Abstract
Using a novel approach for analysis of TRPC channel activity, we report here that NSAIDs are involved into regulation of TRPC channels in the podocytes of the freshly isolated decapsulated glomeruli. Fluorescence and electron microscopy techniques confirmed the integrity of podocytes in the glomeruli. Western blotting showed that TRPC1,3 and 6 are highly expressed in the glomeruli. Single-channel patch clamp analysis revealed cation currents with distinct TRPC properties. This is the first report describing single TRPC-like currents in glomerular podocytes. Furthermore, our data provide a novel mechanism of NSAIDs regulation of TRPC channels, which might be implicated in maintaining the glomerular filtration barrier.
Keywords: TRPC6, podocyte, FSGS, focal segmental glomerulosclerosis
1. Introduction
Filtration of plasma by renal glomeruli is the main function of the kidney. The kidneys, especially the basement membrane of the glomeruli, are damaged by a variety of disorders that cause abnormal excretion of protein in the urine and result in the nephrotic syndrome. Glomerular podocytes are central components of the renal filtration barrier and are thought to play an important role in the pathogenesis of almost all proteinuric glomerulopathies. In the past decade, studies of familial cases of nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) have led to the identification of genes encoding proteins important for glomerular structure and function [1]. Mutations in the gene encoding transient receptor potential canonical channel 6 (TRPC6) protein are the genetic basis for an autosomal dominant form of FSGS whereby the mutated TRPC6 channel causes an increase in calcium current [2; 3].
Non-steroid anti-inflammatory drugs (NSAIDs) are major drugs used in the treatment of inflammation in a wide range of disorders. The best-known mechanism of action of NSAIDs is the inhibition of prostaglandin synthesis secondary to their action on cyclooxygenases (COXs) [4]. A beneficial effect of NSAIDs in reducing proteinuria in patients with primary or recurrent FSGS has been documented. For instance, indomethacin reduces proteinuria in patients with FSGS or other glomerular diseases [5]. However, an effect of NSAIDs on ion channels in the kidney and particularly in the glomerulus is unknown.
Together these observations highlight the need to better understand NSAIDs regulation of TRPC channels. The lack of such studies has not been a result of its unimportance, but rather from the fact that electrophysiological recording of TRPC channels in their native surrounding has not been performed due to the difficulty of studying podocytes function in situ [6]. By modifying a technique of Gloy and colleagues [7] we have succeeded for the first time to perform single channel analysis of endogenous ion channels in the podocytes of isolated decapsulated glomeruli. This technical advance has provided a unique opportunity to evaluate the role of the NSAIDs in the regulation of TRPC channels. The demonstration of a possible involvement of TRPC channels as final effectors of NSAIDs opens new directions of research into FSGS and related kidney diseases.
2. Materials and methods
2.1. Glomeruli isolation
The kidneys of 8-weeks-old male Sprague-Dawley rats were perfused (6 ml/min) through the distal aorta with a Hanks Balanced Salt Solution (Invitrogen) to clear blood from the organs. The kidneys were then decapsulated, and the cortex was isolated, removed and minced using a singled edge razor blade as it was described previously [8–10]. The minced tissue was sequentially pushed through a steel sieve of 100 and then pipetted through a 140 mesh (04-881-5Z and 04-881-5X; Fisher) using the culture medium solution RPMI1640 (Invitrogen, Inc) with 5%BSA which decapsulated the glomeruli. This tissue solution was then pipetted onto a 200 mesh sieve (S4145; Sigma) leaving the glomeruli on the top surface. The glomeruli were rinsed using the RPMI solution into a 15 ml conical tube and let to settle on ice. After sedimentation the excess of RPMI storage solution was removed and the isolated decapsulated glomeruli were used either for patch-clamp, biochemical, or microscopy experiments. For electrophysiological experiments, isolated glomeruli were allowed to settle onto 5 × 5 mm coverglass coated with poly-D-lysine (P4707; Sigma). Cover glasses that contained glomeruli were placed within a perfusion chamber mounted on an inverted Nikon Ti-S microscope and superfused with a physiologic saline solution buffered with HEPES (pH 7.4). Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the IACUC at the Medical College of Wisconsin.
2.2. Electron Microscopy
For the electron microscopy experiments glomeruli were fixed in 2% glutaraldehyde buffered in 0.1M cacodylate (pH 7.4) for 1 h at room temperature and washed three times for 5 min in 0.1M cacodylate buffer. After that, the glomeruli were post-fixed in 1% osmium tetroxide reduced with 1.25% potassium ferricyanide on ice for 2 h. Then, the specimen was washed three times for 5 min in distilled water, dehydrated through graded methanol and embedded in EMBed 812 epoxy resin. After embedding ultrathin sections were cut on a RMC Powertome and stained with uranyl acetate and Reynolds lead citrate. Sections were viewed in a Hitachi HS600 transmission electron microscope and images recorded via an AMT 1K digital imaging camera.
2.3. Staining of the glomeruli with the WGA lectin
The freshly isolated preparation of the rat glomeruli was mounted on the poly-D-lysine-covered glass in a 35 mm culture dish and washed three times for 5 min with the ice-cold PBS. Then the preparation was incubated with 20 μM of the FITC-labelled WGA lectin (FLK-2100, Vector labs) diluted in PBS for 20 min. After the incubation the glomeruli were washed three times with PBS and used for fluorescence microscopy analysis.
2.4. Electrophysiology
Single-channel current data were acquired and subsequently analyzed with a MultiClamp 700B patch clamp amplifier interfaced via a Digidata 1440A (Mol. Devices) with a PC running the pClamp 10.2 suite of software. After a high resistance seal was obtained, cell-attached recording was performed immediately. The membrane resistance was monitored regularly to ensure the quality of recording. For measurements of acute effect only one experiment was performed per dish to avoid any possibility of examining cells whose properties might have been altered by extended exposure to NSAIDs. The bath solution consisted of 135 NaAsp, 1 CaCl2, 10 HEPES, 2 MgCl2, 10 glucose; pH 7.4. The pipette solution contained 126 NaCl, 1.5 CaCl2, 10 HEPES, 10 glucose; pH7.4; plus added directly before the patch-clamp experiments were 100 μM niflumic acid or DIDS (to block Ca2+-activated Cl− channels), 10 mM TEA (to inhibit the large-conductance Ca2+-dependent K+ channel), 10 nM iberiotoxin (to block the Ca2+-activated K+ channels), 10 μM nicardipine (to block N-type Ca2+ channels). SKF-96365 hydrochloride was from Tocris Biosciences (Cat No. 1147).
2.5. Single channel data analysis
All experiments were acquired using pClamp 10.2 software with time and current amplitude data analyzed with this software in conjunction with Origin 7.0 (OriginLab). Currents were low-pass filtered at 300 Hz and digitized at a sampling rate of 1 kHz. Channel activity was determined during at least 1 min recording period before application of NSAIDs. A 50% threshold cross-method was utilized to determine valid channel openings. Open probability (Po) was used to measure the channel activity within a patch. Single-channel unitary current (i) was determined from the best-fit Gaussian distribution of amplitude histograms. Channel activity was analyzed as NPo = I/i, where I is mean total current in a patch and i is unitary current at this voltage. In order to increase accuracy in measurement of Po, only patches containing 5 channels or fewer were used. For presentation, current data from some cell-attached patches were subsequently software filtered at 100 Hz and slow baseline drifts were corrected.
2.6. Statistics
All summarized data is reported as mean ± S.E.M. Data are compared using either the Student's (two-tailed) t-test or a one-way ANOVA and P < 0.05 is considered significant.
3. Results
3.1. Isolation of glomeruli and characterization of the integrity of podocytes in this preparation
After the glomeruli are isolated from the kidneys of Sprague-Dawley rats (as shown in Fig. 1A), the cell bodies of the podocytes appear in the light microscope as oval structures on the surface of the glomerular capillary loops. Fig. 1B shows a representative picture of an isolated decapsulated glomerulus together with a patch clamp pipette. To further confirm that the patched cells were podocytes and to determine that isolated glomeruli were intact, additional control experiments were performed. First, isolated glomeruli were incubated with a specific marker against podocyte sugar residues, WGA lectin from Triticum vulgaris [11]. A representative image of the freshly isolated live rat glomerulus stained with the FITC-labelled WGA lectin is shown in Fig. 1C. In addition, the integrity of the podocytes in the glomeruli preparation was further examined by transmission electron microscopy. A normal configuration of podocytes with coordinated pattern of foot processes was observed. After decapsulation the capillaries of the glomerulus were covered with intact podocytes (Figs. 1D–F). Western blotting showed that TRPC1,3 and 6 are highly expressed in the glomeruli of the Sprague-Dawley rat kidney (Supplementary Fig. S1A). Immunohistochemistry data also demonstrated strong staining of TRPC6 in glomeruli (Supplementary Fig. S1B). Thus, this method of isolation of decapsulated glomeruli is suitable for patch clamp studies of endogenous channels in intact podocytes.
Fig. 1.
Podocytes of the rat glomeruli remain intact after isolation and are available for the patch-clamp pipette. (A) Scheme of the glomeruli isolation protocol. Kidney cortex was isolated from Sprague-Dawley rat kidneys and then homogenized with a blade. The homogeneous cortex fraction was pushed through the steel sieves of the different mesh size and then collected into a Petri dish. (B) A representative image of a decapsulated rat glomelurus in the patch-clamp setup with a patch pipette attached to a podocyte on the edge of the glomerulus (40× and a close-up image). (C) A representative image of the freshly isolated live rat glomerulus (63×) stained with a FITC-labelled WGA lectin as a marker of podocytes. The image shows intact podocyte bodies on the surface of the glomerulus (arrows). The scale bar shown is 50 μm. (D–F) Electron microscopy of freshly isolated decapsulated glomerulus. Fragments of the decapsulated glomerulus visualized with electron microscopy at 1500×, 5000× and 20000× (scale bars are 10 μm, 4 μM and 1 μm for D, E and F, respectively). Different segments of the glomerulus are shown. PB – podocyte body, PN – podocyte nucleus, EF – endothelial fenertrations, FP – foot processes.
3.2. Single-channel biophysical properties of the TRPC-like channels identified in the podocytes of freshly isolated rat glomeruli
Patch clamp electrophysiology was used to assess TRPC activity in the podocytes in freshly isolated glomeruli. TRPC channels typically show low levels of constitutive activity, which most likely depends on a dual glycosylation step [12; 13]. Interestingly, the podocytes in freshly isolated glomeruli exhibited ample spontaneous channel activity under the resting state. When a cell-attached patch was formed, single channel activity was observed in approximately 30% of patches. Figs. 2 A–C demonstrate single channel currents from such preparations at different holding potentials. The data revealed that TRPC channels were active throughout the range of holding potentials tested and Po was not affected by membrane voltage. The identified TRPC-like channels were characterized by different conductances and displayed similar gating properties. Data analysis revealed two major channel populations with the conductance values of 10.3 ± 0.8 pS (n = 11) and 20.1 ± 0.7 pS (n = 10) (Fig. 2D). Both channels had a reversal potential (Erev) of ~0 mV. We termed these channels as “big” and “small” channels, respectively. Different conductances were sometimes found to coexist in the same patch as illustrated in Fig. 2C. At a holding potential of −60 mV, the amplitudes of these single-channel openings were Y1 = 1.25 pA and Y2 = 0.65 pA. There were direct transitions from the closed state (c; zero current) to the level equal to the sum of the current amplitude (Y1 + Y2) (Fig. 2C), providing evidence that the different conductances might represent different sub-states of the same channel, according to the classic scheme of Colquhoun and Sigworth [14].
Fig. 2.
Single-channel biophysical properties of TRPC-like channels identified in podocytes of freshly isolated rat glomeruli. All recordings were performed in cell-attached configuration in voltage clamp mode. Representative current traces of “big” (A) and “small” (B) channels identified in the podocytes. The holding membrane potentials are indicated near the traces. c and o denote closed and open current levels, respectively. (C) Typical current traces held at −60 mV demonstrating co-existence of the two types of the channels in the same patch. Yi denotes the open current levels of channels with different conductances. (D) Single-channel current-voltage relationship for two types of channels identified. Values are means ± SE of at least four experiments. (E) TRPC blocker SKF 96365 rapidly and markedly decreases TRPC activity in isolated glomeruli. Continuous current trace from a representative cell-attached patch that contained TRPC channel was made on podocyte of freshly isolated rat glomerulus before and after treatment with SKF 96365. Areas before (I) and after (II) treatment are shown below with an expanded time scale. This patch was held at a −60-mV test potential during the course of the experiment.
To confirm that identified channels belong to the TRPC-family, a TRPC channel antagonist, SKF 96365 (10 μM) was utilized. As clearly seen in Fig. 2E, SKF 96365 decreased endogenous channels activity in the isolated glomeruli (n=5). Application of vehicle had no effect on TRPC channels activity (data not shown). Since our experimental solutions also contained inhibitors of voltage-gated Ca2+ entry and it was previously shown that podocytes lack L-type Ca2+ channels [15], the data indicate that SKF 96365 inhibits TRPC channels.
3.3. Inhibition of TRPC channels of the rat glomeruli by diclofenac and ibuprofen
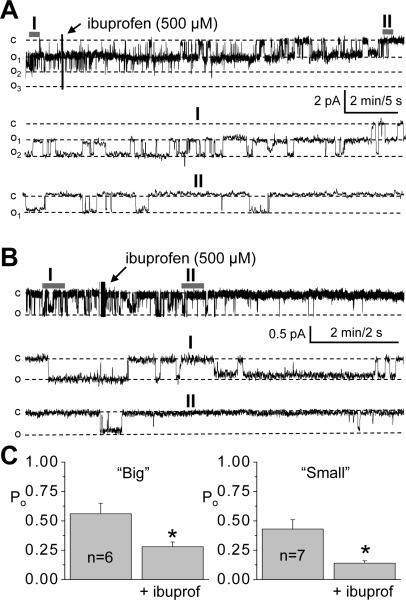
Fig. 3A illustrates a representative time course of TRPC channel current activity in the isolated glomerulus following addition of diclofenac (500 μM). Fig. 3A represents the effect of diclofenac on the “big” channels although similar effects were observed with “small” channels (Fig. 3B). As is apparent from these representative traces and from the summary data in Fig. 3C, application of this NSAID resulted in a rapid decrease in the Po in this native preparation. The channel open probability (Po) decreased from 1.03 ± 0.13 to 0.28 ± 0.11 (n=7). To further investigate effect of NSAIDs on TRPC channels, another well-known NSAID, ibuprofen was studied; ibuprofen yielded results similar to diclofenac with a rapid decrease in Po of both channels as shown in representative traces and on the summary graphs (Fig. 4).
Fig. 3.
Diclofenac decreases the activity of TRPC channels in podocytes of isolated glomeruli. Representative experiments and summary graphs from cell-attached patches containing TRPC channels. Continuous current traces, as well as the fragments of the traces at the expanded time scale for “big” and “small” channels (A and B, respectively) are shown. Arrows demonstrate addition of 500 μM of diclofenac to the external bath solution. The patches were held at a −60 mV test potential during the course of the experiment. The c and oi denote closed and open current levels, respectively. (C) Summary graphs showing the effect of diclofenac on the Po of the channels. *, versus control, the number of the observations is shown.
Fig. 4.
Ibuprofen decreases the activity of TRPC channels in podocytes of isolated glomeruli. Representative experiments and summary graphs from cell-attached patches containing TRPC channels. Continuous current traces, as well as the fragments of the traces at the expanded time scale for “big” and “small” channels (A and B, respectively) are shown. Arrows demonstrate addition of 500 μM of ibuprofen to the external bath solution. (C) Summary graphs showing the effect of ibuprofen on the Po of the channels.
4. Discussion
While an abundance of studies has indicated an important role of TRPC channels in glomeruli and particularly in podocytes, the regulation of these channels has not yet been investigated in freshly isolated glomeruli. Since it is difficult to directly transfer data obtained from cultured glomerular epithelial cells to the responses of podocytes in vivo, we have established an experimental approach that allows us to study podocytes in freshly isolated intact glomeruli. Fluorescence and electron microscopy confirmed the integrity of podocytes in the isolated glomeruli and patch clamp techniques enabled examination of the effects of NSAIDs on native podocyte TRPC channels.
Three independent laboratories reported the identification of several mutations associated with autosomal dominant FSGS in the TRPC6 channel [2; 3; 16]. In addition to gain-of-function mutations in TRPC6 that cause FSGS, changes in channel expression may also contribute to the disease. For instance, the P112Q mutation of TRPC6 demonstrates increased plasma membrane expression [3]. Furthermore, it has been proposed that TRPC6 is also involved in the pathology of nongenetic forms of proteinuric disease since increased TRPC6 expression is found in glomeruli from patients with other proteinuric renal diseases, such as membranous glomerulonephritis and minimal-change disease [17].
We have described here two endogenous channels in the podocytes of freshly isolated glomeruli, which are represented by conductance states of approximately 10 and 20 pS. We cannot exclude the possibility that these states represent the subconductance levels of the same channel since described channels have the additive conductance levels and similar Erev values. Moreover, there is a possibility that the two channels could stand for subconductances of one channel with the conductance of 30 pS.
NSAIDs are widely used as over-the-counter medications to treat inflammation, pain and fever. We propose here that NSAIDs might be useful in FSGS therapy via inhibition of calcium flux through TRPC channels in the podocytes. The pathogenic importance of COXs and especially COX-2 in glomerular diseases has been addressed in a variety of clinical and experimental studies. For example, mice with COX-2 gene deletion exhibit renal alterations, renal dysplasia, and cardiac fibrosis [18]. Furthermore, it was recently shown that COX-2 and derived prostaglandins may mediate basal podocyte differentiation and survival, at least in vitro [19].
Selective inhibition or activation of signaling pathways may be an effective means for modulating proteinuria, although the cellular mechanisms of these processes require further study and the successful therapy of glomerular diseases will probably depend on concurrent targeting of multiple signaling pathways. The ability of podocytes to precisely regulate intracellular Ca2+ levels plays a central role in glomerular disease processes and inhibition of TRPC channels with NSAIDs may be a useful strategy for treating patients with FSGS.
Supplementary Material
Acknowledgments
Glen Slocum, Christine Duris and Clive Wells are recognized for excellent technical assistance with immunohistochemistry and electron microscopy experiments. We are grateful to Drs. David R. Harder and Debebe Gebremedhin (Medical College of Wisconsin) for helpful discussion and initial help with electrophysiological experiments. We also thank Dr. Núria M. Pastor-Soler (University of Pittsburgh School of Medicine) for helpful suggestions.
This research was supported by American Diabetes Association grant 1-10-BS-168, and American Physiological Society S&R Foundation Ryuji Ueno and Lazaro J. Mandel Awards (to A. Staruschenko) and the NIH grants HL-081091 and HL-29587 (to A.W. Cowley, Jr.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Machuca E, Benoit G, Antignac C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum.Mol Genet. 2009;18:R185–R194. doi: 10.1093/hmg/ddp328. [DOI] [PubMed] [Google Scholar]
- [2].Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, vila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- [4].Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCarthy ET, Sharma M. Indomethacin protects permeability barrier from focal segmental glomerulosclerosis serum. Kidney Int. 2002;61:534–541. doi: 10.1046/j.1523-1755.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- [6].Pavenstadt H, Bek M. Podocyte electrophysiology, in vivo and in vitro. Microsc. Res. Tech. 2002;57:224–227. doi: 10.1002/jemt.10078. [DOI] [PubMed] [Google Scholar]
- [7].Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, Schollmeyer P, Greger R, Pavenstadt H. Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J. Clin. Invest. 1997;99:2772–2781. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension. 2005;45:643–648. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- [9].Sharma R, Khanna A, Sharma M, Savin VJ. Transforming growth factor-beta1 increases albumin permeability of isolated rat glomeruli via hydroxyl radicals. Kidney Int. 2000;58:131–136. doi: 10.1046/j.1523-1755.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- [10].Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J. Am. Soc. Nephrol. 1992;3:1260–1269. doi: 10.1681/ASN.V361260. [DOI] [PubMed] [Google Scholar]
- [11].Holthofer H, Virtanen I. Glycosylation of developing human glomeruli: lectin binding sites during cell induction and maturation. J. Histochem. Cytochem. 1987;35:33–37. doi: 10.1177/35.1.3794308. [DOI] [PubMed] [Google Scholar]
- [12].Dietrich A, Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-Linked Protein Glycosylation Is a Major Determinant for Basal TRPC3 and TRPC6 Channel Activity. J. Biol. Chem. 2003;278:47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- [13].Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am. J. Physiol. Renal Physiol. 2010;299:F689–F701. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Colquhoun D, Sigworth FJ. In: Fitting and statistical analysis of single-channel records. Sakmann B, Neher E, editors. Plenum; New York: 1995. pp. 483–587. [Google Scholar]
- [15].Huber TB, Gloy J, Henger A, Schollmeyer P, Greger R, Mundel P, Pavenstadt H. Catecholamines modulate podocyte function. J. Am. Soc. Nephrol. 1998;9:335–345. doi: 10.1681/ASN.V93335. [DOI] [PubMed] [Google Scholar]
- [16].Heeringa SF, Moller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS ONE. 2009;4:e7771. doi: 10.1371/journal.pone.0007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- [18].Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- [19].Cheng H, Fan X, Guan Y, Moeckel GW, Zent R, Harris RC. Distinct roles for basal and induced COX-2 in podocyte injury. J. Am. Soc. Nephrol. 2009;20:1953–1962. doi: 10.1681/ASN.2009010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.