Abstract
Epithelial injury is a central event in the pathogenesis of many inflammatory and fibrotic lung diseases like acute respiratory distress syndrome, pulmonary fibrosis, and iatrogenic lung injury. Mechanical stress is an often underappreciated contributor to lung epithelial injury. Following injury, differentiated epithelia can assume a myofibroblast phenotype in a process termed epithelial to mesenchymal transition (EMT), which contributes to aberrant wound healing and fibrosis. We demonstrate that cyclic mechanical stretch induces EMT in alveolar type II epithelial cells, associated with increased expression of low molecular mass hyaluronan (sHA). We show that sHA is sufficient for induction of EMT in statically cultured alveolar type II epithelial cells and necessary for EMT during cell stretch. Furthermore, stretch-induced EMT requires the innate immune adaptor molecule MyD88. We examined the Wnt/β-catenin pathway, which is known to mediate EMT. The Wnt target gene Wnt-inducible signaling protein 1 (wisp-1) is significantly up-regulated in stretched cells in hyaluronan- and MyD88-dependent fashion, and blockade of WISP-1 prevents EMT in stretched cells. In conclusion, we show for the first time that innate immunity transduces mechanical stress responses through the matrix component hyaluronan, and activation of the Wnt/β-catenin pathway.
Keywords: Epithelial Cell, Hyaluronate, Innate Immunity, MyD88, Wnt Pathway, CD44, Cell Stretch, Epithelial-Mesenchymal Transition, Wound Healing
Introduction
Epithelial injury is now recognized as a primary event in the pathogenesis of lung disease (1). Alveolar cells consist of type I epithelia (AT1),2 which serve gas exchange, and type II epithelia (AT2), which produce surfactant and are now recognized as the critical responders to lung injury. Although AT1 cells usually undergo cell death after critical injury, AT2 cells can present a variety of responses, including proinflammatory response, proliferation, differentiation to AT1 epithelia, or epithelial-to-mesenchymal transition (EMT). The AT2 response to injury may ultimately lead to restoration of pulmonary architecture and function, or to dysregulated repair, fibrosis, and respiratory failure (2). The factors that promote regulated or pathological repair are incompletely understood. However, it is often forgotten that alveolar epithelia are not static cells. Alveolar epithelia undergo continuous cyclic stretch during ventilation; moreover, pathologically high levels of stretch are exerted on alveolar epithelia either as a consequence of injury (anatomical distortion from scarring) or iatrogenically (mechanical ventilation). The response of alveolar epithelia to injury must therefore be seen in the context of mechanical strain. The importance of stretch injury as a cause of lung disease is exemplified in the acute lung injury of critically ill patients, which is estimated at 200,000 cases in the United States annually (3). Mechanical ventilation is crucial for survival of these patients; however, it often leads to ventilator-induced lung injury (VILI) with pulmonary edema, inflammation, and fibrosis, leading to respiratory failure and death.
Epithelial injury is central to the pathogenesis of VILI. Experimental VILI increases epithelial expression of inflammatory mediators TNF-α and IL-6 (4). Furthermore, excessive epithelial cell stretch is a major cause of VILI (5). Lung ventilation volume increases lead to progressive epithelial cell deformation (6), with disruption of the alveolar-capillary barrier and injury to both AT1 and AT2 epithelia. AT2 cells are of particular importance in VILI. AT2 cells constitute the majority of alveolar epithelial cells (7) and are capable of self-renewal, but can also undergo EMT when injured. EMT is indicated by a decrease in epithelial cell markers, such as E-cadherin, and an increase in mesenchymal cell markers such as vimentin and α-smooth muscle actin (α-SMA). EMT is found in healthy tissue during embryogenesis and is observed in wound healing, but it has also been recognized as a source for myofibroblasts in fibrosis and occurs in fibrotic lung in bleomycin-treated mice (8). Additionally, alveolar EMT was shown in idiopathic pulmonary fibrosis (9). EMT is therefore an important part of the aberrant wound healing response and can lead to lung fibrosis and respiratory failure.
Components of the extracellular matrix can act as signaling molecules that contribute to EMT (10, 11). The extracellular matrix glycosaminoglycan hyaluronan (HA) is of particular interest in VILI because HA production is increased in VILI, leading to an inflammatory response (12). HA also plays a central role in EMT in embryonic development, certain types of cancer cells (13), as well as in mammary and kidney epithelial cells (14). These studies suggest that HA may be an important molecule in the mechanical signaling of alveolar cells during ventilation. We therefore hypothesized that mechanical stretch induces EMT via hyaluronan production in alveolar epithelium. We examined our hypothesis using an in vitro stretch injury model of AT2 cells.
EXPERIMENTAL PROCEDURES
Primary Cell Isolation and Cell Stretch
Primary murine AT2 cells were isolated from 6–8-week-old male C57BL/6 mice from Charles River Laboratories, cd44−/− mice backcrossed on a C57BL/6 background for >10 generations (generously provided by T. Mak (15)), or myd88-deficient mice (generously provided by S. Akira (16)) following the methods published by Corti et al. (17). AT2 cells were plated on Flexcell Amino silicone-bottomed plates coated with 0.6 mg/ml collagen I solution (Sigma-Aldrich). AT2 were cultured overnight in bronchial epithelial growth medium (Lonza) with all supplements except hydrocortisone and the addition of 1% anti-biotic anti-mycotic (Invitrogen), 5% fetal bovine serum (FBS; Invitrogen), and keratinocyte growth factor (Sigma-Aldrich). The isolated AT2 population was 92.9 ± 3.2% pure as determined by Pap staining performed as previously published (17) (supplemental Fig. 1A). Static culture of AT2 retained prosurfactant protein C staining throughout the 4-day culture period (supplemental Fig. 1B). AT2 cells were stretched in a Flexcell Tension Plus system (Flexcell International Corporation, Hillsborough, NC) at 15% stretch, 0.86 Hz for 4 consecutive days in bronchial epithelial growth medium supplemented with 0.1% FBS. TGF-β1 (10 ng/ml; R&D Systems, Minneapolis, MN) was added to static cells as a positive control. Wnt-inducible signaling protein 1 (WISP-1) monoclonal anti-mouse antibody (R&D Systems) and isotype rat IgG controls (R&D Systems) were added to stretched culture in some experiments at 20 μg/ml. This WISP-1 antibody has been shown previously to block WISP-1 (8). In other experiments, TGF-β1 blocking antibody (MAB240, R&D Systems) or mouse isotype IgG control was added at a final concentration of 2 μg/ml. Cells were cultured for 4 days statically as controls.
HA Dosing, Blockade, Size Measurement, and Concentration Measurement
In HA-treated statically cultured experiments, Healon (AMO, Santa Ana, CA) was sonicated as described previously (18). Size of sonicated Healon is found in supplemental Fig. 2. Amine group-modified tissue culture plates (BD Primeria, Becton Dickinson) were coated with collagen type I (Sigma-Aldrich) or the sonicated sHA at 0.2 mg/ml overnight at 4 °C prior to cell plating. Additionally, short fragment hyaluronan (sHA) was added to culture medium at the same concentration. AT2 cells were cultured for 4 days with a one-time dose of sHA. In these statically cultured experiments, “collagen” refers to the control cells grown statically on collagen, “collagen + TGF-β1” refers to cells grown on collagen with a one-time TGF-β1 treatment, and “HA” refers to cells grown on sHA-coated plates with a one-time dose of additional sHA. In some experiments, HA-binding peptide (pep-1), scrambled peptide, and dimethyl sulfoxide vehicle control were added to the stretched and static AT2 culture according to methods published previously (19). Pep-1 and scrambled peptide were custom designed (Genscript, Piscataway, NJ). In other experiments, TGF-β1 blocking antibody (MAB240) or mouse isotype IgG control was added at a final concentration of 2 μg/ml. Supernatant was collected from stretched and static AT2 and was used for HA size measurements according to the method published by Aytekin et al. (20). For HA concentration measurement, a commercially available ELISA as used according to the manufacturer's instructions (Echelon, Salt Lake City, UT).
RNA Isolation and Real Time Reverse Transcription-PCR
RNA was isolated using the RNeasy Plus Mini-Kit (Qiagen, Valencia, CA). Purified RNA was treated with DNase and real time reverse transcription-PCR was performed using SYBR Green Master Mix (Applied Biosystems, Foster City, CA) and primers from Integrated DNA Technologies (IDT, Coralville, IA). Primer sequences are shown in supplemental Table 1. Relative gene expression was determined using the ΔΔCt method using the housekeeping gene 18s.
Protein Isolation and Western Blotting
Primary antibodies used for Western blotting were rabbit anti mouse α-SMA (Abcam, Cambridge, MA), rabbit anti-mouse E-cadherin (Cell Signaling, Danvers, MA), and goat anti-mouse vimentin (Chemicon, Billerica, MA). Loading control was rabbit S6 ribosomal protein (Cell Signaling). Immunoprecipitation for CD44 was performed as shown previously (21). Secondary antibodies were anti-goat HRP (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-rabbit HRP (Pierce). HRP was detected using ECL west detection kit (Pierce). Blots were exposed to autoradiography film and processed using a Kodak film developer.
Immunofluorescence
Cells were fixed in ice-cold methanol for HA staining and in 4% paraformaldehyde for F-actin staining. HA staining was done with biotinylated HA-binding protein (Seikagaku, Tokyo, Japan) and streptavidin-conjugated Alexa Fluor 488 (Molecular Probes). F-actin staining was done with Alexa Fluor 488-conjugated phalloidin (Molecular Probes). Images were taken using a confocal scanning microscope at 400× magnification.
Statistical Analysis
Data are presented as means ± S.E. Statistical analysis was performed using Prism GraphPad software. One-way ANOVAs were performed with the Tukey test for post hoc pairwise comparisons. p < 0.05 was considered significant.
RESULTS
Stretch Induces EMT
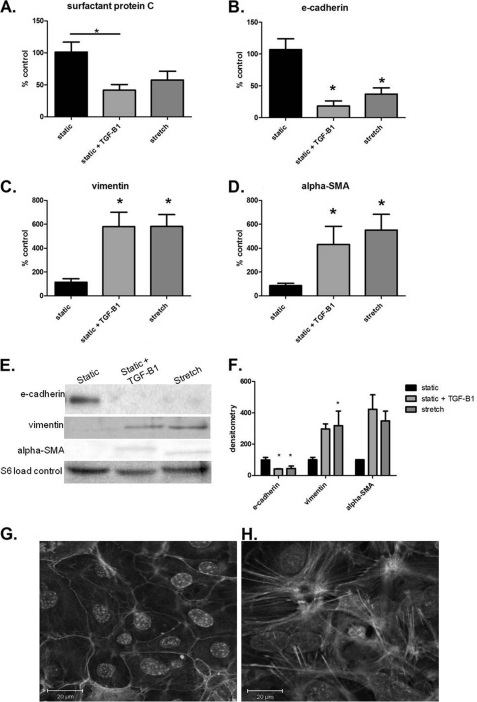
We first examined whether mechanical stretch induces EMT. We evaluated gene expression of surfactant protein C as an AT2 cell marker and established that it was significantly decreased with TGF-β1, but remained statistically unchanged with stretch (Fig. 1A). However, in vitro mechanical stretch of AT2 cells caused significant reduction in gene expression of the epithelial cell marker E-cadherin (Fig. 1B). Furthermore, stretch increased gene expression in mesenchymal genes vimentin and α-SMA (Fig. 1, C and D). As a positive control, TGF-β1 significantly decreased E-cadherin expression and increased a-SMA expression (Fig. 1, B and D). Our stretch-induced gene expression results were confirmed on a protein level by Western blotting (Fig. 1, E and F). F-actin staining showed actin in the cell membrane of statically cultured AT2 (Fig. 1G) whereas actin was polymerized throughout the stretched cells (Fig. 1H). Additionally, cell morphology was altered in the experimental groups compared with static controls. Statically cultured AT2 retained a cobblestone appearance as seen in both F-actin staining (Fig. 1G) and prosurfactant protein C staining (supplemental Fig. 1B), whereas stretch alone caused increased cell spreading and slight spindle-shaped morphology (Fig. 1H and supplemental Fig. 1B). Thus, these results suggest that cell stretch induced EMT in cultured primary AT2 cells.
FIGURE 1.
Stretch ± TGF-β1 induces EMT in AT2 cells. A, TGF-β1 significantly increased surfactant protein C gene expression. B, E-cadherin gene expression is significantly down-regulated in AT2 cells cultured with TGF-β1 or stretched AT2 cells, compared with static controls. C, vimentin gene expression is significantly increased in static + TGF-β1 and stretched AT2 cells compared with static cells. D, α-SMA gene expression is significantly increased in AT2 cells cultured with TGF-β1 or stretched AT2 cells compared with static controls. E, representative immunoblot from three independent experiments is shown. F, immunoblots were quantified by densitometry. E-cadherin protein is significantly diminished in all test groups compared with static. Vimentin in stretched cells is significantly increased compared with static. G, statically cultured cells were stained with F-actin at 4 days. H, stretched cells were stained with F-actin at 4 days. Data are normalized per loading controls and are representative of means ± S.E. (error bars), n = 3–6 per group.*, p < 0.05 ANOVA with Tukey's post hoc test.
Stretch Promotes HA Production
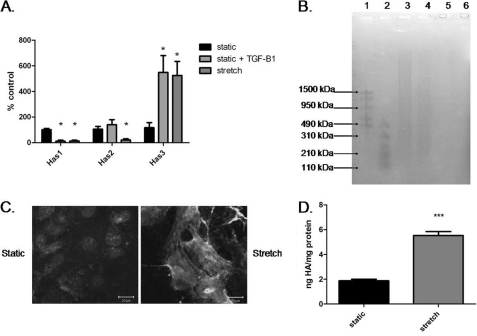
Because mechanical ventilation increases HA synthase 3 expression in a mouse model (12), we examined expression of HA synthase (has) genes 1, 2, and 3 in our in vitro cell stretch injury model. Mechanical stretch significantly increased has3 expression and significantly reduced has1 and has2 expression compared with static control (Fig. 2A). We also determined the size of HA in the supernatant of static and stretched cells with agarose gel electrophoresis. The supernatant of both groups contained HA as indicated by negative staining in hyaluronidase-treated samples (Fig. 2B). Static cultured supernatant contained HA of varying sizes including a larger amount of high molecular mass HA than supernatant from stretched cells. The stretched cell supernatant contained mostly low molecular mass HA (Fig. 2B). Hyaluronan staining of the cells showed that stretch increased HA compared with static control (Fig. 2C). Quantification of HA in supernatant revealed significantly more HA in the stretched cells compared with static (Fig. 2D).
FIGURE 2.
Stretch increased production of low molecular mass HA. A, quantitative RT-PCR of HA synthase genes (has) 1–3. has1 gene expression is significantly decreased with TGF-β1 or stretch compared with static culture. has2 gene expression is significantly decreased in the stretched AT2 cells compared with all other groups. has3 gene expression is significantly increased in static + TGF-β1 and stretched AT2 compared with static treated AT2 cells. B, agarose gel electrophoresis of AT2 supernatant. Lane 1, high molecular mass HA ladder; lane 2, low molecular mass HA ladder; lane 3, static culture supernatant; lane 4, stretch supernatant; lane 5, static supernatant with hyaluronidase treatment; lane 6, stretch supernatant with hyaluronidase treatment. C, immunohistochemistry for HA of statically cultured AT2 (left) and stretched AT2 (right). Nuclei are stained with DAPI. Scale bar, 20 μm. D, ELISA for HA concentration (normalized to protein concentrations) showing statistically significant higher HA in the stretched supernatant. Data are represented as means ± S.E. (error bars), n = 3/group. ***, p < 0.001 ANOVA with Tukey's post hoc test.
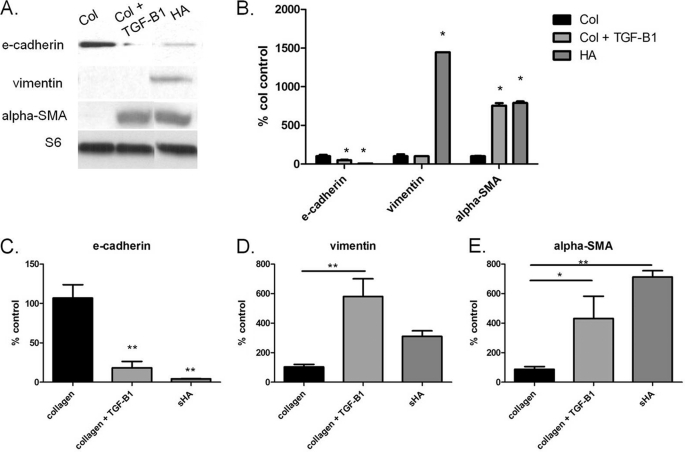
sHA Induces EMT in AT2 Cells in Static Culture and Is Necessary for Stretch-induced EMT
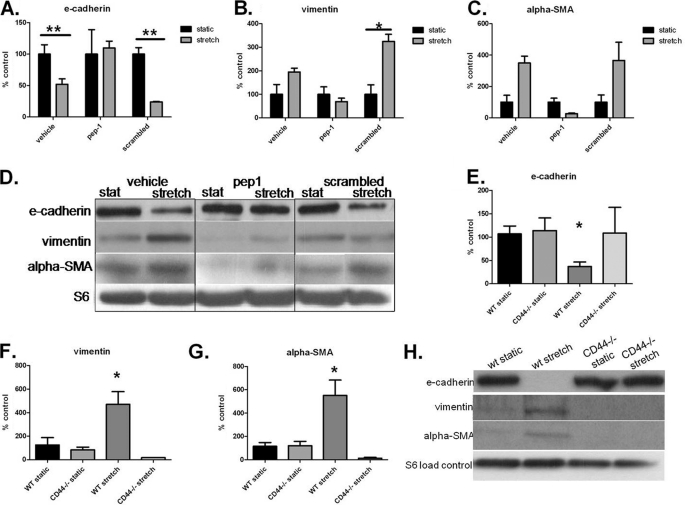
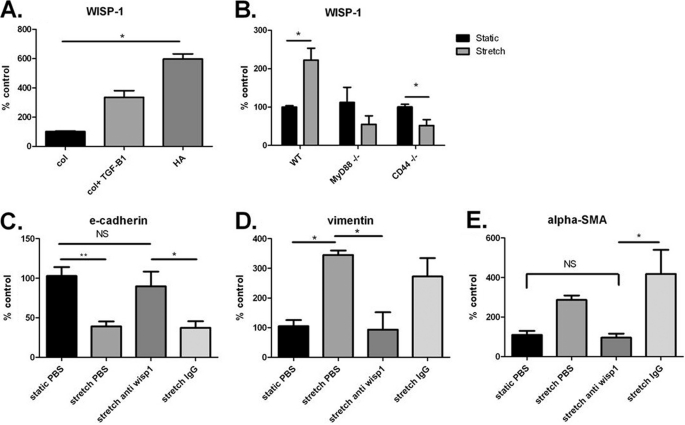
Because we found a significant increase of sHA in our stretched cells, we pursued the question of whether sHA is important in stretch-induced EMT. We added sHA to a static culture of AT2 cells and examined the same epithelial and mesenchymal markers as in our stretch experiments. We found that sHA induced EMT as indicated both by mRNA and protein expression of relevant markers (Fig. 3). We then used the HA antagonist pep-1 during AT2 stretch and examined EMT. Pep-1 diminished the stretch-induced changes in EMT markers shown in RT-PCR (Fig. 4, A–C) and by Western blotting (Fig. 4D). To confirm further that stretch causes EMT via HA, we stretched primary AT2 cells from mice deficient for the HA receptor CD44. We found that CD44-deficient stretched AT2 cells did not undergo EMT. CD44-deficient stretched AT2 cells did not lose E-cadherin expression in protein (Fig. 4E) or mRNA expression (Fig. 4F) and did not gain mesenchymal markers α-SMA and vimentin in either mRNA expression (Fig. 4, E and F) or protein (Fig. 4G).
FIGURE 3.
Addition of short fragment HA to AT2 static culture induces EMT with and without TGF-β1. A, representative immunoblot of three independent experiments. B, densitometry of immunoblots. E-cadherin is significantly decreased with TGF-β1 or HA. Vimentin is significantly greater in HA compared with collagen groups. α-SMA is significantly increased in TGF-β1 and HA groups compared with collagen control. C, mRNA expression of E-cadherin significantly decreased with TGF-β1 and HA compared with collagen control. D, vimentin mRNA expression significantly increased in the TGF-β1-treated group compared with collagen alone. E, α-SMA mRNA expression significantly increased in all groups compared with collagen controls. Data are represented as means ± S.E. (error bars), n = 3/group. *, p < 0.05; **, p < 0.01; ANOVA with Tukey's post hoc test.
FIGURE 4.
HA blockade inhibits stretch-induced EMT. A–C, mRNA expression of EMT markers with HA binding peptide (pep-1) treatment. A, E-cadherin significantly decreased with stretch in vehicle and scrambled groups. B, mRNA expression of vimentin significantly increased with stretch in the scrambled group and unchanged in the vehicle or pep-1 groups. C, mRNA expression of α-SMA significantly increased with stretch in the vehicle and scrambled groups. There is no significant change in the pep-1-treated cells. D, immunoblot of cell lysates from pep-1 experiments. E-cadherin protein is unchanged with stretch when treated with pep-1. pep-1-treated cells have decreased vimentin and α-SMA for both static and stretch compared with other treatments. E, mRNA expression of E-cadherin significantly decreased with stretch in wild-type cells. F, mRNA expression of vimentin significantly increased with stretch in the wild-type cells but not in the CD44−/− cells. G, mRNA expression of α-SMA significantly increased with stretch in the wild-type cells but not in the CD4−/− cells. G and H, immunoblot of cell lysates from CD44−/− experiments showing that E-cadherin is completely diminished with stretch in the wild-type cells but not in CD44−/− cells. Vimentin and α-SMA are increased with stretch in the wild-type cells but not in the CD44−/− cells. Data are represented as means ± S.E. (error bars), n = 3/group. *, p < 0.05; **, p < 0.01, ANOVA with Tukey's post-hoc test.
Because mechanical stretch was shown previously to cause EMT in renal tubular epithelial cells through up-regulation of TGF-β1 (22), we investigated the role of TGF-β1 in our stretch-induced EMT. We performed an ELISA on cell culture supernatants (supplemental Methods). We found no increase in active or latent TGF-β1 in stretched samples compared with static over 4 days of culture (supplemental Fig. 4A). Additionally, we performed experiments adding TGF-β1 to statically cultured cells deficient in CD44 or MyD88. These TGF-β1-treated cells underwent EMT as indicated by gene expression (supplemental Fig. 4,B–G). Finally, we performed blocking experiments of TGF-β1 in either stretched or sHA-treated primary murine AT2 cells. The results suggested that TGF-β1 blockade does not alter expression of EMT markers in these models.
Stretch-induced, HA-mediated EMT Involves Innate Immune Activation
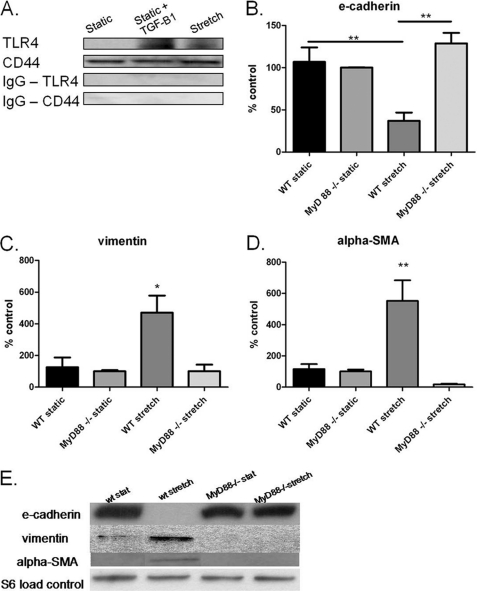
Because HA is a known endogenous activator of the innate immune receptors, TLR-2 and TLR-4, we examined aspects of innate immune activation with stretch. CD44 co-immunoprecipitated with TLR-4 in stretched AT2 and TGF-β1-treated AT2 cells (Fig. 5A). TLR-2 and TLR-4 mediate, in part, their innate immune action through the adaptor molecule MyD88, and MyD88 mediates HA effects on cell fate after bleomycin treatment (23). We therefore used primary AT2 cells from mice deficient in the adaptor molecule MyD88. In these cells, stretch did not significantly decrease gene or protein expression of E-cadherin (Fig. 5, B and E). Additionally, MyD-88 AT2 cells did not exhibit markers of the mesenchymal phenotype vimentin and α-SMA in either mRNA (Fig. 5, C and D) or protein (Fig. 5E).
FIGURE 5.
Cells deficient in the innate immune adaptor molecule MyD88 do not undergo stretch-induced EMT. A, immunoprecipitation with CD44 or IgG control and probed with an antibody to TLR-4 or CD44 as control. Stretch or TGF-β1 causes co-immunoprecipitation of TLR-4 and CD44. B, gene expression of E-cadherin significantly decreased with stretch in wild-type cells but not in MyD88−/− cells. C, gene expression of vimentin not significantly increased in stretched MyD88−/− cells. D, gene expression of α-SMA significantly increased with stretch in wild-type cells but not in MyD88−/− cells. E, representative Western blot of AT2 from wild-type and MyD88−/− static and stretched cells. Data are represented as means ± S.E. (error bars), n = 4/group. *, p < 0.05; **, p < 0.01 ANOVA with Tukey's post hoc test.
Stretch-induced EMT Involves the Wnt/β-Catenin Pathway
Activation of the Wnt/β-catenin pathway is known to cause EMT. We examined mRNA expression of genes downstream of the Wnt/β-catenin pathway including ccnd1, wnt10a, tcf6, srfp1, and wisp-1. We chose to focus on wisp-1 because it is a target gene of Wnt/β-catenin activation and can cause EMT in pulmonary epithelial cells (8). Gene expression of wisp-1 was significantly up-regulated in statically cultured AT2 with sHA (Fig. 6A). Furthermore, expression of wisp-1 and other Wnt-related genes was significantly increased in stretched “wild-type” AT2 cells, but not in myd88- or cd44-deficient AT2 cells compared with static cultures (Fig. 6B and supplemental Fig. 3). We then added a blocking antibody to WISP-1 to our stretched cultures and examined markers of EMT. We found that blockade of WISP-1 prevented the occurrence of EMT in stretched AT2 cells. WISP-1-treated cells were no different from statically cultured cells in the expression of relevant EMT markers, whereas control IgG-treated cells showed EMT, as expected (Fig. 6, C–E). These results suggest that elements of the Wnt pathway, in particular WISP-1, are involved in stretch-induced, HA/MyD88-mediated EMT.
FIGURE 6.
WISP-1 is necessary for EMT. A, gene expression of WISP-1 in static cells was significantly increased with the addition of low molecular mass HA. B, gene expression of WISP-1 was significantly up-regulated in wild-type stretched cells but not in MyD88−/− or CD44−/− cells. C, E-cadherin gene expression was not significantly changed in stretch cells treated with antibody to WISP-1. D, α-SMA gene expression was not significantly changed in stretched cells treated with antibody to WISP-1. Data are represented as means ± S.E. (error bars), n = 3–6/group. *, p < 0.05; **, p < 0.01 as indicated.
DISCUSSION
Epithelial injury is a central event in lung disease, such as acute lung injury (1). Mechanical ventilation is commonly used as support treatment in lung injury, but it compounds the damage by itself causing more lung injury. Alveolar overdistension is a critical mechanism of VILI; even during low tidal volume ventilation, overdistension of the compliant healthy alveoli leads to their damage (24). Our results suggest that abnormal stretch of AT2 cells directly leads to abnormal wound healing via intrinsic innate immune activation and induction of EMT. To our knowledge, this is the first study linking mechanical stress, innate immune activation, and EMT in primary mammalian epithelia. We describe three steps that determine stretch-induced alveolar EMT: (i) overdistension induces sHA production; (ii) extracellular sHA induces EMT; and (iii) at a minimum, stretch/sHA-induced EMT requires the innate immune adaptor molecule MyD88 and the Wnt/β-catenin target gene WISP-1.
HA is emerging as an important regulator of lung cell fate in development and injury. has2-mediated production of higher molecular mass HA is necessary for normal cardiac development in utero (25) and for epithelial survival in lung injury (23). Conversely, VILI (12) and lung injury (26, 27) promote sHA production. The source of sHA may vary. Mechanical stretch causes sHA production in pulmonary fibroblasts, leading to inflammation (12, 28). However, injury also induces sHA production in airway epithelial cells (29). Our work demonstrates that mechanical stretch of AT2 epithelia is sufficient for sHA production, even in the absence of fibroblasts. This effect occurs very early on in stretch injury: at 4 h after initiation of cell stretch, has3 was up-regulated in our model (data not shown). Ultimately, we cannot make a conclusive statement as to the relative contribution of epithelial or mesenchymal sHA origin in VILI. Fibroblasts only constitute <0.2% of alveolar cells (7) but are professional matrix-producers. However, our results are important for two reasons: first, they suggest that sHA in lung injury, regardless of origin, induces EMT in AT2 cells, thus leading to aberrant wound healing; second, our results indicate that treatments targeting solely fibroblasts may not be sufficient to abrogate VILI. AT2 cells are central elements in alveolar regeneration; they replenish the AT1 pool after injury (30), but can also contribute to aberrant wound healing. For example, targeted injury of AT2 cells induces pulmonary fibrosis in a mouse model (31) and AT2 EMT has been demonstrated in a mouse model of pulmonary fibrosis (9). We demonstrate that sHA induces AT2 EMT in an autofeedback loop, highlighting the central role of these cells in VILI and other models of lung injury.
The role of innate immunity in sterile pulmonary injury is now well established (23, 32). HA is a known endogenous activator of the innate immune receptors TLR-2 and TLR-4, is abundantly present in lung injury, and mediates its effects, at least partly, via the innate effector molecule MyD88 (23). In sterile injury HA interacts with a complex of CD44 and TLR-4 in skin injury (21) and in lung injury (32, 33). Our results now suggest that sHA may serve as a mechanotransducer in stretch injury of the lung by binding to CD44, associating CD44 with TLR-4, and signaling through MyD88. Mechanical stretch was previously shown to activate innate immunity in the lung, even at low tidal volume ventilation (34). However, the role of innate immunity in determining cell fate after injury is less well understood. Innate immune signaling through MyD88 can induce apoptosis in some cells (35) or promote cell survival in other cases (23), but to our knowledge this is the first paper to describe innate immune mediation of EMT. Recently, TLR-4 activation was shown to induce hepatic fibrosis via a MyD88-dependent pathway (36). Although the role of HA in TLR-4 activation was not specifically investigated in that paper, HA was increased in that model of hepatic fibrosis (36) as well as in human cirrhosis (37). Our results suggest a possible integrative pathway involving HA activation of innate immunity in fibrotic disease. Activation of innate immunity through exogenous endotoxin has previously been shown to enhance VILI (38). Our results suggest that HA may also activate innate immunity, promote aberrant wound healing, and exacerbate VILI. Furthermore, ventilated patients with acute respiratory distress syndrome often suffer concomitant infections. Our results imply that innate immune activation of AT2 cells in an infectious setting may compound VILI by inducing or potentiating EMT.
We know very little about the biological link between pulmonary innate immune activation and EMT. A common EMT pathway involves the Wnt/β-catenin system. We therefore looked at the Wnt target gene WISP-1. WISP-1 mediates pulmonary fibrosis in mice, causes EMT in cultured AT2 cells, and is up-regulated in human idiopathic pulmonary fibrosis (8). Our results indicate that WISP-1 signaling is downstream of both HA binding to CD44 and innate immune activation. In addition to being a target of Wnt signaling (39), wisp-1 is also a target gene of p38/MAPK signaling (40). Although there is no known connection between the Wnt/β-catenin pathway and MyD88 signaling in the lung, there is a connection between MyD88 activation and p38 activation (41, 42). Stretch activation of TLRs via HA may activate p38, causing expression and signaling of WISP-1 resulting in EMT. Stretch has been shown to activate the Wnt/β-catenin pathway in other cell types, including osteoblasts (43), chondrocytes (44), and myoblasts (45). Because WISP-1 is a target gene of both Wnt and p38, WISP-1 may be the point where the pathways converge to cause EMT (40). Additionally, stretch may disrupt the complexing of E-cadherin and β-catenin leading to activation of the noncanonical Wnt pathway (46). Although we confirmed stretch-induced activation of WISP-1, which is a transducer for the canonical Wnt pathway, we cannot exclude that the noncanonical cadherin/β-catenin pathway may also be activated with stretch.
Although stretch-induced EMT has never been shown in lung epithelium, Sato et al. found that stretch induces EMT in renal tubular epithelial cells through up-regulation of TGF-β1 (22). In contrast to the Sato et al. study, our results show that TGF-β1 is not increased in stretched AT2 cells, and TGF-β1 blockade does not affect stretch- or sHA-induced EMT. Because Bourguignon et al. showed that CD44 is involved in promoting TGF-β1 signaling (47), we further examined TGF-β1 on AT2 cells deficient in CD44 or MyD88. We show that unlike in stretch-induced EMT, AT2 cells do not need CD44 or MyD88 for TGF-β1-induced EMT. This result is interesting because we found that TGF-β1 did cause association of CD44 and TLR-4 (Fig. 5). This contrast in our findings may be due to the many pathways through which TGF-β1 can cause EMT. TGF-β1 can commonly cause EMT through SMAD signaling (48), notch signaling (49), or Wnt/β-catenin signaling, among others (50). TGF-β1 can cause cross-talk between these pathways, resulting in EMT (50). This redundancy in pathways may explain why CD44 is not necessary for TGF-β1-induced EMT in our system. Our results, however, suggest that stretch-induced EMT occurs through innate immune activation and Wnt/β-catenin signaling separate from TGF-β1 signaling. In aggregate, our results would imply that HA-mediated pathways are downstream of TGF-β1 signaling, and they are not redundant for TGF-β1-induced EMT pathogenesis, but that TGF-β1 signaling is not part of the HA pathway in alveolar stretch.
All models of alveolar epithelial stretch injury suffer from methodological weaknesses in their ability to replicate effects in humans. Our in vitro cell stretch model also has limitations. The stretch is performed in two dimensions instead of the complex three-dimensional structure of the alveoli. We stretched only AT2 cells, although there may be interaction between the AT2 and surrounding AT1, macrophages, or other cells in the alveoli that can contribute to the response to stretch. The advantage of our model is that we can study sustained cyclic cell stretch well beyond the 4–8 h of mechanical ventilation in rodents. The stretch level and frequency were chosen based upon ventilation settings used in the mouse lung to induce injury (51). This change in surface area is within values expected in injurious mechanical ventilation (6). Furthermore, 4 days of stretch replicate the time point when VILI is usually observed in ventilated patients (52). We therefore believe that our results have translational relevance in stretch-induced human lung disease.
The stretch injury model of AT2 cells has implications for fibrotic lung disease beyond VILI. Abnormal cell stretch in idiopathic pulmonary fibrosis may occur due to scarring and inhomogeneous lung compliance. Decreased lung compliance in idiopathic pulmonary fibrosis patients may result in exaggerated swings in pleural pressure and preferential ventilation of the lower lung fields (53) where, interestingly, most fibrosis is observed. It is possible that abnormal stretch in diseased lungs can cause changes in cell phenotype leading to progression of fibrosis. EMT may be a source for fibroblasts in idiopathic pulmonary fibrosis (9). Furthermore, HA blockade decreased fibrosis in bleomycin-treated rats, further indicating the importance of HA in fibrosis (54). In that work, HA increased macrophage migration into the lung as well as TGF-β1 expression in the lung. It is therefore probable that in the in vivo fibrotic process, HA produced via epithelial cell stretch promotes macrophage infiltration and macrophage-induced profibrotic responses, thus adding a positive feedback loop to the EMT process. Examination of the relationship among stretch, innate immune activation, and EMT in idiopathic pulmonary fibrosis will be an interesting topic of further study.
In summary, we have found that cyclic stretch promotes EMT in AT2 cells through production of low molecular mass HA which activates the Wnt/β-catenin pathway downstream of MyD88 signaling. This result indicates that AT2 stretch injury may directly lead to EMT, mediated through HA-activated innate immunity, and may directly contribute to the fibrotic response that often follows lung injury.
Supplementary Material
Acknowledgments
We thank Holly Rutledge and Jeff Tucker for assistance with confocal microscopy; Laura Miller and Ligon Perrow for assistance with animal protocols; and Barry Arnett and AMO, Inc., Abbott Park, IL, for providing Healon at no cost.
This work was supported, in whole or in part, by National Institutes of Health Grant ES016126 (to J. W. H.). This work was also supported by intramural funds from the Division of Intramural Research, NIEHS/National Institutes of Health (to S. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Table 1, and Figs. 1–5.
- AT1
- alveolar type I
- AT2
- alveolar type II
- EMT
- epithelial-to-mesenchymal transition
- HA
- hyaluronan
- has
- HA synthase gene
- sHA
- short fragment HA
- α-SMA
- α-smooth muscle actin
- VILI
- ventilator-induced lung injury
- WISP-1
- Wnt-inducible signaling protein 1.
REFERENCES
- 1. Berthiaume Y., Lesur O., Dagenais A. (1999) Thorax 54, 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corvol H., Flamein F., Epaud R., Clement A., Guillot L. (2009) Int. J. Biochem. Cell Biol. 41, 1643–1651 [DOI] [PubMed] [Google Scholar]
- 3. Wurfel M. M. (2007) Proc. Am. Thorac. Soc. 4, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tremblay L. N., Miatto D., Hamid Q., Govindarajan A., Slutsky A. S. (2002) Crit. Care Med. 30, 1693–1700 [DOI] [PubMed] [Google Scholar]
- 5. Lionetti V., Recchia F. A., Ranieri V. M. (2005) Curr. Opin. Crit. Care 11, 82–86 [DOI] [PubMed] [Google Scholar]
- 6. Tschumperlin D. J., Margulies S. S. (1999) J. Appl. Physiol. 86, 2026–2033 [DOI] [PubMed] [Google Scholar]
- 7. Crapo J. D., Barry B. E., Gehr P., Bachofen M., Weibel E. R. (1982) Am. Rev. Respir. Dis. 125, 740–745 [DOI] [PubMed] [Google Scholar]
- 8. Königshoff M., Kramer M., Balsara N., Wilhelm J., Amarie O. V., Jahn A., Rose F., Fink L., Seeger W., Schaefer L., Günther A., Eickelberg O. (2009) J. Clin. Invest. 119, 772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim K. K., Kugler M. C., Wolters P. J., Robillard L., Galvez M. G., Brumwell A. N., Sheppard D., Chapman H. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taliana L., Evans M. D., Ang S., McAvoy J. W. (2006) Mol. Vis. 12, 1233–1242 [PubMed] [Google Scholar]
- 11. Câmara J., Jarai G. (2010) Fibrogenesis Tissue Repair 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai K. J., Spicer A. P., Mascarenhas M. M., Yu L., Ochoa C. D., Garg H. G., Quinn D. A. (2005) Am. J. Respir. Crit. Care Med. 172, 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toole B. P., Zoltan-Jones A., Misra S., Ghatak S. (2005) Cells Tissues Organs 179, 66–72 [DOI] [PubMed] [Google Scholar]
- 14. Zoltan-Jones A., Huang L., Ghatak S., Toole B. P. (2003) J. Biol. Chem. 278, 45801–45810 [DOI] [PubMed] [Google Scholar]
- 15. Schmits R., Filmus J., Gerwin N., Senaldi G., Kiefer F., Kundig T., Wakeham A., Shahinian A., Catzavelos C., Rak J., Furlonger C., Zakarian A., Simard J. J., Ohashi P. S., Paige C. J., Gutierrez-Ramos J. C., Mak T. W. (1997) Blood 90, 2217–2233 [PubMed] [Google Scholar]
- 16. Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. (1998) Immunity 9, 143–150 [DOI] [PubMed] [Google Scholar]
- 17. Corti M., Brody A. R., Harrison J. H. (1996) Am. J. Respir. Cell Mol. Biol. 14, 309–315 [DOI] [PubMed] [Google Scholar]
- 18. Garantziotis S., Li Z., Potts E. N., Kimata K., Zhuo L., Morgan D. L., Savani R. C., Noble P. W., Foster W. M., Schwartz D. A., Hollingsworth J. W. (2009) J. Biol. Chem. 284, 11309–11317 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 19. Mummert M. E., Mohamadzadeh M., Mummert D. I., Mizumoto N., Takashima A. (2000) J. Exp. Med. 192, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aytekin M., Comhair S. A., de la Motte C., Bandyopadhyay S. K., Farver C. F., Hascall V. C., Erzurum S. C., Dweik R. A. (2008) Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor K. R., Yamasaki K., Radek K. A., Di Nardo A., Goodarzi H., Golenbock D., Beutler B., Gallo R. L. (2007) J. Biol. Chem. 282, 18265–18275 [DOI] [PubMed] [Google Scholar]
- 22. Sato M., Muragaki Y., Saika S., Roberts A. B., Ooshima A. (2003) J. Clin. Invest. 112, 1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 24. Treggiari M. M., Martin D. P., Yanez N. D., Caldwell E., Hudson L. D., Rubenfeld G. D. (2007) Am. J. Respir. Crit. Care Med. 176, 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camenisch T. D., Spicer A. P., Brehm-Gibson T., Biesterfeldt J., Augustine M. L., Calabro A., Jr., Kubalak S., Klewer S. E., McDonald J. A. (2000) J. Clin. Invest. 106, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teder P., Vandivier R. W., Jiang D., Liang J., Cohn L., Puré E., Henson P. M., Noble P. W. (2002) Science 296, 155–158 [DOI] [PubMed] [Google Scholar]
- 27. Garantziotis S., Zudaire E., Trempus C. S., Hollingsworth J. W., Jiang D., Lancaster L. H., Richardson E., Zhuo L., Cuttitta F., Brown K. K., Noble P. W., Kimata K., Schwartz D. A. (2008) Am. J. Respir. Crit. Care Med. 178, 939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascarenhas M. M., Day R. M., Ochoa C. D., Choi W. I., Yu L., Ouyang B., Garg H. G., Hales C. A., Quinn D. A. (2004) Am. J. Respir. Cell Mol. Biol. 30, 51–60 [DOI] [PubMed] [Google Scholar]
- 29. Lauer M. E., Erzurum S. C., Mukhopadhyay D., Vasanji A., Drazba J., Wang A., Fulop C., Hascall V. C. (2008) J. Biol. Chem. 283, 26283–26296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lwebuga-Mukasa J. S. (1991) Am. Rev. Respir. Dis. 144, 452–457 [DOI] [PubMed] [Google Scholar]
- 31. Sisson T. H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., Dave A., Engelhardt J. F., Liu X., White E. S., Thannickal V. J., Moore B. B., Christensen P. J., Simon R. H. (2010) Am. J. Respir. Crit. Care Med. 181, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garantziotis S., Li Z., Potts E. N., Lindsey J. Y., Stober V. P., Polosukhin V. V., Blackwell T. S., Schwartz D. A., Foster W. M., Hollingsworth J. W. (2010) Am. J. Respir. Crit. Care Med. 181, 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 33. Muto J., Yamasaki K., Taylor K. R., Gallo R. L. (2009) Mol. Immunol. 47, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaneker M., Joosten L. A., Heunks L. M., Snijdelaar D. G., Halbertsma F. J., van Egmond J., Netea M. G., van der Hoeven J. G., Scheffer G. J. (2008) Anesthesiology 109, 465–472 [DOI] [PubMed] [Google Scholar]
- 35. Bannerman D. D., Goldblum S. E. (2003) Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L899–914 [DOI] [PubMed] [Google Scholar]
- 36. Seki E., De Minicis S., Osterreicher C. H., Kluwe J., Osawa Y., Brenner D. A., Schwabe R. F. (2007) Nat. Med. 13, 1324–1332 [DOI] [PubMed] [Google Scholar]
- 37. Rosenberg W. M., Voelker M., Thiel R., Becka M., Burt A., Schuppan D., Hubscher S., Roskams T., Pinzani M., Arthur M. J. (2004) Gastroenterology 127, 1704–1713 [DOI] [PubMed] [Google Scholar]
- 38. Martin T. R., Frevert C. W. (2005) Proc. Am. Thorac. Soc. 2, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pennica D., Swanson T. A., Welsh J. W., Roy M. A., Lawrence D. A., Lee J., Brush J., Taneyhill L. A., Deuel B., Lew M., Watanabe C., Cohen R. L., Melhem M. F., Finley G. G., Quirke P., Goddard A. D., Hillan K. J., Gurney A. L., Botstein D., Levine A. J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14717–14722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H., Xu Q., Xiao F., Jiang Y., Wu Z. (2008) Mol. Biol. Cell 19, 1519–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burns K., Martinon F., Esslinger C., Pahl H., Schneider P., Bodmer J. L., Di Marco F., French L., Tschopp J. (1998) J. Biol. Chem. 273, 12203–12209 [DOI] [PubMed] [Google Scholar]
- 42. Janssens S., Burns K., Vercammen E., Tschopp J., Beyaert R. (2003) FEBS Lett. 548, 103–107 [DOI] [PubMed] [Google Scholar]
- 43. Jansen J. H., Eijken M., Jahr H., Chiba H., Verhaar J. A., van Leeuwen J. P., Weinans H. (2010) J. Orthop. Res. 28, 390–396 [DOI] [PubMed] [Google Scholar]
- 44. Dell'Accio F., De Bari C., Luyten F. P. (2003) Exp. Cell Res. 287, 16–27 [DOI] [PubMed] [Google Scholar]
- 45. Akimoto T., Ushida T., Miyaki S., Akaogi H., Tsuchiya K., Yan Z., Williams R. S., Tateishi T. (2005) Biochem. Biophys. Res. Commun. 329, 381–385 [DOI] [PubMed] [Google Scholar]
- 46. Arnsdorf E. J., Tummala P., Jacobs C. R. (2009) PLoS One 4, e5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bourguignon L. Y., Singleton P. A., Zhu H., Zhou B. (2002) J. Biol. Chem. 277, 39703–39712 [DOI] [PubMed] [Google Scholar]
- 48. Kasai H., Allen J. T., Mason R. M., Kamimura T., Zhang Z. (2005) Respir. Res. 6, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zavadil J., Cermak L., Soto-Nieves N., Böttinger E. P. (2004) EMBO J. 23, 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Willis B. C., Borok Z. (2007) Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L525–534 [DOI] [PubMed] [Google Scholar]
- 51. Pedreira P. R., García-Prieto E., Parra D., Astudillo A., Diaz E., Taboada F., Albaiceta G. M. (2008) Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L820–827 [DOI] [PubMed] [Google Scholar]
- 52. Acute Respiratory Distress Syndrome Network (2000) N. Engl. J. Med. 342, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 53. Brennan N. J., Morris A. J., Green M. (1983) Thorax 38, 62–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Savani R. C., Hou G., Liu P., Wang C., Simons E., Grimm P. C., Stern R., Greenberg A. H., DeLisser H. M., Khalil N. (2000) Am. J. Respir. Cell Mol. Biol. 23, 475–484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.