Abstract
INTRODUCTION:
Correction of gastroschisis can be accomplished by primary or staged closure. There is, however, no consensus regarding the best approach or criteria to favor one method over the other has been established.
OBJECTIVE:
To compare the outcome of primary and staged closure in newborns with gastroschisis using intravesical pressure (IVP) as the decision criterion.
PATIENTS & METHODS:
We prospectively analyzed 45 newborns with gastroschisis. An IVP with a threshold of 20 cm H2O was used to indicate primary or staged closure, and the outcomes between the two methods were compared.
RESULTS AND DISCUSSION:
Newborns in whom primary closure was feasible were born at a lower gestational age. There was no significant difference in the frequency of complications, time to begin oral feeding, length of parenteral nutrition or length of hospital stay. Compared with previous reports, our data showed higher rates of prenatal diagnosis and cesarean delivery, a lower average birth weight, a higher rate of small gestational age babies and a more frequent association with intestinal atresia. Conversely, our data showed a lower rate of postoperative necrotizing enterocolitis and a lower average length of hospital stay.
CONCLUSION:
No significant difference was observed in the outcome of newborns who underwent primary closure or staged closure of gastroschisis when using an IVP below 20 cm H2O as the criterion for primary closure.
Keywords: Newborn diseases, Gastroschisis, Abdominal wall, Surgical procedures, Operative, Postoperative complications
INTRODUCTION
Gastroschisis is a congenital defect of the abdominal wall that results in the exposure of the abdominal organs to the amniotic cavity. An ideal closure technique for gastroschisis cases has not been established. Primary closure is considered the ideal closure technique, as it is associated with less parenteral nutritional use and a shorter length of hospital stay.1,2 Staged closure has been associated with a higher risk of complications such as infections and fistulae, a slower return to normal bowel function and a longer hospital stay.3,4
The viscero-abdominal disproportion observed in some newborns with gastroschisis and the recognition of abdominal compartment syndrome has allowed staged closure to gain wider acceptance in selected cases.5,6 In addition, the use of preformed silos (which are placed at the bedside) with delayed fascial repair in a more elective setting is a safe technique with low rates of complication and mortality.7-9
Indications for primary or staged closure are unclear and are usually guided individually based on physiological parameters and associated malformations such as intestinal atresia.2,10 Despite the development of a number of different approaches to decide on the closure technique, including intravesical pressure (IVP), splanchnic perfusion pressure, and respiratory function, the ideal parameter has yet to be established.11-13 It has also been suggested that time to closure may be the most significant factor determining length of hospital stay, whereas the closure technique is of secondary importance.14
Considering the high morbidity of infants with gastroschisis and the lack of a clear association between the type of surgical correction and the evolution of these newborns, the aim of this study was to compare the evolution of newborns who underwent primary closure with newborns who underwent staged closure of gastroschisis.
PATIENTS AND METHODS
This study was approved by The Ethics on Human Research Committee of our institution. We prospectively analyzed all of the newborns with gastroschisis admitted at our institution, a high complexity neonatal intensive care unit (NICU), from January 1999 to June 2010.
Newborns with chromosomal defects or other major malformations were excluded from the study. All of the patients were followed from birth until hospital discharge, and no eligible patients were excluded during the study. At our institution, the criterion adopted to perform primary closure is an intraoperative IVP below 20 cm H2O.
General anesthesia was performed with tracheal intubation. As a routine, atropine was administered before induction. Fentanyl (5 µg.kg-1), Propofol (3 mg.kg-1) and sevoflurane sevoflurane (1-2%) were used. At the moment of measurement of the IPV and during surgery, all of the patients were submitted to curarization with the neuromuscular blocker Atracurium (0.5 mg.kg-1). Supplementary local infiltration around the incision with bupivacaine (0.25%) was used for all patients to reduce postoperative pain.
Once the baby was born, a temporary silo was placed under mild sedation in the NICU until surgery and was kept for an average of 2 days.15 The IVP was measured during the surgical procedure. With the newborn in dorsal decubitus, a Foley catheter number 4FR was inserted, and the bladder was completely emptied. A saline solution was then administered through the catheter, which was connected to a plastic tube in the vertical position and considered as zero when the water was at the level of the pubic symphysis.11 Primary closure of the abdominal wall was simulated by holding the borders of the defect with forceps and placing them side-by-side. If the IVP was below 20 cm H2O, primary closure was performed; however, if it was above 20 cm H2O or if it was not feasible to interiorize the abdominal organs, staged closure was performed as described by Miranda et al.16
After closure, the IVP was measured again and was compared to the predicted value. The silo was kept until bowel edema subsided, and clinical evaluation showed that final closure could be feasible as long as the newborn was stable. The IVP was not monitored during the postoperative period because of the risk of infection associated with performing this procedure in the NICU. During the surgical procedure, the CO2 level was also monitored, although it was not used as a criterion for surgical decision.
The data analyzed were descriptive maternal and neonatal data, for example, maternal age, parity, prenatal diagnosis, type of delivery, Apgar scores, birth weight, nutrition, gestational age and gender; and evolution, for example, complications, time to begin oral feeding, time of parenteral nutrition, hospitalization length and survival. Infection was defined as confirmed respiratory or urinary infection and was considered to be associated with the procedure when it occurred up until the seventh postoperative day. In contrast, sepsis was defined as every septic episode observed regardless of the source.
We compared the evolution of the newborns according to the type of surgical closure they had. The continuous variables (time to begin oral feeding, time of parenteral nutrition, and hospitalization length) were analyzed with the unpaired Student's t test with a level of significance of 5% (p≤0.05).
RESULTS
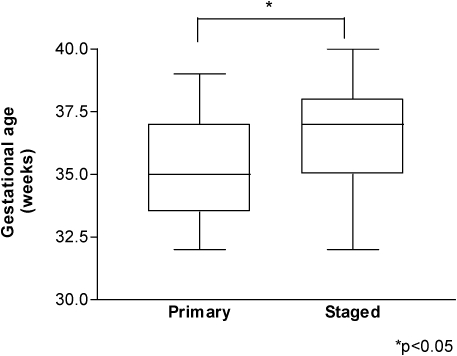
Of the 45 newborns included in our study, 24 were subjected to primary closure and 21 underwent staged closure. The average maternal age was 20±3.6 years for the primary closure group and 19±2.9 years for the staged closure group. All maternal and neonatal data are shown in Table I. All newborns with an IVP below 20 cm H2O also had an expirated CO2 level below 50 mm Hg. Newborns in whom primary closure was feasible were born at a significantly lower gestational age compared with newborns who underwent staged closure (p<0.05) (Figure 1). There were no significant differences in the other maternal and neonatal data between the groups (Table 1). None of the newborns in this study had abdominal compartment syndrome.
Figure 1.
Newborns in whom primary closure was feasible were born at a significantly lower gestational age (p<0.05).
Table 1.
Prenatal and neonatal data for both groups and for all of the newborns included in the study. Newborns in whom primary closure was feasible were born at a significantly lower gestational age (p<0.05).
| Primary closure (n = 24) | Staged Closure (n = 21) | Total (n = 45) | |
| Prenatal diagnosis | 22 (91.7%) | 19 (85.8%) | 45 (100%) |
| Delivery | |||
| Vaginal | 6 (25%) | 5 (23.8%) | 11 (24.4%) |
| Cesarean | 18 (75%) | 16 (76.2%) | 34 (75.6%) |
| Birth weight (g) | 2154±408 | 2237±400 | 2193±401 |
| Gestational age (weeks)* | 35±1.9 | 37±1.7 | 36±2.0 |
| Nutrition | |||
| PIG | 8 (33.3%) | 9 (42.9%) | 17 (37.8%) |
| AIG | 16 (66.7%) | 12 (57.1%) | 28 (62.2%) |
| Associated defects | |||
| Intestinal atresia | 5 (20.8%) | 4 (19.0%) | 9 (20%) |
| Cardiovascular | 2 (8.3%) | 9 (42.9%) | 11 (24.4%) |
| Postoperative complications | |||
| Infection/sepsis | 13 (54.1%) | 9 (42.9%) | 22 (48.9%) |
| Oligury/Anury | 7 (29.2%) | 7 (33.3%) | 14 (31.1%) |
| Necrotizing enterocolitis | 1 (4.2%) | 0 | 1 (2.2%) |
| Beginning of oral feeding (days) | 19.1±3.3 | 26.8±6.4 | 23.5±4.2 |
| Length of parenteral nutrition (days) | 25.7±3.4 | 31.0±6.2 | 28.7±23.8 |
| Length of hospital stay (days) | 34.2±4.1 | 38.4±6.0 | 36.5±23.8 |
*p<0.05.
In all of the newborns included in the study, the values of IVP observed by simulating closure of the abdominal wall were the same as the actual IVP values measured at the end of the procedure. The average length of hospital stay with silo for newborns who underwent staged closure was 6.7±4.5 days. There was no difference in the average number of complications, time to begin oral feeding, length of parenteral nutrition or length of hospital stay between newborns subjected to primary or staged closure (Table 1).
DISCUSSION
The incidence of gastroschisis is increasing worldwide,17,18 and, despite low mortality, it is associated with a high occurrence of morbidity, especially infections, intestinal dysfunction and adhesions.19-21 Prenatal diagnosis and care, surgical management and neonatal intensive care have indisputably improved during the last thirty years; nevertheless, this disease still has high short- and long-term morbidity as well as high hospital costs.22
The influence of the surgical procedure performed and the occurrence of complications is not clear, and the best approach to treat gastroschisis remains controversial.14,23 Primary closure of the abdominal wall is still considered to be the ideal correction of gastroschisis by some authors.1,2 Historically, staged closure has been reported to be associated with increased risk of complications, especially sepsis;24,25 however, delayed closure has also resulted in better outcomes, including fewer days on mechanical ventilation, decreased length of hospital stay, decreased time to full feeding and, consequently, reduced morbidities and associated costs.7,8,9,26,27
IVP has been successfully used as a criterion to gauge whether to perform primary or staged closure. Initially proposed by Nakayama et al., the IVP showed good association with the inferior vena cava pressure, and when kept below 20 mm Hg, it was associated with lower rates of complication.11
In a retrospective study including 48 newborns with abdominal wall defects, Lacey et al. used the IVP to guide surgical correction with a threshold of 20 mm Hg, thereby avoiding renal failure and refractory oliguria.28 In newborns with an intraoperative IVP above 20 mm Hg, Chin et al. observed complications such as ascites leakage, ventral hernia, impaired venous return of the lower extremities and oliguria. In contrast, these complications were not seen in newborns with an intraoperative IVP below 20 mm Hg.29
Olesevich et al. obtained similar results with a faster return to full feeding and a shorter hospital stay in newborns in whom primary closure was accomplished with an IVP below 20 mm Hg.30 Rizzo et al. were the first to report a lower IVP threshold of approximately 15 mm Hg, which is equivalent to 20 cm H20, to guide surgical closure. This technique was associated with more prompt diuresis and a trend toward less ventilator support, shorter total parenteral nutrition time and a shorter hospital stay.31 Banieghbal et al. used respiratory pressure monitoring as an indirect indication of intra-abdominal pressure in newborns with gastroschisis, which correlated well with the IVP.13 At our institution, we use the threshold proposed by Rizzo et al.; however, we did not observe reduction in ventilatory support or in the length of parenteral nutrition.
In our study, infection was the most common complication among newborns in both the primary (54.1%) and staged (42.8%) closure groups. One newborn who underwent primary closure had necrotizing enterocolitis, and there was no significant difference in the frequency of any complications. Among newborns subjected to staged closure, two had a bovine pericardium patch placed during the second surgical procedure to perform complete closure of the abdominal wall. There were no complications related to the patch.
In emerging countries such as ours, mortality from gastroschisis has been reported to be very high, reaching 50%.32,33 In our study, three newborns in the primary closure group and three in the staged closure group died, with an overall mortality of 13%. All of the deaths occurred in the late postoperative period and were attributed to sepsis, not to the surgical procedure.
Another controversy concerning the management of babies with gastroschisis is the ideal gestational age of delivery. It has been suggested that preterm delivery together with cesarean section could improve the outcome. This phenomenon was attributed to limiting the exposure of the bowel to amniotic fluid and its harmful effects and was further supported by other authors;34 however, other studies have not supported this hypothesis, including a prospective trial.35,36 In our study, newborns in whom primary closure was feasible had a significantly lower gestational age compared with the newborns who needed staged closure (35±1.9 vs. 37±1.7). Future studies may help to clarify the impact of early delivery on the management and evolution of newborns with gastroschisis.
Given that there is no consensus in the literature regarding the best closure technique or clear indications and contraindications of the different methods of treatment, the outcomes of newborns with gastroschisis should be analyzed with caution. The differences among studies may be due to the varying criteria adopted by institutions for determining the type of surgical correction to be performed and the technique to be employed. In our study, the outcomes between newborns who underwent primary and staged closure were statistically similar, even though the ones in whom primary closure was feasible were born at a significantly lower gestational age.
CONCLUSION
Primary closure and staged closure were associated with similar outcomes. Primary closure, based on an intraoperative IVP below 20 cm H2O, was associated with a low rate of complications related to increased intraabdominal pressure. Similarly, staged closure, performed to keep the intraabdominal pressure below 20 cm H2O, did not lengthen the hospital stay or increase the frequency of complications compared with the primary closure group. In addition, the association between a lower gestational age and the higher frequency of primary closure must be further evaluated.
REFERENCES
- 1.Alali JS, Tander B, Malleis J, Klein MD. Factors affecting the outcome in patients with gastroschisis: how important is immediate repair. Eur J Pediatr Surg. 2010 doi: 10.1055/s-0030-1267977. Nov 22 In press. [DOI] [PubMed] [Google Scholar]
- 2.McNamara WF, Hartin CW, Escobar MA, Lee YH. Outcome differences between gastroschisis repair methods. J Surg Res. Jun 16 In press. 2010 doi: 10.1016/j.jss.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 3.Canty TG, Collins DL. Primary fascial closure in infants with gastroschisis and omphalocele: a superior approach. J Pediatr Surg. 1983;18:707–12. doi: 10.1016/s0022-3468(83)80009-8. 10.1016/S0022-3468(83)80009-8 [DOI] [PubMed] [Google Scholar]
- 4.Sauter ER, Falterman KW, Arensman RM. Is primary repair of gastroschisis and omphalocele always the best operation. Am Surg. 1991;57:142–4. [PubMed] [Google Scholar]
- 5.Fischer JD, Chun K, Moores DC, Andrews HG. Gastroschisis: a simple technique for staged closure. 1995;30:1169–71. doi: 10.1016/0022-3468(95)90014-4. J Pediatr Surg. [DOI] [PubMed] [Google Scholar]
- 6.Fonkalsrud EW, Smith MD, Shaw KS, Borick JM, Shaw A. Selective management of gastroschisis according to the degree of visceroabdominal disproportion. Ann Surg. 1993;218:742–7. doi: 10.1097/00000658-199312000-00007. 10.1097/00000658-199312000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lansdale N, Hill R, Gull-Zamir S, Drewett M, Parkinson E, Davenport M, et al. Staged reduction of gastroschisis using preformed silos: practicalities and problems. J Pediatr Surg. 2009;44:2126–9. doi: 10.1016/j.jpedsurg.2009.06.006. 10.1016/j.jpedsurg.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Pastor AC, Phillips JD, Fenton SJ, Meyers RL, Lamm AW, et al. Routine use of a SILASTIC spring-loaded silo for infants with gastroschisis: a multicenter randomized controlled trial. J Pediatr Surg. 2008;43:1807–12. doi: 10.1016/j.jpedsurg.2008.04.003. 10.1016/j.jpedsurg.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Allotey J, Davenport M, Njere I, Charlesworth P, Greenough A, Ade-Ajayi N, et al. Benefit of preformed silos in the management of gastroschisis. Pediatr Surg Int. 2007;23:1065–9. doi: 10.1007/s00383-007-2004-9. 10.1007/s00383-007-2004-9 [DOI] [PubMed] [Google Scholar]
- 10.Kronfli R, Bradnock TJ, Sabharwal A. Intestinal atresia in association with gastroschisis: a 26-year review. Pediatr Surg Int. 2010;26:891–4. doi: 10.1007/s00383-010-2676-4. 10.1007/s00383-010-2676-4 [DOI] [PubMed] [Google Scholar]
- 11.Nakayama M, Horikawa D, Nagai H, Tamiya K, Fujita S, Namiki A. Utility of intra-bladder pressure monitoring during closure of abdominal wall defects in newborn infants. Masui. 1992;41:1647–50. [PubMed] [Google Scholar]
- 12.McGuigan RM, Mullenix PS, Vegunta R, Pearl RH, Sawin R, Azarow KS. Splanchnic perfusion pressure: a better predictor of safe primary closure than intraabdominal pressure in neonatal gastroschisis. J Pediatr Surg. 2006;41:901–4. doi: 10.1016/j.jpedsurg.2006.01.007. 10.1016/j.jpedsurg.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 13.Banieghbal B, Gouws M, Davies MR. Respiratory pressure monitoring as an indirect method of intra-abdominal pressure measurement in gastroschisis closure. Eur J Pediatr Surg. 2006;16:79–83. doi: 10.1055/s-2006-924051. 10.1055/s-2006-924051 [DOI] [PubMed] [Google Scholar]
- 14.Banyard D, Ramones T, Phillips SE, Leys CM, Rauth T, Yang EY. Method to our madness: an 18-year retrospective analysis on gastroschisis closure. J Pediatr Surg. 2010;45:579–84. doi: 10.1016/j.jpedsurg.2009.08.004. 10.1016/j.jpedsurg.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 15.Bustorff-Silva JM, Schmidt AF, Gonçalves A, Marba S, Sbragia L. The female condom as a temporary silo: a simple and inexpensive tool in the initial management of the newborn with gastroschisis. J Matern Fetal Neonatal Med. 2008;21:648–51. doi: 10.1080/14767050802178003. 10.1080/14767050802178003 [DOI] [PubMed] [Google Scholar]
- 16.Miranda ME, Tatsuo ES, Guimarães JT, Paixao RT, Lanna JC. Use of a plastic hemoderivative bag in the treatment of gastroschisis. Pediatr Surg Int. 1999;15:442–4. doi: 10.1007/s003830050629. 10.1007/s003830050629 [DOI] [PubMed] [Google Scholar]
- 17.Holland AJ, Walker K, Badawl N. Gastroschisis: an update. Pediatr Surg Int. 2010;26:871–8. doi: 10.1007/s00383-010-2679-1. 10.1007/s00383-010-2679-1 [DOI] [PubMed] [Google Scholar]
- 18.Laughon M, Meyer R, Bose C, Wall A, Otero E, Heerens A, Clark R. Rising birth prevalence of gastroschisis. J Perinatol. 2003;23:291–3. doi: 10.1038/sj.jp.7210896. 10.1038/sj.jp.7210896 [DOI] [PubMed] [Google Scholar]
- 19.Baerg J, Kaban G, Tonita J, Pahwa P, Reid D. Gastroschisis: a sixteen-year review. J Pediatr Surg. 2003;38:771–4. doi: 10.1016/jpsu.2003.50164. 10.1016/jpsu.2003.50164 [DOI] [PubMed] [Google Scholar]
- 20.Vilela PC, Ramos de Amorim MM, Falbo GH, Santos LC. Risk factors for adverse outcome of newborns with gastroschisis in a Brazilian hospital. J Ped Surg. 2001;36:559–64. doi: 10.1053/jpsu.2001.22282. 10.1053/jpsu.2001.22282 [DOI] [PubMed] [Google Scholar]
- 21.Soares H, Silva A, Rocha G, Pissarra S, Correia-Pinto J, Guimarães H. Gastroschisis: preterm or term delivery. Clinics (Sao Paulo) 2010 Feb;65(2):139–42. doi: 10.1590/S1807-59322010000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sydorak RM, Nijagal A, Sbragia L, Hirose S, Tsao K, Phibbs RH, et al. Gastroschisis: small hole, big cost. J Pediatr Surg. 2002;37:1669–72. doi: 10.1053/jpsu.2002.36689. 10.1053/jpsu.2002.36689 [DOI] [PubMed] [Google Scholar]
- 23.Lobo JD, Kim AC, Davis RP, Segura BJ, Alpert H, Teitelbaum DH, et al. No free ride? The hidden cost of delayed operative management using a spring-loaded silo for gastroschisis. J Pediatr Surg. 2010;45:1426–32. doi: 10.1016/j.jpedsurg.2010.02.047. 10.1016/j.jpedsurg.2010.02.047 [DOI] [PubMed] [Google Scholar]
- 24.Sauter ER, Falterman KW, Arensman RM. Is primary repair of gastroschisis and omphalocele always the best operation. Am Surg. 1991;57:142–4. [PubMed] [Google Scholar]
- 25.Driver CP, Bruce J, Bianchi A, Doig CM, Dickson AP, Bowen J. The contemporary outcome of gastroschisis. J Pediatr Surg. 2000;35:1719–23. doi: 10.1053/jpsu.2000.19221. 10.1053/jpsu.2000.19221 [DOI] [PubMed] [Google Scholar]
- 26.Schlatter M, Norris K, Uitvlugt N, DeCou J, Connors R. Improved outcomes in the treatment of gastroschisis using a preformed silo and delayed repair approach. J Pediatr Surg. 2003;38:458–64. doi: 10.1053/jpsu.2003.50079. [DOI] [PubMed] [Google Scholar]
- 27.Minkes RK, Langer JC, Maziotti MV, Skinner MA, Foglia RP. Routine insertion of a Silastic spring-loaded silo for infants with gastoschisis. J Pediatr Surg. 2000;35:834–6. doi: 10.1053/jpsu.2000.6858. [DOI] [PubMed] [Google Scholar]
- 28.Lacey SR, Carris LA, Beyer AJ, 3rd, Azizkhan RZ. Bladder pressure monitoring significantly enhances care of infants with abdominal wall defects: a prospective clinical study. J Pediatr Surg. 1993;28:1370–5. doi: 10.1016/s0022-3468(05)80329-x. 10.1016/S0022-3468(05)80329-X [DOI] [PubMed] [Google Scholar]
- 29.Chin T, Wei C. Prediction of outcome in omphalocele and gastroschisis by intraoperative measurement of intravesical pressure. J Formos Med Assoc. 1994;93:691–3. [PubMed] [Google Scholar]
- 30.Olesevich M, Alexander F, Khan M, Cotman K. Gastroschisis revisited: role of intraoperative measurement of abdominal pressure. J Pediatr Surg. 2005;40:789–92. doi: 10.1016/j.jpedsurg.2005.01.043. 10.1016/j.jpedsurg.2005.01.043 [DOI] [PubMed] [Google Scholar]
- 31.Rizzo A, Davis PC, Hamm CR, Powell RW. Intraoperative vesical pressure measurements as a guide in the closure of abdominal wall defects. Am Surg. 1996;62:192–6. [PubMed] [Google Scholar]
- 32.van Eijck FC, Wijnen RM, van Goor H. The incidence and morbidity of adhesions after treatment of neonates with gastroschisis and omphalocele: a 30-year review. J Pediatr Surg. 2008;43:479–83. doi: 10.1016/j.jpedsurg.2007.10.027. 10.1016/j.jpedsurg.2007.10.027 [DOI] [PubMed] [Google Scholar]
- 33.Maksoud-Filho JG, Tannuri U, Silva MM, Maksoud JG. The outcome of newborns with abdominal wall defects according to the method of abdominal wall defects according to the method of abdominal closure: the experience of a single center. Ped Surg Int. 2006;22:503–7. doi: 10.1007/s00383-006-1696-6. 10.1007/s00383-006-1696-6 [DOI] [PubMed] [Google Scholar]
- 34.Moore TC, Collins DL, Catanzarite V, Hatch EI., Jr Pre-term and particularly pre-labor cesarean section to avoid complications of gastroschisis. Pediatr Surg Int. 1999;15:97–104. doi: 10.1007/s003830050525. 10.1007/s003830050525 [DOI] [PubMed] [Google Scholar]
- 35.Ergun O, Barksdale E, Ergun FS, Prosen T, Qureshi FG, Reblock KR, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424–8. doi: 10.1016/j.jpedsurg.2004.10.013. 10.1016/j.jpedsurg.2004.10.013 [DOI] [PubMed] [Google Scholar]
- 36.Logghe HL, Mason GC, Thornton JG, Stringer MD. A randomized controlled trial of elective preterm delivery of fetuses with gastroschisis. J Pediatr Surg. 2005;40:1726–31. doi: 10.1016/j.jpedsurg.2005.07.047. 10.1016/j.jpedsurg.2005.07.047 [DOI] [PubMed] [Google Scholar]