [77].
Sub-nuclear distribution analyses of LEDGF/p75 and p52 were performed with GFP fusion proteins [64]. Although LEDGF/p52 and p75 might be predicted to display quite similar sub-nuclear distributions, it turns out that these are distinct with respect to the cell cycle [64]. In G1, LEDGF/p52 was found in the nuclear periphery, while p75 was distributed diffusely throughout the nucleus, recapitulating the characteristic dense fine speckled pattern observed with auto-antibodies. During metaphase, LEDGF/p52 was observed to form a cylindrical pattern surrounding chromosomes whereas LEDGF/p75 associated with chromosomes in a striated pattern. At cytokinesis, LEDGF/p75 nuclear localization was diffuse while LEDGF/p52 retained the cylindrical pattern [64]. These observations imply that the respective C-terminal regions govern the finer aspects of intra-nuclear segregation. The localization of LEDGF/p75 is likely to be modulated by interactions with other chromatin bound proteins, some of which are discussed below. Alternatively, the presence of the long C-terminal region of LEDGF/p75 could modify interactions of the shared N-terminal region with chromatin.
2.1 Interactions with cellular and viral proteins
In in vitro assays with recombinant LEDGF proteins and purified general transcription factors, LEDGF/p52 interacted functionally with a number of viral transcriptional activation domains, e.g., VP16, the pseudorabies immediate early protein and adenovirus E1A, as well as with the cellular transcription factor Sp1 and PC4 [34, 35]. In these in vitro systems p52 fulfilled general transcriptional coactivator criteria; however, with the exception of the VP16 activation domain, LEDGF/p75 did so only marginally [34]. In addition, LEDGF/p52, but not p75, has been reported to interact with the essential splicing factor ASF/SF2, thus modulating pre-mRNA splicing [35]. Therefore, LEDGF/p52 has been proposed to be a coordinator between transcription and pre-mRNA splicing [35].
The differential interaction of LEDGF proteins with cellular proteins is informative considering that both proteins share the same N-terminal chromatin-interacting region which corresponds to 97.6% of p52. These examples provide further evidence for the long C-terminal region determining diverse molecular partner interactions and we speculate that they may explain the differences in sub-nuclear localization discussed above [64]. In support of this hypothesis, a polyclonal antibody raised against LEDGF/p52 recognized both p52 and p75 in immunoblots but immunoprecipitated only LEDGF/p52 from cellular extracts, suggesting that native p75 is not recognized, perhaps because amino acids 326–530 or bound cellular interactors mask N-terminal epitopes [34].
The IBD interacts with lentiviral IN proteins, the c-Myc interactor JPO2 [5, 55], the menin/MLL histone methyl transferase complex (Figure 2) [98], and the pogo transposable element with ZNF domain (pogZ) [6]. The IBD surfaces involved are not completely overlapping. For example, mutation of D366, which is located in an interhelical loop that extends into the IN dimer interface, abrogates interaction with lentiviral IN proteins but does not affect interaction with JPO2 or pogZ [5, 6, 55]. However, an F406A mutation in an adjacent loop impairs the binding of these three proteins. Site-directed mutagenesis of LEDGF/p75 IBD residues implicated in IN binding show more overlap with residues required for pogZ than for JPO2 binding. Interestingly, lentiviral INs and pogZ belong to the DDE domain family [6]. The significance of the JPO2 and pogZ interactions is not clear at present. Analogously to its effects on over-expressed HIV-1 IN [47], LEDGF/p75 protects JPO2 from proteolysis and tethers this protein to chromatin during all phases of the cell cycle [5, 55]. By contrast, interaction with pogZ yields a tightly chromatin bound complex in cells in interphase, but no apparent chromosome tethering during mitosis [6].
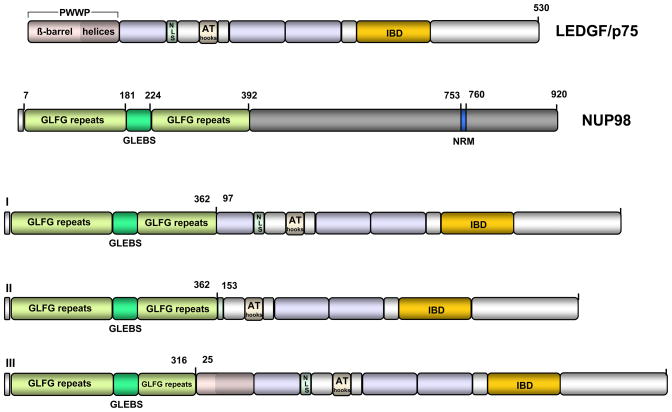
Figure 2. Structure of NUP 98-LEDGF/p75 fusion proteins.
NUP98 contains eight N- terminal GLFG repeats that govern localization of the protein to nuclear GLFG bodies. GLEBS is a sequence within the GLFG repeats that serves as docking site to GLE2p, the yeast ortholog of human RAE1 a protein involved in mRNA nuclear export. Nucleoporin RNA binding motif (NRM) is an octapeptide with partial homology to the ribonucleoprotein motif. In the NUP98-LEDGF/p75 fusion proteins (I–III) described in leukemic patients, the NUP98 GLFG repeats and the GLEBS element fuse to LEDGF/p75 segments that contain the IBD.
LEDGF/p75 interaction determinants are well understood for two protein complexes, the lentiviral IN dimer and the menin/MLL complex. LEDGF/p75 tethers both of them to chromatin [49, 53]. Tethering appears to direct integration of lentiviruses into active transcription units [18, 19, 56, 78]. For Menin/MLL, LEDGF/p75 forms a trimolecular complex that serves to target menin/MLL to genes such as Hoxa9 and so acquire transforming activity [98] (reviewed in [71]).
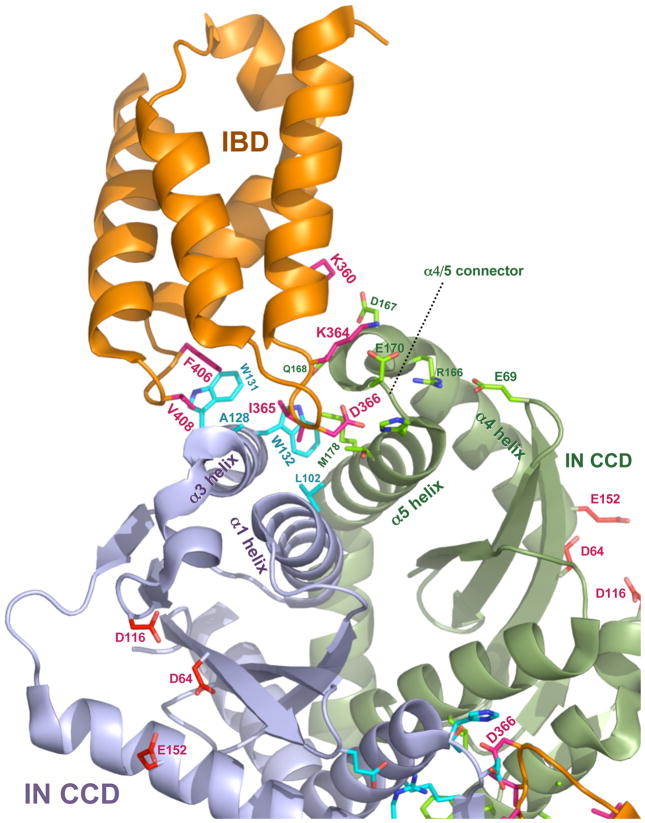
In addition to the catalytic core domain (CCD) [11, 53, 70], the N-terminal domain (NTD) of IN also interacts with the IBD [53]. Detailed structural information is now available for both interactions [13, 16, 38]. As shown in Figure 3, the IBD is a compact structure comprised of a pair of alpha-helical hairpins [16]. The IBD-CCD interface occurs in a pocket that forms between helices α1 and α3 of one IN monomer and helices α4 and α5 in the other IN monomer. In particular, residues connecting IN helices α4 and α5 (the alpha 4–5 connector) and hydrophobic residues in the other monomer engage two inter-helical loops of the IBD respectively (Figure 3) [13]. Alanine-scanning of this region of the IBD indicated that residues I365, D366, F406 and V408 play essential roles in the LEDGF/p75-IN interaction. Mutation of I365, D366, or F406 disrupts LEDGF/p75-IN interaction [16] and the mutants lack HIV-1 cofactor activity [48, 78, 92]. Differences in these regions of other retroviral IN proteins explain their failure to interact with LEDGF/p75 [10, 12, 49]. Within the lentiviral genus of retroviruses, the consistency of IN-LEDGF/p75 interaction despite limited direct sequence homology in key IN residues (Figure 4) suggests a significant selective advantage during viral evolution. The relatively small, deep cleft occupied by the IBD [13] suggests as well that small molecules could interfere with the LEDGF/p75-IN interaction. Recent progress has been made toward this end [23, 42]. The IBD-IN NTD interface involves charged interactions that differ from the more lock-and-key IBD-CCD interaction, with acidic residues in the NTD (E6, E10 and E13) interacting with basic amino acids in the IBD (K401, K402, R404 and R405) [38].
Figure 3. Co-crystal structure of the HIV-1 IN CCD-IBD interface.
The figure was constructed with MacPyMOL from Protein Data Bank file 2BJ4 (www.pdb.org, ref. [13]. LEDGF/p75 contributes most of the amino acid side chains that make direct contact with IN. D366 engages in a pair of essential hydrogen bonds with the main chain amides of E170 and H171 in the alpha-4/5 connector of one IN monomer. Hydrophobic interactions predominate in interactions with the other IN monomer: IN residues W132, W131, A128, L102 form a pocket in the vicinity of IBD residues F406 and V408; this pocket buries IBD residue I365). IN catalytic center resides D64, D116 and E152, the mutation of which produce purely catalytic defects (reviewed in [25]) are shown in the lower half of the figure for each IN monomer. Interaction between the HIV-1 IN NTD and the IBD was previously established by biochemical evidence [53] and Hare et al. have recently solved a co-crystal structure for the LEDGF/p75 IBD complexed with a two-domain fragment of HIV-2 IN (NTD+CCD) [38]. Extensive structural contacts between the IBD and the NTD were identified and characterized [38]. Charged interactions are dominant, with conserved acidic residues in the HIV-2 IN NTD (E6, E10, E13) engaging complementary basic residues in the IBD (K401, R404, R405). Moreover, the NTDs of other lentiviral INs contain the same or closely adjacent glutamic acid residues. Enhancement by LEDGF/p75 of concerted strand transfer activity in vitro was also shown to be impaired by charge-reversing mutation of the basic IBD residues to glutamates in the equine lentivirus (EIAV) IN [38]. This activity could then be partially restored by reciprocal mutation of the acidic IN residues to lysines, verifying that these charged interactions are the main structural feature. See ref. [17] for a recent review of HIV-1 IN structural biology.
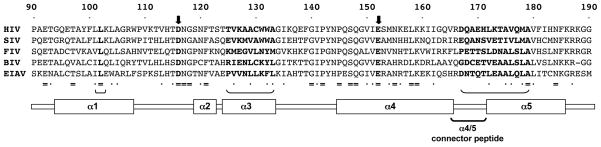
Figure 4. Alignment of the core catalytic domains of different lentiviral IN proteins.
Alignment of the central part of the CCD for IN proteins from the three lentiviral subgenera (primate, feline, ungulate). Identity is indicated by = and residues with conserved biochemical features by dots. IN alpha helices are indicated below and the segments primarily involved in forming the IBD binding pocket are indicated by bold-face font and brackets. The relative lack of sequence conservation in these regions, e.g., the alpha 4/5 connector, is evident. Figure 2 shows the placement of these protein elements for the interface of HIV-1 IN with LEDGF/p75; catalytic center residues (heavy black arrows here) do not contact the IBD. Although its exact oligomerization state in the PIC is not conclusively established, the weight of evidence is in favor of the enzyme acting as a multimer, with a tetramer likely [4, 15, 30, 31, 37, 39, 40, 46, 84, 96]. In vitro, an IN dimer enables 3′ end processing but a tetramer appears needed for DNA strand transfer activity [30, 37, 46]. Of note, higher order multimers were defined in the Hare et al. co- crystal structure of the IBD with HIV-2 INNTD+CCD [38]. Their relevance to the oligmeric state in the virus remains to be determined.
3. A role in transcriptional regulation
As discussed above, p52 and p75 were initially identified by co-purification with PC4 in HeLa cell nuclear extracts and displayed transcriptional coactivator activity in vitro [34]. Over-expression of LEDGF/p75 was also found to augment transcription of a set of stress-related genes [27–29, 45, 57, 73–76, 79, 82, 88] that include heat shock protein 27 [82], αB-crystallin [82], antioxidant protein 2A [29] and involucrin [45]. In all of these cases, LEDGF/p75 induced the expression of the endogenous proteins as well as reporter genes introduced under the control of the homonymous promoters [28, 29, 45, 75, 79]. The transcriptional regulation was mediated by LEDGF/p75 binding to heat shock and stress-related elements in the promoters. Binding to these DNA sequences was identified by electrophoretic mobility shift assays (EMSA) and binding specificity was confirmed using competitor oligonucleotides, supershift induction with antibodies to LEDGF and ablation of interaction following mutagenesis of LEDGF-binding sites in the target oligonucleotides. In addition, the interaction of LEDGF/p75 with some of these promoters was detected by DNase I footprinting. Moreover, mutation of binding sequences in promoters of the LEDGF/p75-responsive genes abrogated the transcriptional coactivator role [28, 29, 45, 75, 79]. In contrast, however, specific binding of LEDGF/p75 to oligonucleotides containing the reported LEDGF/p75-binding sequences was not seen in independent surface plasmon resonance and electrophoretic mobility shift assays. Instead, LEDGF/p75 was reported to bind nonspecifically to DNA through the two AT-hook motifs and the NLS domain [89]. Further studies to clarify the specificity of interaction of LEDGF/p75 with these sequences are necessary.
More recently, LEDGF/p75, but not p52, was implicated in transcriptional regulation of genes involved in the pathogenesis of myeloid leukemias and in the establishment of the embryonic body plan during development [98]. Cytogenetic evidence has identified a translocation that generates a fusion protein between the nucleoporin Nup98 and LEDGF/p75 in patients with acute or chronic myeloid leukemia (Figure 2) [1, 36, 44, 59]. Nup98 translocation occurs commonly in these types of cancers and more than 15 different partners are known to fuse to it. In all these fusion proteins the N-terminal GLFG repeats of Nup98 are linked to the C-terminal region of the partner genes [3, 63, 85]. Expression of the mRNA of Nup98-LEDGF fusions was observed only during active disease. One patient exhibited a fusion of the first 362 residues of Nup98 with a LEDGF/p75 or p52 lacking their first 152 amino acids [1, 36], whereas another chimera was generated by the fusion of exon 8 of Nup98 with exon 4 of PSIP1 such that the first 96 amino acids of LEDGF are missing [59]. The third reported fusion protein contained the first 316 amino acids of Nup98 and all of LEDGF/p75 distal to amino acid 25 [44]. Importantly each of these fusion proteins lack a functional PWWP domain of LEDGF/p75. The presumed loss of the tethering component may lead to alteration of LEDGF/p75-dependent transcriptional regulation.
Nup98-LEDGF/p75 fusions retain the ability of LEDGF/p75 to interact with the menin/MLL histone methylase complex, which may be implicated in leukemogenesis [71, 98]. MLL is targeted by numerous translocations that lead to oncogenic MLL fusion proteins, and LEDGF/p75 normally tethers unrearranged MLL to target genes with Menin essentially serving as a connector between LEDGF/p75 and MLL (Figure 2) [98]. MLL is a nuclear protein containing a SET domain that functions to methylate lysine 4 on histone 3, a genetic modification associated with active transcription. Moreover LEDGF/p75-dependent recruitment of the menin/MLL complex is mediated by the IBD. The recruitment of menin/MLL leads to transcriptional regulation of several genes including Hox genes [98]. Properly regulated expression of the latter genes is necessary for the establishment of the vertebrate body plan during development and altered Hox gene expression during embryogenesis leads to homeotic skeletal transformations similar to those observed in LEDGF/p75 knockout mice [87]. Dysregulation of Hox gene expression has also been observed in LEDGF/p75-deficient cells [19]. Over expression of Hox genes is a salient feature of MLL-associated leukomogenesis as well. Chromosomal translocations that generate MLL fusion proteins have leukomogenic activity and LEDGF/p75-mediated tethering is required during leukemic transformation induced by them [71].
3.1 Effects on cell survival
Over expression of LEDGF/p75 or treatment of cells with recombinant LEDGF/p75 has been reported to rescue a variety of cell types from death induced by diverse environmental insults including serum starvation, oxidative damage, heat shock and UVB irradiation [2, 43, 51, 57, 62, 82, 83, 95]. Protection has in general been ascribed to regulation of stress-responsive gene transcription.
A role for LEDGF/p75 in maintaining lysosomal integrity was also recently proposed [20]. LEDGF/p75 knockdown with siRNAs induced lysosomal-dependent cell death in malignant cell lines but not in immortalized or primary cells. These observations contrast with the normal viability observed in CD4+ T cell leukemic lines in which a 97% reduction of LEDGF/p75 mRNA was achieved by stable expression of specific shRNAs [48]. Proliferative capacity, morphology and other aspects, e.g., susceptibility to infection by gammaretroviral vectors, were indistinguishable from control cells expressing higher levels of LEDGF/p75 [48]. Effects of LEDGF proteins on cell morphology have been reported in other systems however. For example, intracellular over-expression of LEDGF/p52 has recently been shown to stimulate dendritic arborization and axonal elongation in neural cells [99, 100]
A role in cell survival processes was further suggested by evidence that these proteins are targeted by caspases [8, 95]. LEDGF/p75 has been reported to be a substrate for caspase-3 and -7 during apoptosis, which results in protein fragments that lack the pro-survival activity of the full-length protein. Three caspase-cleavage sites were identified at residues 30 and 85 within the PWWP domain, and at amino acid 486 in the C-terminal region.
In contrast to the pro-survival role of LEDGF/p75, p52 over-expression has been reported to promote apoptosis in tumor cells, a phenomenon dependent on the eight amino acids long C-terminal region [8]. However, as noted above, in rat retinal ganglion cells LEDGF/p52 overexpression did not affect viability of these cells and induced neurite growth [100].
3.2 LEDGF/p75 and auto-immunity
The alternative name of DFS70 (nuclear auto-antigen dense fine speckled protein of 70 kDa) arose when LEDGF/p75 was found to be recognized by autoantibodies present in patients with several chronic inflammatory diseases and cancer [32, 33, 65]. A high prevalence (11–22%) of LEDGF/p75-autoantibodies has also been reported in healthy individuals [61, 94]. Their pathogenic significance is not clear but it has been proposed that they are natural auto-antibodies that are overproduced in some disease states [32]. Epitope mapping conducted with LEDGF/p75 auto-antibody-positive serum from 93 patients and 38 healthy controls indicated that 94% of the samples in both groups reacted to a recombinant LEDGF/p75 protein containing residues 349–435, a region that contains the IBD [66]. Reactivity against other regions was considerably underrepresented leading to a conclusion that the IBD is the major auto-immunity determinant.
4. An integration cofactor for HIV-1 and other lentiviruses
Following the discovery that LEDGF/p75 co-immunoprecipitated with over-expressed HIV-1 IN [15], and a series of studies that characterized domain properties and confirmed the necessary and sufficient role for LEDGF/p75 in mediating IN nuclear and chromatin location [10, 13, 14, 16, 24, 47, 49, 50, 52–54, 89, 93], a fundamental role in integration of HIV-1 and other lentiviruses and was demonstrated in several systems [24, 41, 48, 77, 78, 92]. Among the seven genera of retroviruses, this cofactor role has turned out to be strictly lentiviral-specific. Accordingly, LEDGF/p75-deficient cells are resistant to infection by HIV-1, feline immunodeficiency virus (FIV) and equine infectious anemia virus (EIAV) but are permissive to infection by the gammaretrovirus murine leukemia virus (MLV) [48]. Protein interaction studies are consistent with this, showing that LEDGF/p75 interacts directly with all lentiviral INs tested so far but not those of alpha-, beta-, delta and gamma- or spuma-retroviruses [10, 12, 49]. This lentiviral selectivity is notable since this cellular protein, and in particular the IBD, is widely conserved – species-specific positive selection is not evident – suggesting it has been available to diverse retroviruses since at least the emergence of bony fishes. LEDGF/p75 mutants that lack either chromatin or IN interaction capacity can not rescue HIV-1 infection in LEDGF/p75-deficient cells, suggesting that the tethering function is central [48, 78, 92]. This mechanism is also supported by the change in viral integration site distribution observed in LEDGF/p75-deficient cells [18, 19, 56, 78].
HIV-1 IN has two enzymatic activities that are deployed in sequence during viral integration [7]. After reverse transcription, IN removes a dinucleotide from the 3′ end of the viral DNA, a process that appears to occur in the cytoplasm. This 3′ processing step is preserved in LEDGF/p75-null cells [78]. The viral genome (the pre-integration complex or PIC) is then imported into the nucleus where IN carries out the second step, strand transfer. This involves the generation of single strand breaks in the opposite strands of the host DNA 5 bases apart, and concerted ligation of both of the viral 3′ ends to chromosome 5′ ends. Interestingly, PICs isolated from these cells were competent for in vitro integration although in vivo integration was markedly impaired.
In cells, LEDGF/p75 associates with tetrameric IN [15]. The IBD has been demonstrated in vitro to stabilize IN subunit-subunit interactions, promoting the formation of a tetramer [58]. Importantly, tetramers of IN are implicated in the bona fide strand transfer reaction [46]. Therefore, LEDGF/p75 appears to influence higher order structures of IN and this effect may influence its enzymatic activity. Effects of LEDGF/p75 on IN catalysis have been studied using in vitro integration assays that mix designed un-proteinated DNA fragments with purified recombinant LEDGF/p75 and IN proteins [12, 14, 15, 58, 69, 91]. LEDGF/p75 or the IBD significantly increased IN-mediated 3′-processing of relatively short, 21-mer, double-stranded synthetic oligonucleotides [58]. In addition, LEDGF/p75 and to a lesser extent the IBD enhance IN strand transfer activity [12, 14]. The effect of p75 on concerted integration in in vitro assays has differed between different lentiviral IN proteins, enhancing full-site integration (properly concerted insertion of both ends) for EIAV IN but promoting uncoupled (half-site) integration with HIV-1 IN [12]. Promotion of the half-site reaction was corroborated in others studies [58, 69] although the balance of half and full site integration has also appeared to vary with IN protein concentration and other assay parameters [67]. PIC nuclear import is not defective in the absence of LEDGF/p75 nor is nuclear stability of the imported viral genome altered in LEDGF/p75-deficient cells since abundant non-integrated genomes are detected in the form of two-LTR circles [48, 78, 92]. However, in these cells the amount of integrated viral genomes is reduced [48, 78] and the characteristic favoring of active transcription units by HIV-1 [72] is also diminished [19, 56, 78]. These data suggest the possibilities that intra-nuclear trafficking, chromatin attachment, strand transfer or repair of the semi-ligated integration intermediate by host enzymes could be defective in LEDGF/p75-deficient cells. Direct and definitive evaluation of these variables remains to be done.
The effects of IBD over-expression (as GFP-IBD or GFP fused to somewhat longer fragments of the C-terminal region) are interesting and incompletely understood [21, 48]. For example, over-expression of such proteins inhibits HIV-1 integration to roughly the same extent as RNAi. When knockdown is sufficiently stringent to strip LEDGF/p75 from the Triton X-100 resistant chromatin fraction, inhibition of single round HIV-1 reporter virus infection is about 10–30 fold. Roughly the same effect occurs with GFP-IBD over-expression, but combination of the two modalities leads to synergistic (> 500-fold) inhibition [48], and up to approximately 104-fold inhibition has since been observed (our unpublished data). Moreover, passage of HIV-1 in the presence of such dominant interfering IBD proteins leads to selection of adaptive escape mutations in the IN dimer interface, namely A128T and E170G [41].
It remains unclear where these dominant-interfering proteins act in the post-entry series of events that culminate in integration or, more generally, where endogenous LEDGF/p75 first engages the viral complex. HIV-1 and FIV PICs isolated from the cytoplasm of cells could be immunoprecipitated with antibodies to LEDGF/p75 [49]. While no significant pull down was observed in a similar experiment [97], LEDGF/p75 was noted to restore integration activity to salt-stripped cytoplasmic HIV-1 PICs [91]. Whether or not it interacts with IN in the incoming PIC prior to nuclear entry, LEDGF/p75 has not been detected in free virions and it has not been implicated convincingly in PIC nuclear import.
When HIV-1 IN is expressed as a free protein in the absence of other viral components (a situation without a correlate in the viral life cycle) it is readily detected in the nuclei of cells. However, in addition to shifting to the cytoplasm in the absence of LEDGF/p75, IN is also markedly de-stabilized, such that it is difficult to detect by confocal microscopy or immunoblotting [47]. IN mRNA levels are unaffected, however, indicating a post-translational effect. IN was also found to be ubiquitinated and pharmacological inhibition of the proteasome restored wild type levels of the protein [47]. While LEDGF/p75 mutants lacking IN-binding activity fail to shield IN from the proteasome, mutants that selectively impair chromatin binding or nuclear localization of the LEDGF/p75-IN complex still prevent degradation [47]. Recently, it has been reported that IN is targeted for proteasome degradation by binding to the von Hippel-Lindau (VHL) binding protein 1(VBP1), which in turn recruits the Cullin2-based VHL E3 ubiquitin ligase, leading to IN ubiquitination and proteosome degradation [60]. LEDGF/p75 and VBP1 binding sites on IN overlap. While direct evidence is lacking, this suggests that LEDGF/p75 may prevent protesome-mediated degradation of IN by competing with VBP1 binding. The role, if any, of this proteasome shielding mechanism for IN within the HIV-1 PIC is not clear, and it is certainly not sufficient, because chromatin binding mutants that stabilize over-expressed IN do not rescue infection [48].
LEDGF/p75 dependency is observed in murine cells although there is no evidence for a lentivirus that naturally infects rodents [56, 78]. Lentiviral vectors with minimized viral sequences are also dependent [48, 78] which suggests that viral genome complexity, size or nucleotide composition (e.g., A-richness) are not involved. Although the chromatin tethering activity of LEDGF/p75 has been established to play a key role, the molecular mechanism is not completely understood (reviewed in [26, 68]).
4.1 LEDGF/p75 as a therapeutic target
Current HIV therapies utilize combinations of drugs that target viral proteins involved in different steps of the viral life cycle. Toxicity, resistance development and expense remain limitations. The now clearly demonstrated efficacy of strand transfer inhibitors such as raltegravir verifies that integration can be targeted with success [86]. The appropriateness of the LEDGF/p75-IN interaction for therapeutic targeting is supported by the multiple lines of evidence for its role as an HIV-1 dependency factor, the limited physiological effects observed when cells are rendered deficient in this protein, and also by the possibility that resistance potential may be less because one half of the interface is a cellular protein. The interaction appears amenable to small molecule inhibition because of the excellent structural definition that exists, the relatively small size and depth of the IN binding pocket, and the susceptibility of binding to abrogation by single amino acid changes [13]. Cell-based and recombinant protein interaction-based screening systems have been used for such screening [9, 23, 42]. Compounds identified have been reported to have anti HIV-1 activity, providing additional support for the concept that LEDGF/p75 plays a fundamental role in HIV-1 infection [23, 42].
Acknowledgments
We are grateful for support from N.I.H. grants 1SC2GM082301 to M. L. and AI77344 to E.M.P. We thank P. Spearman for editorial advice, and patience.
References
- 1.Ahuja HG, Hong J, Aplan PD, Tcheurekdjian L, Forman SJ, Slovak ML. t(9;11)(p22;p15) in acute myeloid leukemia results in a fusion between NUP98 and the gene encoding transcriptional coactivators p52 and p75-lens epithelium-derived growth factor (LEDGF) Cancer Res. 2000;60(22):6227–9. [PubMed] [Google Scholar]
- 2.Ahuja P, Caffe AR, Holmqvist I, Soderpalm AK, Singh DP, Shinohara T, van Veen T. Lens epithelium-derived growth factor (LEDGF) delays photoreceptor degeneration in explants of rd/rd mouse retina. Neuroreport. 2001;12(13):2951–5. doi: 10.1097/00001756-200109170-00039. [DOI] [PubMed] [Google Scholar]
- 3.Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26(47):6766–76. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- 4.Bao KK, Wang H, Miller JK, Erie DA, Skalka AM, Wong I. Functional oligomeric state of avian sarcoma virus integrase. J Biol Chem. 2003;278(2):1323–7. doi: 10.1074/jbc.C200550200. [DOI] [PubMed] [Google Scholar]
- 5.Bartholomeeusen K, De Rijck J, Busschots K, Desender L, Gijsbers R, Emiliani S, Benarous R, Debyser Z, Christ F. Differential Interaction of HIV-1 Integrase and JPO2 with the C Terminus of LEDGF/p75. J Mol Biol. 2007;372(2):407–21. doi: 10.1016/j.jmb.2007.06.090. [DOI] [PubMed] [Google Scholar]
- 6.Bartholomeeusen K, Gijsbers R, Christ F, Hendrix J, Rain JC, Emiliani S, Benarous R, Debyser Z, De Rijck J. Lens Epithelium Derived Growth Factor/p75 interacts with the transposase derived DDE domain of pogZ. J Biol Chem. 2009 doi: 10.1074/jbc.M807781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown PO, Bowerman B, Varmus HE, Bishop JM. Retroviral integration: structure of the initial covalent product and its precursor, and a role for the viral IN protein. Proc Natl Acad Sci U S A. 1989;86(8):2525–9. doi: 10.1073/pnas.86.8.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown-Bryan TA, Leoh LS, Ganapathy V, Pacheco FJ, Mediavilla-Varela M, Filippova M, Linkhart TA, Gijsbers R, Debyser Z, Casiano CA. Alternative splicing and caspase-mediated cleavage generate antagonistic variants of the stress oncoprotein LEDGF/p75. Mol Cancer Res. 2008;6(8):1293–307. doi: 10.1158/1541-7786.MCR-08-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busschots K, De Rijck J, Christ F, Debyser Z. In search of small molecules blocking interactions between HIV proteins and intracellular cofactors. Mol Biosyst. 2009;5(1):21–31. doi: 10.1039/b810306b. [DOI] [PubMed] [Google Scholar]
- 10.Busschots K, Vercammen J, Emiliani S, Benarous R, Engelborghs Y, Christ F, Debyser Z. The interaction of LEDGF/p75 with integrase is lentivirus-specific and promotes DNA binding. J Biol Chem. 2005;280(18):17841–7. doi: 10.1074/jbc.M411681200. [DOI] [PubMed] [Google Scholar]
- 11.Busschots K, Voet A, De Maeyer M, Rain JC, Emiliani S, Benarous R, Desender L, Debyser Z, Christ F. Identification of the LEDGF/p75 binding site in HIV-1 integrase. J Mol Biol. 2007;365(5):1480–92. doi: 10.1016/j.jmb.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 12.Cherepanov P. LEDGF/p75 interacts with divergent lentiviral integrases and modulates their enzymatic activity in vitro. Nucleic Acids Res. 2007;35(1):113–24. doi: 10.1093/nar/gkl885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherepanov P, Ambrosio AL, Rahman S, Ellenberger T, Engelman A. Structural basis for the recognition between HIV-1 integrase and transcriptional coactivator p75. Proc Natl Acad Sci U S A. 2005;102(48):17308–13. doi: 10.1073/pnas.0506924102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherepanov P, Devroe E, Silver PA, Engelman A. Identification of an evolutionarily conserved domain in human lens epithelium-derived growth factor/transcriptional co-activator p75 (LEDGF/p75) that binds HIV-1 integrase. J Biol Chem. 2004;279(47):48883–92. doi: 10.1074/jbc.M406307200. [DOI] [PubMed] [Google Scholar]
- 15.Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J Biol Chem. 2003;278(1):372–81. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- 16.Cherepanov P, Sun ZY, Rahman S, Maertens G, Wagner G, Engelman A. Solution structure of the HIV-1 integrase-binding domain in LEDGF/p75. Nat Struct Mol Biol. 2005;12(6):526–32. doi: 10.1038/nsmb937. [DOI] [PubMed] [Google Scholar]
- 17.Chiu TK, Davies DR. Structure and function of HIV-1 integrase: an update. Frontiers in Medicinal Chemistry. 2007;3(7):1–20. doi: 10.2174/1568026043388547. [DOI] [PubMed] [Google Scholar]
- 18.Ciuffi A, Bushman FD. Retroviral DNA integration: HIV and the role of LEDGF/p75. Trends Genet. 2006;22(7):388–95. doi: 10.1016/j.tig.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P, Ecker JR, Bushman F. A role for LEDGF/p75 in targeting HIV DNA integration. Nature Medicine. 2005;11:1287–9. doi: 10.1038/nm1329. [DOI] [PubMed] [Google Scholar]
- 20.Daugaard M, Kirkegaard-Sorensen T, Ostenfeld MS, Aaboe M, Hoyer-Hansen M, Orntoft TF, Rohde M, Jaattela M. Lens epithelium-derived growth factor is an Hsp70-2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 2007;67(6):2559–67. doi: 10.1158/0008-5472.CAN-06-4121. [DOI] [PubMed] [Google Scholar]
- 21.De Rijck J, Vandekerckhove L, Gijsbers R, Hombrouck A, Hendrix J, Vercammen J, Engelborghs Y, Christ F, Debyser Z. Overexpression of the lens epithelium-derived growth factor/p75 integrase binding domain inhibits human immunodeficiency virus replication. J Virol. 2006;80(23):11498–509. doi: 10.1128/JVI.00801-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietz F, Franken S, Yoshida K, Nakamura H, Kappler J, Gieselmann V. The family of hepatoma-derived growth factor proteins: characterization of a new member HRP-4 and classification of its subfamilies. Biochem J. 2002;366(Pt 2):491–500. doi: 10.1042/BJ20011811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du L, Zhao Y, Chen J, Yang L, Zheng Y, Tang Y, Shen X, Jiang H. D77, one benzoic acid derivative, functions as a novel anti-HIV-1 inhibitor targeting the interaction between integrase and cellular LEDGF/p75. Biochem Biophys Res Commun. 2008;375(1):139–44. doi: 10.1016/j.bbrc.2008.07.139. [DOI] [PubMed] [Google Scholar]
- 24.Emiliani S, Mousnier A, Busschots K, Maroun M, Van Maele B, Tempe D, Vandekerckhove L, Moisant F, Ben-Slama L, Witvrouw M, Christ F, Rain JC, Dargemont C, Debyser Z, Benarous R. Integrase mutants defective for interaction with LEDGF/p75 are impaired in chromosome tethering and HIV-1 replication. J Biol Chem. 2005;280(27):25517–23. doi: 10.1074/jbc.M501378200. [DOI] [PubMed] [Google Scholar]
- 25.Engelman A. In vivo analysis of retroviral integrase structure and function. Adv Virus Res. 1999;52:411–26. doi: 10.1016/s0065-3527(08)60309-7. [DOI] [PubMed] [Google Scholar]
- 26.Engelman A, Cherepanov P. The lentiviral integrase binding protein LEDGF/p75 and HIV-1 replication. PLoS Pathog. 2008;4(3):e1000046. doi: 10.1371/journal.ppat.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fatma N, Kubo E, Chylack LT, Jr, Shinohara T, Akagi Y, Singh DP. LEDGF regulation of alcohol and aldehyde dehydrogenases in lens epithelial cells: stimulation of retinoic acid production and protection from ethanol toxicity. Am J Physiol Cell Physiol. 2004;287(2):C508–16. doi: 10.1152/ajpcell.00076.2004. [DOI] [PubMed] [Google Scholar]
- 28.Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/−mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ. 2005;12(7):734–50. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- 29.Fatma N, Singh DP, Shinohara T, Chylack LT., Jr Transcriptional regulation of the antioxidant protein 2 gene, a thiol-specific antioxidant, by lens epithelium-derived growth factor to protect cells from oxidative stress. J Biol Chem. 2001;276(52):48899–907. doi: 10.1074/jbc.M100733200. [DOI] [PubMed] [Google Scholar]
- 30.Faure A, Calmels C, Desjobert C, Castroviejo M, Caumont-Sarcos A, Tarrago-Litvak L, Litvak S, Parissi V. HIV-1 integrase crosslinked oligomers are active in vitro. Nucleic Acids Res. 2005;33(3):977–86. doi: 10.1093/nar/gki241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher TM, 3rd, Soares MA, McPhearson S, Hui H, Wiskerchen M, Muesing MA, Shaw GM, Leavitt AD, Boeke JD, Hahn BH. Complementation of integrase function in HIV-1 virions. Embo J. 1997;16(16):5123– 38. doi: 10.1093/emboj/16.16.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganapathy V, Casiano CA. Autoimmunity to the nuclear autoantigen DFS70 (LEDGF): what exactly are the autoantibodies trying to tell us? Arthritis Rheum. 2004;50(3):684–8. doi: 10.1002/art.20095. [DOI] [PubMed] [Google Scholar]
- 33.Ganapathy V, Daniels T, Casiano CA. LEDGF/p75: a novel nuclear autoantigen at the crossroads of cell survival and apoptosis. Autoimmun Rev. 2003;2(5):290–7. doi: 10.1016/s1568-9972(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 34.Ge H, Si Y, Roeder RG. Isolation of cDNAs encoding novel transcription coactivators p52 and p75 reveals an alternate regulatory mechanism of transcriptional activation. Embo J. 1998;17(22):6723–9. doi: 10.1093/emboj/17.22.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge H, Si Y, Wolffe AP. A novel transcriptional coactivator, p52, functionally interacts with the essential splicing factor ASF/SF2. Mol Cell. 1998;2(6):751–9. doi: 10.1016/s1097-2765(00)80290-7. [DOI] [PubMed] [Google Scholar]
- 36.Grand FH, Koduru P, Cross NC, Allen SL. NUP98-LEDGF fusion and t(9;11) in transformed chronic myeloid leukemia. Leuk Res. 2005;29(12):1469–72. doi: 10.1016/j.leukres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Guiot E, Carayon K, Delelis O, Simon F, Tauc P, Zubin E, Gottikh M, Mouscadet JF, Brochon JC, Deprez E. Relationship between the oligomeric status of HIV-1 integrase on DNA and enzymatic activity. J Biol Chem. 2006;281(32):22707–19. doi: 10.1074/jbc.M602198200. [DOI] [PubMed] [Google Scholar]
- 38.Hare S, Shun MC, Gupta SS, Valkov E, Engelman A, Cherepanov P. A novel co-crystal structure affords the design of gain-of-function lentiviral integrase mutants in the presence of modified PSIP1/LEDGF/p75. PLoS Pathog. 2009;5(1):e1000259. doi: 10.1371/journal.ppat.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heuer TS, Brown PO. Photo-cross-linking studies suggest a model for the architecture of an active human immunodeficiency virus type 1 integrase-DNA complex. Biochemistry. 1998;37(19):6667–78. doi: 10.1021/bi972949c. [DOI] [PubMed] [Google Scholar]
- 40.Hindmarsh P, Ridky T, Reeves R, Andrake M, Skalka AM, Leis J. HMG protein family members stimulate human immunodeficiency virus type 1 and avian sarcoma virus concerted DNA integration in vitro. J Virol. 1999;73(4):2994–3003. doi: 10.1128/jvi.73.4.2994-3003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hombrouck A, De Rijck J, Hendrix J, Vandekerckhove L, Voet A, Maeyer MD, Witvrouw M, Engelborghs Y, Christ F, Gijsbers R, Debyser Z. Virus Evolution Reveals an Exclusive Role for LEDGF/p75 in Chromosomal Tethering of HIV. PLoS Pathog. 2007;3(3):e47. doi: 10.1371/journal.ppat.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou Y, McGuinness DE, Prongay AJ, Feld B, Ingravallo P, Ogert RA, Lunn CA, Howe JA. Screening for antiviral inhibitors of the HIV integrase-LEDGF/p75 interaction using the AlphaScreen luminescent proximity assay. J Biomol Screen. 2008;13(5):406–14. doi: 10.1177/1087057108317060. [DOI] [PubMed] [Google Scholar]
- 43.Huang TS, Myklebust LM, Kjarland E, Gjertsen BT, Pendino F, Bruserud O, Doskeland SO, Lillehaug JR. LEDGF/p75 has increased expression in blasts from chemotherapy-resistant human acute myelogenic leukemia patients and protects leukemia cells from apoptosis in vitro. Mol Cancer. 2007;6:31. doi: 10.1186/1476-4598-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussey DJ, Moore S, Nicola M, Dobrovic A. Fusion of the NUP98 gene with the LEDGF/p52 gene defines a recurrent acute myeloid leukemia translocation. BMC Genet. 2001;2(1):20. doi: 10.1186/1471-2156-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubo E, Fatma N, Sharma P, Shinohara T, Chylack LT, Jr, Akagi Y, Singh DP. Transactivation of involucrin, a marker of differentiation in keratinocytes, by lens epithelium-derived growth factor (LEDGF) J Mol Biol. 2002;320(5):1053–63. doi: 10.1016/s0022-2836(02)00551-x. [DOI] [PubMed] [Google Scholar]
- 46.Li M, Mizuuchi M, Burke TR, Jr, Craigie R. Retroviral DNA integration: reaction pathway and critical intermediates. Embo J. 2006;25(6):1295–304. doi: 10.1038/sj.emboj.7601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llano M, Delgado S, Vanegas M, Poeschla EM. LEDGF/p75 prevents proteasomal degradation of HIV-1 integrase. J Biol Chem. 2004;279(53):55570–7. doi: 10.1074/jbc.M408508200. [DOI] [PubMed] [Google Scholar]
- 48.Llano M, Saenz DT, Meehan A, Wongthida P, Peretz M, Walker WH, Teo W, Poeschla EM. An Essential Role for LEDGF/p75 in HIV Integration. Science. 2006;314(5798):461–4. doi: 10.1126/science.1132319. [DOI] [PubMed] [Google Scholar]
- 49.Llano M, Vanegas M, Fregoso O, Saenz DT, Chung S, Peretz M, Poeschla EM. LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral pre-integration complexes. J Virol. 2004;78(17):9524–37. doi: 10.1128/JVI.78.17.9524-9537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llano M, Vanegas M, Hutchins N, Thompson D, Delgado S, Poeschla EM. Identification and Characterization of the Chromatin Binding Domains of the HIV-1 Integrase Interactor LEDGF/p75. Journal of Molecular Biology. 2006;360:760–73. doi: 10.1016/j.jmb.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 51.Machida S, Chaudhry P, Shinohara T, Singh DP, Reddy VN, Chylack LT, Jr, Sieving PA, Bush RA. Lens epithelium-derived growth factor promotes photoreceptor survival in light-damaged and RCS rats. Invest Ophthalmol Vis Sci. 2001;42(5):1087–95. [PubMed] [Google Scholar]
- 52.Maertens G, Cherepanov P, Debyser Z, Engelborghs Y, Engelman A. Identification and characterization of a functional nuclear localization signal in the HIV-1 integrase (IN) interactor LEDGF/p75. J Biol Chem. 2004;279(32):33421–9. doi: 10.1074/jbc.M404700200. [DOI] [PubMed] [Google Scholar]
- 53.Maertens G, Cherepanov P, Pluymers W, Busschots K, De Clercq E, Debyser Z, Engelborghs Y. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J Biol Chem. 2003;278(35):33528–39. doi: 10.1074/jbc.M303594200. [DOI] [PubMed] [Google Scholar]
- 54.Maertens G, Vercammen J, Debyser Z, Engelborghs Y. Measuring protein-protein interactions inside living cells using single color fluorescence correlation spectroscopy. Application to human immunodeficiency virus type 1 integrase and LEDGF/p75. Faseb J. 2005;19(8):1039–41. doi: 10.1096/fj.04-3373fje. [DOI] [PubMed] [Google Scholar]
- 55.Maertens GN, Cherepanov P, Engelman A. Transcriptional co- activator p75 binds and tethers the Myc-interacting protein JPO2 to chromatin. J Cell Sci. 2006;119(Pt 12):2563–71. doi: 10.1242/jcs.02995. [DOI] [PubMed] [Google Scholar]
- 56.Marshall HM, Ronen K, Berry C, Llano M, Sutherland H, Saenz D, Bickmore W, Poeschla E, Bushman FD. Role of PSIP1/LEDGF/p75 in Lentiviral Infectivity and Integration Targeting. PLoS ONE. 2007;2(12):e1340. doi: 10.1371/journal.pone.0001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsui H, Lin LR, Singh DP, Shinohara T, Reddy VN. Lens epithelium-derived growth factor: increased survival and decreased DNA breakage of human RPE cells induced by oxidative stress. Invest Ophthalmol Vis Sci. 2001;42(12):2935–41. [PubMed] [Google Scholar]
- 58.McKee CJ, Kessl JJ, Shkriabai N, Dar MJ, Engelman A, Kvaratskhelia M. Dynamic modulation of HIV-1 integrase structure and function by cellular LEDGF protein. J Biol Chem. 2008 doi: 10.1074/jbc.M805843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morerio C, Acquila M, Rosanda C, Rapella A, Tassano E, Micalizzi C, Panarello C. t(9;11)(p22;p15) with NUP98-LEDGF fusion gene in pediatric acute myeloid leukemia. Leuk Res. 2005;29(4):467–70. doi: 10.1016/j.leukres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Mousnier A, Kubat N, Massias-Simon A, Segeral E, Rain JC, Benarous R, Emiliani S, Dargemont C. von Hippel Lindau binding protein 1-mediated degradation of integrase affects HIV-1 gene expression at a postintegration step. Proc Natl Acad Sci U S A. 2007;104(34):13615–20. doi: 10.1073/pnas.0705162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muro Y, Sugiura K, Morita Y, Tomita Y. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody-positive patients with autoimmune rheumatic disease. Lupus. 2008;17(3):171–6. doi: 10.1177/0961203307086311. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura M, Singh DP, Kubo E, Chylack LT, Jr, Shinohara T. LEDGF: survival of embryonic chick retinal photoreceptor cells. Invest Ophthalmol Vis Sci. 2000;41(5):1168–75. [PubMed] [Google Scholar]
- 63.Nakamura T. NUP98 fusion in human leukemia: dysregulation of the nuclear pore and homeodomain proteins. Int J Hematol. 2005;82(1):21–7. doi: 10.1532/IJH97.04160. [DOI] [PubMed] [Google Scholar]
- 64.Nishizawa Y, Usukura J, Singh DP, Chylack LT, Jr, Shinohara T. Spatial and temporal dynamics of two alternatively spliced regulatory factors, lens epithelium-derived growth factor (ledgf/p75) and p52, in the nucleus. Cell Tissue Res. 2001;305(1):107–14. doi: 10.1007/s004410100398. [DOI] [PubMed] [Google Scholar]
- 65.Ochs RL, Muro Y, Si Y, Ge H, Chan EK, Tan EM. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol. 2000;105(6 Pt 1):1211–20. doi: 10.1067/mai.2000.107039. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa Y, Sugiura K, Watanabe A, Kunimatsu M, Mishima M, Tomita Y, Muro Y. Autoantigenicity of DFS70 is restricted to the conformational epitope of C-terminal alpha-helical domain. J Autoimmun. 2004;23(3):221–31. doi: 10.1016/j.jaut.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Pandey KK, Sinha S, Grandgenett DP. Transcriptional Coactivator LEDGF/p75 Modulates Human Immunodeficiency Virus Type 1 Integrase-Mediated Concerted Integration. J Virol. 2007;81(8):3969–79. doi: 10.1128/JVI.02322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poeschla EM. Integrase, LEDGF/p75 and HIV replication. Cell Mol Life Sci. 2008;65:1403–24. doi: 10.1007/s00018-008-7540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raghavendra NK, Engelman A. LEDGF/p75 interferes with the formation of synaptic nucleoprotein complexes that catalyze full-site HIV-1 DNA integration in vitro: implications for the mechanism of viral cDNA integration. Virology. 2007;360(1):1–5. doi: 10.1016/j.virol.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahman S, Lu R, Vandegraaff N, Cherepanov P, Engelman A. Structure-based mutagenesis of the integrase-LEDGF/p75 interface uncouples a strict correlation between in vitro protein binding and HIV-1 fitness. Virology. 2007;357(1):79–90. doi: 10.1016/j.virol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Roudaia L, Speck NA. A MENage a Trois in leukemia. Cancer Cell. 2008;14(1):3–5. doi: 10.1016/j.ccr.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–9. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 73.Sharma P, Fatma N, Kubo E, Shinohara T, Chylack LT, Jr, Singh DP. Lens epithelium-derived growth factor relieves transforming growth factor-beta1-induced transcription repression of heat shock proteins in human lens epithelial cells. J Biol Chem. 2003;278(22):20037–46. doi: 10.1074/jbc.M212016200. [DOI] [PubMed] [Google Scholar]
- 74.Sharma P, Singh DP, Fatma N, Chylack LT, Jr, Shinohara T. Activation of LEDGF gene by thermal-and oxidative-stresses. Biochem Biophys Res Commun. 2000;276(3):1320–4. doi: 10.1006/bbrc.2000.3606. [DOI] [PubMed] [Google Scholar]
- 75.Shin JH, Piao CS, Lim CM, Lee JK. LEDGF binding to stress response element increases alphaB-crystallin expression in astrocytes with oxidative stress. Neurosci Lett. 2008;435(2):131–6. doi: 10.1016/j.neulet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 76.Shinohara T, Singh DP, Fatma N. LEDGF, a survival factor, activates stress-related genes. Prog Retin Eye Res. 2002;21(3):341–58. doi: 10.1016/s1350-9462(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 77.Shun MC, Botbol Y, Li X, Di Nunzio F, Daigle JE, Yan N, Lieberman J, Lavigne M, Engelman A. Identification and characterization of PWWP domain residues critical for LEDGF/p75 chromatin binding and human immunodeficiency virus type 1 infectivity. J Virol. 2008;82(23):11555–67. doi: 10.1128/JVI.01561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shun MC, Raghavendra NK, Vandegraaff N, Daigle JE, Hughes S, Kellam P, Cherepanov P, Engelman A. LEDGF/p75 functions downstream from preintegration complex formation to effect gene-specific HIV-1 integration. Genes Dev. 2007;21(14):1767–78. doi: 10.1101/gad.1565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh DP, Fatma N, Kimura A, Chylack LT, Jr, Shinohara T. LEDGF binds to heat shock and stress-related element to activate the expression of stress-related genes. Biochem Biophys Res Commun. 2001;283(4):943–55. doi: 10.1006/bbrc.2001.4887. [DOI] [PubMed] [Google Scholar]
- 80.Singh DP, Kimura A, Chylack LT, Jr, Shinohara T. Lens epithelium-derived growth factor (LEDGF/p75) and p52 are derived from a single gene by alternative splicing. Gene. 2000;242(1–2):265–73. doi: 10.1016/s0378-1119(99)00506-5. [DOI] [PubMed] [Google Scholar]
- 81.Singh DP, Kubo E, Takamura Y, Shinohara T, Kumar A, Chylack LT, Jr, Fatma N. DNA binding domains and nuclear localization signal of LEDGF: contribution of two helix-turn-helix (HTH)-like domains and a stretch of 58 amino acids of the N-terminal to the trans-activation potential of LEDGF. J Mol Biol. 2006;355(3):379–94. doi: 10.1016/j.jmb.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 82.Singh DP, Ohguro N, Chylack LT, Jr, Shinohara T. Lens epithelium-derived growth factor: increased resistance to thermal and oxidative stresses. Invest Ophthalmol Vis Sci. 1999;40(7):1444–51. [PubMed] [Google Scholar]
- 83.Singh DP, Ohguro N, Kikuchi T, Sueno T, Reddy VN, Yuge K, Chylack LT, Jr, Shinohara T. Lens epithelium-derived growth factor: effects on growth and survival of lens epithelial cells, keratinocytes, and fibroblasts. Biochem Biophys Res Commun. 2000;267(1):373–81. doi: 10.1006/bbrc.1999.1979. [DOI] [PubMed] [Google Scholar]
- 84.Sinha S, Pursley MH, Grandgenett DP. Efficient concerted integration by recombinant human immunodeficiency virus type 1 integrase without cellular or viral cofactors. J Virol. 2002;76(7):3105–13. doi: 10.1128/JVI.76.7.3105-3113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slape C, Aplan PD. The role of NUP98 gene fusions in hematologic malignancy. Leuk Lymphoma. 2004;45(7):1341–50. doi: 10.1080/10428190310001659325. [DOI] [PubMed] [Google Scholar]
- 86.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Gatell JM, Rockstroh JK, Katlama C, Yeni P, Lazzarin A, Clotet B, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Meibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Nguyen BY, Teppler H. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–54. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 87.Sutherland HG, Newton K, Brownstein DG, Holmes MC, Kress C, Semple CA, Bickmore WA. Disruption of Ledgf/Psip1 results in perinatal mortality and homeotic skeletal transformations. Mol Cell Biol. 2006;26(19):7201–10. doi: 10.1128/MCB.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takamura Y, Fatma N, Kubo E, Singh DP. Regulation of heavy subunit chain of gamma-glutamylcysteine synthetase by tumor necrosis factor-alpha in lens epithelial cells: role of LEDGF/p75. Am J Physiol Cell Physiol. 2006;290(2):C554–66. doi: 10.1152/ajpcell.00398.2005. [DOI] [PubMed] [Google Scholar]
- 89.Turlure F, Maertens G, Rahman S, Cherepanov P, Engelman A. A tripartite DNA-binding element, comprised of the nuclear localization signal and two AT-hook motifs, mediates the association of LEDGF/p75 with chromatin in vivo. Nucleic Acids Res. 2006;34(5):1663–75. doi: 10.1093/nar/gkl052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Maele B, Busschots K, Vandekerckhove L, Christ F, Debyser Z. Cellular co-factors of HIV-1 integration. Trends Biochem Sci. 2006;31(2):98–105. doi: 10.1016/j.tibs.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Vandegraaff N, Devroe E, Turlure F, Silver PA, Engelman A. Biochemical and genetic analyses of integrase-interacting proteins lens epithelium-derived growth factor (LEDGF)/p75 and hepatoma-derived growth factor related protein 2 (HRP2) in preintegration complex function and HIV-1 replication. Virology. 2006;346(2):415–26. doi: 10.1016/j.virol.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 92.Vandekerckhove L, Christ F, Van Maele B, De Rijck J, Gijsbers R, Van den Haute C, Witvrouw M, Debyser Z. Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus. J Virol. 2006;80(4):1886–96. doi: 10.1128/JVI.80.4.1886-1896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vanegas M, Llano M, Delgado S, Thompson D, Peretz M, Poeschla E. Identification of the LEDGF/p75 HIV-1 integrase-interaction domain and NLS reveals NLS-independent chromatin tethering. J Cell Sci. 2005;118(Pt 8):1733–43. doi: 10.1242/jcs.02299. [DOI] [PubMed] [Google Scholar]
- 94.Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EM, Takasaki Y, Tomita Y, Muro Y. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum. 2004;50(3):892–900. doi: 10.1002/art.20096. [DOI] [PubMed] [Google Scholar]
- 95.Wu X, Daniels T, Molinaro C, Lilly MB, Casiano CA. Caspase cleavage of the nuclear autoantigen LEDGF/p75 abrogates its pro-survival function: implications for autoimmunity in atopic disorders. Cell Death Differ. 2002;9(9):915–25. doi: 10.1038/sj.cdd.4401063. [DOI] [PubMed] [Google Scholar]
- 96.Wu X, Liu H, Xiao H, Conway JA, Hunter E, Kappes JC. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. Embo Journal. 1997;16(16):5113–22. doi: 10.1093/emboj/16.16.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan N, Cherepanov P, Daigle JE, Engelman A, Lieberman J. The SET complex acts as a barrier to autointegration of HIV-1. PLoS Pathog. 2009;5(3):e1000327. doi: 10.1371/journal.ppat.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14(1):36–46. doi: 10.1016/j.ccr.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao H, Wang Y, Yin ZQ. A comparison of LEDGFp52 and CNTF on the in vitro growth of rat retinal ganglion cell neurites. Neurosci Lett. 2008;440(1):9–13. doi: 10.1016/j.neulet.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 100.Zhao HS, Chen SJ, Wu N, Wang XQ, Yin ZQ, Wang Y. LEDGFp52 controls rat retinal ganglion cell neurite growth in culture and regulates specific neuronal growth-associated genes and protein production. J Int Med Res. 2008;36(4):815–29. doi: 10.1177/147323000803600425. [DOI] [PubMed] [Google Scholar]