Abstract
Background and Purpose
Multiple studies suggest that statin use prior to acute ischemic stroke (AIS) is associated with improved functional outcome. However, available evidence is conflicting, and several published reports are limited by small sample sizes. We therefore investigated the effect of antecedent use of statins on stroke outcome by performing a meta-analysis of all results from published studies as well as our own unpublished data.
Methods
We performed a systematic literature search and meta-analysis of studies investigating the association between pre-stroke statin use and clinical outcome, and included additional data from 126 pre-stroke statin users and 767 non-users enrolled at our Institution. A total of 12 studies, comprising 2013 statin users and 9682 non- users were meta-analyzed using a random effects model. We also meta-analyzed results for individual TOAST stroke subtypes to determine whether the effect of statin use differed across subtypes, using the Breslow-Day (BD) test.
Results
Meta-analysis of all available data identified an association between pre-stroke statin use and improved functional outcome (Odds Ratio = 1.62, 95% Confidence Interval: 1.39 -1.88), but we uncovered evidence of publication bias. The effect of statin use on functional outcome was found to be larger for small vessel strokes compared to other subtypes (BD p = 0.008).
Conclusions
Antecedent use of statins is associated with improved outcome in AIS patients. This association appears to be stronger in patients with small vessel stroke subtype. However, evidence of publication bias in the existing literature suggests these findings should be interpreted with caution.
Search Terms: Ischemic Stroke, Statins, Outcome, Disability, Meta-Analysis
Introduction
Despite currently available treatment options for acute ischemic stroke (AIS), patients often face the prospect of substantial post-stroke disability.1,2 Results from in vitro and animal model studies suggest that statins have beneficial effects on functional recovery after AIS.3 Pleiotropic effects of statins including their influence on angiogenesis, neurogenesis, synaptogenesis, as well as possible regulation of cerebral blood flow and modulation of the coagulation and immune systems,4 have been proposed as possible biological mechanisms accounting for these observations.
Evidence from human studies investigating the effect of statins on AIS outcome is conflicting, and the interpretation of published results is often complicated by small sample sizes, possible bias, and confounding.5-15 In addition, a recent report suggested that statin effect on outcome might vary depending on stroke etiology14, as determined by Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria.16 We therefore performed a meta-analysis of all available published evidence, as well as our own unpublished data from the Massachusetts General Hospital (MGH). Furthermore, we sought to investigate possible variability in the effect of statins on AIS outcome based on stroke subtype and attempted to replicate and expand on the reported observation in our own cohort.
Methods
MGH Cohort
Patient selection
All AIS subjects were enrolled as part of an ongoing single-center prospective cohort study of consecutive ischemic stroke patients aged ≥18 years admitted to the MGH Stroke Unit after presenting to the Emergency Department (ED) within 24 hours of symptom onset between 2003 and 2009.17 Ischemic stroke was defined as a clinical syndrome of any duration associated with a radiographically proven acute infarct consistent with a vascular pattern of involvement and without radiographic evidence of a demyelinating or neoplastic disease, or other structural disease including vasculitis, subacute bacterial endocarditis, vasospasm due to subarachnoid hemorrhage or cocaine abuse, or primary intracerebral hemorrhage. Diagnosis of acute cerebral ischemia was confirmed for all AIS subjects in the present study by admission DWI MRI completed within 48 hours following symptom onset. The institutional review board approved all aspects of this study, and informed consent for collection of data was obtained for all subjects or their legal guardians.
Data collection and patient follow-up
All patients were evaluated by a neurologist in the ED, where the National Institute of Health Stroke Scale (NIHSS) score was determined, laboratory values were measured, and clinical information was abstracted prospectively by patient or proxy interview, and/or supplemented through medical chart review. Vascular risk factors including hypertension, diabetes, hyperlipidemia, coronary artery disease, and atrial fibrillation were recorded based on existing international guidelines as previously described.17 Medication use prior to admission was also ascertained, including antiplatelet, antihypertensive, and oral hypoglycemic agents, as well as warfarin and statins. AIS subtypes were assigned by stroke neurologists according to TOAST criteria.16 Ischemic stroke infarct size was quantified on DWI MRI according to previously published methods.18
Patients and their caregivers were interviewed by telephone at 3 months post-AIS by certified research staff to assess functional outcome using the modified Rankin Scale (mRS) score. The use or discontinuation of medications after discharge was specifically assessed in this interview. Favorable outcome was defined as mRS ≤ 2 at 90 days, in agreement with previously published studies.5-15
Statistical analysis
All statistical analyses were performed using STATA 10.0. Continuous numerical variables were expressed as mean ± standard deviation (SD) with the exception of DWI volume which was expressed as the median ± inter-quartile range (IQR). Cases and controls were compared in univariate analyses using t-test, Wilcoxon rank sum, chi-square, or Fisher's exact test as appropriate.
Independent predictors of post AIS functional outcome were identified using multivariate logistic regression analysis. Candidate covariates for multivariate modeling included all variables showing a trend in association with outcome in univariate analysis (p < 0·20), as well as variables showing trends towards differential distribution in statin users vs. non users (p < 0·20). Backward elimination of non-significant variables (p > 0·05) was subsequently used to generate a minimal model. We separately analyzed effects of statins on outcome in each TOAST stroke subtype group, and compared them using the Breslow-Day test. Significance threshold was set at p < 0·05 (two-tailed) for all analyses.
Meta-analysis
Literature search and study inclusion criteria
A literature search was performed independently by A.B. and W.J.D. in order to identify human studies in English language exploring the effect of statin use on functional outcome after AIS. Search terms are listed in the Supplementary Appendix (S1) and literature search work-flow is detailed in Supplementary Figure (S2)(please see http://stroke.ahajournals.org). Queried databases included PubMed, Medline, Embase and Ovid. Manual review of references in articles matching searching criteria was conducted to identify potential additional reports. Data were retrieved and meta-analyzed independently by A.B. and W.J.D., and results compared for consistency.
Statistical methods
Results from multivariate regression analyses in individual studies were meta-analyzed using a conservative inverse variance-based random effects pooling method (DerSimonian-Laird).19 Cochran's Q test was used to estimate heterogeneity, followed by calculation of I2 (percentage of effect size attributable to heterogeneity). Effect size heterogeneity was considered significant for heterogeneity p-values < 0.10 or I2 > 0.20.20 Potential study characteristics predicting heterogeneity in effect size determination were identified using a meta-regression approach.21
Publication bias was quantified by inspection of funnel plots and computation of Egger's and Begg's tests p-values.21 Further quantification of the impact of publication bias on meta-analysis results was obtained by applying the “trim and fill” method to estimate the percentage of unpublished negative studies.22
Results
MGH
Of 893 AIS patients, there were 126 subjects with antecedent statin use and 767 subjects without (Table 1). Compared to non statin users, AIS patients who used statins were more likely to have a history of hyperlipidemia and ischemic heart disease, and to be prescribed antihypertensive and glycemic control agents (p<0.003). Ischemic stroke characteristics (including NIHSS and TOAST subtype breakdown) did not differ between groups. Favorable functional outcome at 90-days (mRS 0-2) was not associated with antecedent statin use in univariate analysis (p = 0.10), nor in multivariate analysis (Odds Ratio (OR) = 1.51, 95% Confidence Interval (CI): 0.94 – 2.44), with evidence of a non-significant trend only towards association between statin use and favorable functional outcome (p = 0.08).
Table 1. MGH Cohort Characteristics.
| Variable | Statin (n, %) | Non-Statin (n, %) | p-value |
|---|---|---|---|
| No. of Subjects | 126 (100.0) | 767 (100.0) | |
| Age (Mean ± Standard Deviation) | 65.7 ± 13.0 | 66.1 ± 15.1 | 0.60 |
| Gender (% female) | 42 (0.33) | 316 (0.41) | 0.10 |
| Caucasian | 115 (0.91) | 686 (0.90) | 0.75 |
| Hypertension | 89 (0.71) | 486 (0.63) | 0.13 |
| Type 2 Diabetes | 28 (0.22) | 159 (0.21) | 0.72 |
| Coronary Artery Disease | 34 (0.27) | 142 (0.19) | 0.03 |
| Atrial Fibrillation | 14 (0.11) | 126 (0.16) | 0.15 |
| Previous TIA | 9 (0.07) | 56 (0.07) | 0.99 |
| Previous Ischemic Stroke | 18 (0.14) | 117 (0.15) | 0.89 |
| Hyperlipidemia | 78 (0.62) | 295 (0.38) | < 0.001 |
| Previous History of ICH | 0 (0.00) | 4 (0.05) | 0.99 |
| Antihypertensive Agent Use | 63 (0.50) | 214 (0.28) | < 0.001 |
| Glycemic Control Agent Use | 24 (0.19) | 73 (0.10) | 0.003 |
| Ever Smoker | 80 (0.65) | 392 (0.52) | 0.009 |
| Ever Alcohol User (> 1 drink / week) | 81 (0.64) | 392 (0.51) | 0.007 |
|
| |||
| NIHSS at Admission > 8 | 21 (0.17) | 143 (0.21) | 0.39 |
| Systolic Blood Pressure at Admission (Mean, Standard Deviation in mmHg) | 153 ± 28 | 152 ± 29 | 0.61 |
| Diastolic Blood Pressure at Admission (Mean, Standard Deviation in mmHg) | 80 ± 15 | 79 ± 16 | 0.65 |
| TOAST Ischemic Stroke Subtype | 0.16 | ||
| Cardioembolic | 36 (0.29) | 249 (0.32) | |
| Large Artery | 34 (0.27) | 189 (0.25) | |
| Small Vessel | 10 (0.08) | 92 (0.12) | |
| Other Determined Etiology | 11 (0.09) | 29 (0.04) | |
| Unknown Etiology | 35 (0.27) | 208 (0.27) | |
|
| |||
| 90-day Favorable Outcome (mRS ≤ 2) | 68 (0.54) | 317 (0.41) | 0.10 |
| 90-day Mortality | 8 (0.06) | 84 (0.11) | 0.12 |
In the TOAST subtype subset analysis we identified an association between statin use and favorable outcome among 102 individuals (10 of whom were statin users) with small vessel ischemic stroke: OR = 1.97, 95% CI: 1.02 – 3.79, p = 0.04. The size of the effect of statin on outcome of small vessel strokes was found to be larger than in any other stroke subtype (p = 0.008).
Meta-Analysis
A total of 2013 AIS statin users and 9682 statin non-users were included in meta-analysis of data from our cohort and 11 previously published studies (Table 2). Meta-analysis identified an association between antecedent statin use and favorable outcome: OR = 1.62, 95% CI: 1.39 – 1.88, p < 0.001 (Figure 1). TOAST subtype meta-analysis showed association between statins use and AIS outcome in both large artery (OR = 2.01, 95% CI: 1.14 – 3.54, p = 0.016) and small vessel strokes (OR = 2.11, 95% CI: 1.32 – 3.39, p = 0.002).
Table 2. Individual Studies Included in Meta-analysis.
| Study | Reference | Endpoint | Study Setting | Subjects | Matched Controls | Stroke Subtypes | Cholesterol Levels | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Definition | Time | All | Statin | Non Statin | ||||||
| Reeves | 5 | mRS :0 - 4 | Discharge | Multi-center | 1360 | 309 | 1051 | Yes | No | No |
| Greisenegger | 6 | mRS: 0 - 4 | 7 days | Multi-center | 1691 | 152 | 1539 | No | Yes | Yes |
| Yu | 7 | mRS: 0 - 3 | 10 days | Single Center | 339 | 74 | 265 | No | No | No |
| Yoon | 8 | mRS: 0 - 2 | Discharge | Single Center | 393 | 161 | 232 | No | No | No |
| Arboix | 9 | mRS: 0 - 2 | Discharge | Single Center | 2082 | 381 | 1701 | No | No | No |
| Marti-Fabregas | 10 | Barthel Index: 95-100 | 3 Months | Multi-center | 167 | 30 | 137 | No | Yes | Yes |
| Moonis | 11 | mRS: 0 - 2 | 3 Months | Multi-center | 729 | 129 | 600 | No | Yes | No |
| Elkind | 12 | other | Discharge | Population Based | 570 | 48 | 522 | No | No | No |
| Jonsson | 13 | other | 7 Days | Population Based | 275 | 125 | 150 | Yes | No | No |
| Martinez-Sanchez | 14 | mRS: 0 - 2 | Discharge | Single Center | 2742 | 281 | 2461 | No | Yes | No |
| Goldstein | 15 | mRS: 0 - 2 | 3 Months | Multi-center (Clinical Trial) | 454 | 197 | 257 | Yes | Yes | Yes |
| MGH (Present Study) | -- | mRS: 0 - 2 | 3 Months | Single Center | 893 | 126 | 767 | No | Yes | Yes |
Figure 1. Meta-Analysis Forest Plot.
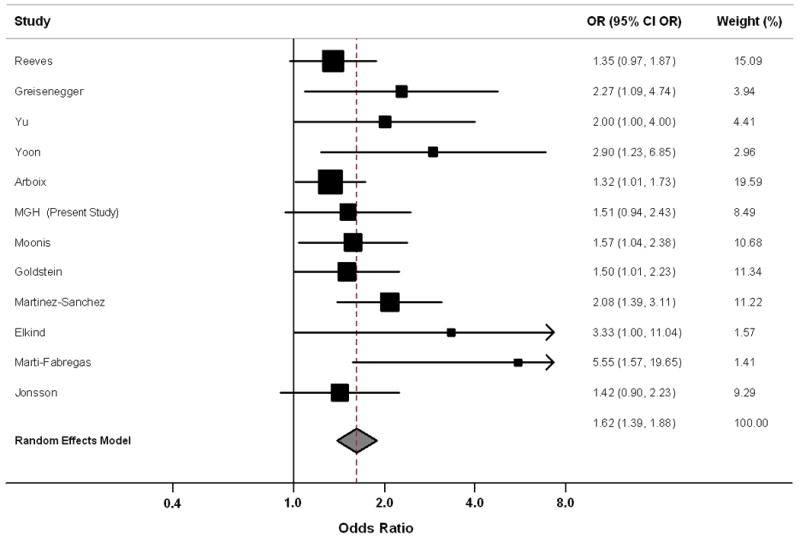
Meta-analysis Heterogeneity
We identified only minor between-study heterogeneity of effect sizes: heterogeneity p-value was 0.36, with I2 = 0.17 (95% CI: 0.00 – 0.57). To further explore meta-analysis heterogeneity, we performed meta-regression to identify associations between study characteristics and reported effect size (Table 3). We identified a significant association between small sample size for AIS statin users and larger reported effect size (p = 0.032), as well as a similar trend for non-statin users sample size (p = 0.078). Conversely, lack of adjustment for TOAST subtypes showed a trend towards association with smaller effect sizes (p = 0.09). Overall, predictors evaluated in meta-regression explained approximately 92% of identified heterogeneity.
Table 3. Meta-regression Results.
| Study Characteristic | Effect Size (log Odds Ratio) Modification | 95% CI Effect Size Modification | p |
|---|---|---|---|
| No. of Statin AIS (for a 100 subjects decrease) | +0.22 | +0.02 / +0.43 | 0.032 |
| No. of Non-Statin AIS (for a 100 subjects decrease) | +0.21 | -0.03 / +0.45 | 0.078 |
| Outcome Captured before 3 months post-stroke | -0.94 | -2.54 / +0.66 | 0.18 |
| Outcome not defined by mRS | +0.90 | -0.52 / +2.30 | 0.15 |
| Single Center vs. Multi-center / Population-Based | +0.84 | -0.48 / +2.16 | 0.15 |
| TOAST Subtypes Not Available | -0.81 | -1.85 / +0.23 | 0.09 |
| Cholesterol Levels Not Available | +0.77 | -0.51 / +2.05 | 0.17 |
Small Study Effect and Publication Bias
As suggested by the meta-regression analysis, we identified evidence of small study effect (i.e. association between smaller sample size and larger reported effect) in our analysis, based on p-values for the Egger test (p < 10-3) and the Begg test (p = 0.003). This finding is most likely reflects publication bias, the presence of which is suggested by the meta-analysis funnel plot (Figure 2). Using the “trim and fill” method to more accurately quantify the impact of publication bias, we estimated at least four studies (33% of published reports) to be unpublished and missing in our meta-analysis. Upon performing meta-analysis including both published and the hypothesized missing studies, the association between antecedent statin use and favorable outcome remained significant: OR = 1.52, 95% CI: 1.28 – 1.82, p < 0.0001 (Figure 3). However, heterogeneity was still apparent in this simulated meta-analysis (p = 0.08, I2 = 0.35).
Figure 2. Meta-analysis Funnel Plot.

Figure 3. Forest Plot for the “Trim and Fill” Meta-analysis.

Discussion
Results from this meta-analysis suggest an association between pre-stroke statin use and improved functional outcome at 90 days. These findings suggest that statin exposure antecedent to acute cerebral ischemia may reduce its severity or improve immediate recovery from it.
Following-up on results from a previous report, we investigated potential differences in statin-related effect on functional outcome based on stroke etiology, as defined by TOAST criteria.14 Meta-analysis of subtype results yielded a beneficial effect of statins in both large artery and small vessel ischemic strokes; however, this meta-analysis of results for TOAST subtypes was limited to two studies. Furthermore, available sample size for the small vessel subtype analysis in our MGH case-control study was relatively small. Further exploration of any subtype-specific effects is necessary to elucidate possible associations with underlying biology of disease.23
Our study has limitations. Most results included in meta-analysis were derived from observational studies. These studies are exposed to several potential sources of bias, including severity bias, recall bias, bias due to statins prescription practices, and propensity to treatment. However, results from the clinical trial included in meta-analysis are in agreement with observational studies. We also found minor effect size heterogeneity across studies; meta-regression results confirmed this finding and indentified small studies as consistently reporting larger effect sizes. It is therefore not surprising that evidence of small study effect is present in our meta-analysis, most likely due to publication bias. We attempted to correct for publication bias using the “trim and fill” method, i.e. by adding missing studies to the meta-analysis, and observed that the updated meta-analysis did not change significantly. However, introduction of missing unpublished studies generated substantial meta-analysis heterogeneity, suggesting caution in interpreting our findings.
Conclusion
Pre-treatment with statins is associated with improved outcome in patients with acute ischemic stroke. This association may be strongest in small vessel stroke patients. However, evidence of publication bias in the existing literature suggests these findings should be interpreted with caution. More clinical trial and experimental model data will be required before the biological mechanisms linking statin use to improved outcome after ischemic stroke are fully elucidated.
Supplementary Material
Acknowledgments
We thank all clinical coordinators and research staff in the Ischemic Stroke Research Group, Department of Neurology, Massachusetts General Hospital.
Funding: NIH-NINDS K23NS064052 (N.S.R.); NIH-NINDS 5R01NS059727, 5P50NS051343, and R01NS063925 (J.R.); NIH-NINDS 5P50NS051343-04 (K.L.F.)
American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N), Deane Institute for Integrative Study of Atrial Fibrillation and Stroke.
Footnotes
Statement of Contribution: Study Design: A.B., W.J.D., N.S.R. Data Acquisition: A.B., W.J.D., L.C., N.S.R., K.L.F., J.R., C.D.A. Data Analysis: A.B., W.J.D. Study Management: L.C., N.S.R., K.L.F., J.R. Manuscript Preparation: A.B., W.J.D. Manuscript Review: A.B., W.J.D., C.D.A., L.C., N.S.R., K.L.F., J.R.
Disclosures: The authors have no Conflict of Interest/Disclosures
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Miida T, Takahashi A, Ikeuchi T. Prevention of stroke and dementia by statin therapy: experimental and clinical evidence of their pleiotropic effects. Pharmacol Ther. 2007;113:378–393. doi: 10.1016/j.pharmthera.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan CJ. Prevention of stroke and dementia with statins: Effects beyond lipid lowering. Am J Cardiol. 2003;91:23B–29B. doi: 10.1016/s0002-9149(02)03270-8. [DOI] [PubMed] [Google Scholar]
- 5.Reeves MJ, Gargano JW, Luo Z, Mullard AJ, Jacobs BS, Majid A. Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. Effect of pretreatment with statins on ischemic stroke outcomes. Stroke. 2008;39:1779–1785. doi: 10.1161/STROKEAHA.107.501700. [DOI] [PubMed] [Google Scholar]
- 6.Greisenegger S, Müllner M, Tentschert S, Lang W, Lalouschek W. Effect of pretreatment with statins on the severity of acute ischemic cerebrovascular events. J Neurol Sci. 2004;221:5–10. doi: 10.1016/j.jns.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Yu AY, Keezer MR, Zhu B, Wolfson C, Côté R. Pre-stroke use of antihypertensives, antiplatelets, or statins and early ischemic stroke outcomes. Cerebrovasc Dis. 2009;27:398–402. doi: 10.1159/000207444. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SS, Dambrosia J, Chalela J, Ezzeddine M, Warach S, Haymore J, Davis L, Baird AE. Rising statin use and effect on ischemic stroke outcome. BMC Med. 2004;2:4. doi: 10.1186/1741-7015-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arboix A, García-Eroles L, Oliveres M, Targa C, Balcells M, Massons J. Pretreatment with statins improves early outcome in patients with first-ever ischaemic stroke: a pleiotropic effect of statins or a beneficial effect of hypercholesterolemia? BMC Neurol. 2010;10:47. doi: 10.1186/1471-2377-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martí-Fàbregas J, Gomis M, Arboix A, Aleu A, Pagonabarraga J, Belvís R, Cocho D, Roquer J, Rodríguez A, García MD, Molina-Porcel L, Díaz-Manera J, Martí-Vilalta JL. Favorable outcome of ischemic stroke in patients pretreated with statins. Stroke. 2004;35:1117–1121. doi: 10.1161/01.STR.0000125863.93921.3f. [DOI] [PubMed] [Google Scholar]
- 11.Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298–1300. doi: 10.1161/01.STR.0000165920.67784.58. [DOI] [PubMed] [Google Scholar]
- 12.Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253–258. doi: 10.1212/01.wnl.0000171746.63844.6a. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson N, Asplund K. Does pretreatment with statins improve clinical outcome after stroke? A pilot case-referent study. Stroke. 2001;32:1112–1115. doi: 10.1161/01.str.32.5.1112. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Sánchez P, Rivera-Ordóñez C, Fuentes B, Ortega-Casarrubios MA, Idrovo L, Díez-Tejedor E. The beneficial effect of statins treatment by stroke subtype. Eur J Neurol. 2009;16:127–133. doi: 10.1111/j.1468-1331.2008.02370.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein LB, Amarenco P, Zivin J, Messig M, Altafullah I, Callahan A, Hennerici M, MacLeod MJ, Sillesen H, Zweifler R, Michael K, Welch A, Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2009;40:3526–3531. doi: 10.1161/STROKEAHA.109.557330. [DOI] [PubMed] [Google Scholar]
- 16.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Conde J, Biffi A, Rahman R, Kanakis A, Butler C, Sonni S, Massasa E, Cloonan L, Gilson A, Capozzo K, Cortellini L, Ois A, Cuadrado-Godia E, Rodriguez-Campello A, Furie KL, Roquer J, Rosand J, Rost NS. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41:437–442. doi: 10.1161/STROKEAHA.109.563502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ay H, Arsava EM, Vangel M, Oner B, Zhu M, Wu O, Singhal A, Koroshetz WJ, Sorensen AG. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke. 2008;39:1171–1176. doi: 10.1161/STROKEAHA.107.502104. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 21.Sutton AJ, Jones DR, Abrams KR, Sheldon TA, Song F. Publication Bias. In: Sutton AJ, Jones DR, Abrams KR, Sheldon TA, Song F, editors. Methods for Meta-analysis in Medical Research. 1st. Chichester, England: John Wiley & Sons; 2000. pp. 109–132. [Google Scholar]
- 22.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–4562. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 23.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


