Abstract
Background
Leishmaniasis is a major parasitic disease in the tropical regions. However, Leishmania infantum has recently emerged as a very important cause of opportunistic infections for individuals positive for human immunodeficiency virus (HIV). However, there is a lack of in vitro tests for assessing the effect of anti-parasitic drugs on the viability and proliferation of Leishmania infantum. The aim of this study is to assess the efficacy of anti-parasitic drugs like allopurinol and Chloralin on the viability and proliferation of L. infantum promastigotes by utilizing two complementary flow cytometric approaches after exposure of the promastigotes to various concentrations of the drugs.
Results
The density of the cultures in the presence and absence of allopurinol was determined by haemocytometer enumeration. The two flow cytometric approaches used to monitor the drug effect were: (i) a quantitative method to measure cell division using 5-,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) staining and (ii) evaluation of cell viability by dual-staining with the membrane-permeable nuclear stain, SBRY-14 and propidium iodide. It was found that concentrations of allopurinol above 50 μg/ml yielded a clear decrease in the proliferation rate of the promastigotes. However, the viability results showed that about 46.8% of the promastigotes incubated in the presence of 800 μg/ml of allopurinol were still alive after 96 hours. In sharp contrast, more than 90% of promastigotes treated with Chloralin 10 μM (2.7 μg/ml) were dead after 48 hours of treatment. These flow cytometric findings suggest that allopurinol has a leishmaniostatic effect while the dinitroaniline compound (Chloralin) has a leishmaniocidal effect against promastigotes.
Conclusions
The flow cytometric data on proliferation and viability were consistent with results obtained from haemocytometer counts and allowed us to develop a model for assessing in vitro the effects of medicaments like allopurinol and chloralin on L. infantum promastigotes on a cellular level.
Introduction
Leishmaniasis is a major parasitic disease. The yearly prevalence is estimated at 12 million people world wide, and 200-350 million people in the tropical and subtropical regions are at risk. In the Mediterranean region, leishmaniasis caused by Leishmania infantum has emerged as an important source of opportunistic infections for individuals infected with the human immunodeficiency virus (HIV) [1,2,3,4,5]. Dogs and wild canids are significant reservoirs and are primarily responsible for the persistence of the disease in this region [6,7]. The seroprevalence of canine leishmaniasis in the mediterranean region ranges between 10 to 37% [6,7]. Even in non-tropical countries like the United States, the leishmaniasis in dogs has been found, with new cases being reported not only in the traditional endemic areas of Oklahoma, Texas and Ohio but also in non-endemic eastern coastal states like Maryland [8,9]. Pentavalent antimonial agents (SbV) in the form of sodium stibogluconate (Pentostam®) or N-methyl-D-glucamine antimoniate (Glucantime®) are still widely used as the drugs of choice against leishmaniasis, despite all the deleterious side effects like cardiac and renal toxicity, the difficulty of administration and high costs. Other drugs utilized include amphotericin B, pentamidine and allopurinol. However, these drugs do not have such a favorable therapeutic index as the antimonials, and they require a long-term therapeutic procedure that often induces toxic side effects. Miltefosine is another potential alternative drug whose efficacy is presently being evaluated [10,11,12,13].
The therapeutic potential of allopurinol for the treatment of canine leishmaniasis alone or in combination with antimonials has been recently published [14,15,16]. However, despite of initial clinical successes, relapses have been documented, and parasite clearance was not achieved in dogs treated solely with allopurinol. Therefore, the development of better and less toxic therapeutic agents is urgently needed. The absence of an alternative chemotherapeutic approach to the treatment of Leishmania infection requires urgent attention. The discovery of the antiprotozoal effects of tubulin inhibitory compounds like the benzimidazoles (albendazole) and the dinitroanilines, have been examined for their leishmaniacidal effects. Although the antiprotozoal effects of the dinitroanilines have received some attention, a more detailed examination of the range of effects of this group of compounds has not been undertaken. Chloralin, like its dinitroaniline parent (trifluralin), targets tubulin. Dinitroanilines are good lead compounds for antiparasitic drugs because they have already been shown to lack activity against mammalian cells and are inexpensive to produce [17,18,19,20,21,22].
Since the present sensitivity assays for antileishmanial drugs have unsatisfactory features, the development of sensitivity assays for in vitro screening of compounds with potential antileishmanial effects (leishmaniocidal)) which have the characteristics of low price, allow high throughput rates, and that yield fast, reliable and reproducible results are urgently required. The currently available assays have several shortcomings, which include the features of being extensively elaborate and very expensive. Since promastigotes are the simplest stage form of Leishmania to cultivate in vitro and therefore used often in drug susceptibility assays [23,24,25,26], we used promastigotes to determine the effect of allopurinol and two other medicaments like chloralin and cycloheximide on this parasite form. Using [3H]-thymidine incorporation assay, Chloralin isomer had showed high efficacy on these promastigotes in an earlier study [18].
The traditional approaches of cell counting and measurement of thymidine incorporation were utilized in the present study, as well as two newly developed flow cytometric methods for assessing cell proliferation and viability. The stable intra-cytoplasmic dye, 5-,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) was used as a quantitative method to measure cell division. CFSE allowed the identification of cell progeny and tracking and analysis of the division history of individual cells that had undergone multiple rounds of division [27]. CFSE attaches to parasite cells by forming stable conjugates with aliphatic amines [28]. Upon subsequent growth, fluorescence per cell is halved per division, providing an ideal tool for monitoring cell proliferation [29]. To determine the viability of promastigotes, the membrane-permeable nuclear stain, SYBR-14, was used in combination with propidium iodide (PI), a positively charged nucleic acid dye unable to cross the intact plasma membranes of living cells [29,30,31,32].
Results
Growth kinetics
The growth kinetics for a period of 7 days is showed in Figure 1. The log phase of the untreated control promastigotes lasted until day 2. Thereafter the parasites gradually entered the stationery phase. The maximum cell concentration of 1.1 × 108/ml was achieved after 72 hours.
Figure 1.
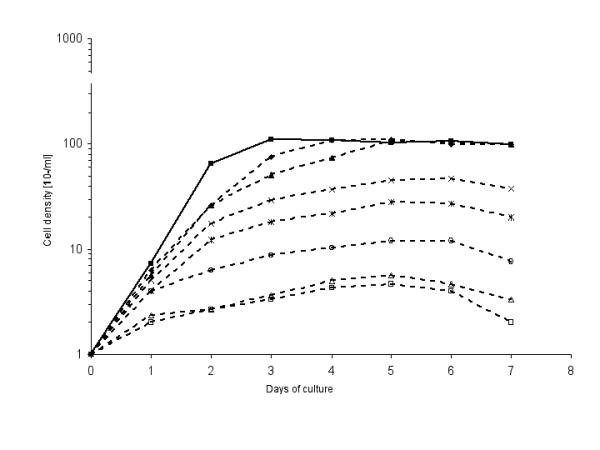
Growth kinetics of p-229 promastigotes exposed to different allopurinol concentrations μg/ml). 0 (control, closed squares), 4 (closed rhombuses), 10 (closed triangles), 50 (crosses) 100 (stars), 200 (open circles), 400 (open triangles) and 800 μg/ml allopurinol (open squares).
Promastigotes treated with 4 and 10 μg/ml of allopurinol, respectively, had a similar 72 hours log phase but reached a maximum cell density comparable to that of the control promastigotes after 4 or 5 days. Allopurinol concentrations higher than 50 μg/ml produced a marked decrease of the proliferation (Figure 1).
Promastigote proliferaton determined by CFSE staining
The proliferation of the promastigotes in the presence and absence of allopurinol was determined by CFSE staining. The results from each treatment were grouped and the histogram overlaid in order to show the decrease in CFSE fluorescence intensity in subsequent days (Figure 2). Eight panels of overlaid histograms show the CFSE fluorescence for control (panel 1) and allopurinol treated promastigotes (panels 2-8). For each treatment, a series of discrete peaks exhibiting progressive decrease of CFSE fluorescence at different time intervals was an indication for on-going cell divisions.
Figure 2.
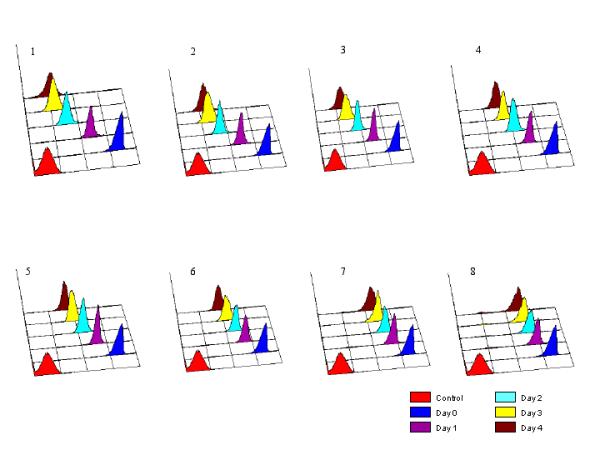
Single parameter histograms of CFSE fluorescence of p-229 promastigotes exposed to 4, 10, 50, 100, 200, 400 and 800 μg/ml allopurinol (panels 2-8, respectively; panel 1, unexposed control). X-axes, CFSE fluorescence intensities (four log scale).
The fluorescence patterns observed in promastigotes treated with 4 and 10 μg/ml allopurinol (panels 2 and 3, respectively) were similar to that of the control promastigotes (panel 1). However, the fluorescence decrease was clearly decelerated by allopurinol concentrations above 50 μg/ml (panels 4-8).
A distinct decrease in the fluorescence intensity in promastigotes treated with 400 and 800 μg/ml allopurinol was observed after 24 hours only, thereafter fluorescence intensities remained almost stable (panel 7 and 8, respectively), suggestive of an almost completely arrested cell proliferation.
Cell divisions and generation times
Each daughter cell inherits approximately half of the CFSE label, hence allowing monitoring and quantification of cell divisions as indicated in Figure 3. The untreated control promastigotes proliferated at a constant rate for the first 48 hours (generation time about 8 hours) and reached a peak cell density at 72 hours. Allopurinol concentrations above 10 μg/ml caused a marked decrease in cell division rates and subsequently increased generation times (e.g. 12 to 15 hours and 20 to 30 hours in presence of 50 μg/ml or 400 μg/ml allopurinol, respectively). During the 96 hours period of observation, the control cells divided 7 times, whereas 6 cell divisions were observed in promastigotes exposed to 50 μg/ml allopurinol and only 3 rounds of divisions were detected when cells were exposed to a drug concentration of 400 μg/ml (Figure 3).
Figure 3.
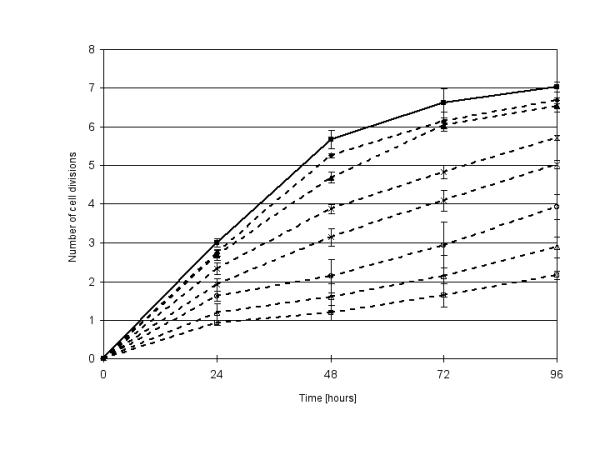
Number of cell divisions of CFSE stained p-229 promastigotes, unexposed control (closed squares), and exposed to 4 (closed rhombuses), 10 (closed triangles), 50 (crosses) 100 (stars), 200 (open circles), 400 (open triangles) and 800 μg/ml allopurinol (open squares).
Promastigote viability
The viability of promastigotes exposed to different concentrations of allopurinol was determined by SYBR-14 and PI dual staining and the results are given as two-parameter density-plots (Figure 4). Table 1 gives the proportions (%) of the promastigotes stained mainly with either PI, SYBR-14 or both (intermediate PI and SYBR-14) after 48 and 96 hours, respectively. After 96 hours only 46.8% of the promastigotes exposed to 800 μg/ml of allopurinol were still alive, whereas 97.1 % of the control promastigotes were alive. There was no significant difference in the proportion of dead or live promastigotes between the control and the 4 or 10 μg/ml allopurinol treated cells. However, allopurinol concentrations above 50 μg/ml led to a clear increase in the proportion of PI stained dead cells (Figure 4 and Table 1) after a 96 hours exposure to the drug. This finding was also observed for the dual stained promastigotes. Consequently, allopurinol treatment led to a clearly decreased proportion of SYBR-14 stained (live) promastigotes.
Figure 4.
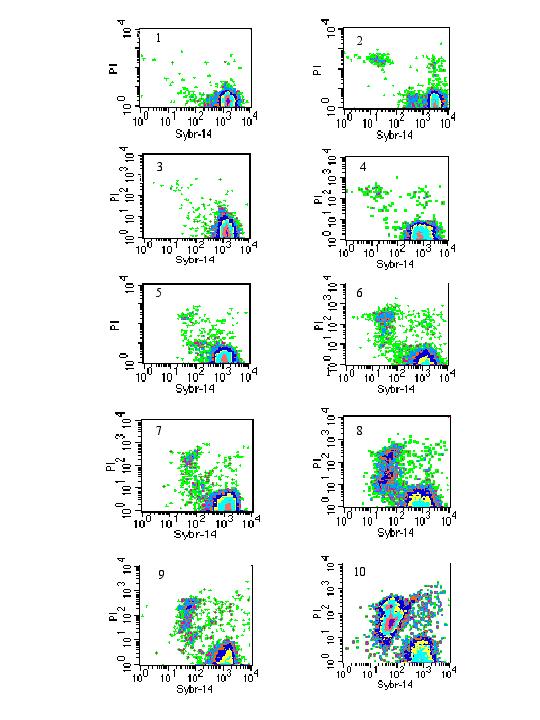
Two-parameter density plots of PI and SYBR-14 stained p-229 promastigotes after 48 hours (left panels) and 96 hours (right panels). Untreated controls (panels 1 and 2), parasites exposed to 4 (panels 3 and 4), 50 (panels 5 and 6), 200 (panels 7 and 8) and 800 μg/ml allopurinol (panels 9 and 10).
Table 1.
Proportion (%) of PI, SYBR-14 and dual (PI/SYBR-14) stained p-229 promastigotes in the presence of various concentrations of allopurinol after 48 and 96 hours
| Allopurinol | 48 hours | 96 hours | ||||
| concentration | ||||||
| PI / | PI / | |||||
| PI | SYBR-14 | PI | SYBR-14 | |||
| (μg/ml) | ||||||
| SYBR-14 | SYBR-14 | |||||
| 0 | 0.15 | 0.09 | 99.28 | 1.63 | 0.78 | 97.12 |
| 4 | 0.27 | 0.30 | 99.16 | 0.69 | 0.39 | 98.76 |
| 10 | 0.66 | 0.27 | 98.74 | 0.60 | 0.33 | 98.88 |
| 50 | 0.84 | 0.33 | 97.93 | 4.99 | 1.24 | 92.26 |
| 100 | 1.19 | 0.32 | 96.92 | 5.25 | 1.07 | 91.19 |
| 200 | 2.09 | 0.42 | 95.72 | 7.25 | 1.49 | 85.29 |
| 400 | 3.39 | 0.76 | 93.50 | 19.72 | 3.59 | 69.56 |
| 800 | 4.74 | 0.76 | 92.33 | 38.85 | 5.79 | 46.80 |
After establishing this flow cytometric model for assessing promastigote susceptibility to allopurinol, it was hypothesized that other antileishmanial drugs could be assessed by this approach and will yield similar results. Thus, it was examined the effect of 10 μM chloralin on the viability of L. infantum promastigotes after 48 hours of exposure. It was found that 91.85 % of the promastigotes were dead and stained with PI while only 7.36% of the promastigotes remained alive and stained with SYBR-14. In contrast, only 0.41% of the untreated promastigotes were dead and stained with PI while 98.61% were alive and stained with SYBR-14. Of note, the increasing of the dose to 100 μM only yielded a similar pattern but with higher promastigote mortality (91.77%) (Table 2 and Figure 5).
Table 2.
Proportion (%) of PI, SYBR-14 and dual (PI/SYBR-14) stained p-229 promastigotes in the presence of various concentrations of Chloralin after 48 hours
| Chloralin | 48 hours | ||
| concentration | |||
| PI / | |||
| PI | SYBR-14 | ||
| (μM/ml) | |||
| SYBR-14 | |||
| 0 | 0.41 | 0.97 | 97.58 |
| 10 | 91.85 | 0.79 | 7.36 |
| 100 | 91.77 | 2.10 | 6.12 |
Figure 5.
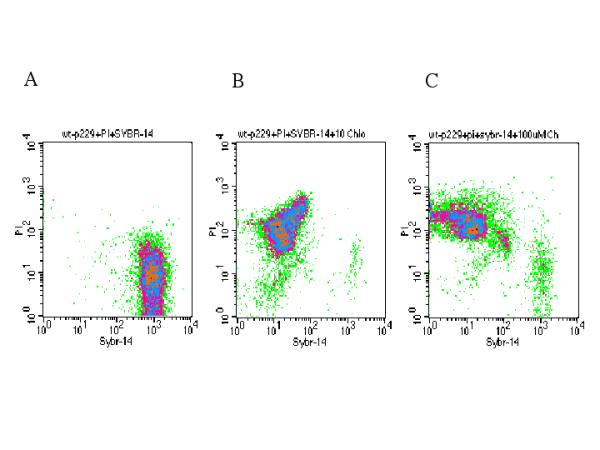
Two-parameter density plots of PI and SYBR-14 stained p-229 promastigotes after 48 hours. Untreated controls (panel A), parasites exposed to 10 (panel B) and 100 μM Chloralin (panel C).
Treatment of promastigotes with 100 μg/ml of cyclohexamide yielded some mortality but it was not to the same magnitude as with 10 μM of chloralin. (Data not shown)
Discussion
The effect of allopurinol on L. infantum (p-229, MCAN/ES/89/IPZ229/1/89, zymodeme MON1) promastigotes was determined by cell counting and flow cytometric approaches. Allopurinol treatment of promastigotes led to a gradual inhibition of cell proliferation with increasing drug concentrations.
The rapid and precise determination of proliferation rates and generation times by CSFE staining is beneficial for the analysis of the effects of antileishmanial compounds. The CFSE results on the growth kinetics were consistent with those determined by cell counting. Until now, uptake of [3H]-thymidine or other radiolabeled compounds have been one of the most common methods for determining cell division, or more specifically, determination of DNA replication. However, these approaches can only give an indication of the overall activity of cell populations and does not give any information on the proliferative activity of individual cells [33,34,35]. Additionally, [3H]-thymidine is only taken up and/or incorporated by cells, which are replicating at the time the culture is pulsed, which underestimates proliferation if the majority of divisions occur at an early phase of the culture [27]. Furthermore, an earlier study indicated that the CFSE method was a more reliable and appropriate method to determine promastigote proliferation, since the [3H]-thymidine incorporation assay seemed to measure the overall [3H]-thymidine uptake by the cells rather than the actual cell proliferation [30]. The combination of SYBR-14 and PI has been used extensively in sperm viability studies [32]. In our study, SYBR-14 stained the nuclei of live promastigotes brilliant green, while PI stained the nuclei of dead promastigotes red. Flow cytometry was effective in quantifying the three fluorescent populations: PI, SYBR-14, and dual-stained promastigotes. Both dyes label DNA, thus avoiding the ambiguity of stains targeting separate cellular organelles [32]. This staining method was advantageous in being rapid (30 min), and the cells did not require extra processing prior to the staining. This study demonstrates that SYBR-14, when used in combination with PI, was effective for simultaneously visualizing both the living and dead populations of Leishmania promastigotes before and after treatment with allopurinol or other drugs.
The distinct increase of dead (PI-stained) and dying (PI- and SYBR-14-stained) promastigotes after exposure to allopurinol concentrations higher than 50 μg/ml generally indicated an allopurinol susceptibility of these cells. The PI and SYBR-14 staining results were consistent with the CFSE and the cell counting results where marked effects of allopurinol were also observed at drug concentrations higher than 50 μg/ml. Furthermore, the presence of more than 45% living promastigotes after exposure to 800 μg/ml of allopurinol for 96 hours, could partly explain the lack of complete inhibition of [3H]-thymidine uptake observed in an earlier study [30]. The various analyses in our study clearly indicated that allopurinol had a partial and not an absolute antileishmanial effect, even at high doses on p229 L. infantum promastigotes. Clinical studies done by Cavaliero et al. [14] showed that allopurinol treatment of L. infantum infected dogs led to clinical cure of the disease, but not to a complete elimination of the parasites, and relapses of the disease were observed in the dogs when treatment was discontinued. Danerolle and Bourdoiseau [16] reported that allopurinol only inhibits the growth of leishmania in vitro and thus acts only as a leishmaniostatic rather than a leishmanicidal drug. Although it might be tempting to explain the clinical observations by the in vitro data observed in our study, more detailed work is required to establish this assumed relationship.
In sharp contrast with the likely leishmaniostastic effect of the allopurinol, the high efficacy of the treatment with 10 μM-chloralin on the viability of L. infantum promastigotes after 48 hours of exposure (the treatment yield more than 90 % lethality on the promastigote) was consistent with earlier results, suggesting that this compound has a significant leishmanicidal effect [17,18].
The CFSE assay and the viability assay with SYBR-14 and PI allow direct and repeated measures on the same cell population resulting in a dynamic picture of the response Leishmania to various concentrations of allopurinol without killing the cells, or having to use radiolabeled isotopes. The assessment of proliferation capacity, viability and cellular changes by flow cytometry proved to be a promising way of evaluating the susceptibility of Leishmania promastigotes to diverse antileishmanial compounds [30]. These assays are relatively new tools in parasitology and pharmacology and have proved to be interesting methods of evaluating susceptibility or resistance of live Leishmania parasites to allopurinol or any other antileishmanial compounds on a cellular level.
Material and Methods
Parasites and drug
Promastigote forms of L. infantum (p-229, MCAN/ES/89/IPZ229/1/89, zymodeme MON 1) were maintained at 27°C in 25 cm2 tissue culture flasks (T25 - Corning) in 5 ml of a liquid medium (pH 7.4) supplemented with 10% heat inactivated calf serum [31,36]. Allopurinol and cycloheximeide (Sigma) was added from a 10 mg/ml stock solution dissolved in 0.1 N NaOH and distilled water respectively. Dr. A Armson kindly provided the Chloralin (Murdoch University, Australia), and it was dissolved in dimethylsulphoxide (DMSO). The recipe for preparation of the liquid medium is listed in the protocols described by Nunez et al [31].
Growth kinetics
1 × 106/ml log phase promastigotes were inoculated into 5 ml fresh medium in T25 culture flasks in the presence of 0, 4, 10, 50, 100, 200, 400 and 800 μg/ml of allopurinol. Assessment of growth by cell counting (Neubauer Chamber) was determined at 24 hours intervals for 8 days. The values obtained were used to determine the relative growth rate and the results were expressed as the mean values of at least three experiments.
Fluorescent probes
All fluorescent stains were purchased from Molecular Probes Europe BV (Leiden, The Netherlands). A 2.8 mg/ml stock solution of 5-,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) was prepared in DMSO and stored at -20°C. Propidium iodide (PI) was supplied as a 1 mg/ml solution in water. SYBR-14 was diluted in DMSO (1 mg/ml).
CFSE as cell division marker
Promastigotes (logarithmic growth phase) were washed (× 3) and resuspended in 2 ml PBS (6 × 107 cells/ml) and 2.8 μg/ml CFSE was added. Cells were incubated at 37°C for 10 minutes during which they were carefully mixed 3 to 4 times. Several volumes of ice-cold medium supplemented with 10% inactivated calf serum were added, and after centrifugation at 1200 g for 10 minutes (4°C) stained cells were resuspended in fresh medium (density adjusted to 5 × 106 cells/ml) and further cultivated in 25 cm2 tissue culture flasks at 27°C in the presence of various concentrations of allopurinol. The CFSE fluorescence was determined immediately after staining and after 24, 48, 72, and 96 hours.
Staining with PI and SYBR-14
Promastigote cultures were initiated at a cell density of 5 × 106 cells/ml medium in presence of different allopurinol concentrations (0 - 800 μg/ml) or in presence of 10 μM (2.7 μg/ml) Chloralin. After an incubation period of 48 and 96 hours at 27°C respectively, approximately 4 × 106 promastigotes were resuspended in 2 ml PBS, and 10 μg/ml PI and 0.1 mg/ml of SYBR-14 were added. The promastigotes were protected from direct light and incubated at 37°C for 30 minutes before flow cytometry analysis.
Flow cytometric analysis
The green fluorescence of CFSE and SYBR-14 and the red fluorescence of PI were excited at 488 nm (FACS Calibur, Becton Dickinson, Heidelberg, Germany). At least ten thousand cells were analyzed per sample and each staining experiment was repeated four times. Data analysis was performed on fluorescence intensities that excluded cell autofluorescence and cell debris. CELLQuest analysis software was used for fluorescence determination and data analysis while WinMDI was used to generate the 3 dimensional histogram overlays.
Acknowledgments
Acknowledgements
The work was partly supported by Foundation Research 3R, Switzerland (grant No.53/96) and by the Swiss National Science Foundation (grant 31-45903). We express our gratitude to Drs. U Müller-Doblies and M. Hurtado for their contributions to the initial studies. Also we thank D. Domingo (Memorial Sloan-Kettering Cancer Center) for her review of the manuscript.
Contributor Information
Sarah W Kamau, Email: skamau@vetparas.unizh.ch.
Rafael Nunez, Email: nunezr@mskcc.org.
Felix Grimm, Email: fgrim@vetparas.unizh.ch.
References
- World Health Organization. Leishmania-HIV co-infection. Epidemiological analysis of 692 retrospective cases. Weekly Epidemiological Records. 1997;72:49–54. [PubMed] [Google Scholar]
- Dedet JP, Lambert M, Pratlong F. Leishmaniasis and human immunodeficiency virus infections. Presse Med. 1995;24(22):1036–40. [PubMed] [Google Scholar]
- Montalban C, Calleja JL, Erice A, Laguna F, Clotet B, Podzamczer D, Cobo J, Mallolas J, Yebra M, Gallego A. Visceral leishmaniasis in patients infected with human immunodeficiency virus. Co-operative Group for the Study of Leishmaniasis in AIDS. J Infect. 1990;21(3):261–70. doi: 10.1016/0163-4453(90)93933-j. [DOI] [PubMed] [Google Scholar]
- Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10(2):298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J, Canavate C, Chamizo C, Laguna F, Alvar J. HIV - Leishmania infantum co-infection: humoral and cellular immune responses to the parasite after chemotherapy. Trans R Soc Trop Med Hyg. 2000;94(3):328–32. doi: 10.1016/s0035-9203(00)90345-6. [DOI] [PubMed] [Google Scholar]
- Baneth G, Dank G, Keren-Kornblatt E, Sekeles E, Adini I, Eisenberger CL, Schnur LF, King R, Jaffe CL. Emergence of visceral leishmaniasis in central Israel. Am J Trop Med Hyg. 1998;59(5):722–5. doi: 10.4269/ajtmh.1998.59.722. [DOI] [PubMed] [Google Scholar]
- Ben Ismail R, Gradoni L, Gramiccia M, Bettini S, Ben Rachid MS, Garraoui A. Epidemic cutaneous leishmaniasis in Tunisia: biochemical characterization of parasites. Trans R Soc Trop Med Hyg. 1986;80(4):669–70. doi: 10.1016/0035-9203(86)90175-6. [DOI] [PubMed] [Google Scholar]
- Sellon RK, Menard MM, Meuten DJ, Lengerich EJ, Steurer FJ, Breitschwerdt EB. Endemic visceral leishmaniasis in a dog from Texas. J Vet Intern Med. 1993;7(1):16–9. doi: 10.1111/j.1939-1676.1993.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Eddlestone SM. Visceral leishmaniasis in a dog from Maryland. J Am Vet Med Assoc. 2000;217(11):1659–1688. doi: 10.2460/javma.2000.217.1686. [DOI] [PubMed] [Google Scholar]
- Ouellette M, Legare D, Papadopoulou B. Microbial multidrug-resistance ABC transporters. Trends Microbiol. 1994;2(10):407–11. doi: 10.1016/0966-842X(94)90620-3. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL. Miltefosine - the long-awaited therapy for visceral leishmaniasis? N Engl J Med. 1999;341(24):1840–2. doi: 10.1056/NEJM199912093412411. [DOI] [PubMed] [Google Scholar]
- Jha TK, Sundar S, Thakur CP, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341(24):1795–800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- Le Fichoux Y, Rousseau D, Ferrua B, Ruette S, Lelievre A, Grousson D, Kubar J. Short- and long-term efficacy of hexadecylphosphocholine against established Leishmania infantum infection in BALB/c mice. Antimicrob Agents Chemother. 1998;42(3):654–8. doi: 10.1128/aac.42.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliero T, Arnold P, Mathis A, Glaus T, Hofmann-Lehmann R, Deplazes P. Clinical, serologic, and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. J Vet Intern Med. 1999;13(4):330–4. doi: 10.1892/0891-6640(1999)013<0330:csapfu>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Liste F, Gascon M. Allopurinol in the treatment of canine visceral leishmaniasis. Vet Rec. 1995;137(1):23–4. doi: 10.1136/vr.137.1.23. [DOI] [PubMed] [Google Scholar]
- Denerolle P, Bourdoiseau G. Combination allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis (96 cases). J Vet Intern Med. 1999;13(5):413–5. doi: 10.1892/0891-6640(1999)013<0413:caaatv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Armson A, Kamau SW, Grimm F, Reynoldson JA, Best WM, MacDonald LM, Thompson RC. A comparison of the effects of a benzimidazole and the dinitroanilines against Leishmania infantum. Acta Trop. 1999;73(3):303–11. doi: 10.1016/S0001-706X(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Callahan HL, Kelley C, Pereira T, Grogl M. Microtubule inhibitors: structure-activity analyses suggest rational models to identify potentially active compounds. Antimicrob Agents Chemother. 1996;40(4):947–52. doi: 10.1128/aac.40.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Edlind TD. Beta-tubulin genes of Trichomonas vaginalis. Mol Biochem Parasitol. 1994;64(1):33–42. doi: 10.1016/0166-6851(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Thompson RC, Reynoldson JA, Seville P. Albendazole: a more effective antigiardial agent in vitro than metronidazole or tinidazole. Trans R Soc Trop Med Hyg. 1990;84(3):375–9. doi: 10.1016/0035-9203(90)90324-8. [DOI] [PubMed] [Google Scholar]
- Chan MM, Grogl M, Chen CC, Bienen EJ, Fong D. Herbicides to curb human parasitic infections: in vitro and in vivo effects of trifluralin on the trypanosomatid protozoans. Proc Natl Acad Sci U S A. 1993;90(12):5657–61. doi: 10.1073/pnas.90.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MM, Grogl M, Callahan H, Fong D. Efficacy of the herbicide trifluralin against four P-glycoprotein-expressing strains of Leishmania. Antimicrob Agents Chemother. 1995;39(7):1609–11. doi: 10.1128/aac.39.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrio J, de Colmenares M, Riera C, Gallego M, Arboix M, Portus M. Leishmania infantum: stage-specific activity of pentavalent antimony related with the assay conditions. Exp Parasitol. 2000;95(3):209–14. doi: 10.1006/expr.2000.4537. [DOI] [PubMed] [Google Scholar]
- Callahan HL, Portal AC, Devereaux R, Grogl M. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41(4):818–22. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno D, Cavaleyra M, Zemzoumi K, Maquaire S, Ouaissi A, Lemesre JL. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob Agents Chemother. 1998;42(12):3097–102. doi: 10.1128/aac.42.12.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno D, Holzmuller P, Lemesre JL. Efficacy of second line drugs on antimonyl-resistant amastigotes of Leishmania infantum. Acta Trop. 2000;74(1):25–31. doi: 10.1016/S0001-706X(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171(1):131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Haugland RP. Handbook of Fluorescent Probes and Research Chemicals. Eugene: Molecular Probes Inc. 1992.
- Ueckert JE, Nebe von-Caron G, Bos AP, ter Steeg PF. Flow cytometric analysis of Lactobacillus plantarum to monitor lag times, cell division and injury. Lett Appl Microbiol. 1997;25(4):295–9. doi: 10.1046/j.1472-765X.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- Kamau SW, Hurtado M, Muller-Doblies UU, Grimm F, Nunez R. Flow cytometric assessment of allopurinol susceptibility in Leishmania infantum promastigote. Cytometry. 2000;40(4):353–60. doi: 10.1002/1097-0320(20000801)40:4<353::AID-CYTO11>3.3.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Nunez R., Kamau S., Grimm F. Flow Cytometric assessment of drug susceptibility in Leishmania infantum Promastigote. Unit 1114 in Robinson JP, Darzynkiewicz Z, Dean P, Orfao A, Rabinovitch P, Tanke H, Wheeless L eds Current protocols in Cytometry New York: John Wiley & Sons, Inc. 2001;Supplement 16:Unit 11.14. doi: 10.1002/0471142956.cy1114s15. [DOI] [PubMed] [Google Scholar]
- Garner DL, Johnson LA, Yue ST, Roth BL, Haugland RP. Dual DNA staining assessment of bovine sperm viability using SYBR-14 and propidium iodide. J Androl. 1994;15(6):620–9. [PubMed] [Google Scholar]
- Marr JJ, Berens RL. Pyrazolopyrimidine metabolism in the pathogenic trypanosomatidae. Mol Biochem Parasitol. 1983;7(4):339–56. doi: 10.1016/0166-6851(83)90016-6. [DOI] [PubMed] [Google Scholar]
- Marr JJ. Purine analogs as chemotherapeutic agents in leishmaniasis and American trypanosomiasis. J Lab Clin Med. 1991;118(2):111–9. [PubMed] [Google Scholar]
- Frayha GJ, Smyth JD, Gobert JG, Savel J. The mechanisms of action of antiprotozoal and anthelmintic drugs in man. Gen Pharmacol. 1997;28(2):273–99. doi: 10.1016/s0306-3623(96)00149-8. [DOI] [PubMed] [Google Scholar]
- Grimm F, Brun R, Jenni L. Promastigote infectivity in Leishmania infantum. Parasitol Res. 1991;77(3):185–91. doi: 10.1007/BF00930856. [DOI] [PubMed] [Google Scholar]


