Abstract
OBJECTIVE:
To determine the impact of periodontal treatment on serum levels of prohepcidin (the prohormone of hepcidin) and systemic inflammation markers, as well as correlations among these markers, in patients with chronic periodontitis and chronic kidney disease who were not undergoing dialysis.
METHODS:
We included 56 chronic periodontitis patients, 36 with chronic kidney disease and 20 without systemic diseases and with normal renal function (control group). Chronic kidney disease was defined as suggested by the clinical practice guidelines in the National Kidney Foundation. Chronic periodontitis was defined through clinical attachment level and by probing pocket depth, according to the American Association of Periodontology. The inflammatory markers ultrasensitive C-reactive protein, interleukin-6, and prohepcidin were evaluated before and 3 months after periodontal treatment.
RESULTS:
The efficacy of periodontal treatment was confirmed by the improvement in clinical parameters of chronic periodontitis in the control and chronic kidney disease groups. Periodontal treatment resulted in significant reductions in ultrasensitive C-reactive protein, interleukin-6 and serum prohepcidin levels in both groups. Moreover, in multivariate linear regression, the reduction in prohepcidin after periodontal treatment was significantly and independently associated with interleukin-6 levels in the control group.
CONCLUSIONS:
By inducing a decline in the systemic inflammatory response and a decrease in serum prohepcidin, successful periodontal treatment may represent an important means of ameliorating the inflammatory burden seen in patients with chronic kidney disease. Trial registration: ISRCTN59866656.
Keywords: Prohepcidin, hronic periodontitis, hronic kidney disease, inflammatory markers, periodontal treatment
INTRODUCTION
Chronic kidney disease (CKD) is considered a worldwide public health problem, mainly due to its high morbidity and mortality. With the progressive and irreversible loss of renal function, several complications arise, anemia being one of the most frequent, due to erythropoietin (EPO) deficiency.1 Treatment of CKD anemia is based on the replacement of exogenous EPO; however, 10% to 20% of patients do not respond adequately to this treatment.2 The two most common reasons for unresponsiveness to EPO therapy are iron deficiency and inflammation. With our increasing understanding of the effect of inflammatory cytokines on erythropoietin and the rapidly evolving knowledge of the changes in iron metabolism that occur with inflammation, it is timely to review the elements of the inflammatory response in patients with CKD. EPO resistance is not unique to patients with CKD, but these patients may be at an increased risk, because inflammation is common in this disease and is associated with increased serum levels of proinflammatory cytokines (for instance, interleukin-6), which may drive the activation of hepcidin synthesis.3,4
Hepcidin is a cationic peptide that is rich in cysteine.5,6 It is synthesized by the liver and excreted by the kidney, and its main function is homeostatic regulation of iron metabolism.7,8 The hormone hepcidin is derived from the two-step conversion of an 84-amino-acid–long peptide, preprohepcidin, first by N-terminal cleavage of a 24-amino-acid signal peptide to give rise to prohepcidin. This step is followed by a second cleavage of a 35-amino-acid peptide to yield the biologically active 25-amino-acid hepcidin (hepcidin 25), which is secreted into the serum. The target for serum hepcidin is the iron exporter ferroportin 1, which is found in the plasma membranes of most body cells and is found at high concentrations in duodenal enterocytes, macrophages, and hepatocytes.3,8 The gene that encodes hepcidin is regulated by iron load, anemia, and, in particular, chronic inflammation.7
Chronic periodontitis (CP) is an immunoinflammatory disease caused by Gram-negative bacteria that destroy the supporting tissues of the teeth,9 induce local inflammation, and are associated with a systemic inflammatory response.10,11 Recent studies have shown an association between high levels of C-reactive protein (CRP) and interleukin-6 (IL-6) and periodontitis, an association that decreases after periodontal treatment (PT).12,13 Due to this association with the systemic inflammatory response, CP has recently been included as a nontraditional risk factor for CKD.14
We hypothesized that part of the chronic inflammatory response seen in CKD patients stems from CP, which, through an increase in the expression of inflammatory markers, such as IL-6, stimulates hepcidin synthesis. Therefore, the aim of this study was to determine the impact of PT on the serum level of prohepcidin (the prohormone of hepcidin) and on systemic inflammation markers, as well as their correlations, in patients with CP and CKD.
MATERIALS AND METHODS
The present study was an interventional, controlled, nonrandomized clinical trial in which the participants, all of whom had a diagnosis of CP, received PT (Trial registration: ISRCTN59866656). The patients with CP were divided into two groups. The first group consisted of CKD patients at stages 3 to 5 who were undergoing conservative treatment. These patients were recruited from the PREVENRIM, a CKD prevention clinic at the Interdisciplinary Nucleus of Studies, Research and Treatment in Nephrology (NIEPEN) of the Universidade Federal de Juiz de Fora, Brazil (UFJF). The second group was a control group that consisted of patients with no systemic disease from the Periodontology Clinic of the School of Dentistry at UFJF. All patients presented moderate to severe CP, which compromised at least two teeth with pocket probing depth (PPD) sites ≥5 mm, at least 1 site with a clinical attachment level (CAL) ≥6 mm, and radiographic evidence of alveolar bone loss.15
The glomerular filtration rate (GFR) was estimated from serum creatinine using an equation from the study “Modification of Diet in Renal Disease” (MDRD).16 The diagnosis and stage of CKD were determined according to the criteria of the United States National Kidney Foundation.2 Patients with two documented diagnoses of proteinuria and/or glomerular hematuria and a GFR <60 mL/min/1.73 m2, measured at least 3 months apart, were diagnosed with CKD.2 This study included patients over 18 years of age, with a minimum of 20 natural teeth and without periapical lesions, who had received no periodontal, antimicrobial, or anti-inflammatory treatment within the last 6 months and who had not used steroids or immunosuppressant drugs. The exclusion criteria were pregnancy or breast-feeding in women and smoking or past history of smoking in people who had quit smoking within the last 10 years.11
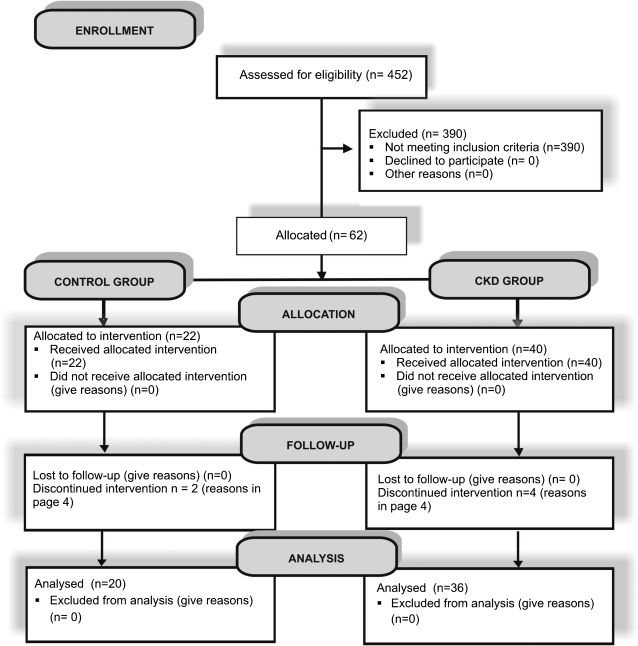
Of a total of 329 patients with CKD, 40 were eligible for the study. Four were excluded due to the need for dialysis treatment (2 patients) or hospitalization (2 patients). In the control group, 22 patients were selected from a total of 123 individuals; however, 2 were excluded: 1 patient became pregnant, and the other had an acute upper respiratory tract infection. Therefore, 36 patients from the CKD group and 20 from the control group completed the study (Figure 1). The groups were selected over a period of 6 months to avoid differences in the results due to variations in sample storage time. After being informed about the study, all participants signed a written consent form approved by the Ethics Committee on Research on Human Beings of the UFJF (#942.248.2006/report # 327/2006). The sample size was defined based on data from an intervention study on CP.11
Figure 1.
Flow diagram of study participants.
Initially, the patients underwent a complete medical examination. Next, a periodontal examination and complementary periapical radiography were performed. The evaluation included the number of teeth, plaque index (PI), gingival index (GI), assessment of bleeding on probing (BOP), PPD, sites with PPD ≥5 mm (PPD≥5), and CAL. The periodontal exam was performed on six different sites (mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual) around each tooth. A millimetered probe was used to obtain these data. All exams were repeated 3 months after the PT.
Both groups received instructions on oral hygiene techniques, including the manual techniques of tooth and interproximal brushing and how to use dental floss and perform supragingival prophylaxis. The nonsurgical periodontal therapy consisted of radicular scraping and subgingival curettage, which used standard instrumentation with Gracey curettes and ultrasound devices and was performed in 1-hour sessions over an average period of 4 weeks. Local anesthesia was used when necessary. Upon concluding the periodontal treatment, participants were followed up after 15, 30, 60, and 90 days. At each return visit, instructions on oral hygiene and supragingival prophylaxis were provided.
Blood samples were collected for biochemical analysis at baseline and 3 months after PT. Venous blood was collected in vacuum tubes between 7:00 am and 9:00 am after 12 hours of fasting. One tube that contained EDTA was analyzed for the following blood parameters: complete hemogram (automated Coulter STKS), serum iron (ferrozine), ferritin (electrochemiluminescence), and transferrin saturation index (Labtest ferrozine). Plasma samples with EDTA/heparin and serum samples were immediately placed in ice, aliquoted within 1 hour, and stored at -80°C until use. Nephelometry was used for the dosage of plasma levels of ultrasensitive CRP (us-CRP). The concentration of IL-6 was evaluated in the plasma using the Human IL-6 ELISA Kit II BD OptEIA (BD Biosciences, CA, USA). The serum level of prohepcidin was evaluated using DRG Hepcidin Prohormone ELISA Kit (DRG International, New Jersey, USA). Reactions were read using a microplate reader (SpectraMax 190, Molecular Devices). The manufacturers' instructions were followed for all kits used in this study.
For statistical analyses, Kolmogorov-Smirnov tests were first performed to evaluate the normality of the sample distribution. In the demographic data, the numerical variables were described using the mean and standard deviation, while the categorical variables were described as percentages. In the comparisons between the CKD and control groups in the first period, Student's t-test for independent samples or the Mann-Whitney nonparametric test was used. For comparisons before and after PT, a paired t-test or Wilcoxon test was used when an abnormal distribution was identified. Correlations among the inflammatory markers and their relationships with the periodontal clinical parameters were analyzed using either the Pearson correlation coefficient for variables with a normal distribution or the Spearman correlation coefficient. A multivariate linear regression analysis was performed using prohepcidin after PT as a dependent variable and the difference of the values (delta = after - before PT) of the independent variables IL-6, us-CRP, estimated glomerular filtration rate (eGFR), CAL, PPD, ferritin, and transferrin saturation (TSat). All results were considered statistically significant at p<0.05. The analyses were performed using the SPSS v. 13.0 computer program.
RESULTS
Patients in the study groups had homogeneous demographic characteristics, and PT was the only variable in both groups. The main cause of CKD was hypertensive nephrosclerosis (30.6%). The comorbidities most frequently found in the CKD group were arterial hypertension (97.2%) and diabetes mellitus (27.8%). It is important to emphasize that no patient used statins or iron replacement therapy during the study. The study was conducted from August 2008 to March 2010 and was finished after the follow-up of the participants was complete.
At baseline, CP was more severe in patients with CKD than in the control group, as documented by significantly higher levels of IL-6 (p = 0.04) and us-CRP (p = 0.03) (Table 1), as well as patients with CKD who had more sites with PPD≥5 mm (p = 0.03) and CAL (p = 0.003) (Table 2). The efficacy of PT was indicated by the significant decreases in the levels of inflammatory markers and the improvement in clinical parameters of CP observed 3 months after completion of PT (Table 2).
Table 1.
Characterization of the study population at baseline.
| Chronic kidney disease (n = 36) | Control (n = 20) | p value | |
| Clinical and demographic characteristics | |||
| Age, mean±SD | 53.17±12.00 | 43.4±0 11.00 | 0.06 |
| Male gender, n (%) | 23 (63) | 9 (45) | 0.17 |
| White race, n (%) | 23 (63.9) | 13 (65) | 0.97 |
| Black race, n (%) | 7(19.4) | 4 (20) | 0.98 |
| Diabetes mellitus, n (%) | 10 (27) | --- | --- |
| BMI (kg/m2), mean±SD | 26.53±4.95 | 26.88±5.10 | 0.8 |
| BP systolic (mm Hg), mean±SD | 143.63±17.80 | 131.16±18.20 | 0.01* |
| BP diastolic (mm Hg), mean±SD | 86.11±9.87 | 81.0±11.34 | 0.08 |
| Serum albumin (g/dL), mean±SD | 4.27±0.34 | 4.37±0.22 | 0.25 |
| Serum creatinine (mg/dL), mean±SD | 2.39±1.11 | 0.77±0.21 | 0.001* |
| eGFR (mL/min/1.73 m2), mean±SD | 34.27±16.02 | 107.60±26.73 | 0.001* |
| CKD stage | |||
| 3 n (%) | 21 (58.30) | --- | --- |
| 4 n (%) | 12 (33.30) | --- | --- |
| 5 n (%) | 3 (8.30) | --- | --- |
| Biochemical characteristics | |||
| Prohepcidin (ng/mL) mean±SD | 166.24±55.70 | 147.39±51.50 | 0.22 |
| IL-6 (pg/mL) mean±SD | 4.04±2.40 | 2.95±2.20 | 0.04* |
| us-CRP (mg/L) mean±SD | 6.18±5.39 | 3.04±3.82 | 0.03* |
| Hemoglobin (g/dL) mean±SD | 12.72±1.96 | 13.30±1.89 | 0.29 |
| Ferritin (ng/dL) mean±SD | 180.60±129.0 | 184.0±154.0 | 0.93 |
| TSat (%) | 29.30 | 33.90 | 0.01* |
*p<0.05.
SD = standard deviation; n = number; BMI = body mass index; BP = blood pressure; eGFR = estimated glomerular filtration rate; CKD = chronic kidney disease; IL-6 = interleukin-6; us-CRP = ultrasensitive C-reactive protein; TSat = transferrin saturation.
Table 2.
Periodontal parameters at baseline and 3 months after periodontal treatment.
| Parameters | CKD | Control | ||
| Before PT | After PT | Before PT | After PT | |
| Number of teeth, mean±SD | 22.97±5.20 | 22.33±5.30 | 23.8±5.6 | 23.15±5.40 |
| BOP % | 24.08 | 5.97* | 31.25 | 5.05* |
| PPD (mm), mean±SD | 2.90±1.13 | 1.99±0.82* | 2.52±0.41 | 1.98±0.40* |
| CAL (mm), mean±SD | 2.92±0.92** | 2.20± 0.65* | 2.37±0.40 | 2.02±0.43* |
| GI score1, mean±SD | 1.36±0.81 | 0.31±0.47* | 1.40±0.59 | 0.41±0.33* |
| PI score2, mean±SD | 1.18±0.69 | 0.36±0.46* | 0.99±0.74 | 0.20±0.14* |
| Sites with PPD ≥5 mm, n | 19.23** | 4.35* | 9.61 | 3.72* |
*p<0.05 (before and after treatment in the same group).
**p<0.05 (comparison between the groups before treatment).
PT = periodontal treatment; BOP = bleeding on probing; PPD = probing pocket depth; CAL = clinical attachment level; GI Score1 = clinically quantifies gingival inflammation: 0 = absence, 1 = little, 2 = moderate, 3 = severe; PI Score2 = quantifies the presence of bacterial plaque on the tooth: 0 = absence, 1 = little, 2 = moderate, 3 = severe; PPD ≥ 5 mm = probing pocket depth ≥ 5 mm; SD = standard deviation; n = number.
Prohepcidin, IL-6, and us-CRP levels decreased significantly after PT in both groups. In the control group, in addition to the decrease in the inflammatory markers, a significant increase was also observed in the levels of hemoglobin and ferritin associated with PT (Table 3).
Table 3.
Parameters of inflammatory and biochemical markers before and 3 months after periodontal treatment.
| CKD mean±SD | Control mean±SD | |||
| Before PT | After PT | Before PT | After PT | |
| Prohepcidin (ng/mL) | 166.24±55.70 | 153.29± 56.90* | 147.39±51.50 | 131.72± 47.10** |
| IL-6 (pg/mL) | 4.04±2.40 | 3.28±2.10** | 2.95±2.20 | 2.09±1.40* |
| us-CRP (mg/L) | 6.18±5.30 | 4.08±3** | 3.04±3.80 | 2.20±2.30* |
| Serum Fe (µg/dL) | 81.60±25.50 | 91.10±24.90 | 99±24.20 | 100.90±18.30 |
| TSat (%) | 29.30 | 30.40 | 33.90 | 30.90* |
| Ferritin (ng/dL) | 180.60±129 | 171.70±125 | 184±154 | 222±205* |
| Hemoglobin (g/dL) | 12.70±5.30 | 12.20±6.20 | 13.30±7.90 | 14.10±6.80* |
*p<0.05, **p<0.01.
CKD = chronic kidney disease; PT = periodontal treatment; IL-6 = interleukin 6; us-CRP = ultrasensitive C-reactive protein; Fe = iron; TSat = transferrin saturation.
In the Pearson correlation, a significant association could be seen between serum prohepcidin after PT and the delta of serum us-CRP in the CKD group, as well as between serum prohepcidin after PT and the deltas of serum IL-6, serum us-CRP, ferritin, and TSat in the control group (Table 4).
Table 4.
Pearson correlations between prohepcidin after periodontal treatment and the deltas of the independent variables.
| IL-6 | us-CRP | eGFR | CAL | PPD≥5 | Ferritin | Transferrin saturation | |
| Prohepcidin CKD | -0.08 | 0.301* | 0,109 | -0.119 | -0.139 | -0.049 | 0.160 |
| Prohepcidin Control | -0.471* | -0.411* | 0,116 | 0.05 | -0.332 | 0.425* | 0.544** |
p<0.05; **p<0.01.
CKD = chronic kidney disease; IL-6 = interleukin 6; us-CRP = ultrasensitive C-reactive protein; eGFR = estimated glomerular filtration rate; CAL = clinical attachment level; PPD ≥ 5 mm = probing pocket depth ≥ 5 mm.
We constructed a multivariate linear regression model, in which the dependent variable was serum prohepcidin after PT and the independent variables were the deltas of IL-6, us-CRP, eGFR, PPD, CAL, ferritin, and TSat. None of the independent variables was significantly and independently associated with the dependent variable in patients with CKD, whereas in the control group, only IL-6 (95% CI -45.40 to -4.49; p = 0.02) was significantly and independently associated with serum prohepcidin.
DISCUSSION
This work evaluated the impact of PT on the systemic inflammatory response and determined, for the first time, a causal association between CP activity and high serum levels of prohepcidin.
Inflammation plays a key role in the pathogenesis of arteriosclerosis, and chronic systemic inflammation has been associated with undesired cardiovascular outcomes in patients with CKD.17 Nevertheless, the nature and the source of inflammation are not always identified. CP is a chronic infectious disease caused by Gram-negative bacteria that determine the systemic inflammatory response.18 Local tissue destruction favors the systemic dissemination of periodontal pathogens and their products (for example, lipopolysaccharides) and locally produced inflammatory mediators, such as interleukin-1, IL-6, tumor necrosis factor-α, and prostaglandin E2, among others.9 It has been documented that CP induces an acute-phase inflammatory response that can be measured by the serum level of CRP.19 We noticed that CP was more severe in patients with CKD not yet on dialysis than it was in patients without systemic disease. Moreover, 3 months after PT, we observed a significant reduction in the CAL and the PPD clinical indices, both of which are markers of the severity of CP, thus confirming the success of the treatment.
Concomitant with the clinical improvement in CP, a reduction was observed in the serum levels of IL-6 and us-CRP, both of which are markers of the systemic inflammatory response, in agreement with the results of other publications.10,13,20,21 Considering that chronic inflammation is a risk factor for atherosclerotic cardiovascular disease in patients with hypertension and diabetes mellitus, the main causes of CKD, it is plausible that the immediate diagnosis of CP followed by PT should constitute an important preventive measure in the course of CKD in the everyday clinic.
Prohepcidin is a prohormonal form of hepcidin, a peptide originally discovered in studies that attempted to identify cationic, antimicrobial peptides in human blood5 and urine.6 The increase in the production of hepcidin in response to inflammation and the decrease in the availability of iron to microorganisms appear to be parts of the host defense mechanism against infection.8 In this study, it was observed for the first time that successful PT results in a decrease in the serum levels of prohepcidin in patients with CP with CKD (CKD group) or without CKD (control group). Due to its size, hepcidin passes through the glomerular membrane, and increased levels of prohepcidin have been associated with GFR in patients with renal diseases.22-24 For instance, an inverse relationship between prohepcidin and GFR was documented in renal transplant patients23 and in patients treated with hemodialysis.24 However, the decreased levels of serum prohepcidin observed in control patients (with CP and normal GFR) seen 3 months after PT suggests a causal association between CP and serum levels of prohepcidin. Moreover, no correlation between the GFR and prohepcidin levels was observed in either of the groups studied.
The synthesis of hepcidin increases during chronic infections. The regulation of prohepcidin and its active form hepcidin is thought to stem from a complex network of stimuli.25 IL-6 is a proinflammatory cytokine that increases during inflammation and is considered an important stimulus for hepatic synthesis of hepcidin and other proteins of the acute phase, such as CRP.3 In fact, in the multivariate linear regression model, IL-6 was the only independent variable that was associated significantly and independently with the dependent variable serum prohepcidin level after PT in the control group.
Anemia is one of the main complications of CKD and results mainly from deficiency in the renal production of EPO.1 Although the majority of patients respond to treatment with erythropoiesis-stimulating agents (ESA), up to 20% of patients have an inadequate therapeutic response.2 The main causes of resistance to treatment with ESA are inflammation and iron deficiency.26 In our patients, although CP was associated with high levels of prohepcidin, us-CRP, and IL-6, the iron parameters at baseline were within the recommended range. It is important to emphasize, however, that the present study was not designed to assess the impact of CP and its treatment on iron reserves and/or erythropoietic responses to ESA. Nonetheless, it is interesting to observe that the patients from the control group, who had CP but no other systemic disorders, showed an increase after PT of almost 1.0 g/dL in hemoglobin levels and an increase in serum ferritin levels. These findings suggest that CP, upon inducing a systemic inflammatory response, including high levels of hepcidin, reduces the availability of iron for erythropoiesis, which is then reversed by PT.
One limitation of this study is that it was not designed for individuals with anemia and therefore could not provide data on the impact of PT on anemia in CKD. In addition, we used an ELISA for prohepcidin, a precursor that gives rise not only to hepcidin-25, but also to hepcidin-20 and hepcidin-22; consequently, it might not precisely reflect hepcidin activity.
In conclusion, this is the first report of an association between CP and serum levels of prohepcidin, the prohormone of hepcidin, in patients with CKD and individuals without systemic diseases. Our findings suggest that CP is more severe in patients with CKD and that it induces a systemic inflammatory response. Successful PT reduces the inflammatory burden and decreases the serum levels of prohepcidin, indicating that it may constitute a therapeutically important intervention during the course of CKD.
ACKNOWLEDGEMENTS
This study was supported by the Fundaç�o Instituto Mineiro de estudos e Pesquisas em Nefrologia (IMEPEN).
REFERENCES
- 1.Abensur H, Bastos MG, Canziani MEF. Aspectos atuais da anemia na doença renal crônica. J Bras Nefrol. 2006;28:104–107. [Google Scholar]
- 2.K/DOQI. National Kidney Foundation. Definition and classification of stages of chronic kidney disease. Am J Kidney Dis. 2002;39:S46–S75. [Google Scholar]
- 3.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 4.Malyszko J, Malyszko JS, Hryszko T, Pawlak K, Mysliwiec M. Is hepcidin a link between anemia, inflammation and liver function in hemodialysed patients. Am J Nephrol. 2005;25:586–90. doi: 10.1159/000089266. [DOI] [PubMed] [Google Scholar]
- 5.Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–50. doi: 10.1016/s0014-5793(00)01920-7. 10.1016/S0014-5793(00)01920-7 [DOI] [PubMed] [Google Scholar]
- 6.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18:394–400. doi: 10.1681/ASN.2006070802. 10.1681/ASN.2006070802 [DOI] [PubMed] [Google Scholar]
- 9.Kshirsagar AV, Offenbacher S, Moss KL, Barros SP, Beck JD. Antibodies to periodontal organisms are associated with decreased kidney function. The dental atherosclerosis risk in communities study. Blood Purif. 2007;25:125–32. doi: 10.1159/000096411. 10.1159/000096411 [DOI] [PubMed] [Google Scholar]
- 10.D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein- associated cardiovascular risk. J Periodontal Res. 2004;39:236–41. doi: 10.1111/j.1600-0765.2004.00731.x. 10.1111/j.1600-0765.2004.00731.x [DOI] [PubMed] [Google Scholar]
- 11.Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol. 2003;30:334–40. doi: 10.1034/j.1600-051x.2003.00282.x. 10.1034/j.1600-051X.2003.00282.x [DOI] [PubMed] [Google Scholar]
- 12.Craig RG, Kotanko P, Kamer AR, Levin NW. Periodontal diseases: a modifiable source of systemic inflammation for the end-stage renal disease patient on haemodialysis therapy. Nephrol Dial Transplant. 2007;22:312–5. doi: 10.1093/ndt/gfl604. 10.1093/ndt/gfl604 [DOI] [PubMed] [Google Scholar]
- 13.Marcaccini AM, Meschiari CA, Sorgi CA, Saraiva MCP, Souza AM, Faccioli LH, et al. Circulation interleukin-6 and high-sensitivity C-reactive protein decrease after periodontal therapy in otherwise healthy subjects. J Periodontol. 2009;80:594–602. doi: 10.1902/jop.2009.080561. 10.1902/jop.2009.080561 [DOI] [PubMed] [Google Scholar]
- 14.Fisher MA, Taylor GW. A prediction model for chronic kidney disease includes periodontal disease. J Periodontol. 2009;80:16–23. doi: 10.1902/jop.2009.080226. 10.1902/jop.2009.080226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machtei EE, Christersson LA, Grossi SG, Dunford R, Zambon JJ, Genco RJ. Clinical criteria for the definition of established periodontitis. J Periodontol. 1992;63:206–14. doi: 10.1902/jop.1992.63.3.206. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Libby P, Ridker PM, Maseri A. Inflamation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 18.Pihlstrom Bl, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. 10.1016/S0140-6736(05)67728-8 [DOI] [PubMed] [Google Scholar]
- 19.Paraskevas S, Huizinga JD, Loss BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–9. doi: 10.1111/j.1600-051X.2007.01173.x. 10.1111/j.1600-051X.2007.01173.x [DOI] [PubMed] [Google Scholar]
- 20.Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51:446–53. doi: 10.1161/HYPERTENSIONAHA.107.101535. 10.1161/HYPERTENSIONAHA.107.101535 [DOI] [PubMed] [Google Scholar]
- 21.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endotelial function. N Eng J Med. 2007;356:911–20. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 22.Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, et al. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–6. doi: 10.2215/CJN.05931108. 10.2215/CJN.05931108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malyszko J, Malyszko JS, Mysliwiec M. A possible role of hepcidin in the pathogenesis of anemia among kidney allograft recipients. Transplant Proc. 2009;41:3056–9. doi: 10.1016/j.transproceed.2009.08.003. 10.1016/j.transproceed.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 24.Kato A, Tsuji T, Luo J, Sakao Y, Yasuda H, Hishida A. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol. 2008;28:115–21. doi: 10.1159/000109968. [DOI] [PubMed] [Google Scholar]
- 25.Young B, Zaritsky J. Hepcidin for clinicians. Clin J Am Soc Nephrol. 2009;4:1384–7. doi: 10.2215/CJN.02190309. 10.2215/CJN.02190309 [DOI] [PubMed] [Google Scholar]
- 26.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–81. doi: 10.1038/ki.2009.21. 10.1038/ki.2009.21 [DOI] [PubMed] [Google Scholar]