Abstract
Increasing evidence supports a role for PKCα in growth arrest and tumor suppression in the intestinal epithelium. In contrast, the Id1 transcriptional repressor has pro-proliferative and tumorigenic properties in this tissue. Here, we identify Id1 as a novel target of PKCα signaling. Using a highly specific antibody and a combined morphological/biochemical approach, we establish that Id1 is a nuclear protein restricted to proliferating intestinal crypt cells. A relationship between PKCα and Id1 was supported by the demonstration that (a) down-regulation of Id1 at the crypt/villus junction coincides with PKCα activation, and (b) loss of PKCα in intestinal tumors is associated with increased levels of nuclear Id1. Manipulation of PKCα activity in IEC-18 nontransformed intestinal crypt cells determined that PKCα suppresses Id1 mRNA and protein via an Erk-dependent mechanism. PKCα, but not PKCδ, also inhibited Id1 expression in colon cancer cells. Id1 was found to regulate cyclin D1 levels in IEC-18 and colon cancer cells, pointing to a role for Id1 suppression in the antiproliferative/tumor suppressive activities of PKCα. Notably, Id1 expression was elevated in the intestinal epithelium of PKCα-knock-out mice, confirming that PKCα regulates Id1 in vivo. A wider role for PKCα in control of inhibitor of DNA binding factors is supported by its ability to down-regulate Id2 and Id3 in IEC-18 cells, although their suppression is more modest than that of Id1. This study provides the first demonstrated link between a specific PKC isozyme and inhibitor of DNA binding factors, and it points to a role for a PKCα → Erk ⊣ Id1 → cyclin D1 signaling axis in the maintenance of intestinal homeostasis.
Keywords: Colon Cancer, Cyclins, ERK, Helix-Loop-Helix Transcription Factors, Intestine, Phorbol Esters, Protein Kinase C (PKC), Signal Transduction, Inhibitor of DNA Binding 1 (Id1), PKCalpha
Introduction
The intestinal epithelium is a continually self-renewing tissue, organized into well defined proliferative and functional compartments (1). Maintenance of tissue integrity relies on tight control of the balance between proliferative activity, differentiation, and apoptosis. Both positive and negative growth regulatory signaling pathways have been implicated in orchestrating the renewal process in this tissue, and disruption of these pathways and/or their downstream targets results in various diseases, including cancer. Increasing evidence points to the PKC enzyme system as a key regulator of intestinal homeostasis (2–6). PKC is a family of phospholipid-dependent serine-threonine kinases, consisting of at least 10 isozymes that act as central players in signal transduction. Members of the family can have tumor promoting (e.g. PKCβII, -ϵ, and -ι) or tumor suppressive (e.g. PKCα and -δ) activity, dependent on the isozyme and tissue context (2, 5, 7, 8). Although several PKC isozymes have been implicated in the regulation of intestinal homeostasis (e.g. PKCα, -βII, -δ, -ϵ, and -ι), increasing evidence points to PKCα as a key negative regulator of proliferation and tumorigenesis in this tissue (4, 9, 10).
PKCα is activated precisely at the point of growth arrest in the crypts of both the small intestine and colon (11, 12). Consistent with a role in growth suppression, activation of PKCα in intestinal crypt-like cells triggers a program of cell cycle withdrawal, mediated by the Ras-Erk pathway and involving down-regulation of cyclin D1, induction of p21Cip1/p27Kip1, activation of pocket proteins, and loss of DNA licensing factors such as cdc6 (9, 13). PKCα-induced down-regulation of cyclin D1 occurs via two apparently independent mechanisms as follows: inhibition of cyclin D1 transcription and blockade of translation initiation, involving protein phosphatase 2A-mediated dephosphorylation of the translational repressor 4E-BP1 (4, 14, 15).
Increasing evidence supports a tumor-suppressive role for PKCα in the intestinal epithelium. The protein is broadly lost in human and murine intestinal tumors (4), and PKCα deficiency is associated with increased crypt cell proliferation and spontaneous intestinal adenoma formation in mice (10). PKCα signaling is disrupted in intestinal tumors with or without perturbation in adenomatous polyposis coli (APC)4/β-catenin signaling, as well as in human colon cancer cell lines that differ in the status of APC, β-catenin, K-Ras, and/or p53 (4). Thus, loss of PKCα signaling is a general characteristic of intestinal tumors regardless of other underlying genetic defects, pointing to the importance of this pathway in the maintenance of intestinal homeostasis and blockade of intestinal tumor formation.
The tumor-suppressive effects of PKCα are likely to involve multiple targets (4, 16–19). Regulation of cyclin D1 appears to play an important role in the intestine because of the following: (a) loss of PKCα in intestinal tumors correlates with marked up-regulation of this cyclin; (b) restoration of PKCα suppresses cyclin D1 levels in colon cancer cells, and (c) forced expression of cyclin D1 partially blocks the ability of PKCα to reverse the transformed phenotype of these cells (4). However, PKCα retains significant in vitro tumor suppressive activity even in the presence of forced expression of cyclin D1 (4), pointing to the involvement of additional as yet unidentified targets.
Inhibitor of DNA binding 1 (Id1) is a member of the Id family of dominant negative antagonists of basic helix-loop-helix transcription factors, which also includes Id2, Id3, and Id4 (20). Id1 and PKCα appear to play opposing roles in the intestinal epithelium. Lacking a DNA-binding motif, Id1 acts by heterodimerizing with other transcription factors (e.g. basic helix-loop-helix factors, Ets2, and Pax family members), preventing their DNA binding and blocking their antiproliferative and differentiation-inducing functions (20, 21). Consistent with this activity, Id1 expression is generally restricted to proliferating/nondifferentiated cells (22, 23). The proliferative activity of Id1 has been attributed to modulation of signal transduction pathways involving TGFβ, vitamin D, and EGF receptor (EGFR) and effects on cell cycle proteins such as cyclin D1, p21Cip1, p27Kip1, and p16Ink4 (20, 24–27). Evidence also points to Id1 as a potential oncogene in the breast, prostate, and ovary (20, 27, 28). Notably, mice with an Id1 transgene targeted to the small intestinal epithelium show an increased incidence of adenomas (29), indicating that, in contrast to PKCα, Id1 has oncogenic properties in the intestine.
This study explored the relationship between PKCα signaling and Id1, and determined, for the first time, that PKCα negatively regulates Id1 expression in intestinal cell lines and in the mouse intestine in vivo.
EXPERIMENTAL PROCEDURES
Mouse Tissues
Experiments involving mice were in accordance with institutional and national guidelines/regulations under an IACUC-approved protocol. APCmin/+ mice (C57BL/6J-ApcMin/J) were from The Jackson Laboratory. PKCα−/− mice (C57Bl/6.SV-129J Prkca−/−), in a mixed C57Bl/6-SV129J background, were obtained from Dr. Jeffery D. Molkentin (Cincinnati Children's Medical Center, Cincinnati, OH) (30). B6129SF2/J mice were used as wild-type controls for PKCα−/− mice (no sex or strain differences in immunostaining were seen between B6129SF2/J, C57Bl/6 mice, KK, or AIJ mice). Intestinal tissues were dissected, rinsed, and immediately fixed in 4% freshly depolymerized paraformaldehyde in PBS and paraffin-embedded for immunohistochemistry or placed in ice-cold Hanks' balanced salt solution (without magnesium or calcium) for isolation of epithelial cells. Azoxymethane-induced tumors, a gift from Drs. Joshua Uronis and David Threadgill, were obtained by administering 10 mg/kg azoxymethane to KK/AIJ mice (2–4 months old, The Jackson Laboratory) in four weekly intraperitoneal injections. Mice were euthanized 5–6 months after the first injection, and intestinal tissue was fixed in 10% neutral buffered formalin overnight and embedded in paraffin.
Immunohistochemistry
Paraffin-embedded tissues were sectioned (5–10 μm), deparaffinized, rehydrated, and incubated in 3% H2O2 (15 min) to block endogenous peroxidase activity. For PKCα and cyclin D1 localization, antigen retrieval involved heating (30–40 min) in DAKO target retrieval solution at 90 °C followed by cooling for 20 min. Sections were “blocked” with 0.03% casein (30 min) and incubated with anti-PKCα (Epitomics 1510-1 rabbit monoclonal antibody at 1:2000–1:5000) or anti-cyclin D1 (Lab Vision Corp. SP4 anti-cyclin D1 antibody at 1:100) antibody for 1 h at room temperature or overnight at 4 °C. Tissues were then incubated with biotinylated anti-rabbit secondary antibody (Vector Laboratories, 1:250), followed by Vectastain Elite ABC reagent (Vector Laboratories) (PKCα) or streptavidin (Zymed Laboratories Inc., 1:20) (cyclin D1) and DAKO DAB chromogen solution (K3466). Counterstaining was performed with hematoxylin. Staining for Id1 using Biocheck BCH-1/195-14-50 rabbit monoclonal anti-mouse/human Id1 antibody at 1:100 was performed similarly except that antigen retrieval involved incubation with EDTA buffer (50 mm Tris buffer containing 1 mm EDTA, pH 8.0) overnight at 60 °C, and primary antibody incubation was followed by addition of EnVisionTM + (Dako K4010) Polymer-HRP goat × rabbit IgG (ready-to-use form). Controls for staining specificity involved omission of primary antibody or replacement of the primary antibody with normal rabbit serum. In the case of PKCα, immunostaining specificity was confirmed using intestinal tissues from PKCα knock-out mice.
Images were obtained using an Olympus BX41 microscope with UPlan F1 lenses (×10–40) and a DP70 digital camera with accompanying software. Linear adjustments to contrast and brightness were performed using Adobe Photoshop and Microsoft PowerPoint. For comparison of wild-type and PKCα−/− intestines, sections were immunostained on the same slide, photographed at the same exposure, and processed identically. Comparison of normal and tumor tissue involved parallel analysis of tissue from the same section.
Isolation of Intestinal Epithelial Cells
Villus/crypt fractions of small intestinal epithelium were isolated as described (31). Briefly, freshly dissected small intestines were rinsed with ice-cold Hanks' balanced salt solution (without magnesium or calcium) containing 0.5 mm DTT (Hanks'/DTT), everted or cut open, and cut into 1–2-cm lengths. Intestinal segments were washed in Hanks'/DTT, and fractions of intestinal epithelial crypt-villus units were obtained by sequential washing with ice-cold chelating buffer (27 mm trisodium citrate, 5 mm Na2HPO4, 96 mm NaCl, 8 mm KH2PO4, 1.5 mm KCl, 0.5 mm DTT, 55 mm d-sorbitol, 44 mm sucrose). Following isolation, washes were pooled into five fractions, and epithelial cells were pelleted by centrifugation at 500 × g and processed for Western blotting. Analysis for the presence of the crypt cell marker, cyclin D1, revealed that fractions 1 and 2 (V1 and V2) contained exclusively villus cells, with crypt cells present in fractions 4 and 5 (C1 and C2). Low levels of cyclin D1 were variably detected in fraction 3 (V3) indicating that crypts began to detach in later washes of this fraction or early washes of fraction 4.
Human Colonic Tissue
Colon tumor tissues were collected from six patients at Roswell Park Cancer Institute, with written informed consent and approval by the Institutional Review Board. Adjacent normal mucosa was available for two of the cases. Tissue was formaldehyde-fixed and paraffin-embedded in the Pathology Core at Roswell Park Cancer Institute.
Cell Culture and Drug Treatment Protocols
IEC-18 nontransformed intestinal crypt cells (ATCC CRL-1589) were maintained in DMEM supplemented with 10 μg/ml insulin, 4 mm glutamine, and 5% fetal bovine serum (FBS). Human colorectal cancer cell lines FET, FET-DNR, GEO (Dr. M. G. Brattain), DLD-1 (Dr. R. J. Bernacki, Roswell Park Cancer Institute), and HCT116 (ATCC) were cultured in RPMI 1640 medium, 10% FBS, and 2 mm l-glutamine. Cells were maintained in a 5% CO2 atmosphere at 37 °C.
PKC isozymes were activated in cells by treatment with 100 nm phorbol 12-myristate 13-acetate (PMA) (LC Labs), 20 μg/ml 1,2-dioctanoyl-sn-glycerol (DiC8) (Sigma), or 100 nm bryostatin (Biomol) for various times. DiC8 was added repeatedly (every hour) in fresh medium to compensate for its rapid metabolism in cells. PMA and bryostatin were dissolved in ethanol (final ethanol concentration in the medium of <0.1%); DiC8 was dissolved in DMSO (final DMSO concentration in the medium of <0.2%). Control cells were treated with the appropriate vehicle alone. Depletion of PKCα, -δ, and -ϵ from IEC-18 cells was accomplished by treatment with 1 μm phorbol 12,13-dibutyrate (PDBu) (Sigma) for 24 h (17).
PKC activity was inhibited using the general PKC inhibitors, Gö6983 (1 μm) or bisindolylmaleimide I (5 μm), or with 2 μm Gö6976 (an inhibitor of classical PKCs that is selective for PKCα in IEC-18 cells). Inhibition of Erk/MAPK activity was achieved using 10 μm U0126 or 50 μm PD098059; PI3K/Akt activity was inhibited using 50 μm LY294002. U0126 and PD098059 were purchased from Alexis; all other inhibitors were from Calbiochem. Cells were pretreated with kinase inhibitors for 30 min (or 1 h for PD098059 and LY294002) prior to addition of PKC agonist. Equivalent volumes of vehicle (DMSO for all inhibitors) were added to control samples.
Western Blot Analysis
Cells were extracted in boiling SDS lysis buffer (10 mm Tris, pH 7.4, 1% SDS) and analyzed by Western blotting using 12% polyacrylamide gels as described (9). Blots were routinely stained with 0.1% Fast Green (Sigma) to ensure equal loading and even transfer. Antibody dilutions were as follows: Id1 (Biocheck, CA, BCH-1/195-14-50), 1:100; Id1 (Santa Cruz Biotechnology, sc-488) 1:100; PKCα (Santa Cruz Biotechnology, sc-8393), 1:1000; PKCα (Epitomics 1510-1), 1:5000; PKCδ (Santa Cruz Biotechnology, sc-213), 1:2000; PKCϵ (Santa Cruz Biotechnology, sc-214), 1:2000; phospho-Erk1/2 (Cell Signaling, 9106), 1:2000; total Erk1/2 (Cell Signaling, 9102), 1:2000; phospho-Akt S473 (Cell Signaling, 9271) 1:500; total Akt (Santa Cruz Biotechnology, sc-8312), 1:2000; cyclin D1 (NeoMarkers, RM-9104-S), 1:1000; p21Cip1 (Pharmingen, 556430), 1:500; actin (Sigma, A2066), 1:20,000; goat anti-rabbit HRP antibody (Millipore, AP132P), 1:2000; goat anti-mouse IgM-HRP antibody (Santa Cruz Biotechnology, sc-2973), 1:5000.
Analysis of RNA
For real time RT-PCR, RNA was isolated using illustra RNAspin (GE Healthcare) or RNeasy Mini (Qiagen) kits, and analysis was performed using the 1-Step Brilliant II SYBR Green quantitative RT-PCR master mix kit (Agilent Technologies), and the 7300 real time PCR system (Applied Biosystems). mRNA levels are expressed relative to 18 S rRNA and normalized to vehicle-treated controls. Primers used were as follows: rat Id1, TGGACGAACAGCAGGTGAAC and TCTCCACCTTGCTCACTTTGC; rat Id2, ATGAAAGCCTTCAGTCCGGTGA and AGCAGACTCATCGGGTCGTC; rat Id3, CTTAGCCTCTTGGACGACATGA and GATTTCCACCTGGCTAAGCTGA; rat Id4, TGCAGTGCGATATGAACGACTG and TGACTTTCTTGTTGGGCGGGAT; 18 S rRNA, CATTGGAGGGCAAGTCTGGTG and CTCCCAAGATCCAACTACGAG. Statistical difference (p < 0.05) between treatment groups was determined by Student's t test using Microsoft Excel. Microarray analysis of RNA was performed by the Genomics Shared Resource at Roswell Park Cancer Institute on an Affymetrix Hu-Ex 1.0 array using an Affymetrix GeneChip System and Scanner 3000. For correlation of Id1 and PKCα expression in published data sets, Affymetrix microarray profiles were obtained from the NCBI GEO data base (DataSet Records GDS389 and GDS2947), and the Pearson correlation coefficient (r) and two-tailed p value for Id1 and PKCα levels were determined. The microarray data analyzed were derived from biopsies of normal and neoplastic human intestine (32) and from normal and neoplastic murine intestinal tissue obtained by laser capture microdissection (33).
Adenovirus Infection
Transduction of cells with adenovirus expressing PKCα, PKCδ, or kinase-dead PKCα was as described previously (4). Adenovirus was used at a multiplicity of infection (m.o.i.) of 10–20, except for kinase-dead PKCα, which was used at an m.o.i. of 500 to ensure equal expression of this unstable protein (4). Cells were harvested after 48 h for Western or RNA analysis.
siRNA-mediated Knockdown
Human Id1 siRNA (target sequence, AACTCGGAATCCGAAGTTGGG) was from Qiagen. Rat Id1 was silenced using Silencer(R) pre-designed siRNA from Applied Biosystems (siRNA ID: 200461, 59151, and 59247). Nonsilencing siRNAs (Qiagen negative control siRNA, Dharmacon siCONTROL nontargeting siRNA) were used as controls. Human colon cancer cell lines were plated at 1.5 × 105 cells/well in 6-well plates 1 day prior to transfection with 50 nm siRNA using Lipofectamine 2000 (Invitrogen); medium was after 16 h. IEC-18 cells were transfected similarly, except that they were plated at 5 × 104 cells/well; 100 nm siRNA was added, and medium was changed after 4 h. Cells were harvested 3 days after transfection, and protein levels were analyzed by Western blotting.
Transfection of IEC-18 Cells with Mouse Id1-EGFP Fusion Protein
IEC-18 cells (5 × 104) were plated 1 day prior to transfection with vector expressing mouse EGFP-Id1 fusion protein (Addgene 20964) or EGFP-N1 control vector (Clontech). Transfection used FuGENE 6 (Roche Applied Science) according to the manufacturer's recommendations, and medium was changed after 4 h. Following selection with 1.8 mg/ml G418 sulfate, EGFP-Id1 and EGFP-expressing cells were sorted by flow cytometry (Roswell Park Core Facility). Experiments were conducted on cell populations that were >90% EGFP-Id1/EGFP-positive, as estimated by fluorescence microscopy (Zeiss Axiovert 25).
RESULTS
Identification of Id1 as a Potential Target of PKCα in Intestinal Epithelial Cells
To identify novel targets of PKCα signaling in the intestinal epithelium, we restored PKCα expression in DLD1 colon cancer cells by adenoviral transduction (4) and explored changes in gene expression by microarray analysis. As in our previous studies (4), effects in PKCα-expressing cells were compared with those in cells transduced with lacZ (control), a kinase-dead mutant of PKCα (34) or PKCδ (another PKC isozyme commonly down-regulated in colon cancer (4)). This analysis identified Id1 as a candidate for further study. As shown in Table 1, Id1 was the second most down-regulated gene in PKCα-transduced cells compared with lacZ-expressing cells. This effect required PKCα kinase activity because transduction with PKCδ or kinase-dead PKCα had only modest effects (<1.6-fold). Indeed, Id1 was the only gene whose down-regulation in PKCα-transduced cells was significantly different from that in cells expressing lacZ (−3.5-fold), kinase-dead PKCα (−2.3-fold), and PKCδ (−2.6-fold), underscoring the potential importance of Id1 as a target for PKCα kinase-dependent regulation.
TABLE 1.
mRNAs down-regulated by PKCα expression in DLD1 cells
Total RNA from cells infected with adenovirus expressing the indicated proteins was subjected to microarray analysis using an Affymetrix Hu-Ex 1.0 array. Numbers show the fold change in expression of the indicated mRNAs relative to their levels in cells infected with lacZ-expressing adenovirus. Data are for known genes that showed >2-fold down-regulation in PKCα-transduced cells and for inhibitor of DNA binding (Id) family members Id2 and Id3. Two independent transductions were analyzed for PKCα; in each case, levels of Id1 were significantly different from those obtained with lacZ-, PKCδ-, and kinase-dead PKCα (KD PKCα)-transduced cells.
| Down-regulated genes | Expressed PKC isozyme |
|||
|---|---|---|---|---|
| PKCα1 | PKCα2 | KD PKCα | PKCδ | |
| CTDSP2 | −3.76 | −4.66 | −4.38 | −2.03 |
| Id1 | −3.50 | −3.56 | −1.53 | −1.36 |
| DHTKD1 | −2.50 | −2.77 | −2.24 | −2.37 |
| DNM1DN11–6 | −2.33 | −2.31 | −2.40 | −2.66 |
| KRTAP10–1 | −2.24 | −2.44 | −2.01 | −2.30 |
| Id2 | −1.50 | −1.80 | 1.06 | 1.19 |
| Id3 | −1.85 | −2.09 | −1.26 | 1.01 |
Inverse Regulation of PKCα Activity and Id1 Expression in Intestinal Tissues
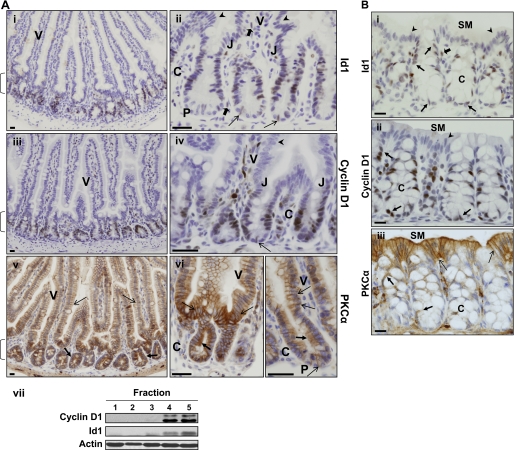
To evaluate Id1 as a potential target of PKCα signaling in the intestinal epithelium in vivo, we compared the expression pattern of these proteins in murine small intestine and colon using immunohistochemistry. Although the known properties of Id1 point to a role in promoting growth and/or inhibiting differentiation in intestinal crypts, previous immunolocalization studies have variably detected Id1 as a cytoplasmic protein, uniformly distributed along the crypt-to-villus axis or up-regulated on the villus (29, 35, 36). The discrepancies in these studies likely result from the polyclonal antibody used, as its suitability for immunolocalization has recently been questioned (37, 38). Thus, we re-evaluated the localization of Id1 in the intestinal epithelium using a recently available, highly specific anti-Id1 rabbit monoclonal antibody from Biocheck (37). Consistent with the role of Id1 as a transcription factor, this antibody localized Id1 to the nucleus of cells in the small intestine and colon (Fig. 1, A, panels i and ii, and B, panel i). Id1 was predominantly detected in the epithelium, with only sporadic stromal cells showing reactivity (Fig. 1A, panel ii, and B, panel i, block arrows). Expression of the protein was largely restricted to crypt cells (Fig. 1A, bracket), and Id1 staining was undetectable in fully differentiated cells of the villus (V) or colonic surface mucosa (SM) (Fig. 1A, panels i and ii, and B, panel i, arrowheads). Although Paneth cells (Fig. 1, P) were negative for Id1, isolated Id1-positive cells, corresponding in shape and localization to the recently identified intestinal stem cells (39), could be seen at the crypt base (Fig. 1A, panel ii, open arrows). The pattern of Id1 staining corresponded to that of the mitogenic protein cyclin D1, whose expression is confined to intestinal crypt cells (Fig. 1, A, panels iii and iv, and B, panel ii). Thus, Id1 expression is restricted to proliferating cells of the crypts in both the small intestine and colon.
FIGURE 1.
Id1 expression inversely correlates with PKCα activation in the intestinal epithelium. A, immunohistochemical analysis of Id1, cyclin D1, and PKCα expression in the mouse small intestine. Panels i and ii, detection of Id1 using a highly specific rabbit monoclonal anti-Id1 antibody. Id1 staining is seen in nuclei of proliferating crypt epithelial cells. Down-regulation of Id1 occurs at the crypt-villus junction (J), and the protein is absent from villus cells (arrowheads) and Paneth cells (P). Open arrows point to the presumptive stem cells at the crypt base, and block arrows indicate Id1-positive stromal cells. Panels iii and iv, staining for the proliferation marker, cyclin D1, parallels that of Id1 (arrows as in panels i and ii). Panels v and vi, PKCα is diffusely distributed/inactive in the proliferating crypt cells (solid arrows) and becomes membrane-associated in nondividing cells of the villus (open arrows) and in Paneth cells (P). Brackets, Crypt compartment; V, villus; C, crypt; J, crypt-villus junction. Panel vii, villus and crypt fractions were isolated by sequential washing of intestinal segments in chelating buffer and subjected to Western blot analysis for the indicated proteins. As confirmed by the crypt marker cyclin D1, fractions 1 and 2 predominantly contain villus cells, and fractions 4 and 5 contain crypt cells. Varying low levels of crypt cell marker were detected in fraction 3. B, immunohistochemical analysis of Id1, cyclin D1, and PKCα expression in the mouse colon. Panels i and ii, Id1 and cyclin D1 staining is seen in nuclei of crypt epithelial cells (arrows) but is absent from the nuclei of the surface mucosa (arrowhead). Block arrow, stromal Id1 staining. Panel iii, PKCα membrane association/activity is only evident in epithelial cells of the surface mucosa (open arrows). Solid arrows indicate the presence of diffuse cytosolic/inactive PKCα in proliferating crypt cells. SM, surface mucosa; C, crypt. Bars, 50 μm. Data are representative of >3 independent experiments.
The pattern of expression of Id1 was also assessed by biochemical analysis of isolated fractions of mouse small intestinal epithelium obtained by successive washes with chelating buffer (31). Immunoblot analysis for the crypt cell marker cyclin D1 (Fig. 1A, panel vii) confirmed that this method yields initial fractions (1 and 2) composed predominantly of villus cells, and later fractions (4 and 5) enriched for crypt cells (fraction 3 showed varying levels of cyclin D1, indicating that crypts begin to be released in this fraction). Id1 was largely detected in fractions 4 and 5, with low levels variably seen in fraction 3 (Fig. 1A, panel vii), confirming the immunohistochemical localization of Id1 to the epithelium of intestinal crypts.
Down-regulation of Id1 in the upper crypt correlated with activation of PKCα. As we have reported previously (9, 11, 12), PKCα is largely inactive in the proliferating cells of the crypt, as reflected in its diffuse cytoplasmic localization (Fig. 1, A, panels v and vi, and B, panel iii, solid arrows). In contrast, the enzyme is predominantly membrane-associated in post-mitotic villus, colonic surface mucosa, and Paneth cells (Fig. 1, A, panels v and vi, and B, panel iii, open arrows), a pattern indicative of enzyme activation (40). Thus, Id1 is appropriately positioned in the intestinal epithelium to be a target for negative regulation by PKCα signaling.
Increased Expression of Id1 in Intestinal Tumors Correlates with Loss of PKCα
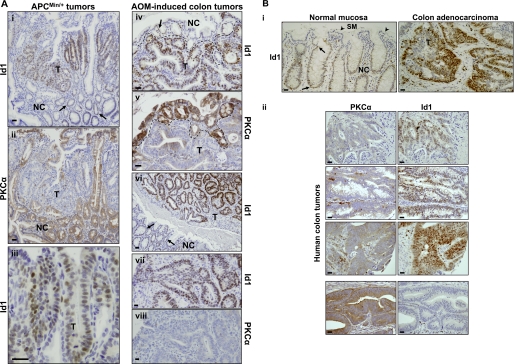
Id1 has been proposed to be a tumor promoter in the intestine (29), whereas PKCα has tumor suppressor activity in this tissue (4, 10). Therefore, the relationship between these proteins was also examined in intestinal neoplasms. Intense nuclear staining for Id1 was detected in intestinal tumors arising in APCmin/+ mice and azoxymethane-treated KK/AIJ mice (Fig. 2A). Notably, levels of Id1 staining were generally higher in tumor cells (Fig. 2A, T) than in adjacent normal crypt cells (Fig. 2A, NC; arrows). As we have shown previously, PKCα expression is uniformly lost in these tumors (Fig. 2A) (4); thus, consistent with the relationship between PKCα and Id1 in the normal intestine, these proteins exhibit an inverse pattern of expression during intestinal tumorigenesis in the mouse. Limited analysis of tissue from patients (Fig. 2B) indicated that Id1 is (a) a nuclear protein restricted to proliferating crypt cells of the colon and (b) is overexpressed in human colon cancer (Fig. 2B, panel i). Because PKCα is also markedly down-regulated in a majority of human colon adenocarcinomas (Fig. 2B, panel ii) (12, 41, 42), the inverse correlation between PKCα and Id1 expression is seen in both murine and human intestinal neoplasms. Collectively, these data establish a clear inverse relationship between PKCα and Id1 in both the normal intestine and during intestinal tumorigenesis.
FIGURE 2.
Id1 is overexpressed in intestinal tumors. A, immunostaining for Id1 and PKCα in small intestinal tissue from APCmin/+ mice (panels i–iii) and colonic tissue from azoxymethane (AOM)-treated mice (panels iv–viii). Panels i and ii, iv and v, vii and viii show serial sections from the same tissue stained for Id1 and PKCα. Id1 is generally overexpressed in the nuclei of tumor (T) cells relative to nuclei of normal crypt (NC) cells (panels i, iv, and vi; arrows), whereas PKCα is lost from the tumors (panels ii, v, and viii). The dashed line in panels iv and v demarcates the boundary between normal and tumor tissue. Higher magnification images in panels iii and vii show nuclear localization of Id1 in tumor cells. Bars, 50 μm. Data are representative of >3 independent experiments. B, immunohistochemical analysis of normal and neoplastic colonic tissue from human patients. Panel i, Id1 expression in normal colonic mucosa and adjacent adenocarcinoma. Tumor (T) and normal tissue are from the same section, and images were processed identically. Nuclear staining for Id1 is seen in the normal crypt (NC) epithelium (solid arrows) but is absent from the surface mucosa (SM; arrowhead), whereas more intense nuclear staining can be seen in tumor cells. Data are representative of analysis of samples from two patients. Panel ii, inverse correlation of PKCα and Id1 expression in colon tumors from human patients. Sections of tumor tissue from four patients were stained for PKCα or Id1. Note the high nuclear expression of Id1 in tumors that lack PKCα and the absence of Id1 in a relatively rare tumor that retains expression of the enzyme (bottom panels). Bars, 50 μm.
PKC/PKCα Activation Leads to Down-regulation of Id1 Protein and mRNA in IEC-18 Intestinal Crypt Cells
The relationship between PKC signaling and Id1 was further explored using the IEC-18 nontransformed rat crypt cell line (43). Id1 was often resolved as a doublet on Western blots of IEC-18 cell extracts (e.g. Fig. 3B, panel ii, and Fig. 4A). The appearance of Id1 as two bands, confirmed by peptide blocking experiments and analysis of Id1 knock-out tissues, has been variably seen in other systems (e.g. HeLa cells, Sertoli cells, lung extracts, and DMS53 cells (44–49)). Although the basis for the doublet and its variable expression is unclear at this time, it has been proposed to reflect differences in O-glycosylation or phosphorylation of the molecule (44, 51). Future studies will address these possibilities, although it should be noted that the modification involved is not affected by PKC signaling in IEC-18 cells because PKC agonist treatment, per se, had no effect on the ratio between the two bands.
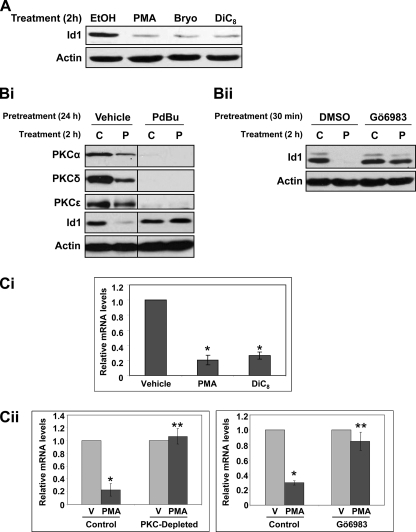
FIGURE 3.
PKC signaling down-regulates Id1 in IEC-18 cells. A, PKC agonist treatment leads to down-regulation of Id1 in IEC-18 cells. Protein extracts from cells treated with PMA (100 nm), bryostatin (Bryo; 100 nm), or DiC8 (20 μg/ml) for 2 h were subjected to immunoblot analysis for Id1 and actin (loading control). B, effects of PKC agonists on Id1 are PKC-dependent. Panel i, PKC agonist-responsive isozymes (PKCα, -δ, and -ϵ) were depleted from IEC-18 cells by treatment with 1 μm PDBu for 24 h. PDBu was removed, and cells were treated with vehicle (C) or 100 nm PMA (P) for 2 h prior to protein extraction and immunoblot analysis for the indicated proteins. Panel ii, cells were pretreated with 1 μm Gö6983 for 30 min prior to addition of vehicle or 100 nm PMA for 2 h. C, PKC activation down-regulates Id1 mRNA. Panel i, cells were treated with PKC agonists or vehicle for 3 h as above, and total cellular RNA was subjected to real time RT-PCR analysis. Levels of Id1 mRNA were normalized to 18 S rRNA and are displayed as relative to vehicle control. Panel ii, cells were depleted of agonist-responsive PKC isozymes by prolonged treatment with PDBU or treated with Gö6983 prior to addition of vehicle (V) or PMA for 3 h. RNA was extracted and analyzed by real time RT-PCR. Data are representative (A and B) or averages ± S.E. (C) of at least three independent experiments. Asterisks signify statistically different (p < 0.05) from vehicle-only control (*) or PMA-treated control (**).
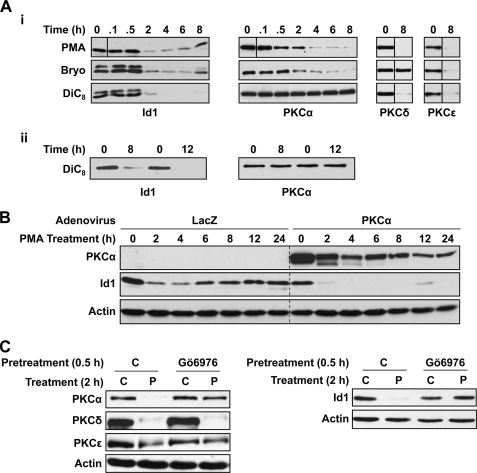
FIGURE 4.
PKCα down-regulates Id1 in intestinal epithelial cells. A, loss of PKCα correlates with restoration of Id1 levels in PKC agonist-treated IEC-18 cells. Panel i, cells were treated with 100 nm PMA, 100 nm bryostatin (Bryo), or 20 μg/ml DiC8 for the indicated times and subjected to Western blot analysis. Each panel shows proteins from a single blot; vertical solid lines indicate where the position of lanes has been changed for clarity. Panel ii, Id1 and PKCα expression in cells treated with DiC8 or vehicle for 8 and 12 h. B, Id1 down-regulation is prolonged in PKCα-overexpressing cells. IEC-18 cells were infected with adenovirus expressing lacZ or PKCα (m.o.i. of 10). After 48 h, cells were treated with PMA and analyzed as in A. The vertical dashed line is included for clarity. Endogenous PKCα is not apparent in lacZ-expressing cells due to lower antibody concentrations and shorter exposures used to detect the exogenous protein. C, inhibition of PKCα activity abrogates PKC agonist-induced Id1 down-regulation. IEC-18 cells were pretreated with vehicle (C) or Gö6976 for 30 min prior to addition of PMA (P) or vehicle (C). After 2 h, protein was extracted and subjected to Western analysis for the indicated proteins. Note that Gö6976 is specific for PKCα in these cells as indicated by its ability to block down-regulation of this isozyme but not that of PKCδ or PKCϵ (enzyme down-regulation is dependent on catalytic activity). Data are representative of at least three independent experiments.
Treatment of IEC-18 cells with a panel of PKC agonists, including the phorbol ester PMA, the macrocyclic lactone bryostatin, and the synthetic diacylglycerol DiC8, led to marked down-regulation of Id1 protein by 2 h (Fig. 3A). PKC agonist-induced Id1 down-regulation was blocked by (a) PDBu-induced depletion of PKC agonist-sensitive isozymes (PKCα, PKCδ, and PKCϵ, see Ref. 9) and (b) treatment with the general PKC inhibitors Gö6983 (Fig. 3B, panels i and ii) or bisindolylmaleimide I (data not shown), confirming the PKC dependence of the effect.
PKC agonist treatment also markedly reduced the levels of Id1 mRNA in IEC-18 cells (Fig. 3C, panel i). Consistent with effects on Id1 protein, Id1 mRNA down-regulation was PKC-dependent, as confirmed using PKC-depleted cells or treatment with the PKC inhibitors Gö6983 (Fig. 1C, panel ii) or bisindolylmaleimide I (data not shown).
Analysis of the time course of Id1 down-regulation by PKC agonists demonstrated that the effects of PMA and bryostatin are transient; although maximal suppression was seen by ∼2 h, reversal of the effect became evident by 6–8 h, and steady-state levels were restored by 12 h (Fig. 4, A, panel i, and B). In contrast, DiC8 (added repeatedly to compensate for its rapid metabolism) induced prolonged suppression of Id1 for at least 12 h (longer times not tested, Fig. 4A, panels i and ii). PKC agonists down-regulate PKC isozymes in an agonist- and isozyme-dependent manner; in IEC-18 cells, PMA down-regulates PKCα, -δ, and -ϵ (Fig. 4A, right panels) (13, 15). In contrast, bryostatin is unable to down-regulate PKCδ (Fig. 4A, panel i), and DiC8 fails to affect PKCα levels (Fig. 4A, panels i and ii). Comparison of the effects of different agonists pointed to a specific role for PKCα in the regulation of Id1; the transient loss of Id1 produced by PMA and bryostatin correlated with down-regulation/desensitization of PKCα (Fig. 4A, panel i), whereas the prolonged suppression induced by DiC8 paralleled the sustained activation of PKCα by this agent (Fig. 4A, panel i, left and center panel; Fig. 4A, panel ii) (17). Consistent with a role for PKCα in modulation of Id1, PMA-induced Id1 suppression was extended to >24 h in PKCα-overexpressing cells (Fig. 4B). Attempts to silence PKCα in IEC-18 cells with several different siRNAs failed to yield sufficient knockdown to block its growth inhibitory effects (data not shown) (52); therefore, an RNAi-based approach could not be used to determine the requirement for this isozyme in the modulation of Id1. However, because PKCα is the only classical PKC expressed in IEC-18 cells, Gö6976, which targets classical PKCs (53), was used to selectively block PKCα activity in this system (the selectivity of this inhibitor for PKCα can be seen in its ability to block agonist-induced/activity-dependent down-regulation of this isozyme but not of PKCδ or PKCϵ; Fig. 4C, left panel). As shown in Fig. 4C (right panel), Gö6976 largely prevented the effects of PKC agonists on Id1. Although these findings point to a predominant role for PKCα in the regulation of Id1 by PKC agonists, blockade by Gö6976 was not always complete; thus, a minor involvement of other isozymes cannot be excluded.
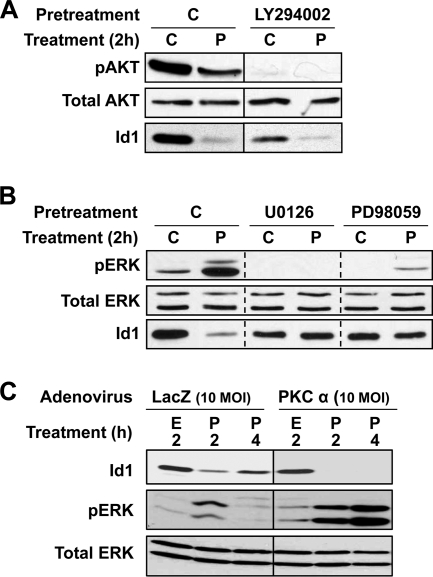
Effects of PKCα on Id1 Are Mediated by the Erk/MAPK Pathway
Our previous studies (13, 14) have determined that PKCα activates the Erk signaling cascade and inhibits the PI3K/Akt pathway in IEC-18 cells (reflected in increased and decreased phosphorylation of Erk and Akt, respectively; Fig. 5). The role of these pathways in Id1 down-regulation was therefore explored. Inhibition of PI3K/Akt with LY294002 led to a reduction in steady-state levels of Id1, suggesting that this pathway is involved in maintaining Id1 expression in intestinal cells (Fig. 5A). However, LY294002 did not block the ability of PMA to induce loss of the protein, indicating that PKCα can suppress Id1 by mechanisms independent of the PI3K/Akt pathway. In contrast, inhibition of Erk signaling with the MEK inhibitors U0126 or PD98095 abrogated the effects of PKC agonists on Id1 (Fig. 5B), supporting a requirement for Erk activity in the effect. Furthermore, the delayed reversal of Id1 down-regulation seen in PMA-treated cells with overexpression of PKCα or following DiC8 treatment (Fig. 4, A, panel ii, and B) was accompanied by prolonged activation of Erk1/2 (Fig. 5C and data not shown). Thus, as seen with the cell cycle effects of PKCα (e.g. cyclin D1 down-regulation and p21Cip1 induction; see Refs. 13, 14), down-regulation of Id1 by PKCα is mediated by Erk signaling.
FIGURE 5.
Erk signaling mediates the effects of PKC activation on Id1 expression. A and B, IEC-18 cells were pretreated with vehicle (C), LY294002 (50 μm), U0126 (10 μm), or PD98059 (50 μm) prior to addition of PMA (P) or vehicle (C). After 2 h, protein was extracted and subjected to Western blotting for the indicated proteins. Each panel shows data from a single blot; vertical solid lines indicate where lanes have been realigned for clarity. Dashed lines are included for clarity. C, IEC-18 cells, infected with adenovirus expressing lacZ (control) or PKCα, were treated with PMA (P) or vehicle (ethanol, E) for the indicated times and subjected to Western blotting for the indicated proteins. Prolonged suppression of Id1 in PKCα-overexpressing cells is associated with sustained Erk activation. Data are representative of at least three independent experiments.
Id1 Is Negatively Regulated by PKCα in Colon Cancer Cells
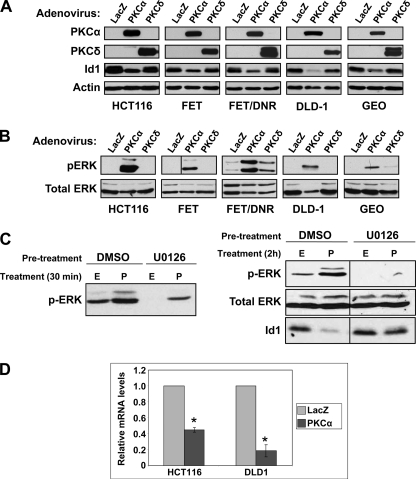
To further examine the relationship between loss of PKCα and Id1 expression in intestinal tumors, PKCα signaling was restored in a panel of colon cancer cell lines by adenoviral transduction. For comparison, cells were transduced with adenovirus expressing lacZ or PKCδ. Consistent with our microarray data (Table 1), levels of Id1 protein were significantly reduced following transduction with PKCα-expressing adenovirus in all cell lines tested (Fig. 6A), demonstrating that, as in nontransformed cells, Id1 is regulated by PKCα in colon tumor cells. The effect showed specificity for PKCα, because re-expression of PKCδ had minimal effects on Id1 protein levels. In keeping with a requirement for Erk activation, expression of PKCα, but not of PKCδ, resulted in increased levels of phospho-Erk in colon cancer cells (Fig. 6B). The requirement for Erk signaling in down-regulation of Id1 in colon tumor cells was tested using HCT116 cells, which express moderate levels of PKCα (4). As shown in Fig. 6C, treatment of these cells with PMA resulted in loss of Id1 (Fig. 6C, right panel), accompanied by rapid activation of Erk signaling (Fig. 6C, left panel). Inhibition of Erk activation with U0126 blocked the down-regulation of Id1 (Fig. 6C, right panel), confirming the requirement for robust activation of Erk signaling for the effect. Real time RT-PCR analysis of adenovirally transduced cells confirmed the ability of PKCα to down-regulate Id1 mRNA seen by microarray analysis (Table 1); transduction with PKCα down-regulated Id1 mRNA in both DLD1 and HCT116 cells (Fig. 6D), with the extent of suppression paralleling that seen for the protein in these cells (Fig. 6A). Thus, PKCα potently regulates Id1 at both the mRNA and protein levels in colon cancer cells as well as in nontransformed intestinal epithelial cells.
FIGURE 6.
PKCα induces Erk activation and down-regulates Id1 in colon cancer cells. A and B, indicated colon cancer cells were infected with adenovirus expressing lacZ, PKCα, or PKCδ at an m.o.i. of 10. After 48 h, protein was extracted and subjected to Western blot analysis for the indicated proteins. Each panel shows data from a single blot; the vertical line on the FET pERK blot indicates where lanes have been rearranged for consistency. Data are representative of at least three independent experiments. C, HCT116 cells were pretreated with vehicle (DMSO) or 10 μm U0126 (in DMSO) for 30 min prior to addition of vehicle (ethanol, E) or 100 nm PMA (P) for 30 min (left panel) or 2 h (right panel). Cells were than processed for Western blot analysis for the indicated proteins. D, HCT116 and DLD1 cells were infected with adenovirus expressing lacZ or PKCα as above and total cellular RNA was subjected to real time RT-PCR analysis. Levels of Id1 mRNA were normalized to 18 S rRNA and are displayed as relative to lacZ-transduced cells. Data are averages (±S.E.) of two (DLD1) or three (HCT116) independent experiments. *, significantly different from lacZ control (p < 0.05).
Id1 Regulates Cyclin D1 in Nontransformed IEC-18 Cells and Colon Cancer Cells
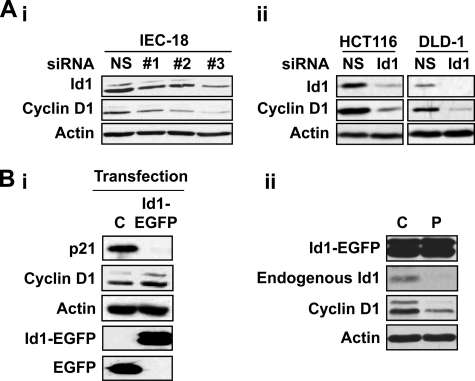
The common signaling pathways engaged by PKCα for its cell cycle effects and for down-regulation of Id1 point to a relationship between these events. Cyclin D1, a major cell cycle target of PKCα in intestinal cells (4, 9, 15), is positively regulated by Id1 in several cell types (25, 26, 54). Thus, the ability of Id1 down-regulation to mediate the effects of PKCα on cyclin D1 was explored by manipulating Id1 levels using siRNA- or exogenous expression-based techniques. siRNA-mediated knockdown of Id1 consistently reduced levels of cyclin D1 in IEC-18, DLD1, and HCT116 cells (Fig. 7A), indicating that endogenous levels of Id1 are limiting, or close to limiting, for cyclin D1 expression in both nontransformed crypt-like cells and colon cancer cells. Thus, the reduction in Id1 levels elicited by PKCα signaling would directly impact cyclin D1 in these cells.
FIGURE 7.
Id1 regulates cyclin D1 in intestinal epithelial cells. A, knockdown of Id1 results in down-regulation of cyclin D1. Indicated cells were transfected with nontargeting siRNA (NS) or siRNA targeting rat (IEC-18) or human (HCT-116 and DLD1) Id1. #1, #2, and #3 designate IEC-18 cells transfected with one of three independent siRNAs targeting rat Id1. 48 h after transfection, cells were harvested and analyzed by Western blotting for the indicated proteins. B, panel i, IEC-18 cells expressing an EGFP-Id1 fusion protein or EGFP (C) were analyzed by Western blotting for the indicated proteins. Panel ii, cells expressing EGFP-Id1 fusion protein were treated with vehicle (C) or 100 nm PMA (P) for 2 h prior to extraction and Western blot analysis. Data are representative of >3 independent experiments.
The role of Id1 in regulation of cyclin D1 was further examined in IEC-18 cells overexpressing an Id1-EGFP fusion protein. Importantly, Western blot analysis of PMA-treated cells determined that the fusion protein was refractory to down-regulation by PKC agonists (Fig. 7B, panel ii). The activity of the fusion protein in these cells was confirmed by its ability to down-regulate p21Cip1 (Fig. 7B), a target of Id1 regulation in multiple systems (55, 56). Consistent with the finding that Id1 levels are limiting for expression of cyclin D1, levels of this cyclin were variably elevated in Id1-EGFP-overexpressing cells compared with controls. However, the presence of the fusion protein did not block the ability of PMA to repress cyclin D1 levels (Fig. 7B, panel ii). These findings are consistent with our previous data demonstrating that PKCα regulates cyclin D1 via multiple mechanisms, including transcriptional and translational repression (4, 14, 15). Thus, although Id1 down-regulation directly affects cyclin D1 steady-state levels in intestinal cells, repression of Id1 represents only one of the mechanisms by which PKCα exerts its cell cycle effects in this system.
Id1 Is Up-regulated in the Intestinal Epithelium of PKCα−/− Mice
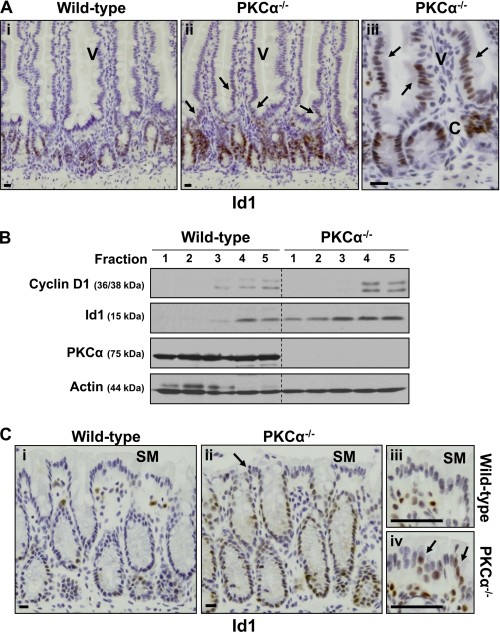
To determine whether the results obtained with cell lines are directly applicable to the intestinal epithelium in vivo, immunohistochemical analysis of Id1 was performed in the small intestine and colon of PKCα−/− mice (30). To ensure identical staining conditions, tissue from wild-type and PKCα−/− mice was processed in parallel, and sections were mounted on the same slide. As seen in wild-type mice, Id1 was expressed predominantly in the nuclei of epithelial cells in the crypts of the small intestine and colon of PKCα−/− mice (Fig. 8, A and C). Notably, however, Id1 staining was consistently increased in crypt cells of PKCα−/− mice relative to those of wild-type mice. The number of Id1-positive cells and the intensity of staining in individual nuclei were both enhanced in PKCα-deficient crypts, indicating that the absence of PKCα signaling was permissive for increased accumulation of Id1 in these cells. Furthermore, in contrast to the restricted pattern of Id1 staining in crypt cells of wild-type mice, Id1 expression was frequently observed in cells of the lower villus (V) and surface mucosa (SM) in PKCα knock-out mice (Fig. 8, A and C, arrows). In some cases, Id1 staining extended as far as the mid-villus (Fig. 8A, right panel).
FIGURE 8.
Id1 is deregulated in the intestine and colon of PKCα−/− mice. A, immunohistochemical analysis of Id1 expression in small intestine of age-matched wild-type and PKCα−/− mice. To allow direct comparison, sections were stained simultaneously on the same slide, and images were processed identically. Arrows indicate Id1 staining on the villus (V) of PKCα−/− mice. C, crypt. Bars, 50 μm. B, Id1 expression in isolated small intestinal epithelial fractions. Fractions, isolated as in Fig. 1A, were subjected to Western blot analysis for the indicated proteins. Cyclin D1 staining confirmed that fractions 1 and 2 contain only villus cells, whereas fractions 4 and 5 contain crypt cells. Dashed lines are included for clarity. C, immunohistochemical analysis of Id1 expression in wild-type and PKCα−/− colon. The arrows in panels ii and iv indicate Id1 staining on the surface mucosa (SM) of PKCα−/− mice. Bars, 50 μm. Data are representative of >3 experiments.
The aberrant expression of Id1 in PKCα−/− mice was confirmed biochemically in isolated fractions of small intestinal epithelium. In agreement with the immunohistochemical data (Fig. 8A, panels i–iii), mucosal fractions from PKCα−/− mice expressed higher levels of Id1 than those from wild-type mice (Fig. 8B). In addition, Id1 protein expression was not restricted to crypt cell fractions but could also be clearly detected in villus cell fractions (fractions 1 and 2) from these animals. Thus, biochemical analysis confirmed both the increased expression of Id1 in the crypt epithelium and the deregulation of its compartmentalization along the crypt-to-villus axis in PKCα−/− mice. Collectively, these data provide direct evidence that PKCα is a negative regulator of Id1 expression in the intestinal epithelium in vivo. It is noteworthy that, consistent with our in vitro data indicating that Id1 is limiting for cyclin D1 expression in intestinal cells (Fig. 7), cyclin D1 levels also appeared to be elevated in the crypts of PKCα−/− mice (e.g. Fig. 8B). (The fact that the aberrant expression of Id1 in villus cells of PKCα−/− mice does not support expression of cyclin D1 presumably reflects the presence of multiple mechanisms of regulation of this protein by PKCα and other signaling pathways in the intestine (4, 14).) Together, these alterations are likely to contribute to the increased number of dividing cells observed in the crypts of PKCα-deficient mice (10).
PKCα Signaling Regulates Expression of Other Id Family Members in Intestinal Epithelial Cells
To determine whether PKCα affects other member(s) of the Id family, RT-PCR analysis of the effects of PKC agonists on Id2, Id3, and Id4 mRNA levels was performed in IEC-18 cells. Consistent with their reported expression in the intestinal epithelium (29, 57), mRNA for Id2 and Id3 was readily detected in IEC-18 cells at levels comparable with those of Id1 mRNA. Although Id4 mRNA was also detected in these cells, levels (based on PCR cycle number) were extremely low (data not shown). These data are consistent with reports of very low Id4 expression in the intestine (29, 57), indicating that this Id is unlikely to play a major role in this tissue. Thus, although PMA induced down-regulation of Id4 mRNA in IEC-18 cells (data not shown), this effect was not further characterized.
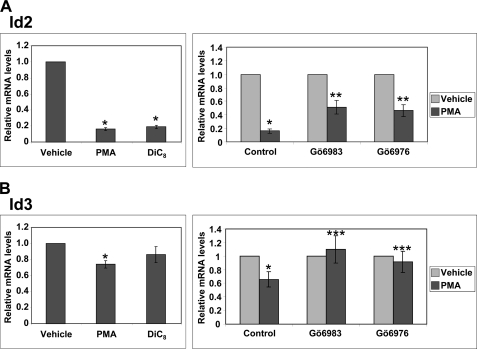
As shown in Fig. 9A, treatment of IEC-18 cells with either PMA or DiC8 led to marked down-regulation of Id2 mRNA. However, in contrast to the effects on Id1, PMA-induced suppression of Id2 mRNA was only partially blocked by the general PKC inhibitor, Gö6983 (Fig. 9A, right panel), and by PKC depletion (data not shown); thus, the effect involved both PKC-dependent and -independent mechanisms. The PKC-dependent portion of the effect appeared to be primarily due to PKCα activity because the extent of PMA-induced down-regulation seen in the presence of the PKCα-selective inhibitor Gö6976 and the general PKC inhibitor Gö6983 was indistinguishable. Id3 was only modestly (albeit consistently) down-regulated by PMA in IEC-18 cells (Fig. 9B). DiC8 also caused a modest decrease in Id3, although this did not reach statistical significance. PMA-induced down-regulation of Id3 was abrogated by treatment with either Gö6983 or Gö6976, indicating that the effect was largely mediated by PKCα. Consistent with these findings, our microarray analysis of adenovirally transduced DLD1 cells pointed to a modest effect of PKCα on Id2 and Id3 mRNA levels (1.5–2-fold decrease), which was not produced by kinase-dead PKCα or PKCδ (Table 1). Collectively, these data indicate that (a) PKCα-induced down-regulation of Id1 does not lead to compensatory changes in expression of other Ids (as noted in Id1 transgenic mice (29)), and (b) Id2 and Id3 are also targets for PKCα regulation in intestinal cells, although the extent of the effect is more modest than that seen for Id1.
FIGURE 9.
PKCα down-regulates Id2 and Id3. IEC-18 cells were treated with vehicle, PMA, or DiC8 for 2 h (left panels) or pretreated with vehicle (control), Gö6983, or Gö6976 for 30 min prior to addition of PMA or vehicle for 2 h (right panels). RNA was extracted and subjected to real time RT-PCR analysis of Id2 (A) or Id3 (B) mRNA. Data, which are normalized to 18 S rRNA levels and expressed relative to vehicle control, are averages of three independent experiments ±S.E. Asterisks signify statistically different (p < 0.05) from control (*), from control and PMA alone (**) or from PMA alone (***).
DISCUSSION
Increasing evidence points to PKCα as an important regulator of homeostasis in the intestinal epithelium, with both antiproliferative and tumor suppressive activity in this tissue (3, 5, 10). This study adds the Id family of transcriptional repressors to the growing list of targets of PKCα signaling. Intestinal epithelial cells express Id1, Id2, and Id3, whereas Id4 is barely detectable (29, 57). Although PKCα signaling can down-regulate Id1–3 at the mRNA level, the effects on Id2 and Id3 are relatively modest; therefore, this study focused on Id1. A combination of in vitro and in vivo analyses revealed that Id1 is negatively regulated by PKCα signaling at the mRNA and protein levels in nontransformed IEC-18 crypt-like cells. Maintenance of Id1 suppression requires sustained PKCα activity, as indicated by the transient effects of PKC agonists that efficiently promote degradation/desensitization of the enzyme (i.e. PMA and bryostatin). The role of PKCα in the regulation of Id1 is further supported by the following evidence: (a) recovery of Id1 levels corresponded to agonist-induced down-regulation of PKCα but not of other PKC isozymes; (b) Id1 suppression was sustained in PKCα overexpressing cells; (c) loss of Id1 was inhibited by selective pharmacological blockade of this isozyme; and (d) Id1 levels were elevated in PKCα-deficient crypt cells in vivo. Although Id1 has been shown to be down-regulated during phorbol ester-induced differentiation in other systems (58, 59), this study is the first, to our knowledge, to link control of Id1 directly to PKC signaling and to identify the specific isozyme involved.
Id1 is a pro-proliferative factor in a number of systems, including the intestine (20, 22, 25, 57, 61). It promotes cell growth by enhancing the activity of mitogenic regulators, such as cyclin D1 and the EGFR pathway, and/or by inhibiting growth-suppressive mechanisms, including expression of cyclin-dependent kinase inhibitors and the TGFβ-signaling pathway (20, 22, 24–27). Decreased expression of Id1 is therefore likely to be an important component of the growth inhibitory program mediated by PKCα in intestinal cells. In this regard, the mechanisms underlying Id1 down-regulation parallel those involved in PKCα-induced cell cycle arrest (9, 13, 14); both require sustained PKCα catalytic activity and Erk signaling but do not depend on changes in the PI3K/Akt pathway. Increased Erk activation was also implicated in PKCα-induced Id1 repression in colon cancer cells. Interestingly, many of the colon cancer cells tested had activating mutations in K-Ras, which can lead to elevated basal Erk activity (62, 63). Nonetheless, analysis of HCT116 cells confirmed that increased Erk signaling was required for PKCα-mediated down-regulation of Id1, even in cells with relatively high levels of basal Erk activation (Fig. 6). The fact that Erk activity is required for hepatocyte growth factor-induced down-regulation of Id1 and cell cycle arrest in human hepatoma cells (64) supports the physiological relevance of these findings.
Importantly, Id1 loss in PKC agonist-treated IEC-18 cells is not simply a consequence of PKC-mediated growth inhibition; effects on Id1 mRNA could be seen at 1 h following PKC activation (data not shown), a time that precedes cell cycle arrest and key growth inhibitory effects of PKCα, including induction of Cip/Kip proteins and hypophosphorylation of pocket proteins (9). Because siRNA-mediated knockdown indicated that Id1 is limiting for cyclin D1 expression in intestinal cells, down-regulation of this cyclin likely represents one mechanism by which loss of Id1 contributes to the growth inhibitory effects of PKCα. However, Id1 binds and modulates a number of growth-regulatory and differentiation-inducing transcription factors (20, 27), including Ets factors (65, 66) and MATH1/HATH1 (67, 68), that may also play a role. Ongoing studies are focused on determining the mechanism by which Id1 regulates cyclin D1 in intestinal cells as well as on identifying additional Id1 targets that mediate the effects of PKCα in this tissue.
Analysis of Id1 expression in the intestine provides support for a role of PKCα in the regulation of this factor in vivo. Using the highly specific Biocheck antibody (37), we show for the first time that Id1 is restricted to nuclei of proliferating crypt cells of the small intestine and colon and absent from post-mitotic cells of the villus/surface mucosa and Paneth cells. Consistent with a role in regulation of cyclin D1 levels, expression of Id1 parallels that of cyclin D1 in intestinal crypts. Importantly, down-regulation of Id1 at the crypt-villus junction in the small intestine, in the upper crypts of the colon, and in Paneth cells coincides with PKCα activation (as indicated by robust association of PKCα with the plasma membrane). Thus, Id1 is positioned both as a regulator of epithelial cell growth/differentiation in the intestine and as a target of PKCα control.
Although our localization data are consistent with known functions of Id1 as a growth-promoting transcription factor, they differ from those of previous studies in which Id1 was reported to be a cytoplasmic protein, either uniformly expressed along the crypt-to-villus axis or present at higher levels in post-mitotic cells (29, 35, 36). These discrepancies likely reflect the lack of specificity of the polyclonal anti-Id1 antibodies used in earlier studies (37, 38). Because biochemical analysis of fractionated intestinal epithelium confirmed that Id1 expression is restricted to crypt cells, it is likely that the cytoplasmic staining detected in previous studies represents cross-reacting proteins. To our knowledge, our study is the first to report Id1 localization in the intestine using the well characterized Biocheck anti-Id1 reagent.
PKCα knock-out mice provided direct evidence that Id1 is regulated by PKCα in the intestine in vivo. Both immunohistochemical and biochemical analyses revealed that Id1 is overexpressed in PKCα-deficient crypt cells relative to corresponding cells in wild-type mice. PKCα loss was also associated with disrupted compartmentalization of Id1 in the intestinal mucosa, with Id1 expression frequently extending onto the villus/surface mucosa in PKCα−/− mice. Together, these data indicate that the regulation of Id1 by PKCα seen in intestinal cell lines is a true reflection of the relationship between these proteins in vivo and support the use of cell lines for studying the underlying mechanism(s) involved.
Although the mechanism of activation of the PKCα-Id1 axis in the intestine remains to be identified, accumulating evidence indicates that PKCα-mediated signaling is likely to interact with the canonical Wnt/β-catenin pathway. Cross-talk between Wnt signaling and pathways involving Notch and EphB/ephrinB plays a critical role in regulation of cell proliferation/differentiation, compartmentalization, and migration in the intestine and is of fundamental importance in intestinal homeostasis and tumor development (69). Notably, activation of PKCα and accompanying down-regulation of Id1 inversely correlate with the activity of the Wnt/β-catenin pathway in intestinal crypts. Furthermore, Wnt5a, which antagonizes canonical Wnt signaling and inhibits intestinal epithelial cell growth, has recently been reported to activate PKCα in a variety of cell types (70–73). In the intestine, Wnt5a is expressed in the mesenchyme underlying the crypt-villus junction and villus/colonic surface mucosa and thus coincides with PKCα activation (74). These findings point to Wnt5a as a potential upstream regulator of PKCα activity in the intestine, initiating a growth arrest and tumor suppression program involving inhibition of Wnt/β-catenin signaling and loss of Id1/cyclin D1. It is also interesting to note that the Wnt/β-catenin target, SOX9, has been implicated in negative regulation of PKCα in intestinal crypts and colon cancer cells (75) and that Id1 can function both as a target and an activator of Wnt signaling (76, 77). Together, these findings place the PKCα-Id1 axis identified here within a network of interacting signaling pathways that cooperate to maintain intestinal homeostasis.
Deregulation of Id1 is likely to contribute to the proliferative defects seen in the intestinal epithelium of PKCα−/− mice (10). Consistent with Id1 being limiting for cyclin D1 expression, the up-regulation of Id1 seen in the context of PKCα deficiency in vivo appeared to be accompanied by increased levels of this potent mitogenic cyclin in crypt cells. In addition, microarray analysis of the PKCα-deficient intestine revealed enhanced EGFR signaling (10), a pathway that is also enhanced with Id1 overexpression (35, 78, 79) and is a critical regulator of crypt cell proliferation (80). Thus, the increase in Id1 expression in PKCα−/− crypts would be expected to be an important mediator of the increased proliferation seen in this model (10).
Forced expression of Id1 promotes adenoma formation in the adult mouse intestinal epithelium (29), pointing to Id1 as a potential oncogene in the intestine. Although it has been reported that Id1 levels are increased in human colon adenocarcinomas (35, 36, 57), this conclusion was based on immunohistochemical analysis using a polyclonal antibody of questionable specificity in the intestine (see above). Our analysis using the Biocheck reagent indicated that (a) Id1 is also a nuclear protein in intestinal tumor cells, and (b) levels of Id1 are consistently higher in tumors than in adjacent normal mucosa in mouse models and human patient samples.
Recent immunohistochemical analysis of a variety of tumor types supports the idea that the oncogenic properties of Id1 are largely mediated by its expression in stromal tissue rather than in tumor cells and that its major role may be in promoting tumor angiogenesis (50, 61). However, our studies clearly demonstrate robust and widespread expression of Id1 in the nuclei of intestinal tumor cells. Therefore, in the intestine, altered Id1 expression appears to be related to increased tumorigenic potential of epithelial cells themselves. Down-regulation of Id1 is therefore likely to contribute directly to the tumor suppressive activity of PKCα in this tissue.
Several lines of evidence point to a role for disruption of PKCα-mediated regulation of Id1 in intestinal tumorigenesis. Our immunohistochemical analysis revealed that Id1 up-regulation coincides with loss of PKCα in both human and mouse tumors. This relationship is also seen in Affymetrix microarray data for normal and neoplastic intestinal tissue from 32 human patients (DataSet Record GDS2947 and Ref. 32) and from the APCmin/+ mouse model (DataSet Record GDS389 and Ref. 33) available in the NCBI GEO data base; in both data sets there was a statistically significant inverse correlation between expression of PKCα and Id1 (r = −0.503, p < 0.001 and r = −0.715, p < 0.005, respectively). The ability of PKCα re-expression to down-regulate Id1 in colon cancer cell lines establishes a direct relationship between expression of these factors during intestinal tumorigenesis. PKCα-induced loss of Id1 was observed in colon cancer cell lines with diverse genetic backgrounds, differing in the status of APC, β-catenin, K-Ras, and/or p53. Thus, the effects of PKCα on Id1 expression parallel its ability to promote tumor suppression in colon cancer cells irrespective of other underlying defects (4). Notably, microarray analysis identified Id1 as the second most down-regulated known gene in colon cancer cells with restored expression of PKCα (Table 1), further highlighting the importance of regulation of this factor in the effects of PKCα signaling.
In summary, we demonstrate that PKCα signaling is an important regulator of Id1 in the intestinal epithelium and that a PKCα → Erk ⊣ Id1 → cyclin D1 signaling axis is likely to be a key player in maintenance of intestinal homeostasis.
Acknowledgments
We thank Dr. Jeffery Molkentin for the PKCα knock-out mice, Drs. Joshua Uronis and David Threadgill for paraffin sections of tissues from azoxymethane-treated mice, and Drs. Carl Morrison, Thaer Khoury, and Angela Omilian for assistance with immunohistochemical analysis of human colon cancer tissues.
Addendum
Since submission of this article, a study has been published demonstrating PKCα-induced, MEK-dependent down-regulation of Id1 mRNA in association with senescence in SSeCKS/Gravin/Akap12-deficient mouse embryonic fibroblasts (60).
This work was supported, in whole or in part, by National Institutes of Health Grants DK60632, DK54909, and CA16056 and Postdoctoral Fellowship CA113048 (to M. A. P.).
- APC
- adenomatous polyposis coli
- DiC8
- 1,2-dioctanoyl-sn-glycerol
- EGFP
- enhanced GFP
- EGFR
- EGF receptor
- Id
- inhibitor of DNA binding
- m.o.i.
- multiplicity of infection
- PDBu
- phorbol 12,13-dibutyrate
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. van der Flier L. G., Clevers H. (2009) Annu. Rev. Physiol. 71, 241–260 [DOI] [PubMed] [Google Scholar]
- 2. Griner E. M., Kazanietz M. G. (2007) Nat. Rev. Cancer 7, 281–294 [DOI] [PubMed] [Google Scholar]
- 3. Black J. D. (2000) Front. Biosci. 5, D406–D423 [DOI] [PubMed] [Google Scholar]
- 4. Pysz M. A., Leontieva O. V., Bateman N. W., Uronis J. M., Curry K. J., Threadgill D. W., Janssen K. P., Robine S., Velcich A., Augenlicht L. H., Black A. R., Black J. D. (2009) Exp. Cell Res. 315, 1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black J. D. (2001) Gastroenterology 120, 1868–1872 [DOI] [PubMed] [Google Scholar]
- 6. Fields A. P., Murray N. R., Gustafson W. C. (2003) Methods Mol. Biol. 233, 539–553 [DOI] [PubMed] [Google Scholar]
- 7. Gorin M. A., Pan Q. (2009) Mol. Cancer 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fields A. P., Regala R. P. (2007) Pharmacol. Res. 55, 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frey M. R., Clark J. A., Leontieva O., Uronis J. M., Black A. R., Black J. D. (2000) J. Cell Biol. 151, 763–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oster H., Leitges M. (2006) Cancer Res. 66, 6955–6963 [DOI] [PubMed] [Google Scholar]
- 11. Saxon M. L., Zhao X., Black J. D. (1994) J. Cell Biol. 126, 747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verstovsek G., Byrd A., Frey M. R., Petrelli N. J., Black J. D. (1998) Gastroenterology 115, 75–85 [DOI] [PubMed] [Google Scholar]
- 13. Clark J. A., Black A. R., Leontieva O. V., Frey M. R., Pysz M. A., Kunneva L., Woloszynska-Read A., Roy D., Black J. D. (2004) J. Biol. Chem. 279, 9233–9247 [DOI] [PubMed] [Google Scholar]
- 14. Guan L., Song K., Pysz M. A., Curry K. J., Hizli A. A., Danielpour D., Black A. R., Black J. D. (2007) J. Biol. Chem. 282, 14213–14225 [DOI] [PubMed] [Google Scholar]
- 15. Hizli A. A., Black A. R., Pysz M. A., Black J. D. (2006) J. Biol. Chem. 281, 14596–14603 [DOI] [PubMed] [Google Scholar]
- 16. Detjen K. M., Brembeck F. H., Welzel M., Kaiser A., Haller H., Wiedenmann B., Rosewicz S. (2000) J. Cell Sci. 113, 3025–3035 [DOI] [PubMed] [Google Scholar]
- 17. Frey M. R., Saxon M. L., Zhao X., Rollins A., Evans S. S., Black J. D. (1997) J. Biol. Chem. 272, 9424–9435 [DOI] [PubMed] [Google Scholar]
- 18. Santiskulvong C., Rozengurt E. (2007) Cell. Signal. 19, 1348–1357 [DOI] [PubMed] [Google Scholar]
- 19. Slosberg E. D., Klein M. G., Yao Y., Han E. K., Schieren I., Weinstein I. B. (1999) Oncogene 18, 6658–6666 [DOI] [PubMed] [Google Scholar]
- 20. Ruzinova M. B., Benezra R. (2003) Trends Cell Biol. 13, 410–418 [DOI] [PubMed] [Google Scholar]
- 21. Bai G., Sheng N., Xie Z., Bian W., Yokota Y., Benezra R., Kageyama R., Guillemot F., Jing N. (2007) Dev. Cell 13, 283–297 [DOI] [PubMed] [Google Scholar]
- 22. Norton J. D. (2000) J. Cell Sci. 113, 3897–3905 [DOI] [PubMed] [Google Scholar]
- 23. Israel M. A., Hernandez M. C., Florio M., Andres-Barquin P. J., Mantani A., Carter J. H., Julin C. M. (1999) Cancer Res. 59, 1726s–1730s [PubMed] [Google Scholar]
- 24. Alani R. M., Young A. Z., Shifflett C. B. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7812–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swarbrick A., Akerfeldt M. C., Lee C. S., Sergio C. M., Caldon C. E., Hunter L. J., Sutherland R. L., Musgrove E. A. (2005) Oncogene 24, 381–389 [DOI] [PubMed] [Google Scholar]
- 26. Caldon C. E., Swarbrick A., Lee C. S., Sutherland R. L., Musgrove E. A. (2008) Cancer Res. 68, 3026–3036 [DOI] [PubMed] [Google Scholar]
- 27. Ling M. T., Wang X., Zhang X., Wong Y. C. (2006) Differentiation 74, 481–487 [DOI] [PubMed] [Google Scholar]
- 28. Fong S., Debs R. J., Desprez P. Y. (2004) Trends Mol. Med. 10, 387–392 [DOI] [PubMed] [Google Scholar]
- 29. Wice B. M., Gordon J. I. (1998) J. Biol. Chem. 273, 25310–25319 [DOI] [PubMed] [Google Scholar]
- 30. Braz J. C., Gregory K., Pathak A., Zhao W., Sahin B., Klevitsky R., Kimball T. F., Lorenz J. N., Nairn A. C., Liggett S. B., Bodi I., Wang S., Schwartz A., Lakatta E. G., DePaoli-Roach A. A., Robbins J., Hewett T. E., Bibb J. A., Westfall M. V., Kranias E. G., Molkentin J. D. (2004) Nat. Med. 10, 248–254 [DOI] [PubMed] [Google Scholar]
- 31. Flint N., Cove F. L., Evans G. S. (1991) Biochem. J. 280, 331–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sabates-Bellver J., Van der Flier L. G., de Palo M., Cattaneo E., Maake C., Rehrauer H., Laczko E., Kurowski M. A., Bujnicki J. M., Menigatti M., Luz J., Ranalli T. V., Gomes V., Pastorelli A., Faggiani R., Anti M., Jiricny J., Clevers H., Marra G. (2007) Mol. Cancer Res. 5, 1263–1275 [DOI] [PubMed] [Google Scholar]
- 33. Paoni N. F., Feldman M. W., Gutierrez L. S., Ploplis V. A., Castellino F. J. (2003) Physiol. Genomics 15, 228–235 [DOI] [PubMed] [Google Scholar]
- 34. Garcia-Bermejo M. L., Leskow F. C., Fujii T., Wang Q., Blumberg P. M., Ohba M., Kuroki T., Han K. C., Lee J., Marquez V. E., Kazanietz M. G. (2002) J. Biol. Chem. 277, 645–655 [DOI] [PubMed] [Google Scholar]
- 35. Meteoglu I., Meydan N., Erkus M. (2008) J. Exp. Clin. Cancer Res. 27, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao Z. R., Zhang Z. Y., Zhang H., Jiang L., Wang M. W., Sun X. F. (2008) Oncol. Rep. 19, 419–424 [PubMed] [Google Scholar]
- 37. Perk J., Gil-Bazo I., Chin Y., de Candia P., Chen J. J., Zhao Y., Chao S., Cheong W., Ke Y., Al-Ahmadie H., Gerald W. L., Brogi E., Benezra R. (2006) Cancer Res. 66, 10870–10877 [DOI] [PubMed] [Google Scholar]
- 38. Gil-Bazo I., Ponz-Sarvisé M., Panizo-Santos A., Calvo A. (2010) Clin. Transl. Oncol. 12, 69. [DOI] [PubMed] [Google Scholar]
- 39. Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., Clevers H. (2007) Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 40. Kraft A. S., Anderson W. B. (1983) Nature 301, 621–623 [DOI] [PubMed] [Google Scholar]
- 41. Kahl-Rainer P., Sedivy R., Marian B. (1996) Gastroenterology 110, 1753–1759 [DOI] [PubMed] [Google Scholar]
- 42. Kahl-Rainer P., Karner-Hanusch J., Weiss W., Marian B. (1994) Carcinogenesis 15, 779–782 [DOI] [PubMed] [Google Scholar]
- 43. Quaroni A., Isselbacher K. J. (1981) J. Natl. Cancer Inst. 67, 1353–1362 [PubMed] [Google Scholar]
- 44. Lingbeck J. M., Trausch-Azar J. S., Ciechanover A., Schwartz A. L. (2005) Oncogene 24, 6376–6384 [DOI] [PubMed] [Google Scholar]
- 45. Lingbeck J. M., Trausch-Azar J. S., Ciechanover A., Schwartz A. L. (2008) FASEB J. 22, 1694–1701 [DOI] [PubMed] [Google Scholar]
- 46. Chaudhary J., Sadler-Riggleman I., Ague J. M., Skinner M. K. (2005) Biol. Reprod. 72, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 47. Chaudhary J., Johnson J., Kim G., Skinner M. K. (2001) Endocrinology 142, 1727–1736 [DOI] [PubMed] [Google Scholar]
- 48. Lowery J. W., Frump A. L., Anderson L., DiCarlo G. E., Jones M. T., de Caestecker M. P. (2010) Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1463–R1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Viñals F., Reiriz J., Ambrosio S., Bartrons R., Rosa J. L., Ventura F. (2004) EMBO J. 23, 3527–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nair R., Junankar S., O'Toole S., Shah J., Borowsky A. D., Bishop J. M., Swarbrick A. (2010) PLoS One 5, e11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pache G., Schäfer C., Wiesemann S., Springer E., Liebau M., Reinhardt H. C., August C., Pavenstädt H., Bek M. J. (2006) Am. J. Physiol. Renal Physiol. 291, F654–F662 [DOI] [PubMed] [Google Scholar]
- 52. Cameron A. J., Procyk K. J., Leitges M., Parker P. J. (2008) Int. J. Cancer 123, 769–779 [DOI] [PubMed] [Google Scholar]
- 53. Martiny-Baron G., Fabbro D. (2007) Pharmacol. Res. 55, 477–486 [DOI] [PubMed] [Google Scholar]
- 54. Ozeki M., Hamajima Y., Feng L., Ondrey F. G., Schlentz E., Lin J. (2007) J. Neurosci. Res. 85, 515–524 [DOI] [PubMed] [Google Scholar]
- 55. Ciarrocchi A., Jankovic V., Shaked Y., Nolan D. J., Mittal V., Kerbel R. S., Nimer S. D., Benezra R. (2007) PLoS ONE 2, e1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prabhu S., Ignatova A., Park S. T., Sun X. H. (1997) Mol. Cell. Biol. 17, 5888–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilson J. W., Deed R. W., Inoue T., Balzi M., Becciolini A., Faraoni P., Potten C. S., Norton J. D. (2001) Cancer Res. 61, 8803–8810 [PubMed] [Google Scholar]
- 58. Kebebew E., Treseler P. A., Duh Q. Y., Clark O. H. (2000) Surgery 128, 952–957 [DOI] [PubMed] [Google Scholar]
- 59. Peddada S., Yasui D. H., LaSalle J. M. (2006) Hum. Mol. Genet. 15, 2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Akakura S., Nochajski P., Gao L., Sotomayor P., Matsui S., Gelman I. H. (2010) Cell Cycle 9, 4656–4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perk J., Iavarone A., Benezra R. (2005) Nat. Rev. Cancer 5, 603–614 [DOI] [PubMed] [Google Scholar]
- 62. Yeh J. J., Routh E. D., Rubinas T., Peacock J., Martin T. D., Shen X. J., Sandler R. S., Kim H. J., Keku T. O., Der C. J. (2009) Mol. Cancer Ther. 8, 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kress T. R., Raabe T., Feller S. M. (2010) Cell Commun. Signal. 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ushio K., Hashimoto T., Kitamura N., Tanaka T. (2009) Mol. Cancer Res. 7, 1179–1188 [DOI] [PubMed] [Google Scholar]
- 65. Ohtani N., Zebedee Z., Huot T. J., Stinson J. A., Sugimoto M., Ohashi Y., Sharrocks A. D., Peters G., Hara E. (2001) Nature 409, 1067–1070 [DOI] [PubMed] [Google Scholar]
- 66. Jedlicka P., Sui X., Gutierrez-Hartmann A. (2009) BMC Cancer 9, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jones J. M., Montcouquiol M., Dabdoub A., Woods C., Kelley M. W. (2006) J. Neurosci. 26, 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Leow C. C., Romero M. S., Ross S., Polakis P., Gao W. Q. (2004) Cancer Res. 64, 6050–6057 [DOI] [PubMed] [Google Scholar]
- 69. de Lau W., Barker N., Clevers H. (2007) Front. Biosci. 12, 471–491 [DOI] [PubMed] [Google Scholar]
- 70. Lee J. M., Kim I. S., Kim H., Lee J. S., Kim K., Yim H. Y., Jeong J., Kim J. H., Kim J. Y., Lee H., Seo S. B., Kim H., Rosenfeld M. G., Kim K. I., Baek S. H. (2010) Mol. Cell 37, 183–195 [DOI] [PubMed] [Google Scholar]
- 71. Yang D. H., Yoon J. Y., Lee S. H., Bryja V., Andersson E. R., Arenas E., Kwon Y. G., Choi K. Y. (2009) Circ. Res. 104, 372–379 [DOI] [PubMed] [Google Scholar]
- 72. Sheldahl L. C., Park M., Malbon C. C., Moon R. T. (1999) Curr. Biol. 9, 695–698 [DOI] [PubMed] [Google Scholar]
- 73. Weeraratna A. T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J. M. (2002) Cancer Cell 1, 279–288 [DOI] [PubMed] [Google Scholar]
- 74. Gregorieff A., Pinto D., Begthel H., Destrée O., Kielman M., Clevers H. (2005) Gastroenterology 129, 626–638 [DOI] [PubMed] [Google Scholar]
- 75. Dupasquier S., Abdel-Samad R., Glazer R. I., Bastide P., Jay P., Joubert D., Cavaillès V., Blache P., Quittau-Prévostel C. (2009) J. Cell Sci. 122, 2191–2196 [DOI] [PubMed] [Google Scholar]
- 76. Lee J. Y., Kang M. B., Jang S. H., Qian T., Kim H. J., Kim C. H., Kim Y., Kong G. (2009) Oncogene 28, 824–831 [DOI] [PubMed] [Google Scholar]
- 77. Nakashima A., Katagiri T., Tamura M. (2005) J. Biol. Chem. 280, 37660–37668 [DOI] [PubMed] [Google Scholar]
- 78. Ling M. T., Wang X., Lee D. T., Tam P. C., Tsao S. W., Wong Y. C. (2004) Carcinogenesis 25, 517–525 [DOI] [PubMed] [Google Scholar]
- 79. Zhang X., Ling M. T., Feng H., Wong Y. C., Tsao S. W., Wang X. (2004) Br. J. Cancer 91, 2042–2047 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80. Howarth G. S., Bastian S. E., Dunbar A. J., Goddard C. (2003) Growth Factors 21, 79–86 [DOI] [PubMed] [Google Scholar]