Abstract
PURPOSE:
To compare the time-of-flight and contrast-enhanced- magnetic resonance angiography techniques in a 3 Tesla magnetic resonance unit with digital subtraction angiography with the latest flat-panel technology and 3D reconstruction in the evaluation of embolized cerebral aneurysms.
INTRODUCTION:
Many embolized aneurysms are subject to a recurrence of intra-aneurismal filling. Traditionally, imaging surveillance of coiled aneurysms has consisted of repeated digital subtraction angiography. However, this method has a small but significant risk of neurological complications, and many authors have advocated the use of noninvasive imaging methods for the surveillance of embolized aneurysms.
METHODS:
Forty-three aneurysms in 30 patients were studied consecutively between November 2009 and May 2010. Two interventional neuroradiologists rated the time-of-flight-magnetic resonance angiography, the contrast-enhanced-magnetic resonance angiography, and finally the digital subtraction angiography, first independently and then in consensus. The status of aneurysm occlusion was assessed according to the Raymond scale, which indicates the level of recanalization according to degrees: Class 1: excluded aneurysm; Class 2: persistence of a residual neck; Class 3: persistence of a residual aneurysm. The agreement among the analyses was assessed by applying the Kappa statistic.
RESULTS:
Inter-observer agreement was excellent for both methods (K = 0.93; 95 % CI: 0.84-1). Inter-technical agreement was almost perfect between time-of-flight-magnetic resonance angiography and digital subtraction angiography (K = 0.98; 95 % CI: 0.93-1) and between time-of-flight-magnetic resonance angiography and contrast-enhanced-magnetic resonance angiography (K = 0.98; 95% CI: 0.93-1). Disagreement occurred in only one case (2.3%), which was classified as Class I by time-of-flight-magnetic resonance angiography and Class II by digital subtraction angiography. The agreement between contrast-enhanced-magnetic resonance angiography and digital subtraction angiography was perfect (K = 1; 95% CI: 1-1). In three patients, in-stent stenosis was identified by magnetic resonance angiography but not confirmed by digital subtraction angiography.
CONCLUSION:
Digital subtraction angiography and both 3T magnetic resonance angiography techniques have excellent reproducibility for the assessment of aneurysms embolized exclusively with coils. In those cases also treated with stent remodeling, digital subtraction angiography may still be necessary to confirm eventual parent artery stenosis, as identified by magnetic resonance angiography.
Keywords: Aneurysm embolization, Follow-up, Flat-panel DSA, 3D TOF-MR, 3T MRI
INTRODUCTION
Intracranial aneurysms are a major health problem worldwide, affecting 3-5% of the population with a prevalence ranging from 1% to 6% in autopsy series in adults1-3 and 0.65% to 7% in angiographic series.3 Their treatment consists of circulatory exclusion, thus avoiding the risk of bleeding.
Initially, endovascular embolization was only considered as an alternative therapy for aneurysms considered too risky4 or inaccessible to conventional surgical treatment.5 However, with the rapid advent of new techniques and materials, the use of endovascular embolization has been gaining ground. This technique was globally recognized in 2002 with the publication of the International Subarachnoid Aneurysm Trial,6 which evaluated the results of conventional surgery and endovascular embolization of ruptured aneurysms and found a reduced risk of death and functional dependence for patients undergoing endovascular treatment.
Despite all of the technical advances in the endovascular field and the development of various endovascular devices, it is known that some embolized aneurysms are still subject to persistence of intra-aneurismal filling due to incomplete initial treatment, re-growth of the aneurysm sac, or reconfiguration of the coils inside the aneurysm. The most accepted classification system for evaluation of recanalization of aneurysms is that of Raymond et al.,7 which indicates the level of recanalization according to degrees: Class 1, excluded aneurysm; Class 2, persistence of residual neck; and Class 3, persistence of residual aneurysm.
When present, the reconfiguration of the coils inside the aneurysm can result in a recurrence of a residual aneurismal neck or body,7,8 which leads to reperfusion of the aneurysm, exposing the patient to the risk of rupture and bleeding.9 According to most studies, recurrence rates are around 14.7% to 33.6%.8 However, retreatment is necessary in only 10% to 15% of cases with embolized aneurysms.9,10 Therefore, imaging surveillance is very important for all patients undergoing endovascular treatment of intracranial aneurysms to identify aneurysm recurrence and to determine the subsequent need for retreatment.
Traditionally, the monitoring of embolized aneurysms consisted of a repeated series of digital subtraction angiography (DSA) at intervals of months to years after embolization. According to the protocol described by Piotin et al.,9 angiographic controls are ideally performed every 6, 18 and 36 months after treatment in the absence of aneurysm recurrence. Since its introduction in 1980, DSA has undergone major innovations, such as the development of flat-panel detector technology and 3D rotational angiography. The use of flat-panel detectors (FPD) made it possible to obtain images with higher resolution and definition. Compared with the conventional imaging intensifier television system, the FPD technology has several theoretical advantages, such as high spatial resolution, wide dynamic range, square field of view, and real-time imaging capabilities with no geometric distortion.11,12 An additional advantage is lower exposure to radiation with the FPD technology.12 In a recent study, Ichida et al.13 assessed the clinical impact of DSA with FPD and concluded that, in general, FPD obtains better image definition and that, particularly in cerebral vascular images, the resolution is far superior than with a conventional imaging intensifier. Therefore, the use of DSA, now with the FDP, might be considered to be a reference method for vascular studies and for imaging surveillance of embolized brain aneurysms, but certain risks should be considered, such as a small but important risk of neurological complications estimated to occur in 0.3 to 1.8 % of cases.14,15 Additional disadvantages include the need for a brief hospitalization after the procedure, exposure to ionizing radiation, allergic and nephrotoxic effects of the iodinated contrast media and the risk of bleeding complications related to the arterial puncture site, all in addition to the high cost of the procedure.14
Analyzing these drawbacks of DSA, many authors have advocated a search for noninvasive imaging methods for the surveillance of embolized aneurysms15-18 and for assessing the feasibility of magnetic resonance imaging (MRI) as a noninvasive method of choice.19-21 Similar studies22-26 have reported a rather low agreement between methods; however, the equipment used in those studies was not the latest technology available.
Our study aimed to compare time-of-flight (TOF) and contrast-enhanced (CE-MRA) magnetic resonance angiography (MRA) techniques in a 3 Tesla (T) magnetic resonance unit with DSA with the latest flat panel technology and 3D reconstruction for the evaluation of embolized cerebral aneurysms.
MATERIALS AND METHODS
All patients were prospectively and consecutively included in the study, following the clinical routine of the interventional neuroradiology service of our institution, from November 2009 until the last patient enrolled in May 2010. At our institution, all patients with cerebral aneurysms treated by embolization undergo angiographic controls by DSA according to the follow-up protocol of 6, 18 and 36 months after embolization;27 therefore, it was not necessary to perform any additional invasive exams for our research. On the day of the scheduled DSA exam, the patient was submitted to an MRI scan as authorized by written informed consent. In the case of recanalization, the next angiographic surveillance exam was shortened or a re-treatment decision was made. The Ethics Committee of our institution approved the study.
Exclusion Criteria
The study excluded patients with a neurosurgical clip located less than 20 mm from the embolized aneurysm. Patients with pacemakers or who were younger than 18 years were also excluded from the study.
Patients and Aneurysms
Forty-three cerebral aneurysms were studied consecutively in 30 patients, 23 women (76.7%) and 7 men (23.3%), ranging from 27 to 74 years of age (mean age of 54.5 years with a standard deviation of 11.2 years). Twenty patients (66.6%) had a single aneurysm, eight (26.6%) patients had 2 aneurysms, 1 (3.3%) patient had three aneurysms and 1 (3.3%) patient had four aneurysms.
Thirty-four (79.07%) of the aneurysms were located in the internal carotid, 6 (13.95%) in the vertebrobasilar system, two (4.65%) in the middle cerebral artery and 1 (2.33%) in the anterior communicating complex.
Of the 43 aneurysms studied, 15 (34.88%) were small (< 7 mm), 12 (27.91%) medium (7 – 12 mm), 12 (27.91%) large (12 – 25 mm) and 4 (9.30%) giant (> 25 mm). The studied aneurysms were treated using simple techniques in 13 cases (30.23%), the balloon remodeling technique in 21 cases (48.83%) and the stent remodeling technique in 9 cases (20.93%). All stents analyzed were Neuroform 3 stents (Boston Scientific, Natick, Massachusetts).
DSA Technique
DSA was performed on a flat-panel detector system, Innova® 4100 (General Electric, Fairfield, Connecticut). Selective catheterization of the vessel nourishing the aneurysm (internal carotid artery or vertebral artery) was performed using a 5F catheter with an approach through the femoral artery. Eight to ten milliliters of nonionic contrast medium (iobitridol, henetix® 300, Guerbet, Roissy, France) were injected into the internal carotid artery or vertebral artery with an automated power injector (Mark V Provis® Medrad, Warrendale, PA) at 4 ml/s. Standardized posterior-anterior and lateral views and the working view used at the previous embolization were acquired. After reconstruction of a 3D rotational acquisition, a final injection was done in the post-processed view, which better depicted the neck of the aneurysm.
MRI Technique
MRI examinations were performed with a 3T unit (Philips Achieva Duo - Philips Medical Systems, Best, The Netherlands), using a standard head coil. MRA TOF 3D was acquired in axial orientation using the following parameters: field of view, 20 cm; TR/TE, 25/3.5 ms; flip angle, 20°; matrix, 528 × 270; voxel size, 0.38 × 0.74 × 1.1 mm; reconstruction voxel size, 0.2 × 0.2 × 0.55 mm; acquisition time, 7 m 5 s. CE-MRA at 3T was performed using a 3D gradient sequence in coronal orientation with the following parameters: field of view, 22 cm; TR/TE, 5.6/2.1 ms; flip angle, 27°; matrix, 512 × 384; voxel size, 0.9 × 0.9 × 0.9 mm; reconstruction voxel size: 0.6 × 0.6 × 0.9 mm; acquisition time, 4 m 27 s.
CE-MRA images were acquired after administration of gadobenate dimeglumine (Viewgam®, Lab. Bacon S.A.I.C., Buenos Aires, Argentina) at a dose of 0.1 mmol/kg (0.2 ml/kg) body weight. Gadobenate dimeglumine was administered to all patients through an antecubital vein at a rate of 2 ml/s using an MR-imaging-compatible power injector (Optistar® LE, Mallinckrodt, Hazelwood, Missouri).
Image Evaluation
Two neuroradiologists (A: _____, B: _____) with experience in DSA and magnetic resonance imaging interpreted the image sets for each method separately and independently. Both readers had access to images of the DSA (2D and 3D rotational DSA) pre- and post-embolization and to the working view used during treatment. Treated aneurysms in each image set were classified as described by Raymond et al.7: class 1 = complete obliteration; class 2 = residual neck; class 3 = residual aneurysm. None of readers participated in any of the DSA procedures, and they only gained access to the obtained angiography after classifying the MRI image sets. Image analysis occurred in the following order for all assessed aneurysms: TOF-MRA, CE-MRA, DSA. This sequence was used to avoid any possible interference from the memory of the DSA, which was used as the reference method, in the assessment of the corresponding image in MRA.
The need for image interpretation to rate the treated aneurysm independently of the methods used to obtain the image in this study allows us to anticipate some of the limitations of the method. The use of equipment of the latest technology, as in this study, produces the most accurate images and minimizes the limitation. Thus, the real status rating by any observer would be achieved only when the images need only reading with no interpretation, which demands either quantification or resolution and specificity.
The DSA images were processed and analyzed on an Advantage Workstation® Volumeshare2™ (General Electric, Fairfield, Connecticut), while the MR images were processed and analyzed on an Extended MR Workspace 2.6.3.1 Systems® workstation (Philips). The MRA images were evaluated using the source images, 3D maximum intensity projection (MIP) and volume-rendered reconstructions targeted on the vessel of interest. The MRA images were also qualitatively evaluated considering the conspicuity of the residual patency, the contrast enhancement achieved, and the presence of artifacts compromising the aneurysm's assessment or parent artery stenosis.
Statistics
Interobserver agreement between the readers was determined using the weighted Kappa statistic (K) with a confidence interval of 95% and following the guidelines suggested by Landis and Koch.28 After the blind study, discrepancies were resolved by consensus. Finally, the consensus data were also assessed using the weighted Kappa statistic, comparing the agreement among methods (TOF-MRA, CE-MRA and DSA). The interpretation of K was as follows28: K = 0 indicated no agreement; K between 0–0.19, poor agreement; K between 0.20–0.39, fair agreement; K between 0.40–0.59, moderate agreement; K between 0.60–0.79, substantial agreement; and K between 0.80–1.00, almost perfect agreement. All of the analyses were performed using SAS (Version 9.2) software (SAS Institute, Cary, North Carolina).
RESULTS
In the studied cases, all of the MRA and DSA images were of high quality. There were no clinical complications from any imaging method.
Classification of Aneurysm Occlusion
Interobserver agreement for the classification of embolized aneurysms was almost perfect (0.80-1.00) for all methods, evaluated with K = 0.93 (95 % CI: 0.84-1) (Figure 1). Disagreement in the Raymond's classification occurred in only three cases: one observer interpreted the 3 aneurysms as class I, class II and class II, whereas the other reader classified them, respectively, as class II, class I and class I.
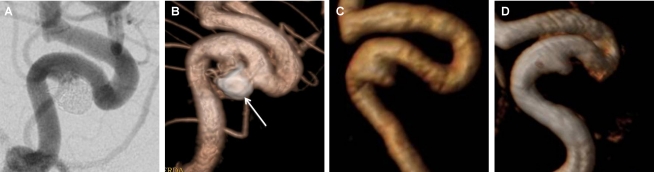
Figure 1.
Post-embolization follow-up of a cavernous carotid artery aneurysm, in working-view incidences, classified in consensus by both readers as class II in Raymond scale. A: DSA of the internal carotid artery showing the residual neck of the treated aneurysm (class II). B: DSA with 3D reconstruction delimiting the aneurysm residual neck (class II) and the coil mesh (arrow). C: CE-MRA with volume-rendered reconstruction showing class II recanalization. D: TOF-MRA with volume-rendered reconstruction showing class II recanalization.
Agreement between the TOF-MRA and DSA in the identification of complete aneurismal occlusion, residual aneurysm or a residual neck was almost perfect, with full agreement in 42 aneurysms (97.6%), K = 0.98 (95% CI: 0.93-1). Of the aneurysms equally classified by both methods, 23 (53.49%) were classified as completely occluded (class I), 5 (11.63%) with a residual neck (class II) and 14 (32.55%) with persistent aneurysm (class III). Disagreement occurred in only 1 case (2.33%), corresponding to a medium aneurysm treated by balloon remodeling that was located in the internal carotid artery; this aneurysm was classified as class I by TOF-MRA and class II by DSA (Table 1). The agreement between CE-MRA and DSA was perfect, with full agreement in all 43 aneurysms (100%), K = 1 (95% CI: 1-1), with the following classifications: 23 aneurysms (53.49%) were classified as class I, 6 (13.95%) as class II, and 14 (32.56%) as class III (Table 2). The correlation between TOF-MRA and CE-MRA in the identification of complete aneurismal occlusion, residual aneurysm or a residual neck was almost perfect, with full agreement in 42 aneurysms (97.6%), K = 0.98 (95% CI: 0.93-1). Of the aneurysms equally classified by both sequences, 23 (53.49%) were classified as class I, 5 (11.63%) as class II, and 14 (32.56%) as class III. Disagreement occurred in only 1 case (2.33%) (Figure 2), which corresponded to the discordant case between TOF-MRA and DSA. None of the 3D rotational DSA added new information to the 2D DSA.
Table 1.
Aneurysm occlusion status as assessed by DSA and TOF-MRA, with Raymond's classification, after interobserver consensus.
| TOF-MRA | DSA | CI 95% | ||||
| Class 1 | Class 2 | Class 3 | K | IL | SL | |
| Class 1 | 23(53.49) | 1(2.33) | 0(0.00) | 0.98 | 0.93 | 1.00 |
| Class 2 | 0(0.00) | 5(11.63) | 0(0.00) | |||
| Class 3 | 0(0.00) | 0(0.00) | 14(32.56) | |||
IL: inferior limit; SL: superior limit; CI: confidence interval; DSA: digital subtraction angiography; CE-MRA: contrast-enhanced magnetic angiography.
Table 2.
Aneurysm occlusion status as assessed by DSA and CE-MRA, with Raymond's classification, after interobserver consensus.
| CE-MRA | DSA | CI 95% | ||||
| Class 1 | Class 2 | Class 3 | K | IL | SL | |
| Class 1 | 23(53.49) | 0(0.00) | 0(0.00) | 1.00 | 1.00 | 1.00 |
| Class 2 | 0(0.00) | 6(11.63) | 0(0.00) | |||
| Class 3 | 0(0.00) | 0(0.00) | 14(32.56) | |||
IL: inferior limit; SL: superior limit; CI: confidence interval; DSA: digital subtraction angiography; CE-MRA: contrast-enhanced magnetic angiography.
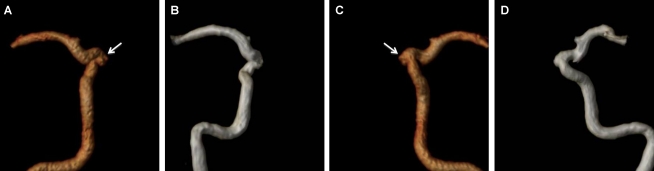
Figure 2.
An internal carotid artery aneurysm with a small neck remnant identified by DSA and CE-MRA but not visible on TOF-MRA. A, B: CE-MRA, with volume-rendered reconstruction of the internal carotid artery, showing a small aneurismal neck remnant (arrow). C, D: TOF-MRA, with volume-rendered reconstruction of the internal carotid artery, without clear identification of the aneurismal neck.
Artifacts
Enhancement of the venous structures was present in all of the CE-MRA images. However, venous overlap did not affect the image interpretation of any studied case.
High signal intensity, secondary to the presence of a clot containing methemoglobin within a thrombosed aneurysm, was identified by TOF-MRA in 2 aneurysms (4.65%). This artifact was not observed close to the aneurysm's neck area in either case, therefore no prejudice in the interpretation of the aneurysm's occlusion status was identified.
In two large aneurysms of the internal carotid artery and one giant basilar aneurysm, type III recanalization was identified by all three methods (TOF-MRA, CE-MRA and DSA). However, the aneurysm remnant was better visualized in the CE-MRA images. In this method, the signal intensity and conspicuity of the recanalization were higher than for TOF-MRA. In these 3 cases, CE-MRA was also better than DSA for visualization of the aneurysm remnant, due to overlapping of the coil mesh repositioned on the periphery of the aneurysm (helmet effect) (Figure 3).
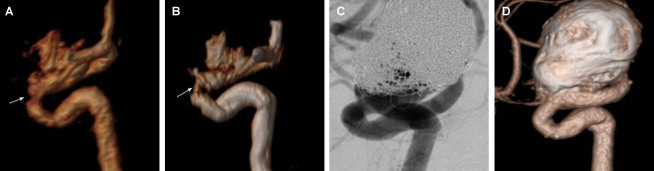
Figure 3.
An ophthalmic aneurysm with an aneurismal remnant (class III), assuming a “helmet” type recanalization. A: DSA demonstrating aneurismal remnant partially superimposed by the coil mesh. B: Angiography, without subtraction, showing the coil mass surrounding the aneurism recanalization. C: CE-MRA with volume-rendered reconstruction showing the aneurysm remnant without interposition of the coil mesh. D: TOF-MRA with volume-rendered reconstruction showing the aneurysm remnant without interposition of the coil mesh but with slight loss of signal in comparison to CE-MRA.
In 3 cases of aneurysms treated with the stent remodeling technique, we observed a decrease in signal intensity in the vessel segment with the stent that assumed an appearance suggestive of stenosis in both the CE-MRA and the TOF-MRA but more pronounced in the latter sequence. None of the three cases had stenosis when assessed by DSA (Figure 4).
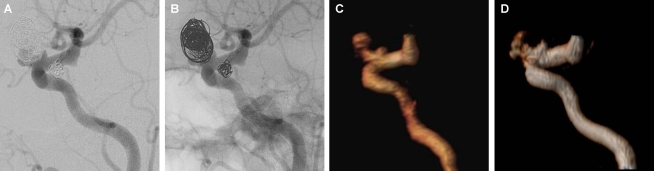
Figure 4.
Follow-up of an aneurysm treated with a stent remodeling technique (Neuroform 3), assessing the patency of the parent artery within the stent. A, B: CE-MRA and TOF-MRA, respectively, with volume-rendered reconstruction showing a focused loss of signal, simulating an in-stent stenosis. C, D: DSA and DSA with 3D reconstruction showing a normal parent artery patency within the stent.
DISCUSSION
Long-term follow-up is required for embolized aneurysms to determine the stability of treatment and identify a possible recanalization, which may require retreatment due to the risk of bleeding. Considering the drawbacks inherent to DSA,15,16 some authors have suggested MRI as a noninvasive method of monitoring embolized cerebral aneurysms.
In our study, we observed that the methods CE-MRA, TOF-MRA and DSA have almost perfect agreement between each other (K = 0.80 to 1.00) with excellent interobserver reproducibility (K = 0.93; 95% CI: 0.84-1).
Interobserver disagreement was seen in only 3 cases (6.9%). Different interpretation of the aneurysm occlusion status between the two readers involved only disagreement between Class 1 (completely occluded) and Class 2 (residual neck) classifications. This interobserver disagreement had no clinical consequence because it did not imply a different therapeutic indication.
Inter-method disagreement was seen in only one case of a medium aneurysm of the internal carotid artery. In this case, DSA and CE-MRA identified a residual neck of less than 2 mm (class II), while TOF-MRA identified the aneurysm as completely occluded (class I). Despite the different inter-method classification for this aneurysm, no difference in retreatment indication was observed. This is in agreement with a study by Pierot et al. 20 where all false negative results on MRI were the results of small residual necks (2 mm or less), which would not have any therapeutic consequences. Again, because the disagreement occurred only with respect to the interpretation of small structures when image resolution, definition, specificity are of utmost importance, the idea of overcoming the limitations related to the observers' procedural protocol by refining the technology gives support to the purpose of this study.
The necessity of having independent observers for image rating raises subjectivity as a limitation in the method. To some extent, the subjectivity was reduced by the high level of experience of the observers with both of the methods involved. However, the Raymond scale, which is widely used, has itself some subjectivity in its classification, as its rating is not based on measurements but rather on descriptions of images. Thus, reducing or eliminating this type of bias depends either on great experience or on high technology that produces images that more faithfully depict the real anatomy.
Therefore, the agreement among methods found within the observers combined with the disagreement between the observers in the Raymond scale seems to demonstrate that the bias is in the Raymond scale rather than in the imaging strategy. Agreement by observers on the results using different methods reinforces this study's presumption that use of the latest technology available reduces the subjectivity of the rating. Even if a time interval was set between the readings of the image sets for each diagnostic exam for a given patient, assuming that in this situation there would be no interference from any recollection of the previous image, the subjectivity of Raymond scale would still exist.
To the authors' knowledge, this is the first study that compares results obtained exclusively from the most advanced equipment with the latest technology for each imaging modality in the assessment of embolized aneurysms.
Several authors have evaluated CE-MRA in comparison to TOF-MRA, and many have reported superiority of the former over the latter.17,19,29,30 CE-MRA has several theoretical advantages relative to TOF-MRA, such as the lower susceptibility to turbulent flow and to saturation effects due to the use of contrast medium, which produces a high-intensity signal within the arterial lumen. Furthermore, contrast enhancement provides increased conspicuity to small vessels in regions of slow flow and improved definition. Another disadvantage of TOF-MRA is the “T1-shine-through” effect, which is intrinsic to the TOF sequence and can appear as a high-intensity signal in subacute or chronic thrombus inside the aneurysm, secondary to the presence of methemoglobin. In rare cases, this effect can obscure the hyperintensity of residual flow within the relevant vessel. CE-MRA is also less sensitive to coil or stent-related artifacts.19
However, CE-MRA also has some disadvantages, such as overlapping of venous structures due to the short time window between both phases, leading to deterioration in image quality, especially in cases of aneurysms located in the middle cerebral artery or near the skull base.20,31 In our study, all of the CE-MRA images had venous enhancement, but there was no prejudice in assessing the status of occlusion of the aneurysm in any case. Moreover, image processing on the workstation removed undesired structures, allowing visualization of the embolized aneurysm at different angles without interposition of venous structures. Another disadvantage is the possibility of false positives for aneurysm recanalization, resulting from peripheral enhancement of an intra-aneurismal organized thrombus or from the vasa-vasorum at the aneurysm wall,19,29 which, however, was not observed in any of our cases. Finally, a negative attribute of the use of gadolinium is the small but important risk of nephrogenic systemic fibrosis.32
Despite the theoretical advantages of CE-MRA over TOF-MRA, Kwee and Kwee,33 in a meta-analysis reviewing the published data on the diagnostic performance of MRA in the follow-up of embolized aneurysms, found no significant statistical differences between both methods. Recently, a multicenter study conducted by Schaafsma et al.34 corroborated the conclusion of the meta-analysis by not identifying an important added value of CE-MRA over TOF-MRA. Since the publication of Marjoie et al.22 reporting the effectiveness of TOF-MRA for monitoring embolized aneurysms, other studies have been published by Urbach et al.,23 Buhk et al.35 and Ferré et al.24 demonstrating the feasibility of this method as a first-line exam for coiled aneurysm follow-up. The last-mentioned study, a recent publication, showed excellent agreement (K = 0.86) between 3T TOF and DSA (non flat panel). Ramgren et al.36 concluded that TOF-MRA at 3T showed better agreement with DSA than TOF-MRA at 1.5T and CE-MRA 3T. In contrast, Anzalone et al.30 also evaluated both MRA techniques in a 3T unit and found better depiction of aneurismal recanalization in CE-MRA than TOF-MRA.
In a recent study, Kaufmann et al.37 concluded that larger, clinically more important aneurysm remnants were better characterized with CE-MRA at both 1.5T and 3T than with TOF-MRA at 1.5T. In the analysis of our results, CE-MRA at 3T provided a better depiction of recanalized aneurysms than TOF-MRA in 3 patients. However, the different appearance of the embolized aneurysm using each method did not imply a disagreement in the therapeutic approach to be conducted. In these 3 cases, CE-MRA was even better than DSA in demonstrating invaginated recanalization into the mass of coils. In these cases, the mesh of spirals around the aneurismal remnant acted as a radiodense “helmet”, which was partially penetrable to X-rays in DSA.25 In fact, other reports have already suggested that MRA can be more accurate than DSA in some circumstances.23,38 Both readers acknowledged preference for the CE-MRA in the evaluation of aneurysms with “helmet” recanalization. However, there was no difference in the status classification of aneurysm occlusion by any of three methods.
A limitation of our study is the small sample size of 43 aneurysms, of which 20 (46%) were incompletely occluded. However, the latest single-center study published analyzing the same methods (3T CE-MRA, 3T TOF-MRA and DSA not performed in a flat-panel unit), enrolled only 12 incompletely occluded aneurysms.39 In this study, TOF-MRA and CE-MRA at 3T were equivalent in evaluating the occlusion status of coiled intracranial aneurysms. Another limitation that should be considered for further studies is that we did not evaluate the 3D rotational angiography with coils digital subtraction, which could especially improve the “helmet” recanalization assessment.
The nitinol self-expandable stent Neuroform evaluated in our series was the first stent used to treat cerebrovascular aneurysms.40 Before the availability of nitinol self-expandable stents, only balloon-expandable coronary stents were available to assist the treatment of cerebral aneurysms by stent remodeling. With the increased use of self-expandable stents due to their better flexibility and navigation relative to coronary stents, it became mandatory to monitor the possibility of in-stent stenosis. Although in-stent stenosis represents a common complication in atherosclerotic peripheral vascular disease, it is not expected at the same rates in self-expandable intracranial stents deployed in a non-stenotic vessel, as in the treatment of aneurysms.41 This is particularly true given the low radial force of the Neuroform stent and the non-use of associated balloon angioplasty, which thus results in a low probability of endothelial trauma during and after deployment. A study41 evaluating the follow-up of 156 cases of cerebral aneurysm treated with Neuroform stent remodeling embolization observed a rate of 5.8% for moderate to severe (> 50%) in-stent stenosis. Although not very frequent, the risk of in-stent stenosis is not negligible. Thus, the imaging method used in the follow-up of aneurysms treated by stent remodeling should also assess the patency of the main vessel.
Nehra et al.42 reviewed the safety and feasibility of 3T MRI in the evaluation of the self-expanding nitinol stent and confirmed the safety and efficacy of MRI in the evaluation of the stent in an animal model. However, in studies involving patients with aneurysms treated using a stent remodeling technique, Lubicz et al.26 observed that the presence of an intracranial stent can compromise the evaluation of patency of the main vessel at 1.5 T MRI. In this study, in-stent signal loss was present in all TOF-MRA cases but was not observed in CE-MRA images. Lovblad et al.43 also found better definition in CE-MRA for evaluating the parent artery lumen within the stent. This study also observed lower susceptibility to artifacts in Neuroform stents than in stainless steel stents. For the detection of residual aneurysms, Lubicz et al.26 found that in five patients with recanalization of the aneurysm treated using a stent remodeling technique, 3 recanalizations were not detected by TOF-MRA, but all were well characterized by CE-MRA and DSA.
In our study of 9 patients treated using a stent remodeling technique, all of the patients were classified under the same status of aneurysm occlusion by all three methods. Regarding the patency of the main vessel within the stent, we had the same impression as Lovblad et al.43 and Lubicz et al.,26 concluding that the parent vessel definition was better in CE-MRA than in TOF-MRA. However, in three cases in our study, both TOF-MRA and CE-MRA showed parent artery stenosis within the stent, which was more pronounced in TOF-MRA, and none of the stenosis was confirmed by DSA.
The necessity of having independent observers for rating of images raises subjectivity as a limitation in the method. To some extent, that subjectivity was reduced by the high level of experience of the observers with both of the methods involved. However, the Raymond scale, which is widely used, itself has some subjectivity in its classification, as its rating is based not on measurements but on descriptions of images. Thus, reducing or eliminating this type of bias depends either on great experience or on high technology that produces images that more faithfully depict the real anatomy.
Therefore, the agreement found within the observers using the different methods, along with the disagreement on the Raymond scale classification, seems to demonstrate that the bias is in the Raymond scale rather than in the method of obtaining the images. Agreement by the observers on the results of the different methods reinforces this study's presumption that the use of the latest technology available reduces the subjectivity. Even when setting a time interval between the readings of the image set for each diagnostic exam for a given patient, assuming that in this situation there would be no interference from any recollection of the previous image, the subjectivity of Raymond scale would still exist.
CONCLUSION
In our study, DSA with flat-panel technology and 3T MRI techniques showed similar assessments of aneurysm occlusion after embolization. However, in cases treated with stent remodeling, DSA may still be necessary to confirm an eventual parent artery stenosis within the stent identified by MRI. In three cases, there was better characterization of the embolized cerebral aneurysms by CE-MRA than by TOF-MRA and even DSA. However, no difference was observed in the indication of retreatment by each method.
REFERENCES
- 1.Stehbens WE. Aneurysms and Anatomical Variation of Cerebral Arteries. Arch Pathol. 1963;75:45–64. [PubMed] [Google Scholar]
- 2.Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol. 1990;34:361–5. doi: 10.1016/0090-3019(90)90237-j. 10.1016/0090-3019(90)90237-J [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa T, Hashi K. The incidence and treatment of asymptomatic, unruptured cerebral aneurysms. J Neurosurg. 1994;80:217–23. doi: 10.3171/jns.1994.80.2.0217. [DOI] [PubMed] [Google Scholar]
- 4.Klein GE, Szolar DH, Leber KA, Karaic R, Hausegger KA. Basilar tip aneurysm: endovascular treatment with Guglielmi detachable coils--midterm results. Radiology. 1997;205:191–6. doi: 10.1148/radiology.205.1.9314984. [DOI] [PubMed] [Google Scholar]
- 5.van der Schaaf IC, Brilstra EH, Buskens E, Rinkel GJ. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke. 2002;33:313–8. doi: 10.1161/hs0102.101479. 10.1161/hs0102.101479 [DOI] [PubMed] [Google Scholar]
- 6.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–74. doi: 10.1016/s0140-6736(02)11314-6. 10.1016/S0140-6736(02)11314-6 [DOI] [PubMed] [Google Scholar]
- 7.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. 10.1161/hs0901.095600 [DOI] [PubMed] [Google Scholar]
- 8.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–82. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 9.Piotin M, Spelle L, Mounayer C, Salles-Rezende MT, Giansante-Abud D, Vanzin-Santos R, et al. Intracranial aneurysms: treatment with bare platinum coils--aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007;243:500–8. doi: 10.1148/radiol.2431060006. 10.1148/radiol.2431060006 [DOI] [PubMed] [Google Scholar]
- 10.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–403. doi: 10.1161/01.STR.0000073841.88563.E9. 10.1161/01.STR.0000073841.88563.e9",-1,"xxx/88563.e9 [DOI] [PubMed] [Google Scholar]
- 11.Kakeda S, Korogi Y, Ohnari N, Hatakeyama Y, Moriya J, Oda N, et al. 3D digital subtraction angiography of intracranial aneurysms: comparison of flat panel detector with conventional image intensifier TV system using a vascular phantom. AJNR Am J Neuroradiol. 2007;28:839–43. [PMC free article] [PubMed] [Google Scholar]
- 12.Hatakeyama Y, Kakeda S, Ohnari N, Moriya J, Oda N, Nishino K, et al. Reduction of radiation dose for cerebral angiography using flat panel detector of direct conversion type: a vascular phantom study. AJNR Am J Neuroradiol. 2007;28:645–50. [PMC free article] [PubMed] [Google Scholar]
- 13.Ichida T, Sasaki S, Shougaki M, Okusako K, Ogawa T, Yokoyama K, et al. [Angiography: clinical evaluation and physical characteristics of FPD - the clinical impact caused by detector characteristics] Nippon Hoshasen Gijutsu Gakkai Zasshi. 2009;65:795–804. doi: 10.6009/jjrt.65.795. [DOI] [PubMed] [Google Scholar]
- 14.Schaafsma JD, Koffijberg H, Buskens E, Velthuis BK, van der Graaf Y, Rinkel GJ. Cost-effectiveness of magnetic resonance angiography versus intra-arterial digital subtraction angiography to follow-up patients with coiled intracranial aneurysms. Stroke. 2010;41:1736–42. doi: 10.1161/STROKEAHA.110.585083. 10.1161/STROKEAHA.110.585083 [DOI] [PubMed] [Google Scholar]
- 15.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke. 1999;30:317–20. doi: 10.1161/01.str.30.2.317. [DOI] [PubMed] [Google Scholar]
- 16.Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522–8. doi: 10.1148/radiol.2272012071. 10.1148/radiol.2272012071 [DOI] [PubMed] [Google Scholar]
- 17.Farb RI, Nag S, Scott JN, Willinsky RA, Marotta TR, Montanera WJ, et al. Surveillance of intracranial aneurysms treated with detachable coils: a comparison of MRA techniques. Neuroradiology. 2005;47:507–15. doi: 10.1007/s00234-005-1375-7. 10.1007/s00234-005-1375-7 [DOI] [PubMed] [Google Scholar]
- 18.Costalat V, Lebars E, Sarry L, Defasque A, Barbotte E, Brunel H, et al. In vitro evaluation of 2D-digital subtraction angiography versus 3D-time-of-flight in assessment of intracranial cerebral aneurysm filling after endovascular therapy. AJNR Am J Neuroradiol. 2006;27:177–84. [PMC free article] [PubMed] [Google Scholar]
- 19.Gauvrit JY, Leclerc X, Caron S, Taschner CA, Lejeune JP, Pruvo JP. Intracranial aneurysms treated with Guglielmi detachable coils: imaging follow-up with contrast-enhanced MR angiography. Stroke. 2006;37:1033–7. doi: 10.1161/01.STR.0000209236.06451.3b. 10.1161/01.STR.0000209236.06451.3b [DOI] [PubMed] [Google Scholar]
- 20.Pierot L, Delcourt C, Bouquigny F, Breidt D, Feuillet B, Lanoix O, et al. Follow-up of intracranial aneurysms selectively treated with coils: Prospective evaluation of contrast-enhanced MR angiography. AJNR Am J Neuroradiol. 2006;27:744–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman J, Nguyen T, Larsen D, Teitelbaum GP. MR artifacts, heat production, and ferromagnetism of Guglielmi detachable coils. AJNR Am J Neuroradiol. 1997;18:497–501. [PMC free article] [PubMed] [Google Scholar]
- 22.Majoie CB, Sprengers ME, van Rooij WJ, Lavini C, Sluzewski M, van Rijn JC, et al. MR angiography at 3T versus digital subtraction angiography in the follow-up of intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol. 2005;26:1349–56. [PMC free article] [PubMed] [Google Scholar]
- 23.Urbach H, Dorenbeck U, von Falkenhausen M, Wilhelm K, Willinek W, Schaller C, et al. Three-dimensional time-of-flight MR angiography at 3 T compared to digital subtraction angiography in the follow-up of ruptured and coiled intracranial aneurysms: a prospective study. Neuroradiology. 2008;50:383–9. doi: 10.1007/s00234-007-0355-5. 10.1007/s00234-007-0355-5 [DOI] [PubMed] [Google Scholar]
- 24.Ferre JC, Carsin-Nicol B, Morandi X, Carsin M, de Kersaint-Gilly A, Gauvrit JY, et al. Time-of-flight MR angiography at 3T versus digital subtraction angiography in the imaging follow-up of 51 intracranial aneurysms treated with coils. Eur J Radiol. 2009;72:365–9. doi: 10.1016/j.ejrad.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Agid R, Willinsky RA, Lee SK, Terbrugge KG, Farb RI. Characterization of aneurysm remnants after endovascular treatment: contrast-enhanced MR angiography versus catheter digital subtraction angiography. AJNR Am J Neuroradiol. 2008;29:1570–4. doi: 10.3174/ajnr.A1124. 10.3174/ajnr.A1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubicz B, Neugroschl C, Collignon L, Francois O, Baleriaux D. Is digital substraction angiography still needed for the follow-up of intracranial aneurysms treated by embolisation with detachable coils. Neuroradiology. 2008;50:841–8. doi: 10.1007/s00234-008-0450-2. 10.1007/s00234-008-0450-2 [DOI] [PubMed] [Google Scholar]
- 27.Abud DG, Nakiri GS, Abud TG, Carlotti CG, Jr, Colli BO, Santos AC. Endovascular therapy for selected (most non-surgical) intracranial aneurysms in a Brazilian university hospital. Arquivos Brasileiros de Neuro-Psiquiatria. 2010 doi: 10.1590/s0004-282x2010000500017. [DOI] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. 10.2307/2529310 [PubMed] [Google Scholar]
- 29.Leclerc X, Navez JF, Gauvrit JY, Lejeune JP, Pruvo JP. Aneurysms of the anterior communicating artery treated with Guglielmi detachable coils: follow-up with contrast-enhanced MR angiography. AJNR Am J Neuroradiol. 2002;23:1121–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Anzalone N, Scomazzoni F, Cirillo M, Righi C, Simionato F, Cadioli M, et al. Follow-up of coiled cerebral aneurysms at 3T: comparison of 3D time-of-flight MR angiography and contrast-enhanced MR angiography. AJNR Am J Neuroradiol. 2008;29:1530–6. doi: 10.3174/ajnr.A1166. 10.3174/ajnr.A1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cottier JP, Bleuzen-Couthon A, Gallas S, Vinikoff-Sonier CB, Bertrand P, Domengie F, et al. Intracranial aneurysms treated with Guglielmi detachable coils: is contrast material necessary in the follow-up with 3D time-of-flight MR angiography. AJNR Am J Neuroradiol. 2003;24:1797–803. [PMC free article] [PubMed] [Google Scholar]
- 32.Grobner T, Prischl FC. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72:260–4. doi: 10.1038/sj.ki.5002338. 10.1038/sj.ki.5002338 [DOI] [PubMed] [Google Scholar]
- 33.Kwee TC, Kwee RM. MR angiography in the follow-up of intracranial aneurysms treated with Guglielmi detachable coils: systematic review and meta-analysis. Neuroradiology. 2007;49:703–13. doi: 10.1007/s00234-007-0266-5. 10.1007/s00234-007-0266-5 [DOI] [PubMed] [Google Scholar]
- 34.Schaafsma JD, Velthuis BK, Majoie CB, van den Berg R, Brouwer PA, Barkhof F, et al. Intracranial aneurysms treated with coil placement: test characteristics of follow-up MR angiography--multicenter study. Radiology. 2010;256:209–18. doi: 10.1148/radiol.10091528. 10.1148/radiol.10091528 [DOI] [PubMed] [Google Scholar]
- 35.Buhk JH, Kallenberg K, Mohr A, Dechent P, Knauth M. No advantage of time-of-flight magnetic resonance angiography at 3 Tesla compared to 1.5 Tesla in the follow-up after endovascular treatment of cerebral aneurysms. Neuroradiology. 2008;50:855–61. doi: 10.1007/s00234-008-0413-7. 10.1007/s00234-008-0413-7 [DOI] [PubMed] [Google Scholar]
- 36.Ramgren B, Siemund R, Cronqvist M, Undren P, Nilsson OG, Holtas S, et al. Follow-up of intracranial aneurysms treated with detachable coils: comparison of 3D inflow MRA at 3T and 1.5T and contrast-enhanced MRA at 3T with DSA. Neuroradiology. 2008;50:947–54. doi: 10.1007/s00234-008-0429-z. 10.1007/s00234-008-0429-z [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann TJ, Huston J, 3rd, Cloft HJ, Mandrekar J, Gray L, Bernstein MA, et al. A prospective trial of 3T and 1.5T time-of-flight and contrast-enhanced MR angiography in the follow-up of coiled intracranial aneurysms. AJNR Am J Neuroradiol. 2010;31:912–8. doi: 10.3174/ajnr.A1932. 10.3174/ajnr.A1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada N, Hayashi K, Murao K, Higashi M, Iihara K. Time-of-flight MR angiography targeted to coiled intracranial aneurysms is more sensitive to residual flow than is digital subtraction angiography. AJNR Am J Neuroradiol. 2004;25:1154–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Sprengers ME, Schaafsma JD, van Rooij WJ, van den Berg R, Rinkel GJ, Akkerman EM, et al. Evaluation of the occlusion status of coiled intracranial aneurysms with MR angiography at 3T: is contrast enhancement necessary. AJNR Am J Neuroradiol. 2009;30:1665–71. doi: 10.3174/ajnr.A1678. 10.3174/ajnr.A1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorella D, Albuquerque FC, Han P, McDougall CG. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6–16. doi: 10.1227/01.neu.0000097194.35781.ea. 10.1227/01.NEU.0000097194.35781.EA [DOI] [PubMed] [Google Scholar]
- 41.Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006l;59:34–42. doi: 10.1227/01.NEU.0000219853.56553.71. 10.1227/01.NEU.0000219853.56553.71 [DOI] [PubMed] [Google Scholar]
- 42.Nehra A, Moran CJ, Cross DT, 3rd, Derdeyn CP. MR safety and imaging of neuroform stents at 3T. AJNR Am J Neuroradiol. 2004;25:1476–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Lovblad KO, Yilmaz H, Chouiter A, San Millan Ruiz D, Abdo G, Bijlenga P, et al. Intracranial aneurysm stenting: follow-up with MR angiography. J Magn Reson Imaging. 2006;24:418–22. doi: 10.1002/jmri.20642. 10.1002/jmri.20642 [DOI] [PubMed] [Google Scholar]