Abstract
UVB irradiation causes characteristic features of skin aging including remodeling of the dermal extracellular matrix. A key feature during this process is the up-regulation of matrix metalloproteinases and cleavage of collagen. Hyaluronic acid (HA), a major component of the dermal matrix, decreases after chronic UVB exposure. However, the factors that govern the decline of HA synthesis during the course of actinic aging are largely unknown. The aim of the present study was to explore whether collagen degradation causes inhibition of HA synthesis in human skin fibroblasts. After treatment of fibroblasts with collagen fragments (CF) in vitro, resolution of the actin cytoskeleton and inhibition of HA secretion occurred because of specific down-regulation of hyaluronan synthase 2 (HAS2) expression. The αvβ3-agonist, RGDS, latrunculin A, and an inhibitor of Rho-activated kinase inhibited HAS2 expression. Conversely, blocking antibodies to αvβ3 abolished the down-regulation of HAS2 and the cytoskeletal effects. Furthermore, inhibition of cofilin phosphorylation in response to CF was prevented by αvβ3-blocking antibodies. The key role of ERK signaling was shown by reduced nuclear accumulation of phosphoERK and of ELK-1 phosphorylation in response to CF. In addition, the ERK inhibitor PD98059 reduced HAS2 expression. Also, UVB irradiation of fibroblasts caused down-regulation of HAS2, which was sensitive to matrix metalloproteinase inhibitors and to αvβ3-blocking antibodies. In conclusion, these data suggest that CF activate αvβ3-integrins and in turn inhibit Rho kinase (ROCK) signaling and nuclear translocation of phosphoERK, resulting in reduced HAS2 expression. Therefore, a novel mechanism is presented how proteolytic collagen cleavage may inhibit HA synthesis in dermal fibroblasts during extrinsic skin aging.
Keywords: Aging, ERK, Extracellular Matrix, Integrin, Signal Transduction, HAS2 Down-regulation, Hyaluronan, Matrix Remodeling
Introduction
UVB irradiation is a key factor during extrinsic skin aging. About 5% of the UVB light reaches the upper dermis and thereby also affects dermal fibroblasts (1). UVB-induced damage accumulates and causes pronounced changes in the appearance and the structure of the skin (2). A hallmark of skin aging is cleavage of collagen by matrix metalloproteinases (MMPs)2 that is not completely reconstituted by de novo collagen synthesis (3). Instead the repeated damage of collagenous networks leaves the skin with accumulating micro defects that cause wrinkling of the skin and impaired elasticity in the long term (2). However, photoaging of the skin also affects the phenotype of embedded cells such as keratinocytes, fibroblasts, and dendritic cells, either by direct effects of irradiation on the cells or indirectly by the remodeled and aged ECM. In contrast to collagen, little is known about the molecular mechanisms that control the remodeling of other ECM molecules within the dermal matrix in response to UVB irradiation. The present study focuses on hyaluronan (HA), which is a major component of the dermal ECM (4). HA is an unbranched polymeric carbohydrate consisting of alternating disaccharide units (d-glucuronic acid β(1–3)-d-N-acetyl-glucosamine β(1–4)). HA is synthesized at the plasma membrane by HA synthases 1–3 (HAS1–3) that assemble activated UDP-glucosamine and glucuronic acid and extrude the growing HA polymer into the extracellular compartment (5). HA serves as a scaffold for the ECM because it allows multiple interactions with HA-binding proteins such as versican, tumor necrosis factor-stimulated gene 6, or serum proteins such as inter-α-trypsin inhibitor (6). Thereby HA is thought to critically determine the extracellular micromilieu and cellular phenotypes. Cellular signaling of HA is mediated by CD44 and RHAMM (receptor for hyaluronan-mediated motility), which are thought to mediate pro-migratory and pro-proliferative effects in fibroblasts (7, 8). On the organ level, HA is a major contributor to the water content of the skin, the turgidity, and the diffusion gradients (4). Loss of HA has been associated with skin aging (9–11). However, little is known about the molecular mechanisms that are responsible for the loss of HA during actinic aging. It has been described before that transforming growth factor β receptor II is down-regulated in the skin of chronically irradiated mice (12). It is therefore possible that decreased growth factor expression or growth factor signaling is an important mechanism of how the decline of HA production occurs. In line with this assumption is the fact that up to now, direct negative regulators of HA synthesis have not been identified in dermal fibroblasts. In the present study, the possibility was considered that cross-talk may exist between collagen fragmentation and loss of HA. Collagen fragments (CF) are known to be bioactive. In smooth muscle cells, CF cause cleavage of focal adhesion kinase (13) and inhibit apoptosis by activation of αvβ3-integrins (14). Interestingly in skin fibroblasts, CF inhibit de novo collagen synthesis (15). Therefore, the specific aim was to investigate whether CF regulate HA synthesis in human dermal fibroblasts and thereby identify a possible link between UVB-induced collagen cleavage and loss of HA during actinic aging.
EXPERIMENTAL PROCEDURES
Materials
Reagents were obtained from the indicated sources: latrunculin A, Y27632, and lysophosphatidic acid from Sigma-Aldrich (Munich, Germany); Streptomyces hyaluronidase from MP Biomedicals Germany (Eschwege, Germany); αvβ3-blocking antibody LM609 and the respective isotype control from Millipore (Schwalbach, Germany); MMP inhibitor I (300 nm/liter), anisomycin (10 μm), and SP600125 (10 μm) from Sigma-Aldrich; and PD98059 and SB203580 (1 μm) from Merck (Darmstadt, Germany). Collagen neoepitopes were detected by immunostaining using collagen 2 3/4Cshort polyclonal rabbit antibody (IBEX; Mont Royal, Canada).
Cell Culture
Human dermal fibroblasts derived from male and female donors were purchased from PromoCell (Heidelberg, Germany) and maintained in monolayer cultures in Dulbecco's modified Eagle's Medium, supplemented with 10% heat-inactivated fetal bovine serum, 2 mmol/liter l-glutamine, and antibiotics (100 units/ml penicillin, 50 mg/ml streptomycin-G). The cells were maintained at 37 °C, 5% CO2 and 95% humidified air.
Type I collagen gels (3.0 mg/ml collagen type I derived from bovine skin) were prepared as described previously (14) by neutralizing the collagen solution (PureCol® Advanced BioMatrix, Tucson, AZ) with DMEM and incubation at 37 °C for 24 h until complete polymerization. CF were prepared by digestion of the collagen gels with 2 mg/ml collagenase type 3 (Worthington Biochemical Corp.) at 37 °C for 30 min. Afterward, collagenase activity was inhibited by the addition of an equal volume of DMEM containing 10% FBS. The solution was diluted with DMEM to a final concentration of 125 μg/ml CF.
Three-dimensional fibroblast cultures were prepared by seeding 100,000 cells into 500 μl of the above described neutralized collagen solution. To prevent attachment to the dish, the cell suspension was incubated in mineral oil (Sigma-Aldrich) for 24 h and transferred to DMEM containing 10% FCS after polymerization. UVB irradiation of the cells was performed with a Bio-Sun irradiation system (Vilbert Lourmat; Munich, Germany) containing two 30-watt UVB sources (312 nm). Dermal equivalents were prepared as described previously (16). Fluorescence-assisted cell sorting (FACS) was performed using annexin V-Alexa Fluor 488 antibody (Cell Signaling Technology, Boston, MA) as described previously (17). Cells were gated according to their scatter properties, and the mean fluorescence of Alexa Fluor 488 was determined. As positive control, staurosporine (Sigma-Aldrich) was used.
Affinity Histochemistry
Cells were fixed in acid-formalin/ethanol (3.7% formaldehyde/PBS, 70% ethanol, and 5% glacial acetic acid, all v/v). After rinsing with PBS, cells were stained for HA using a biotinylated HA-binding protein (bHABP, Seikagaku, Tokyo, Japan) followed by streptavidin-FITC (Dako, Carpinteria, CA) in PBS containing 1% bovine serum albumin as described previously (18). As negative control, cells were digested with Streptomyces hyaluronidase prior to staining, which abolished HA staining (data not shown). Imaging of the cells was performed using a Zeiss Axio Observer Z1 microscope and a 63× objective.
Immunocytochemistry
Cells were fixed in 3.7% formalin and permeabilized with 0.3% Triton X-100 (Sigma-Aldrich). After rinsing with PBS, actin stress fibers were stained by FITC-phalloidin (Sigma-Aldrich; 1:1000). pERK was detected using pERK primary antibody (Cell Signaling Technology; 1:1000) followed by Cy3-conjugated anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Nuclei were counterstained by Hoechst 33342 solution (Invitrogen; Darmstadt, Germany; 1:10000). Nuclear translocation of pERK was detected using a Zeiss LSM 700 microscope and a 63× objective (see Fig. 5C) or Zeiss Axio Observer Z1 ApoTome microscope and a 63× objective (supplemental Fig. S3).
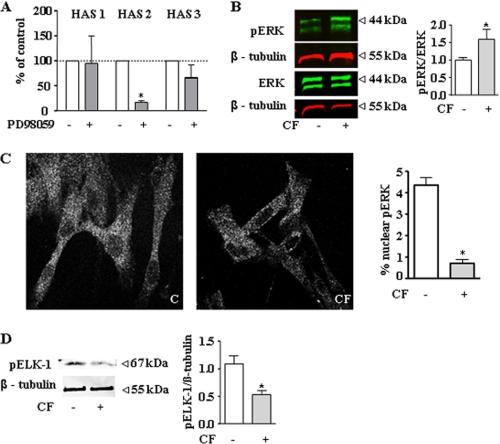
FIGURE 5.
Inhibition of nuclear ERK1/2 activity by CF. A, the ERK inhibitor PD98059 (10 μmol/liter) inhibited HAS2 mRNA expression as determined by real time PCR. B, ERK and phosphoERK in response to CF as determined in total cell lysates after 24 h. C, nuclear translocation of pERK was visualized and quantified by immunostaining and confocal imaging (63× magnification). For quantitative analysis, four images per slide of three independent experiments were analyzed. Using ImageJ, total pERK and nuclear pERK were measured, and the percentage of nuclear pERK was calculated for each image. Panel C indicates control D, the nuclear ERK substrate pELK-1 was detected by immunoblotting of total cell lysates; n = 3–4, mean ± S.E., *, p < 0.05.
Determination of the HA Concentration in Fibroblast Cell Culture Supernatants
HA released into the culture medium was measured with an HABP-based commercial kit according to the manufacturer's instructions (Corgenix, Broomfield, CO) 24 h after stimulation (19). The quantity of HA was calculated as the ratio of HA and total cellular protein.
Size Exclusion Chromatography of HA
Dermal fibroblasts were incubated with 20 μCi/ml [3H]glucosamine for 24 h in the presence and absence of collagen fragments. The conditioned, [3H]glucosamine-labeled medium was divided equally. One-half was digested with hyaluronidase (Streptomyces hyaluronidase, 0.5 units of enzyme/200 μl). Subsequently, both samples were prepurified on a column of Sephadex G50 (Sigma-Aldrich) to separate non-incorporated [3H]glucosamine from the tritiated macromolecular fraction. Comparison of the relative molecular size of secreted HA was performed as described previously (20) using Sephacryl S-1000 (GE Healthcare; Munich, Germany).Only the hyaluronidase-sensitive counts that represent the [3H]glucosamine-labeled HA were plotted.
Real Time RT-PCR
Total RNA from fibroblasts was isolated using RNeasy total RNA kits (Qiagen; Hilden, Germany). The RNA concentration was determined via photometric measurement at 260/280. Total RNA (aliquots of 1000 ng) was transcribed into cDNA using the SuperScript III first strand synthesis system for reverse transcription-polymerase chain reaction (RT-PCR, Invitrogen, Karlsruhe, Germany). To analyze the mRNA expression in human fibroblasts, primers were designed employing Primer Express 3.0 software (Applied Biosystems, Darmstadt, Germany) based on published cDNA sequences. Primer sequences are given in Table 1.
TABLE 1.
Primer sequences used for quantification of gene expression
| Gene | Primer sequence |
|---|---|
| Human HAS1 | 5′-TACAACCAGAAGTTCCTGGG-3′ |
| 5′-CTGGAGGTGTACTTGGTAGC-3′ | |
| Human HAS2 | 5′-GTGGATTATGTACAGGTTTGTGA-3′ |
| 5′-TCCAACCATGGGATCTTCTT-3′ | |
| Human HAS3v1 | 5′-GAGATGTCCAGATCCTCAACAA-3′ |
| 5′-CCCACTAATACACTGCACAC-3′ | |
| Human Hyal1 | 5′-CCAAGGAATCATGTCAGGCCATCAA-3′ |
| 5′-CCCACTGGTCACGTTCAGG-3′ | |
| Human Hyal2 | 5′-GGCTTAGTGAGATGGACCTC-3′ |
| 5′-CCGTGTCAGGTAATCTTTGAG-3′ | |
| Human MMP1 | 5′-TGTGGTGTCTCACAGCTTCC-3′ |
| 5′-CTTGCCTCCCATCATTCTTC-3′ | |
| Human GAPDH | 5′-GTGAAGGTCGGAGTCAACG-3′ |
| 5′-TGAGGTCAATGAAGGGGTC-3′ |
Each real time RT-PCR was performed in triplicate, and the mean value was calculated. PCR was carried out using SYBR Green PCR master mix (Applied Biosystems) as described (12). The 2(−ΔΔC(T)) method was used for comparison of the relative expression in RT-PCR between control and treated cells.
Immunoblotting
For Western blot analysis, whole cell lysates were separated on 10% SDS-PAGE and transferred to nitrocellulose, and the following primary antibodies were used: pERK1/2, ERK1/2, pELK-1, Cofilin, pCofilin, poly(ADP-ribose) polymerase, p38, and pp38 (Cell Signaling Technology) and β-tubulin (Sigma-Aldrich). They were detected by infrared fluorescent-coupled secondary antibodies allowing fluorescent detection on a LI-COR Odyssey infrared imaging system.
Statistical Analysis
All data sets were analyzed either by analysis of variance and the Bonferroni post hoc test or by Student's t test as appropriate. Data are presented as means ± S.E. Statistical significance was assigned at the level of p < 0.05.
RESULTS
Collagen Fragments Inhibit HA Synthesis and HAS2 Expression
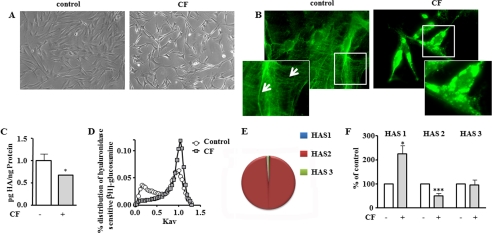
CF were generated by collagenase type 3 digestion of purified bovine collagen type 1. Skin fibroblasts were incubated with these CF for 24 h. In preliminary experiments (supplemental Fig. S1, A–D), a dose-response experiment using CF at 50–250 μg/ml was performed, revealing slightly increased apoptosis starting at 250 μg/ml as evidenced by annexin V FACS analysis. Classical poly(ADP-ribose) polymerase cleavage was not detected up to 250 μg/ml. Fibroblast proliferation measured as [3H]thymidine incorporation was suppressed starting at 150 μg/ml. Therefore, CF were used in all subsequent experiments at 125 μg/ml to avoid anti-proliferative or pro-apoptotic effects. The morphology of fibroblast-treated CF (125 μg/ml) was dramatically changed, characterized by reduced spreading (Fig. 1A) and retraction. However, microscopic surveillance indicated recovery up to 96 h in cells after the addition of CF (supplemental Fig. S1D). Furthermore, pericellular HA strands were markedly reduced (Fig. 1B), and the amount of HA secreted into the conditioned medium was decreased. In addition, the size distribution of secreted HA was shifted to lower molecular mass as evidenced by Sephacryl S-1000 chromatography of [3H]glucosamine-labeled HA. Analysis of the relative amount of HAS mRNA transcripts revealed that HAS2 was the prominent isoform, accounting for more than 95% of HAS transcripts (Fig. 1E). CF caused differential regulation of HAS isoenzymes, as indicated by increased HAS1 mRNA, decreased HAS2 mRNA, and unchanged HAS3 mRNA levels (Fig. 1F). Based on quantitative real time RT-PCR, HAS2 is the most abundant HAS isoform. Therefore, the data suggest that inhibition of HAS2 mRNA is responsible for decreased HA secretion in response to CF. HAS isoforms are thought to generate HA of different chain length. HAS2 and HAS1 synthesize higher molecular weight HA as compared with HAS3 (21). Therefore, the decrease of HAS2 expression likely also explains the loss of the high molecular weight fractions in the supernatants of CF-treated fibroblasts. However, in addition, the mRNA expression of hyaluronidases 1 and -2 was analyzed and revealed 2-fold increased hyaluronidase 2 mRNA (data not shown), hinting toward involvement of HA degradation in the molecular weight shift.
FIGURE 1.
Inhibition of HA synthesis by CF. Human skin fibroblasts were incubated for 24 h with CF (125 μg/ml) generated by digestion of type I collagen gels. A, change in morphology of fibroblasts in response to CF, 63× magnification. B, pericellular HA was preserved by acid-formalin/ethanol fixation and stained with biotinylated HABP and streptavidin-FITC, 63× magnification; insets, at 200× magnification. Arrows point at strands of pericellular HA. C, HA secretion into the medium. D, molecular weight distribution of [3H]glucosamine-labeled HA as determined by Sephacryl S1000 chromatography. E, pie chart of relative mRNA expression of HAS isoforms as determined by real time RT-PCR. F, regulation of HAS isoforms in response to CF as determined by real time RT-PCR; n = 3–6, mean ± S.E., *, p < 0.05; ***, p < 0.01.
αvβ3-Integrin Signaling Inhibits HAS2 Expression
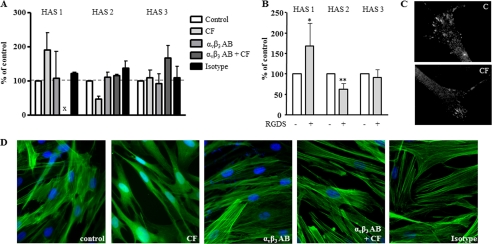
Collagen fragments have been shown to activate αvβ3-integrin signaling in vascular smooth muscle cells (22). Therefore, CF were used in the presence and absence of blocking antibodies to αvβ3. As shown in Fig. 2A, these blocking antibodies abolished both the CF-induced up-regulation of HAS1 and down-regulation of HAS2. Furthermore, the αvβ3-agonist RGDS (23, 24) induced HAS1 and reduced HAS2 expression (Fig. 2B). Immunocytochemistry revealed expression of αvβ3-integrin in a pattern resembling focal adhesions and was not changed by CF (Fig. 2C). CF also caused partial disruption of actin stress fibers, which underlies the morphological change presented in Fig. 1. Interestingly the changes of morphology and actin stress fibers were also prevented by preincubation with blocking αvβ3-antibodies (Fig. 2D). Therefore, the data strongly suggested that αvβ3-integrin signaling mediated the effects of CF on HAS expression, HA synthesis, and the actin cytoskeleton.
FIGURE 2.
αvβ3-Integrin activation mediates HAS regulation by CF. A, preincubation with the αvβ3-blocking antibody LM609 (5 μg/ml, 24 h) inhibited regulation of HAS isoforms by CF as determined by real time RT-PCR, B, HAS isoform expression in response to the αvβ3-agonist RGDS (10 μg/ml); n = 3–5, mean ± S.E., *, p < 0.05; x, not detectable. C, αvβ3 immunostaining of untreated and CF-treated fibroblasts at 24 h. D, actin stress fibers visualized by phalloidin staining; magnification 63×. Non-immune isotype IgG served as control.
Inhibition of ROCK and ERK Signaling Mediates CF Effects on HAS2 Expression
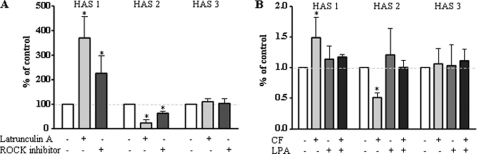
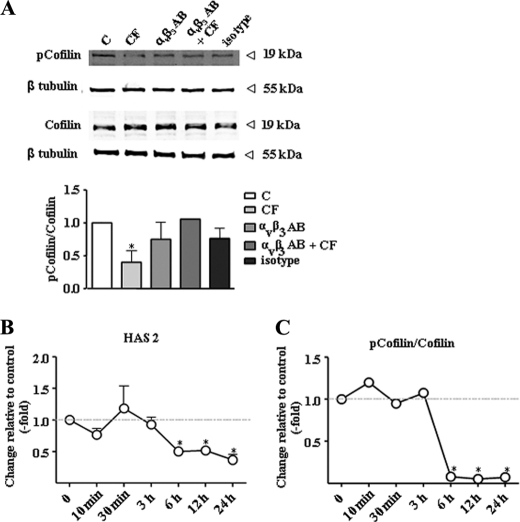
Next the downstream effectors of αvβ3-integrin signaling that might cause down-regulation of HAS2 were investigated. αvβ3 is known to signal through various pathways depending on the cell type and the experimental conditions (25–27). Because CF and αvβ3 had a pronounced effect on the cytoskeleton and HAS2, it was addressed whether these two effects were interrelated. Interestingly, disruption of the actin cytoskeleton by latrunculin A (28) resulted in the same pattern of HAS isoform expression as compared with CF (Fig. 3A). HAS1 was induced, HAS2 was inhibited, and HAS3 was unchanged by latrunculin A. Furthermore, the Rho kinase (ROCK) inhibitor Y27632 increased HAS1, decreased HAS2, and had no effect on HAS3 expression. A role of ROCK was further supported by the use of the ROCK activator lysophosphatidic acid (29, 30), which blocked the effect of CF on HAS expression (Fig. 3B). ROCK is known to cause via LIM-kinase the phosphorylation of cofilin (31), which in the phosphorylated state loses its actin-depolymerizing activity. Actin cytoskeleton in turn is essential for the translocation of phosphorylated ERK1/2 into the nucleus (32). Therefore, it was tested whether cofilin phosphorylation was specifically affected by CF and αvβ3-integrin signaling. Indeed Cofilin phosphorylation was significantly reduced by CF, and this response was abrogated by blocking αvβ3-antibodies (Fig. 4A). Furthermore, the time course of HAS2 down-regulation and inhibition of cofilin phosphorylation were similar (Fig. 4, B and C). Actin filaments contribute to the formation of compartments and translocation pathways to facilitate signaling. In this context, it has been shown that pERK translocation into the nucleus is in part dependent on the cytoplasmic actin network (33). Therefore, it was considered that CF-mediated activation of αvβ3 may inhibit HAS2 expression via the effects on the cytoskeleton and in turn interference with ERK1/2 translocation in the nucleus. In line with this hypothesis, the ERK inhibitor PD98059 specifically inhibited HAS2 expression (Fig. 5A). However, the phosphorylation of ERK1/2 as measured by immunoblotting in total cell lysates was increased in response to CF (Fig. 5B). Therefore, immunocytochemistry and confocal imaging were used to investigate whether translocation of ERK1/2 into the nucleus was affected by CF. As shown in Fig. 5C, nuclear pERK1/2 was significantly reduced in response to CF in human skin fibroblasts. After UVB irradiation of skin fibroblasts, decreased nuclear pERK1/2 was detected by confocal microscopy and the MMP inhibitor restored the nuclear ERK1/2 content (supplemental Fig. S3). In addition to nuclear targeting of pERK1/2, the transcription activator ELK-1 was less phosphorylated in response to CF, supporting the hypothesis that nuclear ERK1/2 activity was in fact reduced in response to CF. In conclusion, these results are consistent with a role of αvβ3-mediated signaling of CF that causes inhibition of ROCK and actin-dependent translocation of ERK1/2 into the nucleus and thus inhibits HAS2 transcription.
FIGURE 3.
Inhibition of ROCK regulates HAS expression. mRNA expression in human fibroblasts was determined by real time RT-PCR. A, mRNA expression after incubation with latrunculin A (3 μmol/liter, 24 h) or the ROCK inhibitor Y27632 (10 μmol/liter, 24 h). B, the ROCK activator lysophosphatidic acid (LPA, 300 nmol/liter) plus or minus CF was added for 24 h; n = 3–5, mean ± S.E., *, p < 0.05.
FIGURE 4.
CF inhibit cofilin phosphorylation. A, phosphorylated cofilin and cofilin were detected by immunoblotting 24 h after the addition of CF plus or minus αvβ3-blocking antibody LM609 (5 μg/ml) or the IgG control (C). B, time course of HAS2 mRNA expression as determined by real time RT-PCR. C, time course of cofilin phosphorylation as evidenced by immunoblotting; n = 3, mean ± S.E., *, p < 0.05.
HAS1 mRNA expression was up-regulated in response to CF in an αvβ3-dependent manner as well as can be concluded by the results shown in Figs. 1F and 2, A and B. To identify the responsible downstream signaling, three different MAPK inhibitors were used. Although inhibition of ERK1/2 and Jun N-terminal kinase had no effect (data not shown), the p38 inhibitor SB203580 inhibited HAS1 mRNA induction by CF (supplemental Fig. S2), and anisomycin, an p38 activator (not shown), increased HAS1 mRNA. Increased p38 phosphorylation in response to CF was detected and occurred at the same time (24 h) as the induction of HAS1 mRNA. Furthermore, blocking antibodies to αvβ3-integrins inhibited CF-induced p38 phosphorylation (supplemental Fig. 2). Collectively these data strongly suggest that HAS1 is induced by αvβ3-integrins via p38 at the same time at which HAS2 is suppressed via inhibition of ROCK.
UVB-induced Collagen Cleavage and αvβ3-Signaling Inhibit HAS2 Expression in Dermal Equivalents
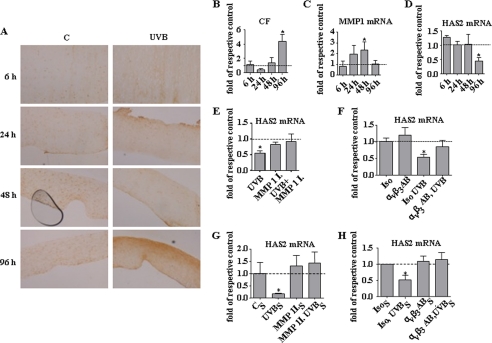
Next it was tested whether exposure of fibroblast cultures and dermal equivalents to UVB irradiation affects HAS2 expression. First collagen fragmentation in response to UVB irradiation was visualized by collagen neoepitope staining in dermal equivalents. After a single dose of UVB, the appearance of collagen neoepitopes (34) was monitored during the course of 96 h (Fig. 6, A and B). In addition, mRNA was isolated from the dermal equivalents during the indicated times after UVB irradiation. Induction of MMP1 mRNA expression was detected 48 h after UVB exposure (Fig. 6C). Notably the decline of HAS2 mRNA expression in response to UVB occurred at 96 h, which was after the peak of MMP1 expression and at the same time when the highest accumulation of collagen neoepitopes was detected (Fig. 6D). In three-dimensional cultures of human fibroblasts in collagen gels, the MMP1 inhibitor abolished UVB-mediated decrease of HAS2 expression (Fig. 6E). To prove that the endogenously generated collagen fragments signal through αvβ3-integrins, the blocking antibody LM609 was applied to the three-dimensional cultures prior to UVB irradiation. As shown in Fig. 6F, the blocking αvβ3-antibody indeed inhibited HAS2 down-regulation.
FIGURE 6.
UVB-induced collagen cleavage inhibits HAS2 via αvβ3. A–D, human fibroblasts were grown in collagen gels to form dermal equivalents. A and B, time course of collagen neoepitope accumulation in response to UVB (10 mJ/cm2) as determined by immunostaining using collagen 2 3/4Cshort polyclonal rabbit antibody. Panel C indicates control. C, MMP1 mRNA expression. D, HAS2 mRNA expression. E and F, three-dimensional cultures of fibroblasts in collagen gels were subjected to irradiation with UVB (100 mJ/cm2). After 24 h, HAS2 mRNA was determined in the presence of the MMP inhibitor I (MMP 1 I., 300 nmol/liter) (E) or the αvβ3-blocking antibody LM609 (αvβ3 AB,5 μg/ml) or isotype control (Iso, 5 μg/ml) (F). G and H, polymeric collagen (120 μg/ml) was added to the medium of monolayer cultures of human skin fibroblasts (CS). Subsequently, the cultures were subjected to UVB irradiation (UVBS, 100 mJ/cm2) in the presence (G) or absence (H) of MMP inhibitor I (MMP 1 I.S, 300 nmol/liter). After 3 days, the conditioned medium was added to a new fibroblast culture in the presence or absence of MMP inhibitor I (300 nmol/liter) (G) or αvβ3-blocking antibody LM609 (αvβ3 ABS, 5 μg/ml) or isotype control (IsoS, 5 μg/ml) (H) for 24 h. Subsequently HAS2 mRNA was determined by real time PCR; n = 3, mean ± S.E., *, p < 0.05.
To further prove that endogenous CF generated in response to UVB down-regulate HAS2, fibroblasts were incubated with collagen and were irradiated to allow cleavage of collagen in response to UVB-induced MMP activity. After 3 days, these supernatants were removed and added without further treatment to fibroblasts to investigate the effect of the endogenous CF in the supernatants. The glucose concentration of this high glucose medium was not reduced significantly by the 3 days of conditioning (data not shown). After 24 h, a significant down-regulation of HAS2 expression was detected. In line with the previous experiments, this down-regulation was sensitive to both the MMP1 inhibitor and the αvβ3-blocking antibodies (Fig. 6, G and H).
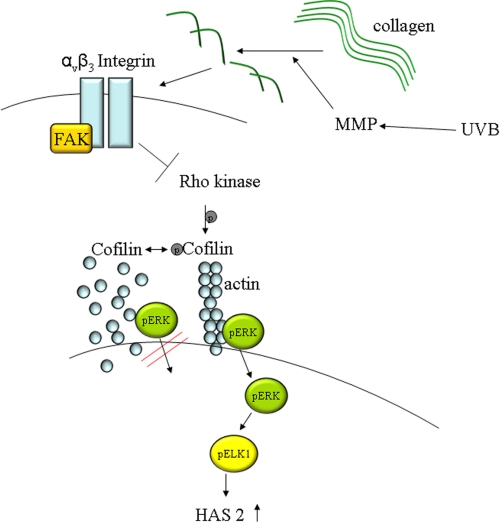
Therefore, the data support the conclusion that UVB-mediated MMP induction and collagen cleavage generate αvβ3-integrin-mediated signals that inhibit ROCK activity and subsequently nuclear activity of ERK1/2. In turn, decreased ERK1/2 activity leads to down-regulation of HAS2 expression and thus decrease of extracellular and pericellular HA (schematic drawing in Fig. 7).
FIGURE 7.
Schematic drawing of the proposed mechanism of HAS2 down-regulation in response to UVB. UVB induces MMP1 expression and degradation of polymeric collagen to CF. The present data suggest that CF activate αvβ3-integrins, which inhibit ROCK activity and cofilin phosphorylation. Consequently translocation of pERK in the nucleus and transcriptional activation of HAS2 are suppressed. FAK, focal adhesion kinase.
DISCUSSION
HA is synthesized by dermal fibroblasts, which incorporate HA as a quantitative and functionally important component into the dermal ECM. HA is found to a lesser extent in the healthy epidermis but is induced in keratinocytes upon wounding (35). The role of HA during skin aging is of interest because loss of HA is thought to be involved in the actinic aging response. However, also other roles of HA have been reported such as the pro-inflammatory action of low molecular weight HA and of HA cables (36, 37). In this regard, it is very interesting that during the acute response to UVB irradiation of the skin, HA synthesis is induced in keratinocytes and low molecular weight HA is detected, which may be involved in the transient inflammatory response during sunburn (38, 39). The regulatory mechanisms that are responsible for the loss of HA during photoaging have not been fully identified yet.
Previous studies have indicated that proteolysis of type I collagen exposes cryptic RGD integrin-binding motifs (39, 40) that attribute new biological activities to CF as compared with native fibrillar collagen. For example, CF have been shown to inhibit apoptosis via αvβ3-integrin signaling in vascular smooth muscle cells (22).
Degradation of fibrillar collagen type I and III occurs in response to UVB irradiation of the skin and likely causes the aging phenotype of chronically sun-exposed skin (2, 41). The degradation of collagen into CF is mediated by MMPs that are up-regulated in response to UVB (2, 3). The induction of MMPs in response to UVB is the result of at least three mechanisms: (i) induction of MMPs in keratinocytes and diffusion of MMPs to the papillary dermis; (ii) induction of cytokines such as IL1 in the epidermis and/or the dermis and subsequent induction of MMPs by IL-1 in a paracrine and autocrine manner; and (iii) induction of MMPs by UVB in fibroblasts in the papillary dermis.
Of note, CF were thought to reduce de novo synthesis of collagen as compared with sun-protected skin (42). This was confirmed in vitro by the finding that de novo synthesis of type I procollagen is indeed inhibited after MMP1-mediated partial degradation of fibrillar collagen in human skin fibroblasts cultured in three-dimensional collagen gels (43, 44). Thus degradation of collagen during actinic aging generates bioactive fragments that induce alternative signaling and contribute to the matrix remodeling and altered cellular phenotypes. In line with these findings, fibroblasts in the dermis are attached to collagen fibrils, are well spread, and display abundant cytoplasmic actin fibrils (45). In contrast, after actinic aging, fibroblasts interact with collagen fragments rather than with fibrils and are characterized by less spreading and less actin (45). In the experiments presented here, a similar effect was shown; CF reduced spreading and cytoplasmic actin in human skin fibroblasts.
The major aim of the present study was to explore whether a cross-talk might exist between collagen degradation by MMPs and regulation of HA synthesis in human skin fibroblasts. Indeed we observed that CF caused loss of the pericellular HA matrix and strongly reduced HA secretion in human skin fibroblasts. CF activate different integrins as compared with polymeric collagen. Specifically activation of αvβ3-integrins has been shown before to mediate CF signaling in vascular smooth muscle cells (22). The use of blocking αvβ3-antibodies in the present study proved that this integrin also mediates the down-regulation of HAS2 and the cytoskeletal effects in response to CF. The ROCK family of Rho-associated protein kinases is essential for F-actin assembly (46). The finding that latrunculin A and the ROCK inhibitor mimicked the effect of CF on HAS isoform expression suggested that the cytoskeleton and ROCK-mediated signals were involved. It is known that αvβ3-integrins are associated with low RhoA-ROCK activity in part through interference with the recycling pathway of α5β1-integrin (47, 48).
ROCK is known to participate in nucleocytoplasmic trafficking of signaling molecules by its effects on assembly of cellular stress fibers (33, 46). Cofilin is essential for turnover of actin filaments, and Cofilin activity is regulated by phosphorylation (49). Specifically, Rho-ROCK signaling activates the LIM-kinase, and LIM-kinase phosphorylates cofilin, which in turn results in stabilization of actin filaments (50). Therefore, it was investigated whether the phosphorylation of cofilin was responsive to CF. Indeed it is shown here that CF induced αvβ3-integrin-dependent phosphorylation of cofilin.
Furthermore, nuclear translocation of ERK1/2 is required for its effects on gene regulation (32, 51). In addition, it has been demonstrated that translocation of ERK1/2 to the nucleus can require RhoA/ROCK signaling (52, 53). Interestingly the content of pERK1/2 in the nucleus was decreased in response to CF, and an ERK1/2 inhibitor inhibited HAS2 expression. As a direct target of the MAP kinase pathways, the transcription factor Elk-1 is known to be coupled to ERK entry into the nucleus (33). Thus as readout for nuclear pERK1/2 activity, the phosphorylation of ELK-1 was detected and found to be decreased.
Downstream of ERK are several nuclear transcription factors, among them cyclic AMP-response element-binding protein (CREB), which is activated via mitogen- and stress-activated kinase (MSK) (54). Because the first 2250 bp of the HAS2 promoter contain three response elements for CREB1 (55), it is likely that HAS2 transcription is induced by pERK1/2-CREB activation and that this is the effector mechanism of HAS2 transcription that is interfered with by CF signaling. In summary, the present data on signaling are consistent with activation of αvβ3-integrins by CF and subsequent inhibition of ROCK and cofilin phosphorylation, which ultimately led to inhibition of pERK translocation and transcriptional activation of HAS2.
HAS2 appeared to be the predominant isoform in skin fibroblasts, based on the results of the quantitative real time RT-PCR. This assumption was further supported by the fact that the extent of HAS2 down-regulation correlated with the decrease of HA secretion. In parallel to HAS2 down-regulation, induction of HAS1 occurred in response to CF. The up-regulation of HAS1 was also dependent on αvβ3-integrins and mediated downstream by activation of p38, as shown by inhibitors. The activation of p38 by αvβ3-integrins has been shown before (56), and induction of HAS1 by p38 activation was reported in human fibroblast-like synoviocytes (57). The induction of HAS1 may represent a counter regulation in response to HAS2 suppression. However, quantitatively and functionally, HAS1 induction is likely less important because HAS1 is expressed at such a low level that the 2–3-fold up-regulation in response to CF may not contribute significantly to total HA synthesis. Therefore, the mechanistic experiments addressing the effects of UVB irradiation were focused mainly on HAS2 expression. The use of dermal equivalents and collagen gels allowed us to investigate the effect of UVB on fibroblasts in a three-dimensional collagen matrix similar to those used before by Varani et al. (43). As a result, down-regulation of HAS2 occurred after induction of MMP1 and at the time of maximal collagen neoepitope accumulation. Furthermore, UVB-induced down-regulation of HAS2 was sensitive to MMP1 inhibition and αvβ3-integrin blockage. Collectively the present data suggest a novel pathway that couples HA synthesis to collagen matrix turnover, similar to inhibition of de novo collagen synthesis by CF (43).
During skin aging, this might be detrimental because collagen cleavage in response to UVB may thereby also induce loss of HA from the skin at the same time at which collagen structure and de novo synthesis are impaired. Because HA is thought to be important for cell proliferation and migration within the skin, this mechanism may contribute to the impaired regenerative capacity of aged skin.
The present finding may also be relevant from the standpoint of HAS regulation in general. Almost all regulatory systems that have been described before induce HA, such as various growth factors and cytokines. Here a direct negative regulatory pathway was identified that might also be important for physiological and pathophysiological processes where extensive remodeling of the collagenous extracellular matrix takes place, such as tumor progression, myocardial, or vascular remodeling.
Supplementary Material
Acknowledgment
We kindly thank Bettina Mausa for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Collaborative Research Center (SFB) 728 (Grants TP C6 and TP C1).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- MMP
- matrix metalloproteinases
- CF
- collagen fragments
- ECM
- extracellular matrix
- HA
- hyaluronic acid
- HAS
- hyaluronan synthase
- HABP
- HA-binding protein
- CREB
- cyclic AMP-response element-binding protein
- ROCK
- Rho kinase
- p
- phospho.
REFERENCES
- 1. Bruls W. A., van Weelden H., van der Leun J. C. (1984) Photochem. Photobiol. 39, 63–67 [DOI] [PubMed] [Google Scholar]
- 2. Fisher G. J., Wang Z. Q., Datta S. C., Varani J., Kang S., Voorhees J. J. (1997) N. Engl. J. Med. 337, 1419–1428 [DOI] [PubMed] [Google Scholar]
- 3. Fisher G. J., Datta S. C., Talwar H. S., Wang Z. Q., Varani J., Kang S., Voorhees J. J. (1996) Nature 379, 335–339 [DOI] [PubMed] [Google Scholar]
- 4. Manuskiatti W., Maibach H. I. (1996) Int. J. Dermatol. 35, 539–544 [DOI] [PubMed] [Google Scholar]
- 5. Itano N., Kimata K. (1996) Biochem. Biophys. Res. Commun. 222, 816–820 [DOI] [PubMed] [Google Scholar]
- 6. Day A. J., Prestwich G. D. (2002) J. Biol. Chem. 277, 4585–4588 [DOI] [PubMed] [Google Scholar]
- 7. Toole B. P., Wight T. N., Tammi M. I. (2002) J. Biol. Chem. 277, 4593–4596 [DOI] [PubMed] [Google Scholar]
- 8. Yoneda M., Yamagata M., Suzuki S., Kimata K. (1988) J. Cell Sci. 90, 265–273 [DOI] [PubMed] [Google Scholar]
- 9. Margelin D., Medaisko C., Lombard D., Picard J., Fourtanier A. (1996) J. Invest. Dermatol. 106, 505–509 [DOI] [PubMed] [Google Scholar]
- 10. Takahashi Y., Ishikawa O., Okada K., Kojima Y., Igarashi Y., Miyachi Y. (1996) J. Dermatol. Sci. 11, 129–133 [DOI] [PubMed] [Google Scholar]
- 11. Takahashi Y., Ishikawa O., Okada K., Ohnishi K., Miyachi Y. (1995) J. Dermatol. Sci. 10, 139–144 [DOI] [PubMed] [Google Scholar]
- 12. Dai G., Freudenberger T., Zipper P., Melchior A., Grether-Beck S., Rabausch B., de Groot J., Twarock S., Hanenberg H., Homey B., Krutmann J., Reifenberger J., Fischer J. W. (2007) Am. J. Pathol. 171, 1451–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carragher N. O., Levkau B., Ross R., Raines E. W. (1999) J. Cell Biol. 147, 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Wnuck Lipinski K., Keul P., Lucke S., Heusch G., Wohlschlaeger J., Baba H. A., Levkau B. (2006) Cardiovasc. Res. 69, 697–705 [DOI] [PubMed] [Google Scholar]
- 15. Varani J., Warner R. L., Gharaee-Kermani M., Phan S. H., Kang S., Chung J. H., Wang Z. Q., Datta S. C., Fisher G. J., Voorhees J. J. (2000) J. Invest. Dermatol. 114, 480–486 [DOI] [PubMed] [Google Scholar]
- 16. Majora M., Wittkampf T., Schuermann B., Schneider M., Franke S., Grether-Beck S., Wilichowski E., Bernerd F., Schroeder P., Krutmann J. (2009) Am. J. Pathol. 175, 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber A. A., Przytulski B., Schumacher M., Zimmermann N., Gams E., Hohlfeld T., Schrör K. (2002) Br. J. Haematol. 117, 424–426 [DOI] [PubMed] [Google Scholar]
- 18. Evanko S. P., Angello J. C., Wight T. N. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 1004–1013 [DOI] [PubMed] [Google Scholar]
- 19. Twarock S., Tammi M. I., Savani R. C., Fischer J. W. (2010) J. Biol. Chem. 285, 23276–23284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkinson T. S., Potter-Perigo S., Tsoi C., Altman L. C., Wight T. N. (2004) Am. J. Respir. Cell Mol. Biol. 31, 92–99 [DOI] [PubMed] [Google Scholar]
- 21. Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A. P., McDonald J. A., Kimata K. (1999) J. Biol. Chem. 274, 25085–25092 [DOI] [PubMed] [Google Scholar]
- 22. von Wnuck Lipinski K., Keul P., Ferri N., Lucke S., Heusch G., Fischer J. W., Levkau B. (2006) Circ. Res. 98, 1490–1497 [DOI] [PubMed] [Google Scholar]
- 23. Ortega-Velázquez R., Díez-Marqués M. L., Ruiz-Torres M. P., González-Rubio M., Rodríguez-Puyol M., Rodríguez Puyol D. (2003) FASEB J. 17, 1529–1531 [DOI] [PubMed] [Google Scholar]
- 24. Marchand-Brynaert J., Detrait E., Noiset O., Boxus T., Schneider Y. J., Remacle C. (1999) Biomaterials 20, 1773–1782 [DOI] [PubMed] [Google Scholar]
- 25. Putnam A. J., Schulz V. V., Freiter E. M., Bill H. M., Miranti C. K. (2009) Cell Commun. Signal 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee D. Y., Yeh C. R., Chang S. F., Lee P. L., Chien S., Cheng C. K., Chiu J. J. (2008) J. Bone Miner Res. 23, 1140–1149 [DOI] [PubMed] [Google Scholar]
- 27. Ahmed M., Kundu G. C. (2010) Mol. Cancer 9, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Oliveira C. A., Mantovani B. (1988) Life Sci. 43, 1825–1830 [DOI] [PubMed] [Google Scholar]
- 29. van Leeuwen F. N., Giepmans B. N., van Meeteren L. A., Moolenaar W. H. (2003) Biochem. Soc. Trans. 31, 1209–1212 [DOI] [PubMed] [Google Scholar]
- 30. Masiero L., Lapidos K. A., Ambudkar I., Kohn E. C. (1999) J. Cell Sci. 112, 3205–3213 [DOI] [PubMed] [Google Scholar]
- 31. Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., Obinata T., Ohashi K., Mizuno K., Narumiya S. (1999) Science 285, 895–898 [DOI] [PubMed] [Google Scholar]
- 32. Pouysségur J., Volmat V., Lenormand P. (2002) Biochem. Pharmacol. 64, 755–763 [DOI] [PubMed] [Google Scholar]
- 33. Aplin A. E., Stewart S. A., Assoian R. K., Juliano R. L. (2001) J. Cell Biol. 153, 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Billinghurst R. C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C., Mitchell P., Hambor J., Diekmann O., Tschesche H., Chen J., Van Wart H., Poole A. R. (1997) J. Clin. Invest. 99, 1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tammi R. H., Tammi M. I. (2009) J. Invest. Dermatol. 129, 1858–1860 [DOI] [PubMed] [Google Scholar]
- 36. Stern R., Asari A. A., Sugahara K. N. (2006) Eur. J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 37. de la Motte C. A., Hascall V. C., Drazba J., Bandyopadhyay S. K., Strong S. A. (2003) Am. J. Pathol. 163, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Averbeck M., Gebhardt C. A., Voigt S., Beilharz S., Anderegg U., Termeer C. C., Sleeman J. P., Simon J. C. (2007) J. Invest. Dermatol. 127, 687–697 [DOI] [PubMed] [Google Scholar]
- 39. Montgomery A. M., Reisfeld R. A., Cheresh D. A. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis G. E. (1992) Biochem. Biophys. Res. Commun. 182, 1025–1031 [DOI] [PubMed] [Google Scholar]
- 41. Fligiel S. E., Varani J., Datta S. C., Kang S., Fisher G. J., Voorhees J. J. (2003) J. Invest. Dermatol. 120, 842–848 [DOI] [PubMed] [Google Scholar]
- 42. Griffiths C. E., Russman A. N., Majmudar G., Singer R. S., Hamilton T. A., Voorhees J. J. (1993) N. Engl. J. Med. 329, 530–535 [DOI] [PubMed] [Google Scholar]
- 43. Varani J., Spearman D., Perone P., Fligiel S. E., Datta S. C., Wang Z. Q., Shao Y., Kang S., Fisher G. J., Voorhees J. J. (2001) Am. J. Pathol. 158, 931–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Varani J., Perone P., Fligiel S. E., Fisher G. J., Voorhees J. J. (2002) J. Invest. Dermatol. 119, 122–129 [DOI] [PubMed] [Google Scholar]
- 45. Varani J., Schuger L., Dame M. K., Leonard C., Fligiel S. E., Kang S., Fisher G. J., Voorhees J. J. (2004) J. Invest. Dermatol. 122, 1471–1479 [DOI] [PubMed] [Google Scholar]
- 46. Narumiya S., Ishizaki T., Watanabe N. (1997) FEBS Lett. 410, 68–72 [DOI] [PubMed] [Google Scholar]
- 47. Danen E. H., Sonneveld P., Brakebusch C., Fassler R., Sonnenberg A. (2002) J. Cell Biol. 159, 1071–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. White D. P., Caswell P. T., Norman J. C. (2007) J. Cell Biol. 177, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moon A., Drubin D. G. (1995) Mol. Biol. Cell 6, 1423–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huveneers S., Danen E. H. (2009) J. Cell Sci. 122, 1059–1069 [DOI] [PubMed] [Google Scholar]
- 51. Brunet A., Roux D., Lenormand P., Dowd S., Keyse S., Pouysségur J. (1999) EMBO J. 18, 664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kawamura S., Miyamoto S., Brown J. H. (2003) J. Biol. Chem. 278, 31111–31117 [DOI] [PubMed] [Google Scholar]
- 53. Liu Y., Suzuki Y. J., Day R. M., Fanburg B. L. (2004) Circ. Res. 95, 579–586 [DOI] [PubMed] [Google Scholar]
- 54. Wiggin G. R., Soloaga A., Foster J. M., Murray-Tait V., Cohen P., Arthur J. S. (2002) Mol. Cell. Biol. 22, 2871–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Makkonen K. M., Pasonen-Seppänen S., Törrönen K., Tammi M. I., Carlberg C. (2009) J. Biol. Chem. 284, 18270–18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen J., Baskerville C., Han Q., Pan Z. K., Huang S. (2001) J. Biol. Chem. 276, 47901–47905 [DOI] [PubMed] [Google Scholar]
- 57. Stuhlmeier K. M., Pollaschek C. (2004) J. Biol. Chem. 279, 8753–8760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.