Introduction
Connexin 43 (Cx43)3 is the predominant isoform of gap junction proteins in the working myocardium. In the heart, MAPKs are implicated in regulating Cx43 remodeling; however, their precise roles remain obscure. Mitogen-activated protein kinase kinase 4 (MKK4) is a critical component of the stress-activated MAPK signaling pathway. We have demonstrated previously that MKK4 antagonizes cardiomyocyte hypertrophy. Herein, we investigate the role of MKK4 in regulating Cx43 expression in cardiomyocytes. We found that knockdown of MKK4 expression or inhibition of its kinase activity in neonatal rat cardiomyocytes (NRCMs) significantly reduced phenylephrine-induced Cx43 expression. Furthermore, two activator protein-1 (AP-1) binding elements in the Cx43 promoter region were identified as being responsible for the MKK4-regulated Cx43 expression. Consistently, we also detected heterogeneously reduced Cx43 expression and attenuated zonula occludens-1 (ZO-1) content in the hearts of MKK4 cardiomyocyte-specific knock-out mice (MKK4cko) following pressure overload. To test whether heterogeneously reduced Cx43 expression contributes to ventricular arrhythmic vulnerability, MKK4cko and control mice were subjected to pressure overload followed by programmed electrical stimulation (PES). Six of 13 MKK4cko mice, but none of the controls, developed ventricular tachycardia. Epicardial activation mapping recorded from the MKK4cko hypertrophied heart showed ventricular activation delay. Mathematical models have simulated that the spatially heterogeneous decrease in Cx43 causes slowed ventricular conduction and fragmented wave propagations leading to re-entrant excitations. Collectively, these data reveal a novel role for MKK4 in regulating Cx43 expression and preventing hypertrophy-associated arrhythmogenesis.
Cx43 is the predominant isoform of gap junction proteins in the working myocardium. It forms low resistance cell-to-cell channels, allowing ions and small molecules to move between adjacent cells and facilitating the orderly spread of the excitation wave responsible for synchronous contraction of the heart (1). Alterations in Cx43 expression may contribute directly to the arrhythmic substrate, as evidenced by studies in mice with a cardiomyocyte-specific deletion of Cx43. Loss of Cx43 expression in the heart results in sudden arrhythmic death due to increased gap junctional resistance and slowed conduction velocity, which form a substrate for re-entrant arrhythmias (2–4). Cx43 has a fast turnover rate with a half-life in the range of 1–5 h, implying a highly regulated process for its synthesis, trafficking, and degradation (1, 5). In the heart, MAPKs are implicated in regulating Cx43 remodeling by either phosphorylation- or transcription-dependent mechanisms (6–11); however, their precise roles are ambiguous. For example, activation of JNK in cardiomyocytes was correlated with the up-regulation of Cx43 after amphetamine treatment (6), whereas Petrich et al. (7, 8) reported that in the hearts of transgenic mice overexpressing constitutive active MKK7 increased JNK activity was accompanied by a substantial reduction in Cx43 expression.
MKK4 is a critical component of the stress-activated MAP kinase signaling pathway, which activates JNKs to regulate diverse physiological processes (12). It was observed in vitro that MKK7 and MKK4 preferentially phosphorylate JNK on threonine 183 and tyrosine 185, respectively (13, 14). The targeted deletion of the mkk4 or mkk7 gene leads to embryonic lethality, providing genetic evidence that MKK4 and MKK7 have nonredundant roles in vivo (12). This concept is underscored by the observation in mice with brain-specific ablation of mkk4 that decreased JNK activity caused a defect in neuronal migration and premature death (15). Furthermore, we have provided substantial evidence demonstrating the functional importance of MKK4 in the heart, where it is required in protecting the heart from maladaptive pathological hypertrophy (16).
In the current study, we investigated the role of MKK4 in regulating Cx43 expression in cardiomyocytes in response to hypertrophic stress. We found in NRCMs that knockdown of endogenous MKK4 expression by siMKK4 or inhibition of its kinase activity by infection of an adenovirus encoding a dominant-negative form of MKK4 (Ad-dnMKK4) caused a substantial reduction in Cx43 expression together with inactivation of the JNK/c-Jun pathway after phenylephrine (PE) stimulation. To gain insight into the mechanism responsible for the decreased Cx43 transcript level, luciferase reporter assays were performed in siMKK4- or Ad-dnMKK4-NRCMs in which blunted Cx43 promoter reporter activity was detected after PE treatment. We also have demonstrated that MKK4 regulates Cx43 transcription most likely by virtue of two AP-1 binding sites in the Cx43 proximal promoter region.
Consistent with these data, we observed decreased transcript and protein levels of Cx43 in the MKK4-deficient myocardium after 1 week of transverse aortic constriction (TAC). Furthermore, the remaining Cx43 was found to be distributed heterogeneously in the MKK4cko-TAC hearts. In addition, we discovered a reduction in ZO-1 protein expression in the MKK4cko-TAC myocardium. To assess whether heterogeneously reduced Cx43 in the MKK4-deficient myocardium contributes to ventricular arrhythmic vulnerability, PES was applied to TAC-treated MKK4cko and MKK4f/f (littermate controls) mice, almost half of the MKK4cko mice (six of 13) compared with none of the controls (0/11) exhibited multiple episodes of ventricular tachycardia. Epicardial activation mapping was recorded in the isolated Langendorff-perfused hearts showing ventricular activation delay in the MKK4cko-TAC heart. Accordingly, mathematical simulation models have demonstrated that the spatially heterogeneous decrease in Cx43 in MKK4cko-TAC mice causes slowed ventricular conduction and fragmented excitation wave fronts leading to re-entry, which may account for the increased susceptibility in ventricular arrhythmias.
Overall, in the present work, we provide new information revealing the role of MKK4 in regulating Cx43 gene expression in cardiomyocytes, implying MKK4 is a critical cardiac protector preventing hypertrophy-associated ventricular arrhythmias through restriction of Cx43 remodeling.
EXPERIMENTAL PROCEDURES
Animal Models
MKK4f/f and their littermates MKK4cko mice were generated previously (16) and used in the present study. All mice were maintained in a pathogen-free facility at the University of Manchester. The animal studies were performed in accordance with the UK Home Office and institutional guidelines.
Quantitative Real-time PCR
Total RNA was prepared from NRCMs or ventricular tissues. Real-time quantitative PCRs were performed using the SYBR Green I Core kit (Eurogentec). The primers used for detection of Cx43 and GAPDH expression were obtained from Qiagen.
Immunoblot Analysis
Protein extracts (50 μg) were subjected to immunoblot analysis with antibodies against Cx43, N-cadherin, β-catenin, plakoglobin, and tubulin (Sigma); ZO-1 (Zymed Laboratories Inc.); MKK7, JNK, c-Jun, phospho-JNK, and phosho-cJun (Cell Signaling); and MKK4 (BD Pharmingen).
Immunohistochemical Analyses
Fresh cryosections of ventricular tissues were used to analyze Cx43, N-cadherin, β-catenin, and plakoglobin by indirect immunofluorescence. As secondary antibodies, goat anti-mouse or goat anti-rabbit antibodies, conjugated to Alexa Fluoro 488 (Invitrogen) or Alexa Fluoro 568 (Invitrogen), respectively, were used.
Adenovirus Vector Construction
Using the vector containing the rat Cx43 (rCx43) promoter region (−148, +280) as a template, we created a single mutation at either the AP-1 (−47, −39) site, or the AP-1 (−122, −112) site, or double mutations at both AP-1 sites using the QuikChange site-directed mutagenesis kit (Stratagene). Adenovirus expressing wild type Cx43 promoter-Luc or various mutations was generated using ViraPower Adenoviral Expression System (Invitrogen).
Luciferase Reporter Assay
48 h post-transfection of siRNA, NRCMs were infected with Ad-Cx43APwt-Luc for 24 h. Following PE treatment (100 μm for 24 h), aliquots of NRCM lysates were assayed for luciferase activity using a luciferase assay kit (Promega). To measure rCx43 promoter-luciferase activity after blocking MKK4 activation, NRCMs were infected with Ad-dnMKK4 for 24 h prior to the infection of Ad-Cx43APwt-Luc for a further 24 h. Then, NRCMs were treated with 100 μm PE for 24 h followed by the luciferase assay. To determine whether MKK4-regulated Cx43 transcription was dependent on AP-1 transcriptional activity, NRCMs were first infected with either Ad-GFP or Ad-caMKK4 for 24 h, Ad-Cx43APwt-Luc, or Ad-Cx43APma-Luc, or Ad-Cx43APmb-Luc, or Ad-Cx43APdm-Luc was added to either Ad-GPF-NRCMs or Ad-caMKK4-NRCMs for a further 24 h. Luciferase activity in each experimental group was then measured described as above.
ECG
8–10 week old male MKK4f/f and MKK4cko mice were subjected to either sham or TAC operation for 1 week as described previously (16). To monitor cardiac rhythms at baseline and in hypertrophic conditions, we carried out in vivo ECG analysis on anesthetized mice. RR interval, P wave duration, PR interval, QRS, JT and QT durations were recorded.
PES
To assess propensity to ventricular arrhythmias, 1 week TAC-treated mice were subjected to PES. A pacing train of eight stimuli (S1) was delivered at a basic cycle length of 100 ms, with a single (S2) premature extrastimulus introduced at progressively shorter intervals until an arrhythmia was induced or the ventricular refractory period was reached.
Epicardial Activation Mapping
Epicardial activation mapping of the left ventricular free wall was recorded in isolated Langendorff-perfused hearts using custom-made 64 separated electrodes (8 × 8 grids, 0.55-mm spacing) at a basic cycle length of 100 ms. The activation time was determined as the point of maximal negative slope and displayed in a grid representing the layout of the original recording array. All activation times were related to the timing of the first detected waveform. Isochrones were drawn manually around areas activated in steps of 1 ms.
Statistical Analysis
Data are expressed as mean ± S.E. and analyzed using two-way analysis of variance followed by Bonferonni's post test where appropriate. Comparisons between two groups were performed using Student's t test. p values <0.05 are considered statistically significant. An expanded “Materials and Methods” section is available in the supplemental data.
RESULTS
MKK4 Regulates Cx43 Gene Expression via AP-1 Binding Sites
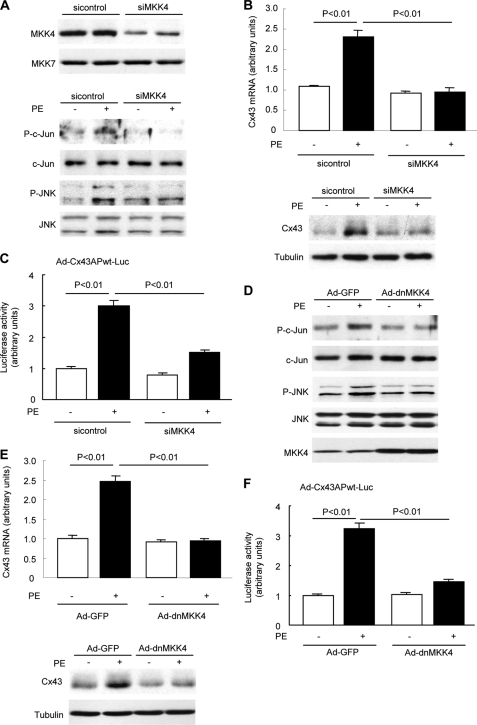
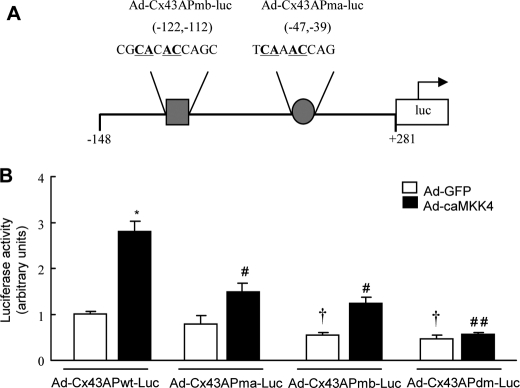
Transcriptional regulation is a key mechanism dictating Cx43 expression. The down-regulation of Cx43 ascribable to altered transcriptional regulation has been reported in many forms of heart disease (17, 18). To investigate whether MKK4 regulates Cx43 expression in response to hypertrophic stress, we first examined Cx43 mRNA level and protein expression in NRCMs in which endogenous MKK4 protein was knocked down by 70% using siMKK4 (Fig. 1A). Blunted phosphorylation of JNK and c-Jun after PE stimulation was observed when MKK4 expression was deficient (Fig. 1A). In response to PE stimulation (100 μm, 48 h), siMKK4-NRCMs showed a significant reduction in Cx43 mRNA level, and a corresponding substantial decrease in its protein expression also was detected (Fig. 1B). We performed a systematic promoter-reporter study in NRCMs to determine the regulation of the rCx43 proximal promoter by MKK4. NRCMs were pretreated with siMKK4 prior to infection with a recombinant adenovirus encoding the rCx43 promoter region extending 148 nucleotides upstream to 281 nucleotides downstream relative to the transcription initiation site (−148 to +281, containing two AP-1 binding sites, Ad-Cx43APwt-Luc). Following PE stimulation (100 μm, 24 h), a significant decline in rCx43 promoter-luciferase activity was detected in siMKK4-NRCMs versus that in the control siRNA-NRCMs (Fig. 1C). To corroborate these data, we then infected NRCMs with a recombinant adenovirus expressing a dominant-negative form of MKK4 (Ad-dnMKK4) to block activation of the JNK/c-Jun pathway (Fig. 1D). Similarly, significantly reduced Cx43 mRNA and protein levels were observed in Ad-dnMKK4-NRCMs (Fig. 1E). Moreover, PE stimulation failed to induce a similar extent of rCx43 promoter-luciferase activity in Ad-dnMKK4-NRCMs, compared with that in Ad-GFP-NRCMs (Fig. 1F). To determine whether MKK4-regulated Cx43 transcription is dependent on AP-1 transcriptional activity, we generated various recombinant adenoviruses containing either a single mutation at the AP-1 (−47 to −39) site, referred to as Ad-Cx43APma-Luc; or mutation at the AP-1 (−122 to −112) site, referred to as Ad-Cx43APmb-Luc, or double mutations at both AP-1 binding sites (Ad-Cx43APdm-Luc) (Fig. 2A). As shown in Fig. 2B, infection of adenovirus encoding constitutively active MKK4 (Ad-caMKK4) significantly increased the Ad-Cx43APwt-Luc activity in NRCMs. Conversely, Ad-Cx43APdm-Luc activity proved to be considerably decreased even at basal level, co-infection of Ad-caMKK4 into NRCMs failed to restore its luciferase activity. Co-infection of Ad-caMKK4 with either Ad-Cx43APma-Luc or Ad-Cx43APmb-Luc could not render a similar level of luciferase activity as Ad-Cx43APwt-Luc. Together, these results indicate that MKK4 is required for Cx43 transcription, acting through two AP-1 binding sites in the Cx43 proximal promoter via JNK/c-Jun activation. Decreased Cx43 protein expression is likely attributable to reduced its mRNA level.
FIGURE 1.
MKK4 regulates Cx43 expression via JNK/c-Jun activation in cardiomyocytes. A, NRCMs were transfected with MKK4 siRNA or control siRNA for 72 h prior to immunoblotting for MKK4 expression. MKK7 protein expression was examined to determine the specificity of MKK4 knockdown. siMKK4-NRCMs were treated with PE (100 μm) for 30 min before detecting phosphorylation of JNK and c-Jun by immunoblotting. B, quantitative real-time PCR analyses of Cx43 transcript levels (upper panel) and immunoblot analysis of Cx43 protein expression (lower panel) in siMKK4-NRCMs following PE treatment (100 μm, 48 h). Tubulin expression is the protein loading control. C, siRNA-transfected NRCMs were infected with AdCx43APwt-Luc (multiplicity of infection, 25) for 24 h followed by PE treatment (100 μm, 24 h). The Cx43 promoter-dependent luciferase activity was measured by the luciferase reporter assay system. Data are mean ± S.E. (n = 3 per group). D, Ad-dnMKK4-NRCMs were treated with PE (100 μm) for 30 min before detecting activation of c-Jun and JNK by immunoblotting. Immunoblot analysis shows MKK4 expression in Ad-dnMKK4-NRCMs. E, quantitative real-time PCR analyses of Cx43 transcript levels (upper panel) and immunoblot analysis of Cx43 protein expression (lower panel) in Ad-dnMKK4-NRCMs. Ad-GFP is a control virus. Tubulin expression is the protein loading control. F, infection of Ad-dnMKK4 in cardiomyocytes decreased the PE-induced Cx43 promoter-dependent luciferase activity. Data are mean ± S.E. (n = 3 per group).
FIGURE 2.
MKK4-regulated Cx43 transcription is dependent on AP-1 transcriptional activity. A, schematic diagram of mutated rCx43 promoter-luciferase constructs (mutated nucleotides are underlined and in boldface). B, the infection of Ad-caMKK4 significantly increased the AdCx43APwt-Luc activity. *, p < 0.01 versus Ad-GFP + Ad-Cx43APwt-Luc. Ad-Cx43APdm-Luc activity was decreased even at the basal level. †, p < 0.05 compared with Ad-GFP + Ad-Cx43APwt-Luc. Co-infection of Ad-caMKK4 and Ad-Cx43APdm-Luc could not ameliorate the reduction. ##, p < 0.001 versus Ad-caMKK4 + Ad-Cx43APwt-Luc. Moreover, co-infection of Ad-caMKK4 failed to restore the reduced luciferase level induced by Ad-Cx43APma-Luc or Ad-Cx43APmb-Luc. #, p < 0.01 versus Ad-caMKK4 + Ad-Cx43APwt-Luc (n = 6 per group). Data are mean ± S.E.
Heterogeneous Reduction of Cx43 in MKK4cko-TAC Mice
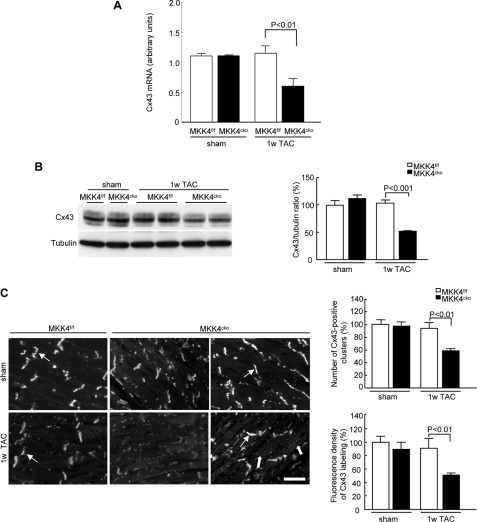
Prompted by the above findings, we then analyzed Cx43 mRNA levels, protein content, and distribution in the MKK4-deficient myocardium. As shown in Fig. 3A, a down-regulation of the Cx43 transcript was detected in the MKK4cko hearts 1 week after TAC, which was applied to induce a pressure overload and subsequent hypertrophy on the heart. Accordingly, we observed a decreased Cx43 protein level (∼50% of controls) in the MKK4cko heart following 1 week TAC (Fig. 3B) and heterogeneity in Cx43 distribution shown by the absence of Cx43 labeling in some patches of the MKK4cko ventricular free wall, whereas in some patches of the myocardium, Cx43 labeling was scattered in the cytoplasm (Fig. 3C). Quantification of the Cx43 immunofluorescence revealed reduced aggregate number by 42% and intensity by 49% in the MKK4cko-TAC hearts compared with the littermate controls (MKK4f/f-TAC), thus confirming heterogeneous Cx43 expression (Fig. 3C). In addition to Cx43, we also examined Cx40 and Cx45 expression in the MKK4cko ventricles. Cx40 and Cx45 are important connexins in the heart. Cx40 is expressed predominantly in atrium, whereas Cx45 is the major gap junction isoform expressing in myocytes of the sinoatrial and atrioventricular nodes. By immunoblotting and immunohistochemistry, no detectable levels of Cx40 and Cx45 expression were observed in the ventricles of either MKK4cko or MKK4f/f mice (data not shown). Together, these data show that under hypertrophic stress MKK4 deficiency causes reduced and heterogeneous Cx43 expression.
FIGURE 3.
Reduced and spatially heterogeneous distribution of Cx43 in MKK4cko-TAC mice. A, quantitative real-time PCR analyses of Cx43 transcript levels. The data are derived from three independent experiments performed in triplicate and are normalized to the GAPDH content (n = 5 per group). B, immunoblot analyses demonstrate significantly decreased Cx43 content. Tubulin expression is the protein loading control. The ratios of Cx43/tubulin are represented by the bar graphs (n = 6 per group). C, immunohistochemical staining of Cx43 in MKK4cko mice. Thick arrows point to diffuse Cx43 labeling in the cytoplasm, whereas thin arrows show Cx43 distributed in intercalated discs. Scale bar, 5 μm. The number of Cx43-positive clusters and fluorescence intensity of Cx43 labeling are quantified and expressed by the bar graphs (n = 6 per group). Data are mean ± S.E. 1w TAC, 1-week TAC.
ZO-1 Reduction in MKK4cko-TAC Mice
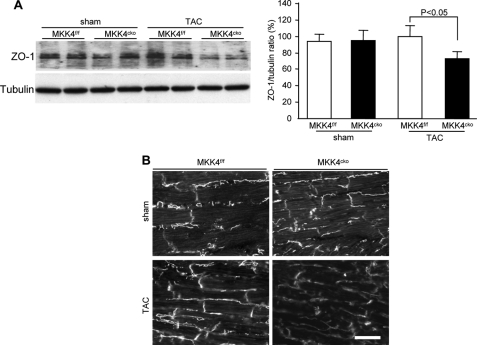
Cx43 is localized primarily at the intercalated disc where gap junctions are in close proximity to zonula adherens junctions and desmosomes (19). ZO-1, a component of tight junctions, is known to be an important adaptor protein for Cx43 (20). Because we found diffused Cx43 labeling in the MKK4cko-TAC cardiomyocytes, we then analyzed whether changes in ZO-1 expression or localization occurred in MKK4-deprived myocardium after pressure overload. Indeed, immunoblot analysis showed an ∼30% diminution in ZO-1 protein expression in the MKK4cko-TAC heart (Fig. 4A); this result was further confirmed by immunohistochemistry (Fig. 4B). In addition, we analyzed N-cadherin, β-catenin, and plakoglobin expression, all of which are important for the assembly and maintenance of Cx43 at the plasma membrane (1, 21). Expression and localization of these junctional proteins were not different visibly between the controls and MKK4cko-TAC mice as shown by immunoblotting and immunohistochemistry (supplemental Fig. I). Examination of the structure of MKK4-decificent cardiomyocytes by transmission electron microscopy surprisingly showed that the structure of myofibrils, sarcomeres, and Z-lines appeared normal in the MKK4cko-TAC cardiomyocytes relative to the controls (supplemental Fig. II). Adherens junctions and demosomes characterized as submembranous electron dense zones in the vicinity of intercellular spaces were visible in both genotypes (supplemental Fig. II). Combined, these results suggest that overall cytoarchitecture of the MKK4cko cardiomyocytes appears normal and intact compared with the controls. As such, the patchy reduction in Cx43 expression is due most likely to loss of MKK4 and is not caused by any disruption in the intercalated disc. Moreover, a reduction in ZO-1 expression might play a role in the diffused positioning of cellular Cx43.
FIGURE 4.
Decreased ZO-1 content in MKK4cko-TAC mice. A, immunoblot analyses of ZO-1 expression show a moderate but significant decrease in the MKK4cko-TAC heart. The ratios of ZO-1/tubulin are expressed in the bar graphs. B, immunohistochemical staining for ZO-1 confirmed decreased ZO-1 in the mutants (scale bar, 5 μm). Data are mean ± S.E (n = 6 per group).
Disruption of MKK4 in Cardiomyocytes Sensitizes Mice to Ventricular Arrhythmias
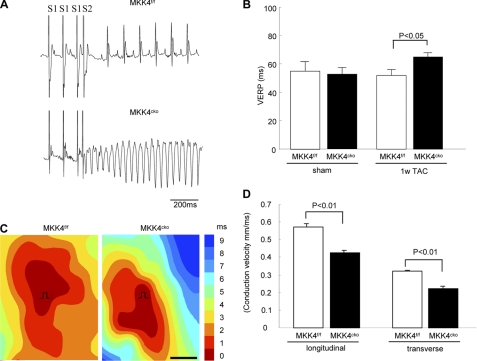
Previous studies suggest that patchy Cx43 reduction likely forms a proarrhythmic substrate (2–4). Herein, we first examined surface ECG on anesthetized mice. The ECG recordings revealed marked abnormalities in ventricular conduction in MKK4cko-TAC mice, reflected by wider QRS durations (16.02 ± 0.62 ms) compared with 10.37 ± 0.46 ms (QRS) in the controls (Table 1). QRS/QTc ratio also was significantly greater in the MKK4cko-TAC heart compared with that in the control heart. However, no significant differences in JT intervals were observed in the two TAC groups (Table 1). These ECG parameters indicate slowed ventricular conduction in the MKK4cko-TAC heart. Next, we performed PES to evaluate whether the heterogeneous decrease in Cx43 and slowed ventricular conduction sensitized MKK4cko mice to ventricular arrhythmias. A train of eight stimuli (S1) was delivered at a basic cycle length of 100 ms, with a single extrastimulus (S2) added at progressively shorter intervals to induce ventricular arrhythmias. This S1-S2 pacing protocol induced multiple (more than six) episodes of ventricular tachycardia in six of 13 MKK4cko-TAC mice, which lasted 1333 ± 182 ms (Fig. 5A). The ventricular effective refractory period also was determined by the same S1-S2 protocol. Consistent with the wider QRS duration, we noted a prolonged the ventricular effective refractory period in the MKK4cko-TAC heart (Fig. 5B). To examine whether heterogeneously reduced Cx43 expression in MKK4-decifient myocardium affects ventricular electrical propagation, epicardial mapping of electrical activity of the left ventricular free wall was performed on the isolated Langendorff-perfused hearts. As illustrated in Fig. 5C, ventricular activation was significantly delayed in the MKK4cko-TAC heart, as shown by slowed conduction velocity (CV) at both longitudinal and transverse directions in the MKK4cko-TAC heart, compared with that in the control heart (Fig. 5D).
TABLE 1.
Electrocardiographic assessment of MKK4f/f and MKK4cko mice one week after TAC
P-wave duration, QRS complex duration, and intervals of RR, PR, JT, and QT were recorded from anesthetized mice. QTc was obtained by correction for the heart rate. Data are presented as mean ± S.E. *, p < 0.01 versus MKK4f/f-TAC mice.
| ECG parameters | Sham |
TAC |
||
|---|---|---|---|---|
| MKK4f/f (n = 7) | MKK4cko (n = 6) | MKK4f/f (n = 11) | MKK4cko (n = 13) | |
| Heart rate (bpm) | 376 ± 12 | 403 ± 13 | 391 ± 17 | 398 ± 15 |
| P duration (ms) | 10.2 ± 0.9 | 10.1 ± 1.0 | 11.3 ± 1.1 | 10.5 ± 0.7 |
| RR interval (ms) | 161.36 ± 6.1 | 150.15 ± 5.4 | 157.00 ± 6.07 | 158.20 ± 10.15 |
| PR interval (ms) | 39.99 ± 1.90 | 38.38 ± 1.70 | 37.92 ± 1.99 | 37.36 ± 2.28 |
| QRS duration (ms) | 10.27 ± 0.75 | 10.50 ± 0.51 | 10.37 ± 0.46 | 16.02 ± 0.62* |
| QTc (ms) | 69.47 ± 4.34 | 76.59 ± 3.30 | 69.95 ± 3.64 | 90.69 ± 2.54* |
| JT (ms) | 17.63 ± 1.28 | 19.10 ± 1.60 | 17.19 ± 1.33 | 19.44 ± 0.71 |
| QRS/QTc | 0.15 | 0.14 | 0.15 | 0.18* |
FIGURE 5.
MKK4cko-TAC mice are more susceptible to ventricular arrhythmias and slowed conduction velocity. A, subjected to a train of eight stimuli (S1 at 100 ms) with a premature stimulus at 90 ms (S2). No episodes of arrhythmias were induced in the MKK4f/f-TAC heart (upper panel); however, ventricular tachycardia was induced in the MKK4cko-TAC heart (lower panel) (scale bar: 200 ms). B, the ventricular effective refractory period was measured using the same S1-S2 protocol, showing a prolonged ventricular effective refractory period in the MKK4cko-TAC heart (n = 6–13 per group). C, representative epicardial activation maps recorded from the MKK4f/f and MKK4cko hearts of TAC-treated mice. Earliest activation is found in red, the latest activation is shown in blue, and numbers indicate activation time (ms). The slowing of conduction and propagation of excitation was seen in the MKK4cko-TAC heart. D, summary data illustrate that both longitudinal CV and transverse CV were significantly slower in the MKK4cko heart (n = 3 per group). Data are presented as mean ± S.E.
Computational Simulations of Cx43 Remodeling
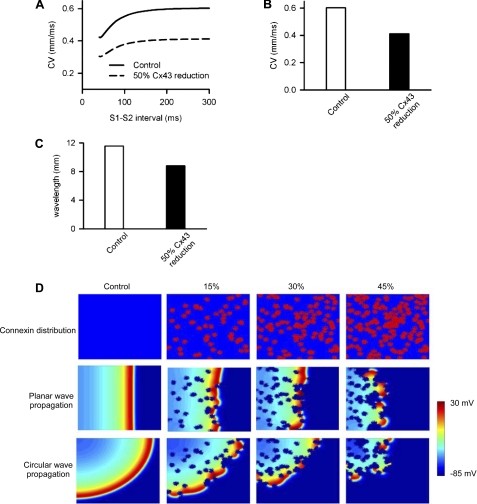
To substantiate the data from epicardial activation mapping, computer simulations were performed to address whether or not patched Cx43 reduction is proarrhythmic as suggested in this and previous studies (3, 21–23). First, we implemented a mathematical model of mouse ventricular myocytes to study CV in homogeneous tissue consisting of 100 cells. In this model, the changes in CV and wavelengths were studied based on an equivalent percentage of intercellular electrical coupling reduction uniformly across the strand to the reduced level of Cx43 expression observed in the MKK4cko-TAC heart. As expected, the control CV of solitary wave in strands of mycoytes was 0.603 mm/ms at an S1-S2 interval of 100 ms; however, a diminution in intercellular electrical coupling by 50% reduced the solitary wave CV to 0.415 mm/ms. Accordingly, the control wavelength (wavelength = CV × averaged action potential duration at 90% repolarization across the strand) of the solitary wave was 11.6 mm, and when intercellular electrical coupling was reduced by 50%, the wavelength was reduced to 8.8 mm, which is attributable to the decreased CV (Fig. 6, A–C). Further simulations were performed in a two-dimensional model of homogeneous ventricular tissue (ventricular tissue has a size of 10 mm × 10 mm with 200 × 200 cells) to study the effects of an arbitrarily and randomly distributed pattern of patched reduction in the intercellular electrical coupling on CV of planer and circular waves. With patched reduction of gap junctional coupling, break-up of the wavefronts was observed in all cases (Fig. 6D). These patches gave distorted wavefronts of the planer and circular waves, leading to fragmented wavefronts forming multiple re-entrant excitation wavelets. Together, the simulations show that heterogeneously reduced intercellular electrical coupling due to heterogeneously reduced expression of Cx43 most likely contributes to slowed conduction velocity, fragmented wavefronts of excitation waves, which increased propensity for arrhythmias.
FIGURE 6.
Computational simulations of the effects of Cx43 remodeling on increased propensity for arrhythmias. A, CV under control (solid line) and 50% homogeneous reduction of Cx43 (dashed line). B, conduction velocity when the S1-S2 interval is equal to 100 ms. C, the effect of reduced Cx43 coupling on wavelength of a solitary wave. D, effects heterogeneous reduction of gap junctional coupling on the propagation of ventricular excitation waves in a two-dimensional model of the ventricular sheet. The top rows show a color-coded distribution of gap junctional coupling (blue, D = 0.011 mm2/ms; red, D = 0), the middle and bottom rows show snapshots of planer wave conduction and circular wave conduction, respectively. Snapshots of ventricular excitation wave conduction were shown for the control conduction (first columns), a 15% reduction of the gap junctional coupling (second columns), a 30% reduction (third columns), and a 45% reduction (fourth columns).
DISCUSSION
In the present study, we provide several lines of evidence demonstrating that MKK4 regulates Cx43 expression in cardiomyocytes. Knockdown of MKK4 or blocking its kinase activity in primary rat neonatal cardiomyocytes resulted in a reduction in Cx43 expression. By promoter-reporter assays, we have shown that MKK4 modulates Cx43 expression through AP-1 activity. Study of the MKK4cko heart provided in vivo evidence demonstrating functional effects of MKK4 deficiency-induced Cx43 alteration. A deficiency of MKK4 in cardiomyocytes caused patchy Cx43 reduction in response to pressure overload. This alteration in Cx43 expression/distribution may constitute an arrhythmogenic substrate in MKK4cko-TAC mice.
Regulation of Cx43 Expression by MKK4
Assembly of gap junctions is a multiphase process, including connexin synthesis, trafficking, and formation of intercellular channels, of which connexin synthesis is the rudimentary step. It has been shown that the Cx43 promoter region contains several putative transcription factor binding sites for AP-1, cAMP response element, and specific protein-1 (SP-1), all of which are thought to be involved in up-regulation of Cx43 transcription (24, 25). On the other hand, muscle segment homeobox genes Msx1/2 can function in concert with T-box factors Tbx2/3 to repress Cx43 expression (26). Despite this progress in understanding the transcriptional regulation of Cx43, the genetic control mechanisms underlying the spatial and temporal expression pattern of Cx43 remain exclusive. In the current study, we have provided several lines of evidence to demonstrate MKK4 regulation of Cx43 gene expression: 1) knockdown of endogenous MKK4 expression by siMKK4 or inhibition of its kinase activity by infection of Ad-dnMKK4 caused a substantial reduction in Cx43 expression; 2) luciferase reporter assays showed blunted Cx43-promoter reporter activity in siMKK4- or Ad-dnMKK4-NRCMs after PE treatment; 3) increased MKK4 activation in NRCMs resulted in elevated Cx43 promoter-reporter activity; and 4) luciferase assays using mutations in the Cx43 promoter region demonstrated that MKK4-regulated Cx43 transcription is dependent on AP-1 transcriptional activity. Consistent with these data, decreased Cx43 expression was also detected in the MKK4cko-TAC heart. Of note, there was a similar reduction of Cx43 at both the mRNA and protein level. We thus believe that the MKK4/JNK/c-Jun pathway regulates Cx43 transcription via AP-1 activity in cardiomyocytes under hypertrophic stress and that decreased Cx43 protein expression is likely attributable to its reduced mRNA level.
Cx43 is a highly phosphorylated and regulated protein. It has been proposed that phosphorylation of Cx43 is related to its internalization (5); however, previous studies have also demonstrated that dephosphorylated Cx43 is associated with trafficking/endocytosis in the cytoplasm, whereas those gap junctions remaining in the intercalated disc contain phosphorylated Cx43 (27, 28). In our experimental setting, we presently cannot rule out that (de)phosphorylation of Cx43 contributes to its reduction and heterogeneous expression in MKK4cko-TAC cardiomyocytes. However, the migratory pattern of Cx43 on SDS-PAGE gel electrophoresis appeared similar in both controls and MKK4cko-TAC hearts. Alterations in Cx43 phosphorylation status may not always be reflected in its mobility on polyacrylamide gels, and, therefore, additional studies employing antibodies specific for different phosphorylated isoforms of Cx43 are necessary to resolve this issue.
Roles of Junctional Proteins in MKK4cko-TAC Mice
It has become apparent that molecular “cross-talk” exists between gap junctions and adherens junctions or desmosomes. For example, cardiac-specific perturbation of N-cadherin leads to the formation of an arrhythmogenic substrate by an alteration in Cx43 (29, 30). β-Catenin, in addition to scaffolding N-cadherin to actin, also contributes to transcriptional regulation of Cx43 (31). Mutations in plakoglobin are linked to a rare recessive disorder, Naxos disease (32). Interestingly, Cx43 levels are decreased remarkably in the hearts of Naxos disease patients, who present with right ventricular cardiomyopathy accompanied by a high incidence of arrhythmias and sudden cardiac death (32). ZO-1, in fact, physically associates with Cx43 acting as an adaptor, which anchors gap junctions to the cytoskeleton (20, 33). Abolition of the association of ZO-1 and Cx43 results in a reduction in the number of gap junction plaques and an increase in mean plaque size (33). In the present study, we did not observe visible abnormalities in the expression and distribution of N-cadherin, β-catenin, and plakoglobin in the MKK4cko heart. However, ZO-1 expression proved to be significantly decreased in the MKK4cko hypertrophic heart. This reduction of ZO-1 expression may underlie the observed diffused expression of Cx43, part of which was detected in the cytoplasm. It is presently unclear whether MKK4 deficiency is directly responsible for the decreased ZO-1 expression or whether it is secondary to the reduction of Cx43 expression.
Loss of MKK4 Couples Hypertrophic Signals to Arrhythmogenesis
Cell-to-cell coupling and myocardial tissue architecture are thought to be major factors dictating normal impulse propagation through the heart (34). QRS duration is determined by impulse conduction velocity and cardiomyocyte size (35). One week after TAC, MKK4cko mice displayed increased cardiac hypertrophy as indicated by enlarged cross-sectional area (346.5 ± 3.26 μm2) compared with 252.4 ± 6.88 μm2 cross-sectional area in the MKK4f/f cardiomyocyte (16). In the present study, ECG recordings showed prolonged QRS duration and greater QRS/QTc ratio in the MKK4cko-TAC heart, whereas JT intervals did not appear significantly different between the two TAC groups. Epicardial activation mapping demonstrated delayed ventricular electrical propagation in the MKK4cko-TAC heart. Together, these results indicate that the MKK4cko-TAC heart had slowed ventricular conduction, which is likely attributed to patchy Cx43 reduction together with increased cardiomyocyte size. Furthermore, in the hypertrophied MKK4cko heart, heterogeneity in Cx43 expression is thought to result in cardiomyocyte uncoupling, which creates the nonuniformities required for initiating breaks in a propagating wavefront. The mathematical models in this study have simulated this phenomenon. Computational simulations demonstrate that this spatially heterogeneous decrease in Cx43 accounts for slowed ventricular conduction and fragmented wave propagations, a critical factor for arrhythmogenesis. Previous studies have shown that ventricular myocardium is not homogeneous; it has electrophysiologically distinct cell types, which give rise to transmural voltage gradients and a dispersion of repolarization (36). Heterogeneity in Cx43 distribution has been correlated with increased transmural dispersion in refractoriness (37), and the hypertrophic response of enlarged myocardium mass, is believed to add additional factors to transmural dispersion of repolarization, augmenting electrical heterogeneities intrinsic to the ventricular myocardium. As such, it is plausible that heterogeneously reduced Cx43 expression together with increased hypertrophy predispose MKK4cko mice to ventricular arrhythmias.
We have noticed that although a significant decrease was seen in Cx43 expression at both the mRNA and protein level in all MKK4cko-TAC hearts; however, six of 13 mutant mice developed ventricular tachycardia when in vivo ventricular programmed pacing was introduced. The possible reasons for such individual discrepancy in occurrence of ventricular tachycardia could be due to the heterogeneous reduction and distribution of Cx43 in MKK4cko-TAC mice, or due to individual difference in the extent of hypertrophy induced by TAC hence leads to a nonuniform reduction and/or distribution of Cx43 in MKK4cko-TAC mice, or due to the nature of the different response (changes in Cx43 expression) of individual mouse to TAC stress and genetic modification of MKK4.
Of note, apart from cardiomyocytes, interstitial collagen deposition is also responsible for cardiac tissue architecture. Increased interstitial fibrosis, especially reparative fibrosis, which replaces zones of dead cardiomyocytes with collagen within muscle bundles, can disrupt impulse propagation. This type of fibrotic change is expected to amplify the effect of conduction slowing and electrical uncoupling in the areas of Cx43 remodeling. Interestingly, 1 week of TAC treatment did not cause apparent apoptosis and fibrosis in MKK4cko mice (16); thus, involvement by interstitial fibrosis in ventricular tachycardia occurrence in MKK4cko mice can be excluded.
Conclusions
In summary, we have identified a new role for MKK4 in regulating Cx43 expression in cardiomyocytes. MKK4 is a critical regulator in the heart for maintaining Cx43 expression/distribution, which are important for preservation of normal cardiac electrical function. The recognition of the functional significance of MKK4 in preventing arrhythmogenesis, together with our previous findings that MKK4 antagonizes pathological hypertrophy, may lead to the development of better therapies for treating hypertrophy-associated ventricular arrhythmias.
Supplementary Material
This work was supported by British Heart Foundation Grants PG/07/055/23144 (to X. W. and E. J. C.) and PG/09/052/27833 (to X. W., E. J. C. and M. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods,” Figs. 1 and 2, and additional references.
- Cx43
- connexin 43
- MKK4
- mitogen-activated protein kinase kinase 4
- NRCM
- neonatal rat cardiomyocyte
- PE
- phenylephrine
- AP-1
- activator protein-1
- ZO-1
- zonula occludens-1
- PES
- programmed electrical stimulation
- Luc
- luciferase
- TAC
- transverse aortic constriction
- ECG
- electrocardiography
- CV
- conduction velocity
- SP-1
- specific protein-1
- QTc
- corrected QT
- rCx43
- rat Cx43
- Ad
- adenovirus
- caMKK4
- constitutively active MKK4
- dnMKK4
- dominant negative MKK4.
REFERENCES
- 1. Saffitz J. E., Kléber A. G. (2004) Circ. Res. 94, 585–591 [DOI] [PubMed] [Google Scholar]
- 2. Gutstein D. E., Morley G. E., Tamaddon H., Vaidya D., Schneider M. D., Chen J., Chien K. R., Stuhlmann H., Fishman G. I. (2001) Circ. Res. 88, 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gutstein D. E., Danik S. B., Sereysky J. B., Morley G. E., Fishman G. I. (2003) Am. J. Physiol. Heart. Circ. Physiol. 285, H1091–H1096 [DOI] [PubMed] [Google Scholar]
- 4. van Rijen H. V., Eckardt D., Degen J., Theis M., Ott T., Willecke K., Jongsma H. J., Opthof T., de Bakker J. M. (2004) Circulation 109, 1048–1055 [DOI] [PubMed] [Google Scholar]
- 5. Laird D. W. (2005) Biochimica. et Biophysica. Acta 1711, 172–182 [DOI] [PubMed] [Google Scholar]
- 6. Shyu K. G., Wang B. W., Yang Y. H., Tsai S. C., Lin S., Lee C. C. (2004) Cardiovasc. Res. 63, 98–108 [DOI] [PubMed] [Google Scholar]
- 7. Petrich B. G., Eloff B. C., Lerner D. L., Kovacs A., Saffitz J. E., Rosenbaum D. S., Wang Y. (2004) J. Biol. Chem. 279, 15330–15338 [DOI] [PubMed] [Google Scholar]
- 8. Petrich B. G., Gong X., Lerner D. L., Wang X., Brown J. H., Saffitz J. E., Wang Y. (2002) Circ. Res. 91, 640–647 [DOI] [PubMed] [Google Scholar]
- 9. Cameron S. J., Malik S., Akaike M., Lerner-Marmarosh N., Yan C., Lee J. D., Abe J., Yang J. (2003) J. Biol. Chem. 278, 18682–18688 [DOI] [PubMed] [Google Scholar]
- 10. Ogawa T., Hayashi T., Kyoizumi S., Kusunoki Y., Nakachi K., MacPhee D. G., Trosko J. E., Kataoka K., Yorioka N. (2004) J. Cell Sci. 117, 2087–2096 [DOI] [PubMed] [Google Scholar]
- 11. Salameh A., Schneider P., Mühlberg K., Hagendorff A., Dhein S., Pfeiffer D. (2004) Eur. J. Pharmacol. 503, 9–16 [DOI] [PubMed] [Google Scholar]
- 12. Wang X., Destrument A., Tournier C. (2007) Biochim. Biophys. Acta 1773, 1349–1357 [DOI] [PubMed] [Google Scholar]
- 13. Brancho D., Tanaka N., Jaeschke A., Ventura J. J., Kelkar N., Tanaka Y., Kyuuma M., Takeshita T., Flavell R. A., Davis R. J. (2003) Genes Dev. 17, 1969–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tournier C., Whitmarsh A. J., Cavanagh J., Barrett T., Davis R. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 7337–7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X., Nadarajah B., Robinson A. C., McColl B. W., Jin J. W., Dajas-Bailador F., Boot-Handford R. P., Tournier C. (2007) Mol. Cell Biol. 27, 7935–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu W., Zi M., Jin J., Prehar S., Oceandy D., Kimura T. E., Lei M., Neyses L., Weston A. H., Cartwright E. J., Wang X. (2009) Circ. Res. 104, 905–914 [DOI] [PubMed] [Google Scholar]
- 17. Peters N. S., Green C. R., Poole-Wilson P. A., Severs N. J. (1993) Circulation 88, 864–875 [DOI] [PubMed] [Google Scholar]
- 18. Dupont E., Matsushita T., Kaba R. A., Vozzi C., Coppen S. R., Khan N., Kaprielian R., Yacoub M. H., Severs N. J. (2001) J. Mol. Cell Cardiol. 33, 359–371 [DOI] [PubMed] [Google Scholar]
- 19. Saffitz J. E. (2005) Ann. N.Y. Acad. Sci. 1047, 336–344 [DOI] [PubMed] [Google Scholar]
- 20. Toyofuku T., Yabuki M., Otsu K., Kuzuya T., Hori M., Tada M. (1998) J. Biol. Chem. 273, 12725–12731 [DOI] [PubMed] [Google Scholar]
- 21. Severs N. J., Bruce A. F., Dupont E., Rothery S. (2008) Cardiovasc. Res. 80, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danik S. B., Liu F., Zhang J., Suk H. J., Morley G. E., Fishman G. I., Gutstein D. E. (2004) Circ. Res. 95, 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gutstein D. E., Morley G. E., Vaidya D., Liu F., Chen F. L., Stuhlmann H., Fishman G. I. (2001) Circulation 104, 1194–1199 [DOI] [PubMed] [Google Scholar]
- 24. Teunissen B. E., Jansen A. T., van Amersfoorth S. C., O'Brien T. X., Jongsma H. J., Bierhuizen M. F. (2003) Gene 322, 123–136 [DOI] [PubMed] [Google Scholar]
- 25. Mitchell J. A., Lye S. J. (2005) Endocrinology 146, 2048–2054 [DOI] [PubMed] [Google Scholar]
- 26. Boogerd K. J., Wong L. Y., Christoffels V. M., Klarenbeek M., Ruijter J. M., Moorman A. F., Barnett P. (2008) Cardiovasc. Res. 78, 485–493 [DOI] [PubMed] [Google Scholar]
- 27. Matsushita T., Oyamada M., Fujimoto K., Yasuda Y., Masuda S., Wada Y., Oka T., Takamatsu T. (1999) Circ. Res. 85, 1046–1055 [DOI] [PubMed] [Google Scholar]
- 28. Lampe P. D., Cooper C. D., King T. J., Burt J. M. (2006) J. Cell Sci. 119, 3435–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kostetskii I., Li J., Xiong Y., Zhou R., Ferrari V. A., Patel V. V., Molkentin J. D., Radice G. L. (2005) Circ. Res. 96, 346–354 [DOI] [PubMed] [Google Scholar]
- 30. Li J., Patel V. V., Kostetskii I., Xiong Y., Chu A. F., Jacobson J. T., Yu C., Morley G. E., Molkentin J. D., Radice G. L. (2005) Circ. Res. 97, 474–481 [DOI] [PubMed] [Google Scholar]
- 31. Ai Z., Fischer A., Spray D. C., Brown A. M., Fishman G. I. (2000) J. Clin. Invest. 105, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKoy G., Protonotarios N., Crosby A., Tsatsopoulou A., Anastasakis A., Coonar A., Norman M., Baboonian C., Jeffery S., McKenna W. J. (2000) Lancet 355, 2119–2124 [DOI] [PubMed] [Google Scholar]
- 33. Maass K., Shibayama J., Chase S. E., Willecke K., Delmar M. (2007) Circ. Res. 101, 1283–1291 [DOI] [PubMed] [Google Scholar]
- 34. Kléber A. G., Rudy Y. (2004) Physiol. Rev. 84, 431–488 [DOI] [PubMed] [Google Scholar]
- 35. Wiegerinck R. F., Verkerk A. O., Belterman C. N., van Veen T. A., Baartscheer A., Opthof T., Wilders R., de Bakker J. M., Coronel R. (2006) Circulation 113, 806–813 [DOI] [PubMed] [Google Scholar]
- 36. Antzelevitch C. (2001) Cardiovasc. Res. 50, 426–431 [DOI] [PubMed] [Google Scholar]
- 37. Wiegerinck R. F., van Veen T. A., Belterman C. N., Schumacher C. A., Noorman M., de Bakker J. M., Coronel R. (2008) Heart Rhythm. 5, 1178–1185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.