Abstract
The neurofibroma, a common feature of neurofibromatosis type 1 (NF1), is a benign peripheral nerve sheath tumor that contains predominantly Schwann cells (SC). There are reports that neurofibroma growth may be affected by hormonal changes, particularly in puberty and pregnancy, suggesting an influence by steroid hormones. This study examined the effects of estrogen and progesterone on proliferation and apoptosis in a panel of NF1 tumor xenografts. SC-enriched cultures derived from three human NF1 tumor types [dermal neurofibroma, plexiform neurofibroma and malignant peripheral nerve sheath tumor (MPNST)] were xenografted in sciatic nerves of ovariectomized scid/Nf1−/+ mice. At the same time, mice were implanted with time-release pellets for systemic delivery of progesterone, estrogen or placebo. Proliferation and apoptosis by the xenografted SC were examined two months after implantation, by Ki67 immunolabeling and TUNEL. Estrogen was found to increase the growth of all three MPNST xenografts. Progesterone was associated with increased growth in two of the three MPNSTs, yet decreased growth of the other. Of the four dermal neurofibroma xenografts tested, estrogen caused a statistically significant growth increase in one and progesterone did in another. Of the four plexiform neurofibroma SC xenografts, estrogen and progesterone significantly decreased growth in one of the xenografts, but not the other three. No relationship of patient age or gender to steroid response was observed. These findings indicate that human NF1 Schwann cells derived from some tumors show increased proliferation or decreased apoptosis in response to particular steroid hormones in a mouse xenograft model. This suggests that antiestrogen or anti-progesterone therapies may be worth considering for specific NF1 neurofibromas and MPNSTs.
Key words: NF1, neurofibroma, steroid hormones, estrogen, xenograft, MPNST, progesterone
Introduction
Neurofibromatosis type 1 (NF1) is a common autosomal dominant disorder with a wide variety of features, the hallmark feature being neurofibromas.1 Neurofibromas are benign peripheral nerve tumors that contain more than 50% Schwann cells (SC). Smaller, cutaneous or subcutaneous neurofibromas are termed “dermal” here and have no potential for malignancy. However, neurofibromas involving larger nerves can become large and have a risk of transformation to malignant peripheral nerve sheath tumor (MPNST).1 Some of the neurofibroma SCs are clonally expanded from a SC whose normal NF1 gene allele was mutated, fulfilling the tumor suppressor two-hit system. Neurofibromas often first appear around puberty and develop throughout adulthood. Pregnancy is often associated with increased size, number and malignant potential of neurofibromas.2–6 Lammert et al.7 reported a survey of 59 NF1 patients that found that while oral contraceptive pills did not stimulate subjective growth of neurofibromas in the majority of patients, two receiving high dose Depo Provera contraceptive did report significant tumor growth. Thus, neurofibroma growth often parallels hormonal changes. McLaughlin and Jacks8 examined 59 human neurofibromas for the expression of estrogen (ER alpha) and progesterone receptors (PR) with immunohistochemistry. They found that the majority (75%) of neurofibromas had at least a few cells that expressed PR, but these cells were S100 negative (therefore not Schwann cells). They did not see detectable ER alpha staining. A similar study in our lab showed that 54% of neurofibromas were PR-positive and that cultured tumor Schwann cells do express steroid hormone receptor transcripts.9
Steroid hormones are cholesterol derivatives with important roles in growth, metabolism, differentiation and reproduction. They have been implicated in some cancers, especially breast and prostate cancer. A few biochemical studies have been done on rodent SCs,10,11 however, only recently has data been published regarding hormone effects in normal human or NF1-tumor derived Schwann cells. Recent in vitro data indicated that steroid hormone receptor expression correlated with neurofibromin levels in mouse ES-derived Schwann cells and two human tumor cultures (from our group).5,6 The plexiform culture did not significantly respond to hormones in a proliferation assay, but the MPNST line showed increase in proliferation rate in response to estrogen and progesterone. Our group showed that neurofibromin-deficient tumor SC responded to hormones in culture.9 Overdiek et al.12 also showed an increase in proliferation in neurofibroma SC in response to progesterone. To survey the effect of estrogen and progesterone on proliferation and apoptosis in a series of human neurofibroma Schwann cells in vivo, we employed a sciatic nerve xenograft model in ovariectomized scid/Nf1 mice treated with progesterone or estrogen. Human SC derived from three NF1 tumor types (dermal neurofibroma, plexiform neurofibroma and MPNST) were tested by assaying proliferation and apoptosis of the xenografted SC using immunohistochemistry. This is the first such in vivo NF1 study.
Results
Eleven SC cultures (from four dermal neurofibromas, three MPNSTs and four plexiform neurofibromas) (five from females, six from males) were tested using a total of 104 sciatic nerve xenografts. The xenograft results were consistent for each culture; there were virtually no statistical outliers. As indicated previously, MPNST sNF96.2 grew the most dramatically (Fig. 1) with the sciatic nerve massively enlarged under estrogen treatment (and still visibly enlarged in placebo and progesterone treatment).17 However, most of the xenografts did not produce grossly enlarged nerves. Figure 2 shows an example of one of the plexiform SC cultures and Figure 3 shows an example of xenograft immunostaining. hGST staining was detected in multiple cells in each xenograft, indicating that human cells were present in the sciatic nerve 2 months after the xenograft injection. Proliferation and apoptosis were measured by Ki67 staining and TUNEL staining of xenograft sections, respectively. All xenografts had at least some Ki67-positive hGST-positive cells except for cNF04.9a, a dermal neurofibroma-enriched SC culture which failed to form a mass in one nerve each in two mice (one placebo, one estrogen). For all xenografts, the average ratios of Ki67/TUNEL positive cells in the same size area from serial sections were compared between the treatment groups (estrogen versus placebo, progesterone versus placebo). Results are illustrated by graphs in Figure 4. An increase in this ratio (i.e., increased survival and/or increased proliferation) is consistent with increased cell mass in treatment relative to placebo. Similarly a decrease indicates reduction in cell mass relative to placebo.
Figure 1.
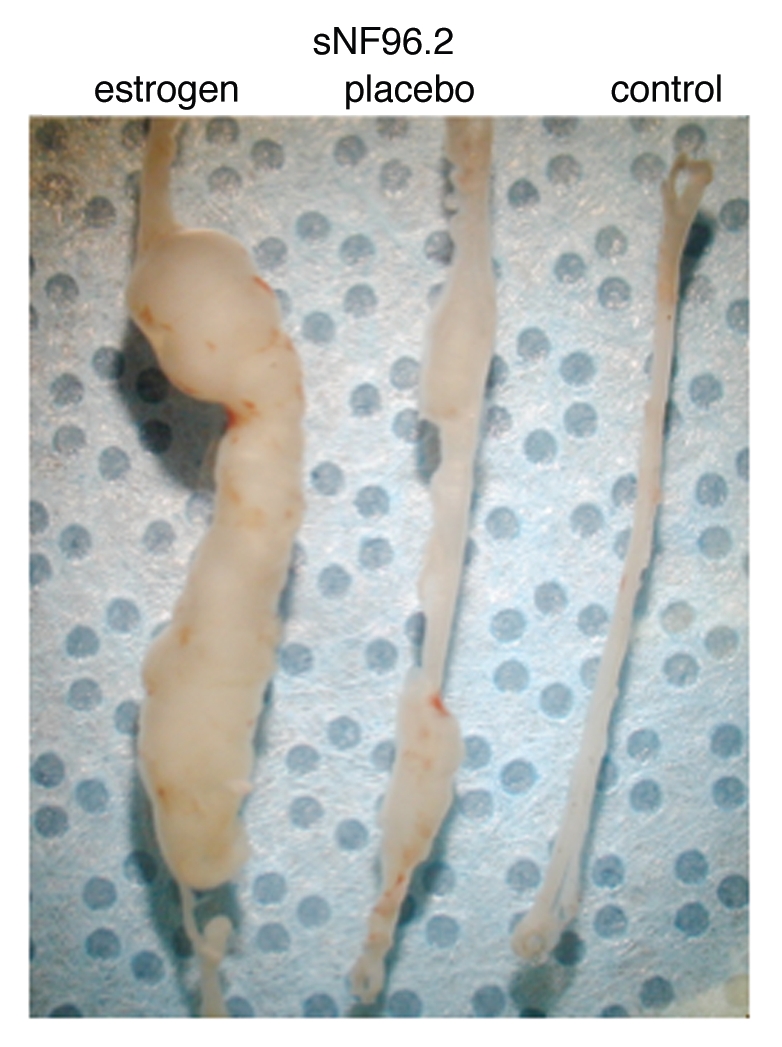
Representative sNF96.2 xenograft whole sciatic nerves show visible additional growth under influence of estrogen. The leftmost nerve is from a mouse treated with estrogen. The middle nerve is from a mouse treated with placebo pellet. The rightmost nerve is from an untreated mouse, where the nerve was injected with PBS as injection control.
Figure 2.
Cultured plexiform pNF01.1 Schwann cells are shown, stained with S-100, at x40 magnification. This culture shows characteristic enrichment for SC.
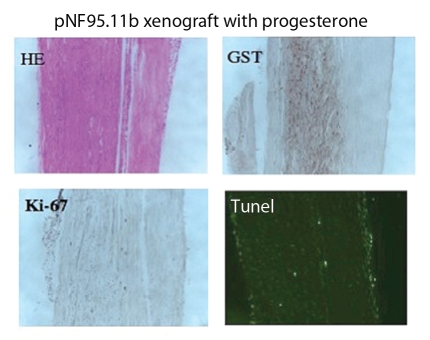
Figure 3.
Immunohistochemical staining of plexiform pNF95.11b xenograft with progesterone hormone treatment. (A) H/E stain. (B) hGST immunostain (brown cells are engrafted human SC). (C) Ki-67 immunostain for proliferation. (D) TUNEL stain for apoptosis. Magnification x200.
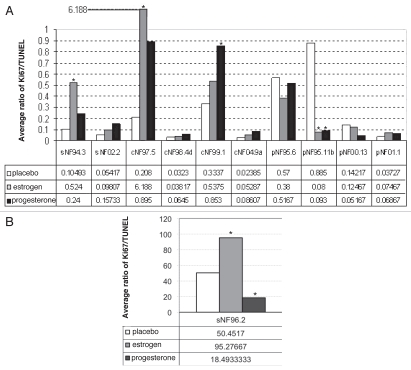
Figure 4.
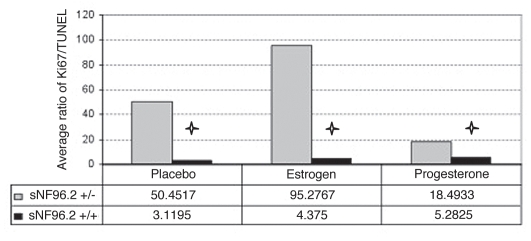
(A) Effect of hormones on xenograft compared to placebo. The star indicates p ≤ 0.05. Open bar, placebo; gray bar, estrogen; black bar, progesterone. Xenografts of sNF94.3 and cNF97.5 showed statistically significant increase in response to estrogen, whereas the pNF95.11b xenograft showed significant inhibition with both hormones, compared to placebo. Progesterone was associated with an increase in the cNF99.1 xenograft. (B) Xenograft of sNF96.2 showed statistically significant inhibition in response to progesterone, but increase in xenograft size in response to estrogen.
In all four dermal-derived SC culture xenografts (where human cells were evident), there is suggestion of increased tumor cell mass in presence of estrogen and/or progesterone (Fig. 4A). This met statistical significance (p ≤ 0.05) in dermal culture cNF97.5 with estrogen (p = 0.0189) and dermal culture cNF99.1 with progesterone (p = 0.0108) (Fig. 4A). In the presence of progesterone, cNF97.5 had a growth trend but it was not statistically significant (p = 0.895). Figure 5 shows sections of the dermal neurofibroma cNF97.5 xenograft, illustrating the significant increase in cell mass with estrogen but not progesterone. For the plexiform neurofibroma SC xenografts, the ratio of proliferation: apoptosis showed a decrease trend in the presence of estrogen (for pNF00.13, pNF95.6 and pNF95.11b) and progesterone (same three cultures) (Fig. 4A). This was statistically significant for estrogen and progesterone in pNF95.11b, while both hormones were associated with increase in pNF01.1 (not statistically significant, but the number of surviving cells for pNF01.1 was very small and thus most subject to error) (Fig. 4A). For MPNST-derived SC xenografts, there was a trend of increasing cell mass in the presence of estrogen for all three cultures, which was statistically significant in sNF96.2 and sNF94.3 (Fig. 4). Progesterone was associated with a trend of increasing cell mass in sNF94.3 and sNF02.2 xenografts relative to placebo, but decreasing cell mass compared to placebo in sNF96.2 (Fig. 4).
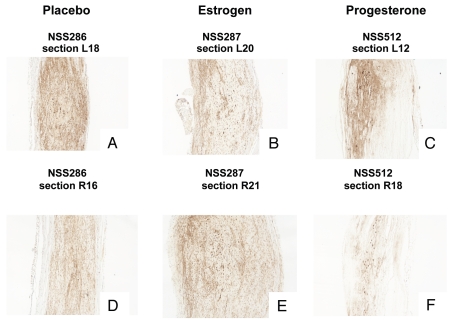
Figure 5.
Example of hormone effects on xenografts at a gross morphology level. Photomicrographs are shown for the dermal neurofibroma cNF97.5 xenograft immunostained with hGST antibody to show cells of human origin. Photos (A and D) are placebo sections from left side and right side nerves, respectively; (B and E) are estrogen-associated sections; (C and F) are progesterone-associated sections. An increase in xenograft size can be seen in the presence of estrogen, but not progesterone. The magnification is x200.
MPNST sNF96.2 produced the most interesting results due to its massive expansion in vivo. This xenograft best resembles an MPNST,17 while another MPNST xenograft (sNF94.3, which did not grow as robustly) better fits histology criteria for a plexiform neurofibroma.16 This culture was remarkable in that estrogen was associated with tremendous growth, greater than under placebo conditions (Figs. 1 and 4B). Yet progesterone was associated with failure of the xenografts to achieve placebolevel growth (Fig. 4B). Immunohistochemistry of sNF96.2 xenografts for progesterone receptor (PR) and estrogen receptor (ERα & ERβ) showed a negative result for all three receptors with DAB detection, compared to positive control slides (tonsil for PR, breast cancer for ERα, colon cancer for ERβ, data not shown). In addition, we also xenografted sNF96.2 into ovariectomized/pellet-implanted female scid mice lacking the Nf1 heterozygous background. In comparison to the Nf1(+/−)/scid results, the scid-only xenografts grew less robustly, regardless of hormone treatment (statistically significant) (Fig. 6).
Figure 6.
Comparison of the effects of hormone on sNF96.2 xenograft in Nf1+/−, scid mice versus Nf1+/+, scid mice. The star * indicates statistically significant results with p ≤ 0.05. Gray bar, Nf1+/−; black bar, Nf1+/+.
Discussion
The neurofibroma clonal tumorigenic cell is the Schwann cell, containing somatic loss of function of the remaining NF1 allele.21–23 Thus, these tumors (and MPNSTs) are heterogeneous in terms of different combinations of germline/somatic NF1 (and in some cases, other genes) mutations, cytogenetics, expression array results and presence of a number of antigens.8,9,14,21,22,24–46 Given this, and variable patient reports about whether their neurofibromas were affected by pregnancy, it is not surprising that this study found heterogeneous results.
MPNST sNF96.2 showed robust response to estrogen.
The MPNST sNF96.2 data suggest that at least for this culture, a germline background of heterozygosity for a disruptive Nf1 mutation in the recipient mouse is more permissive for increased growth under hormone influence compared to the scid-only background (wild-type at Nf1 locus). Interestingly, this MPNST xenograft grows less robustly in male recipient mice, even smaller than female-placebo.17 Estrogen induced a greater xenograft growth than placebo and progesterone actually somewhat inhibited growth compared to placebo. Thus, this cell line is clearly differentially responsive to hormones and genetic background. Such complexity may be true of many neurofibroma clonal SC, but the sNF96.2 cell line amplified sufficiently in vivo to clearly observe these properties. Although progesterone and estrogen receptors were not detected in the xenograft, they may still be present at low levels as seen in some SC in vitro previously (or there may be additional receptors not yet identified).5 Consistent with this is the previous observation that these receptor transcripts are undetectable in this cell line and barely detectable in RNA from primary tumor (using real-time PCR) and treatment with ligands had no effect on proliferation or apoptosis in culture.9 Alternatively, it may be that the hormones are exerting effects through non-genomic mechanisms, regardless of karyotype, a phenomenon increasingly under study.47
Some xenografts of all three tumor types respond to hormones: clinical significance.
Although estrogen and progesterone showed variable effects on xenograft growth, there were consistent results specific to each tumor culture. Responses are not related to patient age or tumor type, although most of the significant responding cultures were from males, and/or were from MPNSTs. The data support a functional role of steroid hormones in tumor growth in some individuals and/or tumors (3/11 positive for response to estrogen; 2/11 positive response to progesterone; 1/11 negative response to estrogen; 2/11 negative response to progesterone). These responses were in independent cell cultures except for sNF96.2 (described above) and pNF95.11b, which showed inhibition by both hormones compared to placebo. Overall, these observations suggest that hormone-related therapies may be of potential use in controlling growth of some NF1 tumors. This is feasible even in the absence of hormone receptors since some such agents (e.g., tamoxifen) can exert effects independent of estrogen receptor status.48–51 Future work could involve expression array or proteomic studies comparing responding and non-responding tumors, to develop a profile that might predict response to hormones and thus suggest possible efficacy of anti-hormone therapies. If the mechanisms through which the hormones act in these tumors are characterized, we may be able to identify alternative therapeutic targets that are not directed at the hormones/receptors themselves, such as underway in breast cancer.52 Although the two month interval was sufficient to show proof of principle in this experiment, extended xenograft time beyond 2 months would yield larger tumors, which could be useful for preclinical studies to measure treatment efficacy. This xenograft system could also be used to measure effects of hormone-related (or other) therapies, especially those aimed at the hormones known to stimulate certain tumor cell cultures. This work contributes to the growing evidence that steroid hormones can play an important role in NF1 tumorigenesis.
Materials and Methods
Neurofibroma SC culture.
NF1 tumor-derived Schwann cells were isolated from surgical specimens of neurofibromas or MPNSTs, from patients (age 1–35 years) meeting NF1 diagnostic criteria, under IRB approval.13 Our SC culture protocol that favors NFl−/− cells has been described previously.14 To briefly review, viable tumor was isolated from surgically resected neurofibromas and single cell suspensions were created. The cells were then cultured in Dulbecco's Modified Eagle's Medium supplemented with 15% fetal bovine serum, 50 ng/ml neuregulin (GGF2) and antibiotics (Pen/Strep) on laminin-coated plates. Schwann cells were enriched to 70–95% by differential detachment over several passages, using mild trypsinization. Cultures were characterized with S-100, P-75 (NGFR) and Ki-67 immunostaining. The three MPNST cultures we established are available at ATCC and do not require laminin or neuregulin.
Xenograft.
The Nf1Fcr mutation on a C57BL/6 background15 was bred to B6.CB17 immunodeficient scid mice (Jackson Laboratories # 001913) through several generations to produce the desired “NSS” mice (Nf1 heterozygote and scid homozygote) for xenograft. This xenograft system is well-established in our lab.16,17 As approved by UF IACUC, bilateral ovariectomy, hormone pellet implant and sciatic nerve xenograft were performed on 8-week-old female mice. Under anesthesia, a 1.0 cm incision was made in the skin to expose the back muscles. A small 0.2 cm incision was made in the muscles overlying the ovaries on both sides. The ovaries were isolated, tied off with sterile sutures and removed. The muscles and the skin were sutured separately. Ovariectomized females were then implanted with 60-day release pellets of either17β-estradiol (0.72 mg/pellet, cat # SE-121), progesterone (25 mg/pellet, SP-131) or placebo (SC-111) (Innovative Research of America). This is the established system for providing steady-state physiologic blood levels of these hormones in isolation.18–20 Human tumor derived Schwann cells [5 × 105/5 ul (see below)] were then injected intra-fascicularly in the surgically-exposed sciatic nerve of each leg. This was sutured and the animals were returned to SPF housing for 2 months. We used three mice per treatment group, for a total of six xenografts for each treatment. All xenografts were performed on the same day for each culture, using the same batch of cells.
Analysis of xenograft.
Sciatic nerves were harvested two months after surgery, fixed in 4% paraformaldehyde and paraffin embedded longitudinally. Serial sections (7 µm) were cut from each block through the entire nerve, and staining/immunohistochemistry was performed with H&E (hematoxylin and eosin staining), hGST (human glutathione transferase), Ki67 and TUNEL as described below. Every 7th section was H&E stained for light microscopy examination. Based on this, three sets of three serial sections across the widest area of the graft were used to determine proliferation-to-apoptosis ratio.
For hGST staining (using human-specific antibody), endogenous peroxidases were quenched with 1% hydrogen peroxide in methanol for 30 minutes. After washing in PBS, the rabbit polyclonal anti-human hGST antibody (Cat# 107; Dako, Carpinteria, CA) was added at a 1:100 dilution. Staining was detected with 1:500 dilution of swine anti-rabbit biotinylated conjugated-secondary antibody and ABC, 3,3′-diaminobenzidine as a substrate for peroxidase. For Ki67 staining, to identify proliferating cells, endogenous peroxidases were quenched with 1% H2O2 in methanol for 30 minutes. To unmask antigens, samples were pre-treated in Target Retrieval Solution (10 mM citrate buffer, pH 6.0, Dako Cat# S-1700; Carpinteria, CA) at 95–99°C for 30 minutes. The rabbit polyclonal antihuman Ki67 antibody (Cat # 21174418, Zymed, San Francisco, CA) was used at a 1:100 dilution. Staining was detected with a 1:500 dilution of swine anti-rabbit biotinylated conjugated-secondary antibody and ABC, 3,3′-diaminobenzidine as a substrate for peroxidase. For TUNEL (TdT-mediated dUTP Nick-End Labeling) staining of cells undergoing apoptosis, the DeadEnd™ Fluorometric Tunel System Kit (Cat# G3250, Promega, Madison, WI) was used according to the manufacturer's instructions. This system used fluorescent detection. PR immunohistochemical staining has been previously described.9 ERalpha and ERbeta staining (DAB detection) were done by the Shands Diagnostic Resource Lab and the UF Molecular Pathology Core, respectively.
Statistical analysis.
For most of the xenografts, sciatic nerves weren't visibly enlarged or enlarged in a uniform shape, so volumetric analysis was not possible. Instead, human (hGST positively-stained) cells within entire xenograft sections were counted. However, for the large xenograft developed from culture SNF96.2, we chose three representative non-adjacent areas in the GST-positive region for counting, at x400 magnification. The ratio of Ki67/TUNEL positive staining cells in the same area was compared from serial sections in each group (estrogen, progesterone, placebo). The average number of Ki67-positive cells and TUNEL-positive cells from three slides each was calculated for each sample. Data from all six xenografted nerves were averaged for each culture and condition, with and without Dixon outlier analysis. Ki67/TUNEL ratios were calculated for each group (placebo, estrogen and progesterone). The results were analyzed with a two-tailed unpaired t-test to compare effects for estrogen versus placebo and progesterone versus placebo.
Acknowledgements
This work was supported by US Department of Defense NF Research Program grants [M.R.W. (170110707), D.M. (170010549)], an NIH NRSA fellowship (L.F., 1F30NS43951) and the Hayward Foundation (M.R.W.). We thank Debbie Neubauer and Beth Fisher for technical assistance.
Abbreviations
- MPNST
malignant peripheral nerve sheath tumor
- NF1
neurofibromatosis 1
- SC
schwann cell
- PR
progesterone receptor
- ER
estrogen receptor
- hGST
human glutathione S transferase
- TUNEL
terminal deoxynucleotidal transferase dUTP nicked end labeling
- DAB
3,3′-diaminobenzidine
- scid
severe combined immunodeficiency
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12878
References
- 1.Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, et al. Guidelines for diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81–88. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puls LE, Chandler PA. Malignant schwannoma in pregnancy. Acta Obstet Gynecol Scand. 1991;70:243–244. doi: 10.3109/00016349109006218. [DOI] [PubMed] [Google Scholar]
- 3.Dugoff L, Sujansky E. Neurofibromatosis type 1 and pregnancy. Am J Med Genet. 1996;66:7–10. doi: 10.1002/(SICI)1096-8628(19961202)66:1<7::AID-AJMG2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146:1624–1633. doi: 10.1002/ajmg.a.32301. [DOI] [PubMed] [Google Scholar]
- 5.Roth TM, Ramamurthy P, Muir D, Wallace MR, Zhu Y, Chang L, et al. Influence of hormones and hormone metabolites on the growth of Schwann cells derived from embryonic stem cells and on tumor cell lines expressing variable levels of neurofibromin. Dev Dyn. 2008;237:513–524. doi: 10.1002/dvdy.21430. [DOI] [PubMed] [Google Scholar]
- 6.Roth TM, Ramamurthy P, Muir D, Wallace MR, Zhu Y, Roelen KF. Influence of hormones and hormone metabolites on the growth of schwann cells derived from embryonic stem cells and on tumor cell lines expressing variable levels of neurofibromin. Dev Dyn. 2008;237:1. doi: 10.1002/dvdy.21430. [DOI] [PubMed] [Google Scholar]
- 7.Lammert M, Mautner VF, Kluwe L. Do hormonal contraceptives stimulate growth of neurofibromas? A survey of 59 NF1 patients. BMC Cancer. 2005;5:16. doi: 10.1186/1471-2407-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin ME, Jacks T. Progesterone receptor expression in neurofibromas. Cancer Res. 2003;63:752–755. [PubMed] [Google Scholar]
- 9.Fishbein L, Zhang X, Fisher LB, Li H, Campbell-Thompson M, Yachnis A, et al. In vitro studies of steroid hormones in neurofibromatosis 1 tumors and Schwann cells. Mol Carcinogen. 2007;46:512–523. doi: 10.1002/mc.20236. [DOI] [PubMed] [Google Scholar]
- 10.Jung-Testas I, Schumacher M, Bugnard H, Baulieu EE. Stimulation of rat Schwann cell proliferation by estradiol: synergism between the estrogen and cAMP. Brain Res Devel Brain Res. 1993;72:282–290. doi: 10.1016/0165-3806(93)90194-f. [DOI] [PubMed] [Google Scholar]
- 11.Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Demonstration of progesterone receptors in rat Schwann cells. J Steroid Biochem Mol Biol. 1996;58:77–82. doi: 10.1016/0960-0760(96)00009-x. [DOI] [PubMed] [Google Scholar]
- 12.Overdiek A, Winner U, Mayatepek E, Rosenbaum T. Schwann cells from human neurofibromas show increased proliferation rates under the influence of progesterone. Pediatr Res. 2008;64:40–43. doi: 10.1203/PDR.0b013e31817445b8. [DOI] [PubMed] [Google Scholar]
- 13.Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–57. [PubMed] [Google Scholar]
- 14.Muir D, Neubauer D, Lim IT, Yachnis AT, Wallace MR. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am J Pathol. 2001;158:501–513. doi: 10.1016/S0002-9440(10)63992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 16.Perrin GQ, Fishbein L, Thomson SA, Thomas SL, Stephens K, Garbern JY, et al. Plexiform-like neurofibromas develop in the mouse by intraneural xenograft of an NF1 tumor-derived Schwann cell line. J Neurosci Res. 2007;85:1347–1357. doi: 10.1002/jnr.21226. [DOI] [PubMed] [Google Scholar]
- 17.Perrin GQ, Li H, Fishbein L, Thomson SA, Hwang MS, Scarborough MT, et al. An orthotopic xenograft model of intraneural NF1 MPNST suggests a potential association between steroid hormones and tumor cell proliferation. Lab Invest. 2007;87:1092–1102. doi: 10.1038/labinvest.3700675. [DOI] [PubMed] [Google Scholar]
- 18.Zhou JR, Yu L, Mai Z, Blackburn GL. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int J Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang AC, Nakazawa M, Romeo RD, Reeb BC, Sisti H, McEwen BS. Effects of long-term estrogen replacement on social investigation and social memory in ovariectomized C57BL/6 mice. Horm Behav. 2005;47:350–357. doi: 10.1016/j.yhbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.McAsey ME, Cady C, Jackson LM, Li M, Randall S, Nathan BP, et al. Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp Neurol. 2006;197:197–205. doi: 10.1016/j.expneurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Colman SD, Williams CA, Wallace MR. Benign neurofibromas in type 1 neurofibromatosis (NF1) show somatic deletions of the NF1 gene. Nat Genet. 1995;11:90–92. doi: 10.1038/ng0995-90. [DOI] [PubMed] [Google Scholar]
- 22.Serra E, Rosenbaum T, Winner U, Aledo R, Ars E, Estivill X, et al. Schwann cells harbor the somatic NF1 mutation in neurofibromas: evidence of two different Schwann cell subpopulations. Hum Mol Genet. 2000;9:3055–3064. doi: 10.1093/hmg/9.20.3055. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabibi D, Zerilli M, Caradonna G, Schillaci L, Belmonte B, Rodolico V. Diagnostic and prognostic value of CD10 in periphernal nerve sheath tumors. Anticancer Res. 2009;29:3149–3155. [PubMed] [Google Scholar]
- 25.Holtkamp N, Mautner VF, Friedrich RE, Harder A, Hartmann C, Theallier-Janko A, et al. Differentially-expressed genes in neurofibromatosis 1-associated neurofibromas and malignant peripheral nerve sheath tumors. Acta Neuropathol. 2004;107:159–168. doi: 10.1007/s00401-003-0797-8. [DOI] [PubMed] [Google Scholar]
- 26.Perry A, Kunz SN, Fuller CE, Banerjee R, Marley EF, Liapis H, et al. Differential NF1, p16 and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically-similar spindle cell neoplasms. J Neuropathol Exp Neurol. 2002;61:702–709. doi: 10.1093/jnen/61.8.702. [DOI] [PubMed] [Google Scholar]
- 27.McCarron KF, Goldblum JR. Plexiform neurofibroma with and without associated malignant peripheral nerve sheath tumor: a clinicopathologic and immunohistochemical analysis of 54 cases. Mod Pathol. 1998;7:612–617. [PubMed] [Google Scholar]
- 28.Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008;35:1014–1019. doi: 10.1111/j.1600-0560.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 29.Cunha KS, Barboza EP, Fonseca EC. Identification of growth hormone receptor in plexiform neurofibromas of patients with neurofibromatosis type 1. Clinics (Sao Paulo) 2008;63:39–42. doi: 10.1590/s1807-59322008000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedrich RE, Keiner D, Hagel C. Expression of insulin-like growth-factor-1 receptor (IGF-1R) in peripheral nerve sheath tumors in neurofibromatosis type 1. Anticancer Res. 2007;27:2085–2090. [PubMed] [Google Scholar]
- 31.Kweh F, Zheng M, Kurenova E, Wallace M, Golubovskaya V, Cance WG. Neurofibromin interacts with the N-terminal domain of focal adhesion kinase. Molec Carcinogen. 2009;48:1005–1017. doi: 10.1002/mc.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Rao PK, Wen R, Song Y, Muir D, Wallace P, et al. Notch and Schwann cell transformation. Oncogene. 2004;23:1146–1152. doi: 10.1038/sj.onc.1207068. [DOI] [PubMed] [Google Scholar]
- 33.Muir DF. Differences in proliferation and invasion by normal, transformed and NF1 Schwann cell cultures are influenced by matrix metalloproteinase expression. Clin Exp Metastasis. 1995;13:303–314. doi: 10.1007/BF00133486. [DOI] [PubMed] [Google Scholar]
- 34.Mawrin C, Schulz S, Hellwig-Patyk A, Kirches E, Roessner A, Lendeckel U, et al. Expression and function of somatostatin receptors in peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 2005;64:1080–1088. doi: 10.1097/01.jnen.0000190065.36182.25. [DOI] [PubMed] [Google Scholar]
- 35.Cunha KS, Barboza EP, Da Fonseca EC. Identification of growth hormone receptor in localised neurofibromas of patients with neurofibromatosis type 1. J Clin Pathol. 2003;56:758–763. doi: 10.1136/jcp.56.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirose T, Tani T, Shimada T, Ishizawa K, Shimada S, Sano T. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293–298. doi: 10.1097/01.MP.0000062654.83617.B7. [DOI] [PubMed] [Google Scholar]
- 37.Boyle JL, Haupt HM, Stern JB, Multhaupt HA. Tyrosinase expression in malignant melanoma, desmoplastic melanoma and peripheral nerve tumors. Arch Pathol Lab Med. 2002;126:816–822. doi: 10.5858/2002-126-0816-TEIMMD. [DOI] [PubMed] [Google Scholar]
- 38.DeClue JE, Heffelfinger S, Benvenuto G, Ling B, Li S, Rui W, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest. 2000;105:1233–1241. doi: 10.1172/JCI7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman LS, Atit R, Rosenbaum T, Cox AD, Ratner N. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem. 2000;275:30740–30745. doi: 10.1074/jbc.M001702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashour GA, Ratner N, Khan GA, Wang HL, Martuza RL, Kurtz A. The angiogenic factor midkine is aberrantly expressed in NF1-deficient Schwann cells and is a mitogen for neurofibroma-derived cells. Oncogene. 2001;20:97–105. doi: 10.1038/sj.onc.1204026. [DOI] [PubMed] [Google Scholar]
- 41.Upadhyaya M, Spurlock G, Kluwe L, Chuzhanova N, Bennett E, Thomas N, et al. The spectrum of somatic and germline NF1 mutations in NF1 patients with spinal neurofibromas. Neurogenetics. 2009;10:251–263. doi: 10.1007/s10048-009-0178-0. [DOI] [PubMed] [Google Scholar]
- 42.Miller SJ, Jessen WJ, Mehta T, Hardiman A, Sites E, Kaiser S, et al. Integrative genomic analyses of neurofibromatosis tumours identify SOX9 as a biomarker and survival gene. EMBO Mol Med. 2009;1:236–248. doi: 10.1002/emmm.200900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantripragada KK, Spurlock G, Kluwe L, Chuzhanova N, Ferner RE, Frayling IM, et al. High-resolution DNA copy number profiling of malignant peripheral nerve sheath tumors using targeted microarray-based comparative genomic hybridization. Clin Cancer Res. 2008;14:1015–1024. doi: 10.1158/1078-0432.CCR-07-1305. [DOI] [PubMed] [Google Scholar]
- 44.Fishbein L, Eady B, Sanek N, Muir D, Wallace MR. Analysis of somatic NF1 promoter methylation in plexiform neurofibromas and Schwann cells. Cancer Genet Cytogenet. 2005;157:181–186. doi: 10.1016/j.cancergencyto.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Harder A, Rosche M, Reuss DE, Holtkamp N, Uhlmann K, Friedrich R, et al. Methylation analysis of the neurofibromatosis type 1 (NF1) promoter in peripheral nerve sheath tumours. Eur J Cancer. 2004;40:2820–2828. doi: 10.1016/j.ejca.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Upadhyaya M, Spurlock G, Majounie E, Griffiths S, Forrester N, Baser M, et al. The heterogeneous nature of germline mutations in NF1 patients with malignant peripheral serve sheath tumours (MPNSTs) Hum Mutat. 2006;27:716–720. doi: 10.1002/humu.9429. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Li X, Zhao L, Zhang L, Zhang G, Wang J, Wei L. Nongenomic effect of estrogen on the MAPK signaling pathway and calcium influx in endometrial carcinoma cells. J Cell Biochem. 2009;106:553–562. doi: 10.1002/jcb.22017. [DOI] [PubMed] [Google Scholar]
- 48.Colletta AA, Wakefield LM, Howell LM, van Roozendaal KE, Danielpour D, Ebbs SR, et al. Antioestrogens induce the secretion of active TGFβ from human fetal fibroblasts. Br J Cancer. 1990;62:405–409. doi: 10.1038/bjc.1990.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butta A, MacLennan K, Flanders KC, Sacks NP, Smith I, McKinna A, et al. Induction of transforming growth factor β1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. 1992;52:4261–4264. [PubMed] [Google Scholar]
- 50.Todorova VK, Kaufmann Y, Luo S, Suzanne Klimberg V. Tamoxifen and raloxifene suppress the proliferation of estrogen receptor-negative cells through inhibition of glutamine uptake. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-010-1316-y. In press. [DOI] [PubMed] [Google Scholar]
- 51.Bolanz KA, Kovacs GG, Landowski CP, Hediger MA. tamoxifen inhibits TRPV6 activity via estrogen receptor-independent pathways in TRPV6-expressing MCF-7 breast cancer cells. Mol Cancer Res. 2009;7:2000–2010. doi: 10.1158/1541-7786.MCR-09-0188. [DOI] [PubMed] [Google Scholar]
- 52.Speers C, Tsimelzon A, Sexton K, Herrick AM, Gutierrez C, Culhane A, et al. Identification of novel kinase targets for the treatment of estrogen receptor-negative breast cancer. Clin Cancer Res. 2009;15:6327–6340. doi: 10.1158/1078-0432.CCR-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]