Abstract
Fibroblast proliferation is an early feature of progressive tissue fibrosis and is largely regulated by the cytokine transforming growth factor-β1 (TGF-β1). In the oral mucosa, fibroblasts have a unique phenotype and demonstrate healing with no fibrosis/scarring. Our previous studies show that whereas dermal fibroblasts proliferate in response to TGF-β1, oral fibroblasts have an antiproliferative response to this cytokine. Hyaluronan (HA) was directly linked to this TGF-β1-dependent response. The aim of this study was to understand the underlying mechanism through which HA regulates TGF-β-dependent responses. Using patient-matched oral and dermal fibroblasts, we show that TGF-β1-dependent proliferation is mediated through the HA receptor CD44, whereas the TGF-β1-mediated antiproliferative response is CD44-independent. Furthermore, overexpression of HAS2 (HA synthase-2) in oral cells modifies their response, and they subsequently demonstrate a proliferative, CD44-dependent response to TGF-β1. We also show that epidermal growth factor (EGF) and its receptor (EGFR) are essential for TGF-β1/HA/CD44-dependent proliferation. Increased HA levels promote EGFR and CD44 coupling, potentiating signal transduction through the MAPK/ERK pathway. Thus, in a HA-rich environment, late ERK1/2 activation results from EGFR/CD44 coupling and leads to a proliferative response to TGF-β1. In comparison, in a non-HA-rich environment, only early ERK1/2 activation occurs, and this is associated with an antiproliferative response to TGF-β1. In summary, HA facilitates TGF-β1-dependent fibroblast proliferation through promoting interaction between CD44 and EGFR, which then promotes specific MAPK/ERK activation, inducing cellular proliferation.
Keywords: ERK, Fibroblast, Hyaluronate, MAP Kinases (MAPKs), Transforming Growth Factor beta (TGFbeta), CD44, Epidermal Growth Factor Receptor, Proliferation
Introduction
The ability of tissues to heal rapidly following injury or inflammation is an important mechanism for survival in nature. However, in many instances, healing is either impaired or continues unabated, leading to progressive tissue fibrosis. In the skin and internal organs, progressive fibrosis can lead to a multitude of clinical conditions, ranging from scarring and disfiguration to chronic diseases, such as liver disease, chronic kidney disease, and heart failure (1–3). In contrast, certain injuries result in a defective wound matrix and display failure of re-epithelialization, leading to deficient healing. This is a feature of chronic non-healing wounds, which are prominent in diabetics and in the elderly. The achievement of rapid and efficient healing without scarring and fibrosis is therefore the focus of much ongoing study.
In adults, the oral mucosa is the only tissue that demonstrates rapid healing without any discernable scar formation. Thus, investigating healing in the context of oral mucosal injuries may contribute toward improved understanding of the mechanisms required to procure efficient and scar-free healing in other tissues. In all tissues, fibroblasts are considered to be the primary source of reparative matrix. In response to tissue injury, they proliferate, migrate to the site of injury, and differentiate into their activated form, myofibroblasts (4–6). These myofibroblasts are then involved in wound contraction and extracellular matrix generation (7, 8). Increased activation and proliferation of resident fibroblasts is therefore an important early step that is central to the wound healing process. Numerous studies have shown that fibroblasts exhibit a degree of phenotypic plasticity with respect to their size, migration, proliferation, differentiation, and collagen turnover (7, 9–11). In line with this, we have previously shown that fibroblasts derived from the non-scarring oral mucosa have a distinct cellular phenotype. They demonstrate increased migration and extracellular matrix reorganization as compared with other fibroblasts (12–14). Of significance, they also demonstrate a differential response to the profibrotic cytokine transforming growth factor-β1 (TGF-β1), as compared with normal fibroblasts (15, 16). We have recently shown that oral fibroblasts are resistant to TGF-β1-driven myofibroblastic differentiation. Furthermore, we demonstrated that the matrix polysaccharide hyaluronan (HA)2 plays a pivotal role in regulating TGF-β1-driven cellular differentiation in that it facilitates fibroblast-myofibroblast transition (15). We have also previously shown that although most fibroblasts proliferate when exposed to TGF-β1, fibroblasts derived from the oral mucosa demonstrate an antiproliferative response to this cytokine (16). HA was also found to be linked to the TGF-β1-driven proliferative response.
HA is a linear non-sulfated glycosaminoglycan that is a key component of vertebrate connective tissue matrix. It is involved in a range of cellular functions, including cell-cell adhesion, migration, proliferation, and differentiation and therefore plays an important role in wound healing and tissue repair (17–24). The biosynthesis of HA is regulated by three mammalian HA synthase isoenzymes, of which HAS2 (hyaluronan synthase 2) demonstrates the greatest expression in fibroblasts (15, 25–27). It signals through interaction with several cell surface receptors, and the adhesion molecule CD44 is the best characterized of these (28). Using a library of patient-matched oral mucosal and dermal fibroblasts, we previously demonstrated the relationship between HA generation and fibroblast-specific variation in TGF-β1-dependent proliferation (16). Dermal fibroblasts with high levels of HA generation and high HAS2 expression proliferate in response to TGF-β1, whereas oral mucosal fibroblasts with low HAS2 expression and low levels of HA generation demonstrate an antiproliferative response to TGF-β1. Moreover, we have also shown that by altering the levels of HA generated by the fibroblasts, we can effectively modify the resultant response to TGF-β1. However, although it has been shown that HA promotes a proliferative response to TGF-β1, the mechanism through which it regulates TGF-β1-dependent responses and thereby potentially influences the fibrotic response has not yet been fully understood.
It is widely accepted that the cytokine TGF-β1 is a mediator of tissue repair, and its aberrant expression is strongly implicated in progressive tissue fibrosis (29–32). Several studies have indicated that epidermal growth factor (EGF) enhances the profibrotic effects of TGF-β1, suggesting that it also plays an important role in progressive tissue fibrosis (33–36). In our recent studies, we have demonstrated that fibroblasts lose their ability to achieve TGF-β1-driven differentiation as they undergo cellular senescence, affecting their ability to attain appropriate wound closure and tissue repair (25). We have shown that this age-related resistance to TGF-β1 is associated with loss of the EGF receptor (EGFR) and furthermore that the EGFR is essential for signal transduction through CD44 and the MAPK/ERK signaling pathway necessary for differentiation (37). In this study, we sought to identify the mechanisms underlying the HA-dependent regulation of fibroblast proliferation and furthermore sought to investigate if the resistance to TGF-β1-driven proliferation seen in oral mucosal fibroblasts is also related to EGFR expression or function.
EXPERIMENTAL PROCEDURES
Materials
All reagents were from Sigma or Invitrogen unless otherwise stated. Reverse transcription, siRNA transfection reagents, and quantitative PCR (QPCR) primers and reagents were purchased from Invitrogen and Applied Biosystems (Cheshire, UK). Other reagents used were recombinant TGF-β1, goat anti-human EGF antibody from R&D Systems (Oxford, UK), EGF receptor inhibitor AG1478 from Invitrogen, and ERK (MEK) inhibitor PD98059 from Calbiochem.
Cell Culture
Donor-matched samples of oral mucosal and dermal fibroblasts were obtained by biopsy from consenting adults undergoing routine minor surgery, and ethical approval for the biopsies was obtained from the South East Wales Research Ethics Committee. The cells were isolated as described previously (12, 15) and cultured in Dulbecco's modified Eagle's medium and F-12 medium containing 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin supplemented with 10% fetal calf serum (FCS) (Biologic Industries Ltd., Cumbernauld, UK). The cells were maintained at 37 °C in a humidified incubator in an atmosphere of 5% CO2, and fresh growth medium was added to the cells every 3–4 days until confluent. The cells were incubated in serum-free medium for 48 h before use in experiments, and all experiments were done under serum-free conditions unless otherwise stated. All experiments were undertaken using cells at passages 6–10 and performed on confluent cultures except for those experiments examining proliferation. These used subconfluent cells to allow for cell growth.
Analysis of Cell Proliferation
The commercially available alamarBlueTM proliferation assay (BIOSOURCE) was used to assess cell growth. The assay utilizes an oxidation-reduction indicator that fluoresces in response to chemical reduction of growth medium resulting from cell growth and metabolism and demonstrates a linear relationship between the magnitude of fluorescence and cell number and viability. For this assay, the cells were grown in 35-mm dishes and assessed at subconfluence following a 24- or 48-h period of growth arrest as indicated. Following appropriate stimulation of cells (as indicated in Fig. 1–12 individual legends), 10% alamarBlueTM was added to the cell culture medium for 1 h at 37 °C. 100-μl aliquots of the conditioned medium were then removed and added to a clear 96-well plate. Subsequently, fluorescence was measured in a FLUOstar Optima fluorescence meter (BMG Lab Technologies) with excitation wavelength at 540 nm and emission wavelength at 590 nm, and the results are expressed as arbitrary fluorescence units.
FIGURE 1.
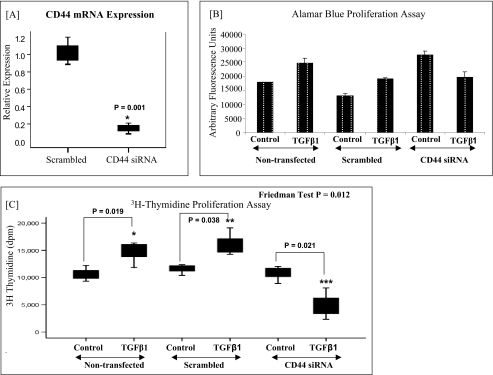
Involvement of CD44 in TGFβ1-driven proliferation in dermal fibroblasts. A, CD44 mRNA expression. Dermal fibroblasts were transfected with CD44 or negative control (scrambled) siRNA and incubated for 24 h. To confirm CD44 knockdown, mRNA extraction was carried out, and CD44 mRNA expression was assessed by RT-QPCR. Ribosomal RNA was used as an endogenous control, and gene expression was assessed relative to the scrambled samples. The comparative CT method was used for relative quantification of gene expression, and the results are expressed as box plots. For each box plot, the box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Wilcoxon signed-rank test, and the results are representative of three experiments. B, alamarBlueTM proliferation assay. In parallel experiments, the effect of CD44 knockdown on TGF-β1-driven proliferation in dermal fibroblasts was assessed using an alamarBlueTM assay (as described under “Experimental Procedures”). As above, the siRNAs were incubated with the cells in serum-free medium for 24 h. Subsequently, 10 ng/ml TGF-β1 was incubated with the cells for a further 24 h prior to analysis. The results are expressed as the means ± S.E. (error bars) for three experiments. C, [3H]thymidine proliferation assay. The effect of CD44 knockdown on TGF-β1 driven proliferation in dermal fibroblasts was also assessed using a [3H]thymidine assay (as described in “Experimental Procedures”). As above, the siRNAs were incubated with the cells in serum-free medium for 24 h. Subsequently, 10 ng/ml TGF-β1 was incubated with the cells for a further 24 h prior to analysis. The results are expressed as box plots. For each box plot, the box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05. The results are representative of three experiments.
FIGURE 2.
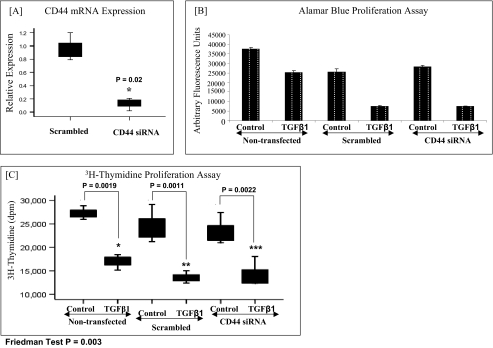
Involvement of CD44 in the TGFβ1-driven antiproliferative response in oral fibroblasts. A, CD44 mRNA expression. Oral mucosal fibroblasts were transfected with CD44 or negative control (scrambled) siRNA and incubated for 24 h. To confirm CD44 knockdown, mRNA extraction was carried out, and CD44 mRNA expression was assessed by RT-QPCR. Ribosomal RNA was used as an endogenous control, and gene expression was assessed relative to the scrambled samples. The comparative CT method was used for relative quantification of gene expression, and the results are expressed as box plots. For each box plot, the box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Wilcoxon signed-rank test, and the results are representative of three experiments. B, alamarBlueTM proliferation assay. In parallel experiments, the effect of CD44 knockdown on the TGF-β1-driven antiproliferative response in oral fibroblasts was assessed using an alamarBlueTM assay (as described under “Experimental Procedures”). As above, the siRNAs were incubated with the cells in serum-free medium for 24 h. Subsequently, 10 ng/ml TGF-β1 was incubated with the cells for a further 24 h prior to analysis. The results are expressed as the means ± S.E. (error bars) for three experiments. C, [3H]thymidine proliferation assay. The effect of CD44 knockdown on TGF-β1-driven proliferation in oral fibroblasts was also assessed using a [3H]thymidine assay (as described under “Experimental Procedures”). As above, the siRNAs were incubated with the cells in serum-free medium for 24 h. Subsequently, 10 ng/ml TGF-β1 was incubated with the cells for a further 24 h prior to analysis. The results are expressed as box plots. For each box plot, the box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05. The results are representative of three experiments.
FIGURE 3.
Effect of HAS2 overexpression in oral fibroblasts. A, HAS2 mRNA expression. Oral mucosal fibroblasts were transfected with either HAS2-pCR3.1 (HAS2-transfected) or PCR3.1 alone (mock-transfected) and incubated for 24 h. The medium was then replaced with serum-free medium for another 24 h before incubating the cells with either 10 ng/ml TGF-β1 or unstimulated medium (control) for a further 24 h. Total mRNA was then extracted, and RT-QPCR was performed to confirm HAS2 overexpression. Ribosomal RNA was used as an endogenous control, and gene expression was assessed relative to the control mock-transfected cells. The comparative CT method was used for relative quantification of gene expression, and the results are represented as the means ± S.E. (error bars) for three experiments. B, alamarBlueTM proliferation assay following HAS2 overexpression. In parallel experiments, oral mucosal fibroblasts transfected with either pCR3.1 (mock-transfected) or HAS2-pCR3.1 (HAS2-transfected) were stimulated with either 10 ng/ml TGF-β1 or unstimulated medium (control) for 24 h, and the alamarBlueTM assay was used to assess fibroblast proliferation (as described under “Experimental Procedures”). The results are expressed as the means ± S.E. for three experiments. C, [3H]thymidine proliferation assay following HAS2 overexpression. Oral mucosal fibroblasts transfected with either pCR3.1 (mock-transfected) or HAS2-pCR3.1 (HAS2-transfected) were stimulated with either 10 ng/ml TGF-β1 or unstimulated medium (control) for 24 h, and the [3H]thymidine assay was used to assess fibroblast proliferation (as described under “Experimental Procedures”). The results are expressed as the median ± IQR of three experiments. For each box plot, median values are represented by the line within the box. The box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05.
FIGURE 4.
Involvement of CD44 in the TGFβ1-driven proliferative response in HAS2-overexpressed oral fibroblasts. Oral mucosal fibroblasts were co-transfected with pCR3.1 and either CD44 siRNA or scrambled siRNA (mock-transfected). Alternatively, oral fibroblasts were co-transfected with HAS2-pCR3.1 and either CD44 siRNA or scrambled siRNA (HAS2-transfected). The cells were then incubated in serum-free medium for 24 h. They were subsequently incubated with either 10 ng/ml TGFβ1 or unstimulated medium (control) for a further 24 h. Analyses were then performed to confirm up-regulation of HAS2 expression (A), confirm down-regulation of CD44 expression (B), and assess the effect of HAS2 overexpression and CD44 down-regulation on TGF-β1-driven proliferation (C). mRNA expression of HAS2 and CD44 was assessed using RT-QPCR. A [3H]thymidine assay was used to assess proliferation (as described under “Experimental Procedures”). In A and B, the results are expressed as means ± S.E. (error bars) and represent the results for three experiments. In C, the results are expressed as the median ± IQR of three experiments. For each box plot, median values are represented by the line within the box. The box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test, followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05.
FIGURE 5.
Expression of EGFR (A) and EGF (B) in dermal and oral fibroblasts. Dermal and oral mucosal fibroblasts were grown in culture as described under “Experimental Procedures.” They were growth-arrested by incubation in serum-free medium and then incubated in either medium containing 10 ng/ml TGFβ1 or unstimulated medium for 24 h. Total mRNA was extracted, and mRNA expression of EGFR (A) and EGF (B) were assessed using RT-QPCR. Ribosomal RNA was used as an endogenous control, and the comparative CT method was used for relative quantification of gene expression. The results are represented as the means ± S.E. (error bars) and are representative of three experiments. Statistical analysis was performed using the paired Student's t test, and statistical significance was taken as p < 0.05.
FIGURE 6.
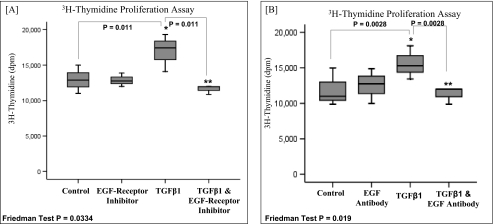
Involvement of EGFR and EGF in TGF-β1-driven proliferation in dermal fibroblasts. A, subconfluent monolayers of dermal fibroblasts were growth-arrested in serum-free medium for 48 h. The cells were then incubated with either serum-free medium alone (control), serum-free medium containing 10 ng/ml TGF-β1, serum-free medium containing EGFR inhibitor AG1471 (10 μm) alone, or serum-free medium containing 10 ng/ml TGFβ1 and EGFR inhibitor AG1471 (10 μm). The incubations were continued for a further 24 h, and analysis of cell growth was performed using the [3H]thymidine assay (as described under “Experimental Procedures”). The results are expressed as the median ± IQR (error bars) of three experiments. For each box plot, median values are represented by the line within the box. The box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05. B, subconfluent monolayers of dermal fibroblasts were growth-arrested in serum-free for 48 h. The cells were then incubated with either serum-free medium alone (control), serum-free medium containing 10 ng/ml TGF-β1, serum-free medium containing 10 μg/ml anti-EGF antibody, or serum-free medium containing 10 ng/ml TGF-β1 and 10 μg/ml anti-EGF antibody. The incubations were continued for a further 24 h, and analysis of cell growth was performed using the [3H]thymidine assay (as described under “Experimental Procedures”). The results are expressed as the median ± IQR of three experiments. For each box plot, median values are represented by the line within the box. The box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05.
FIGURE 7.
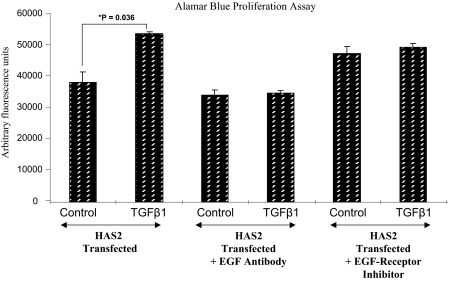
Effect of EGFR and EGF inhibition in TGF-β1-driven proliferation in HAS2-overexpressed oral fibroblasts. Oral mucosal fibroblasts were transfected with HAS2-pCR3.1 and incubated for 24 h. The medium was then replaced with serum-free medium for another 24 h before incubating the cells with either unstimulated medium (control), medium containing 10 ng/ml TGF-β1, medium containing 10 μg/ml anti-EGF antibody, medium containing 10 ng/ml TGF-β1 and 10 μg/ml anti-EGF antibody, medium containing 10 μm EGFR inhibitor AG1471, or medium containing 10 ng/ml TGF-β1 and 10 μm EGFR inhibitor AG1471. The incubations were continued for a further 24 h, and analysis of cell growth was subsequently performed using the alamarBlueTM assay (as described under “Experimental Procedures”). The results are represented as the means ± S.E. (error bars) and are representative of three experiments.
FIGURE 8.
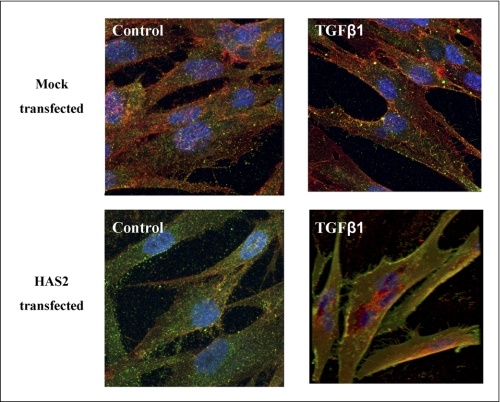
Effect of HAS2 overexpression on CD44 and EGFR co-localization in oral fibroblasts. Oral mucosal fibroblasts were transfected with either HAS2-pCR3.1 (HAS2-transfected) or PCR3.1 alone (mock-transfected) and incubated for 24 h. The medium was then replaced with serum-free medium for another 24 h before incubating the cells with either 10 ng/ml TGF-β1 or unstimulated medium (control) for a further 24 h. The cells were then fixed, and the expression of CD44 (red) and EGFR (green) was examined by immunocytochemistry, and their association was examined by merging individual images (yellow). DAPI staining was used to visualize nuclei (blue). Original magnification was ×400.
FIGURE 9.
Involvement of ERK1/2 signaling in TGF-β1-driven proliferation in fibroblasts. A, subconfluent monolayers of dermal fibroblasts were growth-arrested in serum-free medium for 48 h. The cells were then incubated with either serum-free medium alone, serum-free medium containing 10 ng/ml TGF-β1, serum-free medium containing 10 μm ERK inhibitor PD98059, or serum-free medium containing both 10 ng/ml TGF-β1 and 10 μm ERK inhibitor PD98059. The incubations were continued for a further 24 h, and analysis of cell growth was performed using the [3H]thymidine assay (as described under “Experimental Procedures”). The results are expressed as the median ± IQR (error bars) of three experiments. For each box plot, median values are represented by the line within the box. The box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05. B, subconfluent monolayers of oral fibroblasts were transfected with HAS2-pCR3.1 and incubated for 24 h. The cells were then incubated with either serum-free medium alone, serum-free medium containing 10 ng/ml TGF-β1, serum-free medium containing 10 μm ERK inhibitor PD98059, or serum-free medium containing both 10 ng/ml TGF-β1 and 10 μm ERK inhibitor PD98059. The incubations were continued for a further 24 h, and analysis of cell growth was performed using the [3H]thymidine assay (as described under “Experimental Procedures”). The results are expressed as the median ± IQR of three experiments. For each box plot, median values are represented by the line within the box. The box represents 50% of the values (the 25th and 75th percentiles), with the bars presenting the highest and lowest values. Statistical analysis was performed using the Friedman test followed by the Wilcoxon signed-rank test, and statistical significance was taken as p < 0.05.
FIGURE 10.
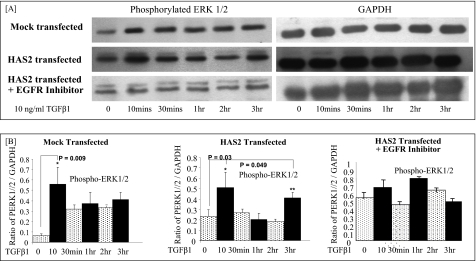
ERK1/2 signaling in dermal and oral fibroblasts. A, confluent monolayers of dermal and oral fibroblasts were growth-arrested in serum-free medium for 48 h. Subsequently, they were incubated with 10 ng/ml TGF-β1 for up to 3 h, and phosphorylation of ERK1/2 proteins was assessed by Western blot analysis at the indicated times. Phosphorylation of ERK1/2 in dermal fibroblasts following incubation with 10 ng/ml EGFR inhibitor AG1471 was also assessed. Western blot analysis for GAPDH was performed to ensure equal loading of protein samples. B, following scanning densitometry, alteration in phosphorylated ERK1/2 expression was corrected for the expression of GAPDH and is represented as the mean ± S.D. of three separate experiments. Statistical analysis was performed using the paired Student's t test, and statistical significance was taken as p < 0.05.
FIGURE 11.
ERK1/2 signaling in oral fibroblasts following HAS2 overexpression. A, oral mucosal fibroblasts were transfected with either HAS2-pCR3.1 (HAS2-transfected) or PCR3.1 alone (mock-transfected) and incubated for 24 h. The medium was then replaced with serum-free medium for another 24 h before incubating the cells with 10 ng/ml TGF-β1 for up to 3 h. Phosphorylation of ERK1/2 proteins was assessed by Western blot analysis at the indicated times. Phosphorylation of ERK1/2 in HAS2-transfected oral fibroblasts following incubation with 10 ng/ml EGFR inhibitor AG1471 was also assessed. Western blot analysis for GAPDH was performed to ensure equal loading of protein samples. B, following scanning densitometry, alteration in phosphorylated ERK1/2 expression was corrected for the expression of GAPDH and is represented as the mean ± S.D. of three separate experiments. Statistical analysis was performed using the paired Student's t test, and statistical significance was taken as p < 0.05.
FIGURE 12.
Proposed mechanism for TGF-β1-driven proliferation in fibroblasts. The diagram illustrates the proposed model for Smad and MAPK/ERK activation necessary to induce fibroblast proliferation following TGF-β1 stimulation. P, phosphorylation; TGF-βR, TGF-β1 receptor. For a detailed explanation of the proposed pathway, see “Results.”
[3H]Thymidine Assay
As a comparison, the incorporation of d-[3H]thymidine into DNA was also used to assess fibroblast proliferation. Cells were grown in 35-mm dishes and assessed at subconfluence. Metabolic labeling was performed by incubation of the cells with 1 μCi/ml d-[3H]thymidine for 24 h. The incubation with d-[3H]thymidine was performed at the same time as stimulation of the cells with serum-free medium, TGF-β1, EGF antibody, ERK inhibitor, or the EGFR inhibitor (as indicated in Fig. 1–4, 6, 7, and 9 individual legends). The medium was then discarded, and the cells were washed repeatedly with PBS containing 1 mm thymidine prior to fixing with 500 μl of 5% trichloroacetic acid containing 1 mm thymidine at 4 °C for 1 h. The cell layer was extracted by incubation with 1 ml of 0.1 m NaOH at 20 °C for 24 h and neutralized with 0.1 m HCl. The radioactivity was determined by β counting on a Packard Tri-Carb 1900 liquid scintillation analyzer, and the results are represented as disintegrations/min (dpm).
Reverse Transcription (RT) and QPCR
RT and QPCR were used to assess CD44, HAS2, EGF, and EGFR mRNA expression in oral mucosal and dermal fibroblasts. The cells were grown in 35-mm dishes and washed with PBS prior to lysis with tri-reagent and RNA purification according to the manufacturer's protocol. Reverse transcription was performed using the High Capacity cDNA reverse transcription kits according to the manufacturer's protocol (Applied Biosystems). This uses the random primer method for initiating cDNA synthesis. As a negative control, RT was performed with sterile H2O replacing the RNA sample. QPCR was performed as in our previous studies (37) using the 7900HT fast real time PCR system from Applied Biosystems.
Small Interfering RNA (siRNA) Transfection
Transient transfection of fibroblasts was performed with specific siRNA nucleotides (Applied Biosystems) targeting CD44. Transfection was performed in 35-mm dishes using Lipofectamine 2000 transfection reagent (Invitrogen) in accordance with the manufacturer's protocol as described in our previous work (37). As a control, cells were transfected with negative control siRNA (a scrambled sequence that bears no homology to the human genome) (Applied Biosystems).
Overexpression of HAS2
HAS2 open reading frame (ORF) (25, 37) was inserted into the vector pCR3.1 using a standard ligation reaction with Promega T4 DNA ligase. Amplification of the cloned vector was performed via bacterial transformation (JM109 competent Escherichia coli, Promega) as described previously (37). Transient transfection was performed with the aid of Nucleofector technology (Amaxa Biosystems) in accordance with the manufacturer's protocol for transfection of primary mammalian fibroblasts (37). The cells were transfected either with HAS2-pCR3.1 or pCR3.1 (mock-transfected) and were incubated for 24 h in serum-free medium prior to experimentation. pmaxGFP transfection was performed in parallel and used to calculate transfection efficiency (41.3% following 48 h and 39.1% following 96 h).
Simultaneous Overexpression of HAS2 and CD44 Knockdown
In one of the experiments (Fig. 4) HAS2-pCR3.1 and CD44 siRNA were transfected simultaneously into oral mucosal fibroblasts. Nucleofector technology as described above was used for transfection of both HAS2-pCR3.1 and CD44 siRNA. The final concentration of CD44 siRNA used was 30 nm, and 1 μg of HAS2-pCR3.1 DNA was used as in previous experiments. Mock transfection with pCR3.1 and transfection with scrambled siRNA were performed as controls. RT and QPCR were performed to assess overexpression of HAS2 and CD44 knockdown.
Immunoblotting/Western Blot Analysis
Western blot analysis was used to assess expression of phosphorylated ERK1 and ERK2 (p44/42). Cells were grown to confluence in 35-mm dishes, and Western blot analysis was carried out as described in our previous work (37). The primary antibody used was monoclonal mouse anti-phosphorylated p44/42-mitogen-activated protein kinase antibody for ERK1 and ERK2, dilution 1:1000 from Cell Signaling Technology (Beverly, MA). Expression of GAPDH was analyzed as a control to ensure equal loading (anti-GAPDH, 1:1000 dilution in TBS, host/mouse). The secondary antibody uses was anti-mouse IgG/horseradish peroxidase, 1:12,000 dilution in TBS, purchased from Sigma.
Immunocytochemistry
Cells were grown to 70% confluence in 8-well Permanox chamber slides. The culture medium was removed, and the cells were washed with sterile PBS prior to fixation in acetone-methanol (1:1, v/v) for 5 min at room temperature. Following fixation, slides were blocked with 5% bovine serum albumin (BSA) for 20 min prior to a further washing step with PBS. Subsequently, the slides were incubated with the primary antibody diluted in 0.1% BSA/PBS for 2 h at room temperature (rat monoclonal anti-CD44 antibody (A020) (dilution, 1:200) Calbiochem; polyclonal rabbit anti-EGFR antibody (1005:sc-03) (dilution, 1:1000) Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Following a further washing step, slides were incubated with FITC-conjugated and/or TRITC-conjugated secondary antibodies for 1 h at room temperature. (DAKO, Cambridgeshire, UK) Cells were then mounted and analyzed by fluorescent microscopy.
Statistical Analysis
The results are expressed as the mean ± S.D. when normally distributed and median ± interquartile range (IQR) when not normally distributed. For normally distributed data, the paired Student's t test was used to identify statistical significance. For non-parametric data, the Wilcoxon signed rank test was used to test statistical significance. For experiments with multiple variables, the Friedman test was used for global comparison of different groups, followed by the Wilcoxon signed rank test for subgroup analysis. All data were analyzed using the software SPSS version 14.0 (SPSS Inc., Chicago, IL), and p < 0.05 was considered significant.
RESULTS
TGF-β1-dependent Proliferation in Fibroblasts Is Mediated through the HA Receptor, CD44, whereas the TGF-β1-dependent Antiproliferative Response Is Independent of CD44
The involvement of the principal HA receptor, CD44, in the regulation of TGF-β1-dependent proliferation in dermal fibroblasts was assessed by the use of siRNA to down-regulate CD44 levels (Fig. 1). Fig. 1A demonstrates the successful knockdown of CD44 expression in dermal fibroblasts following transient transfection with CD44 siRNA, as compared with dermal fibroblasts transfected with the scrambled control. In parallel experiments, the alamarBlueTM assay (Fig. 1B) and the [3H]thymidine assay (Fig. 1C) were used to assess the effect of CD44 knockdown on TGF-β1-dependent proliferation in dermal fibroblasts. As we have previously demonstrated (16), in the non-transfected cells and the cells transfected with scrambled controls, stimulation with TGF-β1 promotes proliferation in dermal fibroblasts. However, down-regulation of CD44 in these cells resulted in loss of TGF-β1-dependent proliferation and an antiproliferative response to TGF-β1 ensued.
In oral mucosal fibroblasts, TGF-β1 normally promotes an antiproliferative response (16). The involvement of CD44 in regulation of the TGF-β1-driven antiproliferative response in these cells was also assessed using siRNA to knock down CD44 levels (Fig. 2). Despite potent down-regulation of CD44 expression (Fig. 2A), there was no effect on the TGF-β1 driven antiproliferative response in oral cells using either the alamarBlueTM assay (Fig. 2B) or the [3H]thymidine assay (Fig. 2C).
In our previous studies, the differential effects of TGF-β1 on dermal and oral fibroblasts were shown to be related to the levels of HA generated by these cells (15, 16). In this study, the central role of HAS2 in the regulation of proliferation in fibroblasts was investigated using HAS2 overexpression vectors (Fig. 3). HAS2 overexpression in oral fibroblasts was initially confirmed by using RT and QPCR (Fig. 3A). Subsequent assessment of cell growth following HAS2 overexpression demonstrated that the oral fibroblasts then behaved like dermal fibroblasts and demonstrated a proliferative response to TGF-β1 (Fig. 3, B and C). Thereafter, the involvement of CD44 in TGF-β1-driven proliferation in HAS2-transfected oral fibroblasts was assessed. Initially successful overexpression of HAS2 and knockdown of CD44 were confirmed by RT and QPCR (Fig. 4, A and B, respectively). Following this, the [3H]thymidine assay was used to assess the effects on TGF-β1-dependent cell proliferation (Fig. 4C). The results revealed that oral fibroblasts transfected with mock or scrambled controls have an antiproliferative response to TGF-β1 as expected. In confirmation of the results shown in Fig. 3, B and C, oral fibroblasts transfected with the HAS2 overexpression vector demonstrated a proliferative response to TGF-β1. Of significance, following combined transfection with HAS2 and CD44 siRNA, the oral fibroblasts demonstrated an antiproliferative response to TGF-β1, indicating the important regulatory role of CD44 in this system.
EGF and EGFR Are Essential for TGF-β1/HA/CD44-dependent Proliferation in Dermal Fibroblasts and HAS2-overexpressed Oral Fibroblasts
We have previously shown that resistance to TGF-β1-driven fibroblast differentiation associated with cellular senescence is associated with loss of the EGFR (37). Comparison of EGFR and EGF expression was therefore performed in both oral and mucosal fibroblast populations using RT-QPCR to investigate their potential role in resistance to TGF-β1-driven proliferation (Fig. 5, A and B, respectively). However, both the scarring and non-scarring fibroblast phenotypes expressed EGFR (Fig. 5A) and EGF (Fig. 5B) at similar levels. Of note, TGF-β1 stimulation is shown to increase EGF mRNA expression in both fibroblast phenotypes (Fig. 5B).
To investigate the possible participation of either EGFR or EGF in TGF-β1-dependent proliferation, dermal cells were treated with TGF-β1 in the presence of either the EGFR inhibitor (AG1478) or an EGF antibody (Fig. 6, A and B, respectively), and cell proliferation was assessed using the [3H]thymidine assay. The addition of either the chemical inhibitor (AG1478) or anti-EGF neutralizing antibodies appeared to inhibit TGF-β1-driven proliferation in dermal fibroblasts. Furthermore, inhibiting either EGF or its antibody was also shown to inhibit the TGF-β1-driven proliferative response in oral HAS2-transfected cells (Fig. 7).
In the Presence of HA, EGFR and CD44 Appear to Co-localize
Signaling mediated by EGFR has been shown to be diversified by interaction with a number of proteins, including the HA receptor, CD44 (37, 39). These interactions have been shown to have fundamental importance in subsequent cell signaling cascades (40, 41). Given the decisive role played by both EGFR and CD44 in TGF-β1-driven proliferation, we sought to investigate the potential for interaction between these two proteins in our system using immunocytochemistry to stain for these proteins (Fig. 8). The results demonstrated increased yellow color depicted at the cell periphery in the images with HAS2 overexpression and TGF-β1 stimulation, suggesting increased EGFR and CD44 co-localization in these circumstances. In comparison, co-localization was not clearly apparent in either the mock-transfected oral fibroblasts or in the unstimulated HAS2 transfected oral fibroblasts.
TGF-β1/HA/CD44-dependent Fibroblast Proliferation Signals through the MAPK/ERK Pathway
The classical signaling pathway for TGF-β1 involves the Smad family of transcriptional proteins (42). However, several studies have shown that TGF-β1 can also signal in a Smad-independent fashion, recruiting pathways such as the MAPK pathway (42). In a recent paper, we have demonstrated the importance of MAPK/ERK signaling in TGF-β1-driven phenotypic activation of fibroblasts (37). We have therefore investigated a possible role for ERK signaling in TGF-β1-driven proliferation using the chemical ERK inhibitor PD98059. We initially showed the importance of ERK signaling in TGF-β1-driven proliferation in dermal fibroblasts because the addition of the ERK inhibitor was shown to attenuate the proliferative response (Fig. 9A). We then showed that ERK signaling is also essential for TGF-β1-driven proliferation in HAS2-overexpressed oral fibroblasts (Fig. 9B).
In light of the above results, we subsequently assessed the ability of the two fibroblast populations to activate ERK1 and ERK2 in response to TGF-β1 stimulation (Fig. 10). Western blot analysis in dermal fibroblasts demonstrated increased ERK1/2 phosphorylation at an early time point of 20 min following the administration of TGF-β1. ERK1/2 phosphorylation then returned to basal levels by 40 min. In dermal fibroblasts, a second peak of ERK1/2 activation was also demonstrated at the later time points of 1–3 h post-TGF-β1 stimulation. In comparison, Western blot analysis in oral fibroblasts also demonstrated increased ERK1/2 activation at the early time point of 10 min post-TGF-β1 stimulation. In contrast to dermal cells, however, oral fibroblasts did not demonstrate a second peak of ERK1/2 activation. The effect of inhibiting EGFR activation by using the AG1478 compound on ERK1/2 activation led to loss of both early and late ERK1/2 activation, indicating the importance of TGF-β1-dependent EGFR activation in MAPK/ERK signaling. These results were quantified and confirmed using scanning densitometry (Fig. 10B). We then investigated the effects of HAS2 overexpression in oral fibroblasts on ERK1/2 signaling (Fig. 11). The control or mock-transfected oral fibroblasts showed only early ERK1/2 activation at 10 min following TGF-β1 stimulation as previously. However, oral fibroblasts transfected with the HAS2 overexpression vector also demonstrated a second wave of ERK1/2 activation at 3 h post-TGF-β1 stimulation, similar to the pattern of ERK activation seen in dermal fibroblasts. Furthermore, the incubation of HAS2-transfected oral cells with the EGFR inhibitor (AG1478) again showed loss of both peaks of ERK1/2 activation. These results have also been quantified and confirmed by the use of scanning densitometry (Fig. 11B).
DISCUSSION
Fibroblast proliferation is a process central to tissue repair, and factors that govern fibroblast proliferation play an important role in determining wound-healing outcomes. The cytokine TGF-β1 is a known mediator of fibroblast proliferation, and several reports have established that increased expression of this cytokine directly correlates with progression of tissue fibrosis (31, 32, 43, 44). A better understanding of factors that are involved in the regulation of TGF-β1-mediated fibroblast proliferation is therefore crucial in determining the pathogenic mechanisms that lead to either impaired or excessive healing. During the search for regulators of fibroblast proliferation, hyaluronan was identified as a prime candidate. Many researchers have shown its involvement in processes fundamental to tissue repair (45–47). Our previous research demonstrated its central role in the regulation of TGF-β1-dependent fibroblast to myofibroblast differentiation (15, 48). We then proceeded to show that it also plays a key role in determination of fibroblast proliferative response to TGF-β1 (16). Notably, we showed that oral fibroblasts, with a non-scarring phenotype, generate low HA levels and show an inhibitory growth response to TGF-β1, whereas dermal fibroblasts, that can produce scarring, generate larger amounts of HA and show an accelerated growth response to this cytokine. Furthermore, we showed that the increased HA generation in dermal fibroblasts is causally related to TGF-β1-driven proliferation in these cells and that inhibiting HA synthesis in dermal fibroblasts resulted in an antiproliferative response to TGF-β1. Thus, we have previously demonstrated that modulating HA levels in dermal fibroblasts alters their function to behave in a manner similar to non-scarring oral mucosal fibroblasts. In this study, we provide mechanistic insights into how HA can modify TGF-β1-dependent proliferation in fibroblasts and thereby present a number of amenable targets for the control of fibroblast proliferation and ultimately for the regulation of tissue repair.
HA effects its biological responses in part through its interaction with a number of cell surface receptors (49, 50). In this work, we initially sought to determine if the principal HA receptor, CD44, was involved in either the TGF-β1-driven proliferative or antiproliferative responses in fibroblasts. The results indicated that knockdown of CD44 in the HA-rich dermal fibroblasts had a profound effect on TGF-β1-driven proliferation, leading to a complete reversal of this response with an ensuing antiproliferative response. Meanwhile, CD44 knockdown in oral fibroblasts did not change the TGF-β1 response. These results are in accordance with previous research from our group demonstrating that both HA and CD44 promote the profibrotic actions of TGF-β1 (15, 16, 48). To further verify these data, we overexpressed HAS2 in the non-scarring oral mucosal fibroblast phenotype. We have previously shown that the HAS2 overexpression vector increases HA production in fibroblasts, thus generating a HA-rich environment similar to that seen in dermal fibroblasts (25). This HA-rich environment subsequently led to a proliferative response to TGF-β1 in the oral cells, similar to the response seen in the scar-forming dermal fibroblasts. Furthermore, once the oral cells were in the presence of an HA-rich environment, CD44 once again became crucial in mediating this response. These results again assert that the levels of HA are decisive in determining the outcome of TGF-β1-dependent cell responses. It is not known whether the observed changes are due to the presence of HA bound to HA synthase, secreted HA, or increased HA actually bound to CD44; however, the data suggests that it is HA-CD44 binding that is necessary for TGF-β1-driven proliferation because the presence of HA as well as CD44 was necessary for this response to occur. Hence, these data suggest that in the presence of an HA-rich matrix, HA binds to CD44 and alters a fibroblast's proliferative response to TGF-β1, whereas in a non-HA-rich environment, TGF-β1 mediates its responses through other CD44-independent pathways, such as the Smad pathway (16). These results are consistent with our previous data showing that inhibition of HA synthesis in dermal fibroblasts results in an antiproliferative response to TGF-β1 (16).
The EGF receptor is a transmembrane glycoprotein that is a member of the ErbB family of tyrosine kinase receptors (51). It binds EGF as well as several other peptide growth factors and is involved in the activation of multiple signal transduction pathways, including MAPK (52). As such, it is known to be a robust regulator of cellular processes, such as proliferation (53). Signaling mediated by the EGFR is diversified by its interaction with a number of non-ErbB proteins, and the CD44 receptor has been reported to be one of these proteins (39). A number of reports have implicated EGF and its receptor in fibroproliferative processes in humans and in animal models of lung and kidney disease (35, 36, 54). Furthermore, some studies have demonstrated that synergistic action of EGF/EGFR and TGF-β1 can induce epithelial to mesenchymal transition, the process through which epithelial cells may transform into fibroblasts and promote fibrosis (35). Our group has previously demonstrated that EGF and EGFR signaling through the MAPK/ERK pathway are essential for HA-mediated TGF-β1-driven fibroblast to myofibroblast differentiation, and loss of either HA, EGF, or EGFR results in failure of fibroblast differentiation and suboptimal wound closure (37). In this study, we have investigated whether EGF and its receptor are also involved in TGF-β1-dependent fibroblast proliferation. The results demonstrated that although EGF and its receptor are both equally expressed in scarring and non-scarring fibroblast phenotypes, blocking either EGF or EGFR activity effectively attenuates TGF-β1/HA/CD44-dependent proliferation in the dermal cells as well as in the HA-rich oral mucosal cells, thus indicating that EGF and its receptor are both important targets for the manipulation of TGF-β1-driven fibroblast proliferation in an HA-rich environment.
As described above, CD44 can function as a co-receptor, physically co-localizing with other membrane-bound proteins, resulting in modulation of intracellular signal transduction pathways and facilitating the formation of specialized signaling complexes (39). In light of previous reports indicating interactions between CD44 and EGFR in other cell types, we sought to determine if this interaction occurs in our system. We have previously reported that in dermal fibroblasts, CD44 and EGFR co-localization occurs following TGF-β1 stimulation (37). Here we report that in oral fibroblasts, EGFR and CD44 co-localization only occurs in HAS2-transfected cells stimulated with TGF-β1. These data indicate that the presence of HA is required for CD44/EGFR coupling following TGF-β1 stimulation. These data are consistent with research performed in the cancer field that demonstrates that HA promotes clustering of EGFR and CD44, forming a functional unit in the cell membrane of tumor cells (55). Many studies have shown that HA itself is able to bind and activate the EGFR and thus potentiate downstream signaling processes (40). However, in our system, this does not appear to be the case because increasing HA levels through overexpression of HAS2 alone are insufficient to drive EGFR-CD44 coupling because TGF-β1 stimulation is also essential. We have also shown that TGF-β1 is required for EGF induction in both fibroblast phenotypes. Furthermore, the use of EGF blocking antibodies in HAS2-transfected oral cells eliminates the TGF-β1-driven proliferative response. This indicates that the proliferative response is also EGF-dependent and that HA does not substitute as a ligand for EGF in EGF/EGFR binding. Taken together, these data suggest that TGF-β1-mediated induction of EGF may be necessary to promote CD44/EGFR coupling, and EGF/EGFR as well as HA/CD44 binding is necessary to drive the CD44/EGFR interaction.
In our previous work, we have shown that both oral and dermal fibroblast populations demonstrate strong activation of the Smad signaling pathway in response to TGF-β1 (16). In addition, there was a critical role for the involvement of Smad3 in the regulation of both the proliferative response in dermal fibroblasts and the antiproliferative response in oral fibroblasts, suggesting that synergistic activity of Smad and other signaling intermediates dictate the final functional outcome of TGF-β1 stimulation. It is well known that TGF-β1 can also induce activation of the MAPK signaling pathway, and several studies have proposed a contributory role of MAPK/ERK signaling in the promotion of the fibrotic response (56, 57). We therefore sought to examine the potential involvement of MAPK/ERK signaling in TGF-β1-mediated proliferation in fibroblasts. The results reported here illustrate that inhibition of ERK1/2 activation significantly attenuated TGF-β1-driven proliferation in both dermal fibroblasts and in HA-rich oral mucosal fibroblasts. Comparison of ERK1/2 signaling in the two fibroblast populations demonstrated that in dermal fibroblasts, TGF-β1 stimulation led to two peaks of ERK1/2 activation, whereas oral fibroblasts only demonstrated early ERK1/2 phosphorylation in response to TGF-β1. Subsequent overexpression of HAS2 in oral fibroblasts, however, negated the demonstrated differences in ERK1/2 signaling between the two fibroblast phenotypes. Both peaks of ERK1/2 activation appear to be dependent on a functional EGFR. However, our results suggest that it is the late ERK1/2 activation that is HA- and CD44-dependent and leads to TGF-β1-driven proliferation, whereas the function of the early wave of ERK1/2 activation is not clear. Combining these data with our previous work, we propose that in fibroblasts with an HA-rich matrix, TGF-β1 stimulation further increases HA levels (15, 16) and promotes the following responses. First, it induces Smad3 activation (which has also been shown to be central to up-regulation of HAS2 and therefore increased HA levels) (37). In addition, it increases levels of EGF, which binds to EGFR and subsequently promotes early ERK1/2 activation, the functional significance of which remains unclear. Second, the increased HA levels enhance HA-CD44 binding, and the increased EGF binds to EGFR. Binding of these two ligands to their receptors promotes coupling of some of the EGFR with the CD44 receptors, and this promotes late ERK1/2 activation, which is then necessary to promote proliferation. In a non-HA-rich environment, Smad3 activation and only early ERK1/2 activation occurs, and this is associated with an antiproliferative response to TGF-β1. Although this model has many similarities to the model proposed for TGF-β1-dependent fibroblast differentiation, a number of key differences exist. First, Smad3 is the principal receptor-regulated Smad that is essential for TGF-β1-dependent fibroblast proliferation, whereas Smad2 was shown to be vital to fibroblast differentiation. Second, interaction between the Smad and MAPK pathway is probably necessary for TGF-β1-dependent proliferation, as discussed below. Furthermore, in the model proposed here, late activation of ERK1/2 is shown to be vital for TGF-β1-dependent proliferation to take place. In contrast, only early ERK1/2 activation appears to be necessary to induce cell differentiation (37).
Proteins from the MAPK pathway have been shown to modify the outcome of Smad signaling through several means. They are capable of altering phosphorylation of the Smads (58). However, this is unlikely to occur in our system because we have previously shown that Smad phosphorylation is similar in both fibroblast phenotypes. MAPK proteins may serve to regulate the capacity of Smad proteins to transolocate to the nucleus (59). They are also capable of physically interacting with transcription factors, thus regulating transcription of Smad target proteins in the nucleus (38, 60). Further investigation is therefore required to elucidate potential interaction between the MAPK/Smad pathways and to determine signaling events downstream of Smad3 and ERK1/2 phosphorylation to delineate the kinetics involved in the regulation of fibroblast proliferation by TGF-β1.
The mechanisms that control the transition between scar-free tissue repair and fibrosis are complex and not well understood. Our study provides mechanistic insights into this process. The data presented here extend the findings from our previous work that demonstrate the important regulatory role of an HA-rich pericellular matrix in the coordination of TGF-β1-dependent fibroblast proliferation (16). Using our previous reports together with our current findings, we propose that stimulation of proliferation in response to TGF-β1 requires an HA-rich matrix as well as functionality of CD44, EGF, and EGFR. Our proposed model of the relationship between TGF-β, HA, and fibroblast proliferative responses is illustrated in Fig. 12. In this model, HA facilitates TGF-β1-dependent fibroblast proliferation through promoting interaction between the CD44 and EGF receptor. This then promotes specific intracellular signal transduction through the MAPK/ERK pathway, which acts complementary to the Smad pathway, resulting in cell proliferation.
Acknowledgments
We acknowledge the help of Prof. D. W. Thomas and Prof. P. Stephens (Wound Biology Group, School of Dentistry, Cardiff University) for the provision of fibroblasts.
Footnotes
- HA
- hyaluronan
- EGFR
- EGF receptor
- QPCR
- quantitative PCR
- IQR
- interquartile range
- CD44
- cluster of differentiation
- Smad
- homologue of Drosophila protein mothers against decapentaplegic
- ECM
- extracellular matrix
- HAS2
- Hyaluronan Synthase 2
- siRNA
- small interfering RNA
- cDNA
- complementary DNA
- ErbB
- erythroblastosis oncogene B
- IQR
- interquartile range.
REFERENCES
- 1. Eddy A. A. (1994) J. Am. Soc. Nephrol. 5, 1273–1287 [DOI] [PubMed] [Google Scholar]
- 2. Bedossa P., Paradis V. (2003) J. Pathol. 200, 504–515 [DOI] [PubMed] [Google Scholar]
- 3. Francis G. S., McDonald K., Chu C., Cohn J. N. (1995) Am. J. Cardiol. 75, 11A–16A [DOI] [PubMed] [Google Scholar]
- 4. Grinnell F. (1994) J. Cell Biol. 124, 401–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabbiani G. (2003) J. Pathol. 200, 500–503 [DOI] [PubMed] [Google Scholar]
- 6. Krieg T., Heckmann M. (1989) Recenti Prog. Med. 80, 594–598 [PubMed] [Google Scholar]
- 7. Chipev C. C., Simon M. (2002) BMC Dermatol. 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002) Nat. Rev. Mol. Cell Biol. 3, 349–363 [DOI] [PubMed] [Google Scholar]
- 9. Armstrong J. R., Ferguson M. W. (1995) Dev. Biol. 169, 242–260 [DOI] [PubMed] [Google Scholar]
- 10. Chang H. Y., Chi J. T., Dudoit S., Bondre C., van de Rijn M., Botstein D., Brown P. O. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van den Bogaerdt A. J., van Zuijlen P. P., van Galen M., Lamme E. N., Middelkoop E. (2002) Arch. Dermatol. Res. 294, 135–142 [DOI] [PubMed] [Google Scholar]
- 12. Stephens P., Davies K. J., Occleston N., Pleass R. D., Kon C., Daniels J., Khaw P. T., Thomas D. W. (2001) Br. J. Dermatol. 144, 229–237 [DOI] [PubMed] [Google Scholar]
- 13. Stephens P., Davies K. J., al-Khateeb T., Shepherd J. P., Thomas D. W. (1996) J. Dent. Res. 75, 1358–1364 [DOI] [PubMed] [Google Scholar]
- 14. al-Khateeb T., Stephens P., Shepherd J. P., Thomas D. W. (1997) J. Periodontol. 68, 1063–1069 [DOI] [PubMed] [Google Scholar]
- 15. Meran S., Thomas D., Stephens P., Martin J., Bowen T., Phillips A., Steadman R. (2007) J. Biol. Chem. 282, 25687–25697 [DOI] [PubMed] [Google Scholar]
- 16. Meran S., Thomas D. W., Stephens P., Enoch S., Martin J., Steadman R., Phillips A. O. (2008) J. Biol. Chem. 283, 6530–6545 [DOI] [PubMed] [Google Scholar]
- 17. Kosaki R., Watanabe K., Yamaguchi Y. (1999) Cancer Res. 59, 1141–1145 [PubMed] [Google Scholar]
- 18. Legg J. W., Lewis C. A., Parsons M., Ng T., Isacke C. M. (2002) Nat. Cell. Biol. 4, 399–407 [DOI] [PubMed] [Google Scholar]
- 19. Itano N., Atsumi F., Sawai T., Yamada Y., Miyaishi O., Senga T., Hamaguchi M., Kimata K. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito T., Williams J. D., Al-Assaf S., Phillips G. O., Phillips A. O. (2004) Kidney Int. 65, 823–833 [DOI] [PubMed] [Google Scholar]
- 21. Camenisch T. D., Spicer A. P., Brehm-Gibson T., Biesterfeldt J., Augustine M. L., Calabro A., Jr., Kubalak S., Klewer S. E., McDonald J. A. (2000) J. Clin. Invest. 106, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zoltan-Jones A., Huang L., Ghatak S., Toole B. P. (2003) J. Biol. Chem. 278, 45801–45810 [DOI] [PubMed] [Google Scholar]
- 23. Brecht M., Mayer U., Schlosser E., Prehm P. (1986) Biochem. J. 239, 445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evanko S. P., Angello J. C., Wight T. N. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 1004–1013 [DOI] [PubMed] [Google Scholar]
- 25. Simpson R. M., Meran S., Thomas D., Stephens P., Bowen T., Steadman R., Phillips A. (2009) Am. J. Pathol. 175, 1915–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spicer A. P., McDonald J. A. (1999) Glycoforum, www.glycoforum.gr.jp/science/hyaluronan/HA07/HA07E.html
- 27. Spicer A. P., McDonald J. A. (1998) J. Biol. Chem. 273, 1923–1932 [DOI] [PubMed] [Google Scholar]
- 28. Ponta H., Sherman L., Herrlich P. A. (2003) Nat. Rev. Mol. Cell Biol. 4, 33–45 [DOI] [PubMed] [Google Scholar]
- 29. Border W. A., Noble N. A. (1994) N. Engl. J. Med. 331, 1286–1292 [DOI] [PubMed] [Google Scholar]
- 30. Border W. A., Noble N. A. (1995) J. Clin. Invest. 96, 655–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anscher M. S., Peters W. P., Reisenbichler H., Petros W. P., Jirtle R. L. (1993) N. Engl. J. Med. 328, 1592–1598 [DOI] [PubMed] [Google Scholar]
- 32. Border W. A., Noble N. A. (1997) Kidney Int. 51, 1388–1396 [DOI] [PubMed] [Google Scholar]
- 33. Ellis I. R., Schor A. M., Schor S. L. (2007) Exp. Cell Res. 313, 732–741 [DOI] [PubMed] [Google Scholar]
- 34. Samarakoon R., Higgins S. P., Higgins C. E., Higgins P. J. (2008) J. Mol. Cell Cardiol. 44, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Docherty N. G., O'Sullivan O. E., Healy D. A., Murphy M., O'neill A. J., Fitzpatrick J. M., Watson R. W. (2006) Am. J. Physiol. Renal. Physiol. 290, F1202–F1212 [DOI] [PubMed] [Google Scholar]
- 36. Hardie W. D., Davidson C., Ikegami M., Leikauf G. D., Le Cras T. D., Prestridge A., Whitsett J. A., Korfhagen T. R. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L1217–L1225 [DOI] [PubMed] [Google Scholar]
- 37. Simpson R. M., Wells A., Thomas D., Stephens P., Steadman R., Phillips A. (2010) Am. J. Pathol. 176, 1215–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Javelaud D., Mauviel A. (2005) Oncogene 24, 5742–5750 [DOI] [PubMed] [Google Scholar]
- 39. Pályi-Krekk Z., Barok M., Kovács T., Saya H., Nagano O., Szöllosi J., Nagy P. (2008) Cancer Lett. 263, 231–242 [DOI] [PubMed] [Google Scholar]
- 40. Bourguignon L. Y., Gilad E., Brightman A., Diedrich F., Singleton P. (2006) J. Biol. Chem. 281, 14026–14040 [DOI] [PubMed] [Google Scholar]
- 41. Kim Y., Lee Y. S., Choe J., Lee H., Kim Y. M., Jeoung D. (2008) J. Biol. Chem. 283, 22513–22528 [DOI] [PubMed] [Google Scholar]
- 42. Attisano L., Wrana J. L. (2002) Science 296, 1646–1647 [DOI] [PubMed] [Google Scholar]
- 43. Beanes S. R., Dang C., Soo C., Ting K. (2003) Expert Rev. Mol. Med. 5, 1–22 [DOI] [PubMed] [Google Scholar]
- 44. Connor T. B., Jr., Roberts A. B., Sporn M. B., Danielpour D., Dart L. L., Michels R. G., de Bustros S., Enger C., Kato H., Lansing M. (1989) J. Clin. Invest. 83, 1661–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen P. Y., Huang L. L., Hsieh H. J. (2007) Biochem. Biophys. Res. Commun. 360, 1–6 [DOI] [PubMed] [Google Scholar]
- 46. Chen W. Y., Abatangelo G. (1999) Wound Repair Regen. 7, 79–89 [DOI] [PubMed] [Google Scholar]
- 47. Jiang D., Liang J., Noble P. W. (2007) Annu. Rev. Cell Dev. Biol. 23, 435–461 [DOI] [PubMed] [Google Scholar]
- 48. Webber J., Meran S., Steadman R., Phillips A. (2009) J. Biol. Chem. 284, 9083–9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knudson C. B., Knudson W. (2000) Glycoforum, www.glycoforum.gr.jp/science/hyaluronan/HA10a/HA10aE.html
- 50. Turley E. A., Harrison M. (1999) Glycoforum, www.glycoforum.gr.jp/science/hyaluronan/HA11/HA11E.html
- 51. Poumay Y., Mitev V. (2009) Folia Med. 51, 5–17 [PubMed] [Google Scholar]
- 52. Dong J., Ramachandiran S., Tikoo K., Jia Z., Lau S. S., Monks T. J. (2004) Am. J. Physiol. Renal. Physiol. 287, F1049–F1058 [DOI] [PubMed] [Google Scholar]
- 53. Pandiella A., Lehvaslaiho H., Magni M., Alitalo K., Meldolesi J. (1989) Oncogene 4, 1299–1305 [PubMed] [Google Scholar]
- 54. Terzi F., Burtin M., Hekmati M., Federici P., Grimber G., Briand P., Friedlander G. (2000) J. Clin. Invest. 106, 225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bourguignon L. Y., Gilad E., Peyrollier K. (2007) J. Biol. Chem. 282, 19426–19441 [DOI] [PubMed] [Google Scholar]
- 56. Ma F. Y., Sachchithananthan M., Flanc R. S., Nikolic-Paterson D. J. (2009) Front. Biosci. (Schol. Ed.) 1, 171–187 [DOI] [PubMed] [Google Scholar]
- 57. Deng Z. Y., Li J., Jin Y., Chen X. L., Lü X. W. (2009) Chin. Med. J. 122, 1449–1454 [PubMed] [Google Scholar]
- 58. Funaba M., Murakami M. (2008) J. Biochem. Biophys. Methods 70, 816–819 [DOI] [PubMed] [Google Scholar]
- 59. Engel M. E., McDonnell M. A., Law B. K., Moses H. L. (1999) J. Biol. Chem. 274, 37413–37420 [DOI] [PubMed] [Google Scholar]
- 60. Zhang Y., Feng X. H., Derynck R. (1998) Nature 394, 909–913 [DOI] [PubMed] [Google Scholar]