Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common reproductive dysfunction in premenopausal women. PCOS is also associated with increased risk of cardiovascular disease at the time of PCOS and later in life. Hypertension, a common finding in women with PCOS, is a leading risk factor for cardiovascular disease. The mechanisms responsible for hypertension in women with PCOS has not been elucidated.
Objectives
To characterize the cardiovascular-renal consequences of hyperandrogenemia in a female rat model.
Methods
Female Sprague Dawley rats, aged 4–6 weeks, were implanted with DHT or placebo pellets lasting 90 days. Following 10–12 weeks, blood pressure (by radiotelemetry), renal function (glomerular filtration rate, morphology, protein and albumin excretion), metabolic parameters (plasma insulin, glucose, leptin, cholesterol, oral glucose tolerance test), inflammation (plasma TNF-α), oxidative stress (mRNA expression of NADPH oxidase subunits, p22phox, p47phox, gp91phox, and NOX4, nitrate/nitrite excretion), and mRNA expression of components of the renin-angiotensin system (RAS) (angiotensinogen, angiotensin-I-converting enzyme (ACE), AT1 receptor) were determined.
Results
Plasma DHT was increased 3-fold in hyperandrogenemic female 1 rats, whereas plasma estradiol levels were not different compared to control females. HAF rats exhibited estrus cycle dysfunction. They also had increased food intake and body weight, increased visceral fat, glomerular filtration rate, renal injury, insulin resistance and metabolic dysfunction, oxidative stress, and increased expression of angiotensinogen and ACE and reduced AT1 receptor expression.
Conclusions
The HAF rat is a unique model that exhibits many of the characteristics of PCOS in women and is a useful model in order to study the mechanisms responsible for hypertension.
Keywords: angiotensinogen, leptin, insulin resistance, cholesterol, oxidative stress
Polycystic ovary syndrome (PCOS) was first described by Stein and Levanthal in 1934 2, and is the most common endocrine disorder in women of reproductive age, affecting 5–10% 3. PCOS often starts with menarche in adolescents 4. PCOS is also associated with increased risk of cardiovascular disease at the time of PCOS diagnosis and later in life 5. Hypertension, a common finding in women with PCOS 6, is a leading risk factor for cardiovascular disease. The mechanisms responsible for hypertension in women with PCOS has not been elucidated.
The major criteria for a diagnosis of PCOS are clinical or biochemical, oligoanovulation, and polycystic ovarian morphology 7. However, the presence of one or more of these symptoms is considered, by definition, a possible criteria for the PCOS syndrome 7. For example, in a large study of women with hyperandrogenism, 72.1% were considered to have PCOS 8. In women with hirsutism, 78.4% were considered to have PCOS 9. In women with menstrual and ovulatory dysfunction, the percentage of women with PCOS was considered to be approximately 27.1% 7. Finally, approximately 20–30% of women of reproductive age have polycystic ovaries, but only 20% of them actually have defined PCOS, which is three fold higher than in the general population 7. Women with PCOS are at increased risk for endometrial carcinoma (3 fold higher odds 10), obesity (50% incidence 7), type II diabetes and insulin resistance (50–70 % incidence7, 11), dyslipidemias and hyperleptinemia (70% incidence7). Women with PCOS also have hypertension and cardiovascular disease 7. The presence of PCOS also has genetic or familial implications as well since sisters and daughters of women with PCOS are more likely to develop the syndrome than non-related individuals 7. 7, 11 The mechanisms responsible for PCOS development are not clear, nor have the mechanisms responsible for the increased risk of hypertension and cardiovascular disease risk been elucidated.
The study into the mechanisms responsible for the cardiovascular complications of PCOS have not been elucidated in part due to lack of a suitable animal model that adequately exhibits the symptoms found in women with PCOS. Models of estradiol valerate-induced PCO have been described, but plasma testosterone levels were lower in the treated rats than controls, and the treated rats did not exhibit insulin sensitivity 12. Shi and colleagues also reported that the JCR:LA-cp rat develops features of PCOS, such as obesity, insulin resistance, elevated serum testosterone levels, but this model has a defect in the ObR gene leading to loss of leptin receptor function 13. Therefore, evaluation of the possible role of leptin in mediating some of the features of PCOS, such as sympathetic activation, cannot be studied in this animal model. Since the increase in androgens has been implicated as playing a major role in mediating both the infertility problems and the metabolic abnormalities in women with PCOS, Manneras and colleagues developed a model using low doses of dihydrotestosterone supplements in young female Wistar rats 14. These investigators characterized the ovarian changes that occur in this model, and found that the DHT treatment increased the number of cystic follicles 14. Since androgen excess is a major component of the polycystic ovary syndrome, we used a modification of this hyperandrogenemic female 1 model to evaluate their metabolic features, blood pressure and kidney function.
The purpose of the present study then was to characterize the cardiovascular complications that occur in the DHT-treated female 1 rat, to evaluate its utility as a model of the cardiovascular complications of PCOS in women.
Methods
Animal model
Thirty two female Sprague Dawley rats were obtained from the vendor (Harlan Sprague Dawley, Indianapolis, IN) at 3 weeks of age. Rats were maintained throughout on standard rat chow. Rats were housed in temperature-controlled rooms with a constant light/dark cycle (12 hr/12 hr) and free access to water and food. At 4–6 wks of age, rats were implanted subcutaneously on the back of the neck with non-aromatizable, continous-release dihydrotestosterone pellets (DHT 7.5 mg/90 days (daily dose= 83 μg)), Innovative Research, Sarasota, FL) or placebo pellets (Innovative Research). Rats were allowed to age to 14–16 weeks of age before study. One set of HAF and control rats (n=5–6/grp) was used to measure body weights, blood pressure. The other rats were used for renal function, metabolic function (oral glucose tolerance tests, etc), and molecular studies. For the initial studies in which blood pressure was measured, rats were weighed daily and food intake was measured daily. In subsequent studies rats were weighed weekly. The protocols complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health, and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Measurement of sex steroids
Plasma testosterone and estradiol were measured in all HAF and control rats,15 using commercially available radioimmunoassay kits (Coat-A-Count testosterone kit (Diagnostic Products Corporation, Los Angeles, CA) and Ultrasensitive Estradiol kit (Diagnostic Products) 15. Plasma DHT was measured using a radioimmunoassay kit following oxidation/extraction as suggested by the manufacturer (Diagnostic Systems Laboratories, Inc, Webster, TX). Vaginal smears to determine estrus cycling were performed daily in the first set of control and HAF rats.
Measurement of blood pressure
Under gas anesthesia with isoflurane (Malinkrodt Veterinary, Hazelwood, CA), and with aseptic technique, HAF rats and controls (n=5–6/grp)16 were implanted at 15 weeks of age with radiotelemetry transmitters (TA11PA-C40; Data Sciences International, St. Paul, MN) into the abdominal aorta below the renal arteries, as we previously described 17. The transmitter was secured to the abdominal muscle. Rats were placed into individual cages above a receiver (RLA-3000) and allowed two weeks of recovery. Thereafter, mean arterial pressure (MAP) was monitored continuously for 3 days. Telemetry blood pressure measurements were obtained during a 10-second sampling period (500 Hz), recorded and averaged every 5 min for 24 hrs per day. Data are presented as mean +/− SEM, and averaged for each group of rats.
Measurement of renal function
Left femoral arterial and venous catheters were placed in rats, using isoflurane anesthesia and aseptic technique. Catheters were exteriorized at the nape of the neck. Rats (n= 5/grp) were allowed to recover for three days and then renal function was measured in conscious, unrestrained rats in their home cages. Briefly, 3H-inulin (20 μCi/ml 0.9% NaCl) at 2 ml/hr was infused into rats throughout the study. After 2 hr equilibration, 3 blood samples (50 μl each) were taken at 30 min intervals. GFR was calculated as cpm for infusate × infusion rate divided by cpm for plasma samples. Data are expressed as mean ml/min/g kidney weight +/− SEM.
Urinary protein and albumin excretion
Rats (n=8/grp) were placed in plastic metabolic cages and urine was collected for 24 hrs. Urinary protein excretion was measured using the Bradford method using a commercially available reagent (BioRad, Richmond, CA) and urinary albumin excretion was measured using the Nephrat ELISA (Exocell, Philadelphia, PA).
Assessment of glomerular sclerosis
Kidney sections from HAF and control rats (n=6/grp) were examined by a pathologist (LCR) who was not aware of the identity of the groups. Kidneys were embedded in paraffin and cut into 5-μm sections. The sections were stained with methinamine silver and periodic acid–Schiff reagent. Between 200–300 glomeruli from each kidney were examined, and each was graded for injury as follows: <25% of the glomerulus damaged; 25% to 50% damaged; 50% to 75% damaged; >75% damaged; and global sclerosis. The data from all rats in a group were averaged and expressed as a percentage of glomeruli from each kidney exhibiting the 5 levels of injury.
Measurement of metabolic factors
Body weights were measured weekly beginning with DHT implantation. Blood glucose was determined, from blood obtained from the tail vein of the rats, using a glucometer (Accu-check Advantage; Roche). Plasma insulin and leptin were measured by radioimmunoassay, according to the manufacturer’s recommendations using commercially available kits (Linco Research, St. Charles, MO). Plasma cholesterol was measured using an enzymatic colorimetric method following the manufacturer’s protocol (Wako Pure Chemical Industries, Ltd., Richmond, VA). Perirenal fat was removed at the time of sacrifice of the rats and weighed. Oral glucose tolerance test was performed in rats (n=6 per group) fasted for 18 hrs. Briefly, rats were given an oral glucose load (D-(+)-glucose in water; Sigma, St. Louis, MO) by gavage (2 g/kg body weight; total volume =500 μl). Prior to and every 30 min after the glucose load, a drop of blood was obtained from a tail cut, and glucose levels were measured by Accu-check Advantage glucometer. Data were evaluated as area under the curve and shown graphically.
Measurement of indicators of oxidative stress
Renal mRNA expression of NADPH oxidase subunits, gp91, p22phox, p47phox and NOX4, was measured by real time reverse transcription polymerase chain reaction (RT-PCR) in cortex of kidneys from HAF and control rats (n=6/grp), as previously described 18. Urinary nitrate/nitrite excretion was measured in 24 urine collections, as we previously described 1920.
Measurement of TNF-α
TNF-α was measured by ELISA in HAF and control rats (n=6/grp), using a commercially available kit (Quantikine, R&D Systems, Minneapolis, MN).
Expression of intrarenal renin-angiotensin system components
mRNA expression of angiotensinogen, angiotensin converting enzyme (ACE) and AT1 receptor in kidneys from HAF and control rats (n=6/grp) was measured by real time reverse transcription polymerase chain reaction (RT-PCR) using methods and primers that as previously described 21. Elongation factor-1 (EF-1) was used as the internal control.
Statistical analyses
All data are expressed as mean ± S.E.M. Results from the two groups were compared by t-test. Differences were considered statistically significant at p<0.05. Statistical analyses were performed with SigmaStat software package version 3.1 (Systat software Inc., San Jose, CA).
Results
Sex steroid levels
As shown in Figure 1, in HAF rats plasma DHT was increased by approximately 3 fold, whereas plasma estradiol levels were unchanged compared to untreated females. Testosterone levels were below detection by RIA. Vaginal smears revealed that HAF rats were not estrus cycling.
Figure 1. Plasma dihydrotestosterone and estradiol in HAF rats and placebo controls.
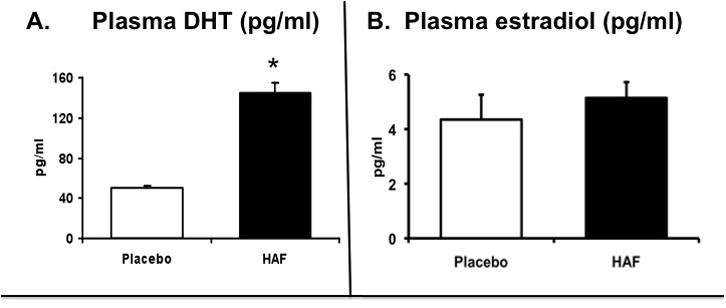
(n=8–10/grp). Data are mean ± SEM. *, p<0.01, HAF vs controls.
Body weights
As shown in Figure 2A, DHT caused an increase in food consumption in HAF rats (approximately 3 gr/d), and caused subsequent increases in body weight (Figure 2B). By 16 weeks of age, body weight was 28% higher in HAF rats than controls.
Figure 2. Effect of hyperandrogenmia on food intake, body weight, peri-renal fat and oral glucose tolerance test in HAF and placebo control rats.
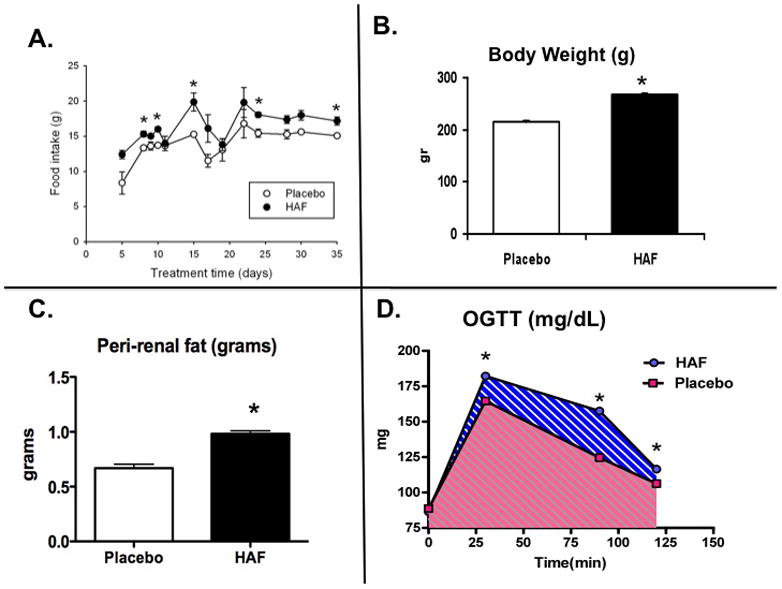
Panel A: Changes in food intake in HAF and placebo rats (n=8/grp). Panel B. Body weight at 16 wks of age in HAF and placebo rats (n=8/grp). Panel C. Peri-renal fat weight at 18 wks of age in HAF and placebo control rats (n=8/grp). Panel D. Oral glucose tolerance (OGTT) in HAF and placebo control rats (n=5/grp). Data are mean ± SEM. *, p<0.01, HAF vs controls.
Mean arterial pressure (MAP) and renal function and morphology
As shown in Figure 3A, by 18 weeks of age, MAP was significantly higher in HAF rats than controls. GFR was also higher in HAF rats (Figure 3B). Urinary protein excretion was 3 fold higher (35.1 ± vs. 11.3 ± mg/24 hr; p<0.01), and urinary albumin excretion was 5 fold higher in HAF rats than controls (Figure 3C). Kidneys from rats were assessed for the levels of glomerular injury (Figure 3D). There was no injury noted in control females, but HAF rats had significantly higher levels of renal injury.
Figure 3. Effect of hyperandrogenemia on mean arterial pressure, kidney function, albuminuria and renal injury.
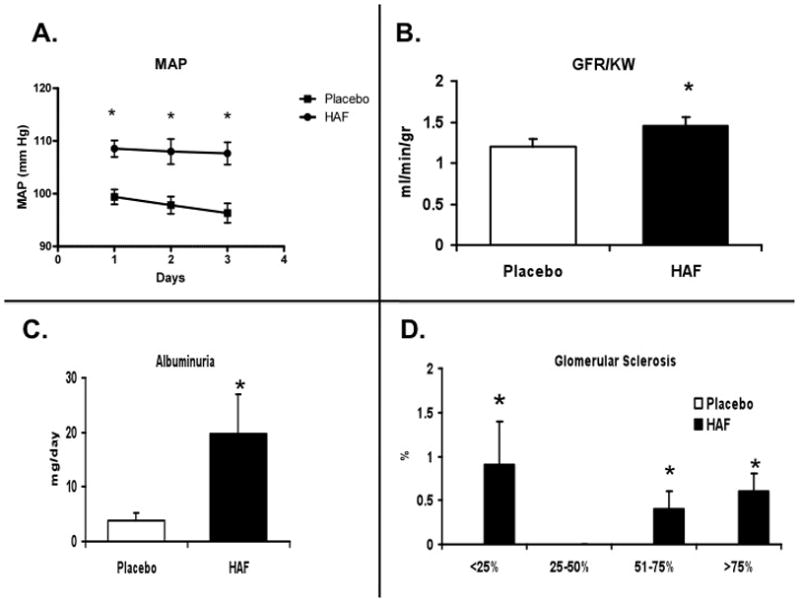
Panel A. Mean arterial pressure (MAP, in mm Hg) was measured by telemetry in HAF (n=6) and placebo control (n=5) rats. Panel B. Glomerular filtration rate factored for kidney weight (GFR/KW; ml/min/g KW) in HAF and placebo control rats (n=5/grp). Panel C. Albumin excretion over 24 hrs (ml/d) in HAF and placebo control rats (n=8/grp). Panel D. Percentage of glomeruli with injury at each level in HAF and placebo control rats (n=6/grp). Data are mean ± SEM. *, p<0.01, HAF vs controls.
Parameters of metabolic syndrome
In addition to the increase in body weight and hypertension, we determined if the HAF rats exhibited any other characteristics of metabolic syndrome. Peri-renal fat weight was higher in HAF rats (Figure 2C). Unfasted blood glucose levels were higher in HAF rats than controls (Figure 4A). In addition, unfasted plasma insulin, leptin, and cholesterol were higher in HAF rats (Figures 4B, C, D) than controls. In response to an oral glucose tolerance test, HAF rats had greater area under the curve 22 than controls (Figure 2D), suggesting insulin resistance.
Figure 4. Effect of hyperandrogenmia on plasma glucose, insulin cholesterol and leptin.
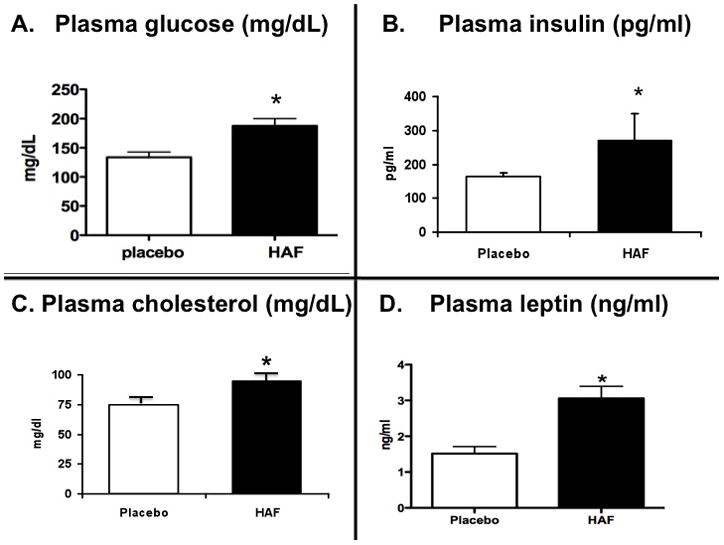
Plasma glucose (non-fasting, Panel A), plasma insulin (Panel B), plasma cholesterol (Panel C) and plasma leptin (Panel D) in HAF and placebo control rats (n=6/grp). Data are mean ± SEM. *, p<0.01, HAF vs controls.
Measurements of oxidative stress
In order determine possible mechanisms that could account for higher blood pressure in HAF rats, the mRNA expression of various NADPH oxidase subunits were measured. As shown in Figure 5A-D, pg91phox, p47phox, p22phox, and NOX4 mRNA expression was higher in renal cortex of HAF rats than controls. Urinary excretion of nitrate/nitrite, an index of nitric oxide, tended to be lower in HAF rats, but was not significantly different than controls (11.7 ± 1.2 (n=11) vs. 16.7 ± 2.9 (n=8); p=0.3).
Figure 5. Effect of hyperandrogenemia on mRNA expression of renal cortical NADPH oxidase subunits.
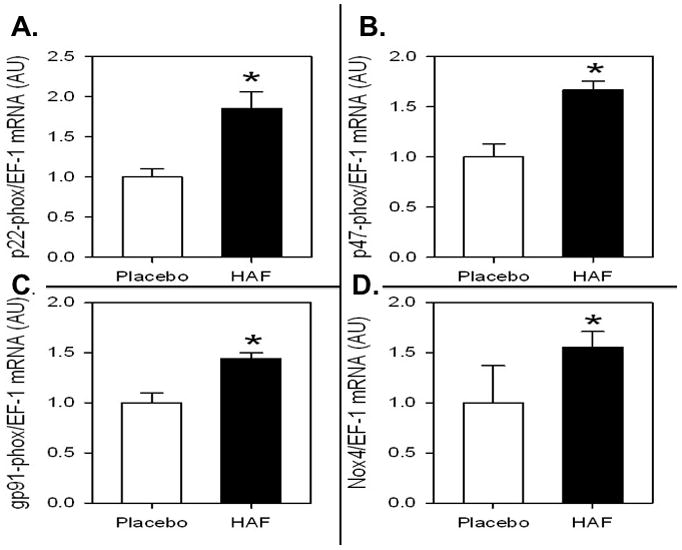
mRNA expression of p22phox (Panel A), p47phox (Panel B), gp91phox (Panel C), and NOX4 (Panel D). mRNA expression was determined by renal time RT-PCR, factored for EF-1 mRNA expression, and presented a arbitrary units (AU). Data are mean ± SEM; n=8/grp). *, p<0.01, HAF vs controls.
Measurements of TNF-α
Plasma TNF-α was significantly higher in HAF rats than control females (see Figure 6A).
Figure 6. Effect of hyperandrogenmia on plasma TNF-alpha, and intrarenal mRNA expression of angiotensinogen, ACE, and AT1 receptor.
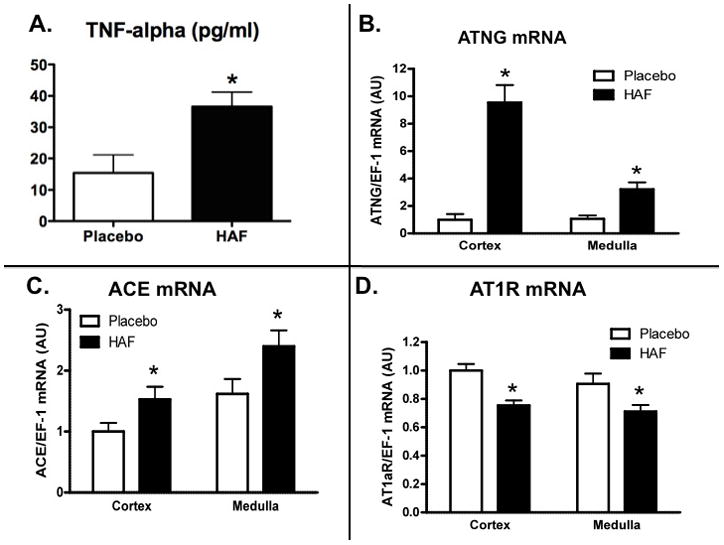
Panel A. TNF-α in plasma. Panels B-D: mRNA expression of angiotensinogen (ATNG, Panel B), ACE (Panel C), and AT1 receptor (AT1R, Panel D) in cortex and medulla was determined by renal time RT-PCR, factored for EF-1 mRNA expression, and presented a arbitrary units (AU). Data are mean ± SEM (n=8/grp) *, p<0.01, HAF vs controls.
Expression of RAS components
As shown in Figure 6B, renal cortical mRNA expression of angiotensinogen was 10 fold higher in HAF rats than controls. Similarly, renal cortical expression of ACE was also increased in HAF rats (Figure 6C). However, AT1 receptor mRNA expression was lower in both cortex and medulla In HAF rats than controls (Figure 6D). Medullary mRNA expression of angiotensinogen, and ACE were increased and AT1 receptor was decreased in HAF rats compared to controls.
Discussion
In this manuscript we characterized some of the cardiovascular consequences of increased androgens in a rat model of hyperandrogenemia. The major findings of the study are that increasing DHT by approximately 3 fold, a level that is consistent with androgen levels in women with PCOS, causes increases in blood pressure and mild renal injury. In an attempt to identify possible mechanisms by which androgens may cause hypertension and renal injury in this model, we found that the DHT-treated rats exhibited characteristics of the metabolic syndrome and inflammation. In addition, intrarenal mRNA expression of components of the RAS were also elevated. Although expression of several of the subunits of NAPH oxidase were increased in renal cortex of HAF rats, whether oxidative stress was increased in them was not clear from our studies.
A common finding in women with PCOS is that they exhibit elevated blood pressure. The mechanisms responsible are not clear. Hypertension is a significant risk factor for cardiovascular disease. Defects in the kidney’s ability to excrete salt and water have been shown to occur in all forms of hypertension studied to date 23. Thus we evaluated kidney function, protein and albumin excretion and glomerular sclerosis index. We found that chronic DHT caused a slight increase in GFR. It is possible that the increase in circulating glucose in the HAF rats could have increased GFR 24. In addition, individuals and animals with increases in body weight also show increases in GFR 24, 25, and thus the increase in body weight with DHT could have played a role as well. We also found that DHT caused a 3-fold increase in urinary protein excretion and a 5-fold increase in urinary albumin excretion compared to control rats. It is possible that the increase in blood pressure with DHT contributed to the increased protein and albumin excretion, but since there was slight glomerular injury present in the HAF rats, it is also likely that both renal injury and the elevated blood pressure contributed to proteinuria and albuminuria.
Women with PCOS often exhibit insulin resistance even in the absence of overt obesity 11. Diamanti-Kandarakis reports that approximately 50–70% of women with PCOS have insulin resistance 11. Furthermore, female-to-male transsexuals who receive high doses of androgens have an increased incidence of PCOS and metabolic syndrome disorders 26. In the present study we found that DHT caused an increase in food intake and body weight in the HAF rats. We did not measure blood pressure as the rats were increasing body weight and thus cannot determine if the increase in blood pressure with DHT mirrored the increase in body weight. However, increases in body weight have been shown previously to increase blood pressure 24. Along with the increase in body weight, we found increases in blood glucose and insulin and abnormal oral glucose tolerance test, all of which are indications of insulin resistance. In addition, we found that DHT caused increases in cholesterol and leptin and increases in peri-renal fat, as well. Taken with the increases in body weight and hypertension, these factors are all characteristics of the metabolic syndrome.
The mechanisms by which obesity increases blood pressure are not entirely clear. However, there is evidence that increases in body weight and visceral fat (as we found in the HAF rats) lead to increases in leptin (which we also found in HAF rats) which activates the melanocortin-4 receptor in the hypothalamus and causes sympathetic activation 27. Women with PCOS have increased sympathetic activity 28. Manneras and colleagues and Stener-Victorin also found that sympathetic activity was increased in a Wistar model of PCOS 12, 16. It is not clear whether increased sympathetic activity contributes to the elevated blood pressure in HAF rats..
It is important to note that androgens in men have different effects than in women. For example, in men an increase in androgens is associated with increases in lean body mass 29, 30, not increases in visceral fat as we found in our rats. In addition, in men, a reduction in androgen levels is associated with obesity and characteristics of the metabolic syndrome and insulin resistance 29, 30, not an increase in androgens, as in women with PCOS and our HAF rats. Androgen supplements in men also improve insulin resistance 31. A reduction in androgens in men is associated with inflammation, and androgen supplements in men reduce inflammation 32, 33. In contrast, women with PCOS have increases in inflammatory cytokines and inflammation 34., and our hyperandrogenemic rats have increases in TNF-α. Thus while decreases in androgens in men are associated with insulin resistance, type II diabetes, hypertension and renal disease, increases in androgens produce these symptoms in our hyperandrogenemic female rats. The reasons why different levels of androgens similar effets in males and females is not clear and shoul be further studied.35–37
In some situations, TNF-α has been shown to increase blood pressure. High levels of TNF-α, as found in sepsis or endotoxic shock, are associated with reductions in blood pressure 38, 39. LaMarca and colleagues reported that infusion of low doses of TNF-α in ovariectomized female rats replete with estradiol or progesterone did not increase their blood pressure 40. Similar findings were made in normal pregnant female rats. However, in pregnant rats in which TNF-α is similarly increased, such as the model of reduced uterine perfusion pressure (RUPP) that is hypertensive, etanercept, the TNF-α soluble receptor, significantly reduced BP 41. Future studies will be necessary to determine if the slight increases in TNF-α found in our HAF rats contribute to their hypertension.
In order to determine other possible mechanisms by which blood pressure could be increased in our rat model of hyperandrogenemia, we measured mRNA expression of components of the RAS. The RAS is activated in women with PCOS, and telmisartan, an angiotensin AT1 receptor antagonist, reduces their blood pressure 42, 43. We found that angiotensinogen mRNA expression was increased 10 fold in HAF rats. This is consistent with previous studies in rats showing that androgens stimulate intrarenal synthesis of angiotensinogen 44, 45. We also found that ACE synthesis was increased with DHT. In contrast, AT1 receptor expression in both cortex and medulla was decreased in our DHT-treated females. Increases in Ang II can downregulate AT1 receptor expression, but upregulate ACE 46, 47. There is also evidence that estradiol upregulates AT1 receptor expression 48, but not androgens 49. However, estradiol levels were not different in HAF rats and controls. Thus our data suggest that Ang II levels may be increased in HAF rats. However, the caveat to this is that we have not measured protein expression of the RAS components in this study and thus cannot be certain that Ang II levels are increased. Future studies will be necessary to confirm this hypothesis.
An additional mechanism by which blood pressure could be elevated with DHT in our females is via increases in oxidative stress. We found that DHT increased expression of 4 of the subunits of NADPH oxidase. Ang II has been shown to increase oxidative stress and intrarenal expression of NADPH oxidase subunits 50, 51. Thus an increase in Ang II with DHT could have caused an increase in expression of the NADPH oxidase subunits. However, whether oxidative stress was increased in the HAF rat was not conclusive in our present studies, since the levels of nitrate/nitrite excretion, an index of NO, which should decrease as oxidative stress and superoxide increase, were not different than controls. Therefore, future studies with antioxidants and specific inhibitors of NADPH oxidase will be necessary to determine if oxidative stress plays a role in mediating the hypertension in HAF rats. This being said, we and others have shown previously that oxidative stress is important in mediating the hypertension in male spontaneously hypertensive rats and Dahl salt sensitive rats 52, 53. However, we have been unable to support a role for oxidative stress in controlling blood pressure in female rats of these strains 54.
Summary
In summary, PCOS is the most common reproductive dysfunction in young women. PCOS with increases in androgens predisposes women to increased risk of cardiovascular disease when they are young as well as after menopause. Hypertension is one of major risk factors for cardiovascular disease. We have characterized the metabolic and cardiovascular-renal consequences of hyperandrogenemia in a female rat model that mimics many of the changes that occur in women with PCOS. For example, HAF rats exhibit increases food intake, body weight, blood pressure, and GFR. They have insulin resistance with increases in non-fasting glucose and insulin levels, and abnormal oral glucose tolerance test. They also exhibit factors associated with the metabolic syndrome, such as increases in leptin, cholesterol, peri-renal fat weight, and TNF-α. Expression of some of the components of the RAS were also elevated. Use of this model will allow for the further study of the pathways involved in mediating the hypertension in HAF rats, and hopefully, allow us and other investigators to shed light on possible mechanisms by which androgens cause cardiovascular disease in women with PCOS.
Acknowledgments
The authors acknowledge the support of the National Institutes of Health RO1s HL66072 and HL69194 and PO1 HL51971, and the American Heart Association Scientific Development Award #0830239N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez-Torres I, Roque P, El Hafidi M, Diaz-Diaz E, Banos G. Association of renal damage and oxidative stress in a rat model of metabolic syndrome. Influence of gender. Free Rad Biol Med. 2009;43:761–71. doi: 10.1080/10715760903045296. [DOI] [PubMed] [Google Scholar]
- 2.Stein IF, Levanthal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–91. [Google Scholar]
- 3.Diamanti-Kandarakis E. Polycystic ovarian syndrome; pathophysiology, molecular aspects and clinical implications. Exp Rev Mol Med. 2008;10:1–21. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- 4.Witchel SF. Puberty and polycystic ovary syndrome. Mol Cell Endocrinol. 2006;254–255:146–53. doi: 10.1016/j.mce.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Christakou CD, Diamanti-Kandarakis E. Role of androgen excess on metabolic aberrations and cardiovascular risk in women with polycystic ovary syndrome. Women’s Health. 2008;4:583–694. doi: 10.2217/17455057.4.6.583. [DOI] [PubMed] [Google Scholar]
- 6.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Position statement: Criteria for defining polycystic ovary syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–5. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R, Carmina D, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HW, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Carmina E, Rosato F, Jannì A, Rizzo M, Longo RA. Extensive clinical experience: relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab. 2006;91:2–6. doi: 10.1210/jc.2005-1457. [DOI] [PubMed] [Google Scholar]
- 9.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrin Metab. 2004;89:453–62. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 10.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine. 2006;30:13–7. doi: 10.1385/ENDO:30:1:13. [DOI] [PubMed] [Google Scholar]
- 12.Stener-Victorin E, Ploj K, Larsson B-M, Holmang A. Rats with steroid-induced polycystic ovaries develop hypertension and increased sympathetic nervous system activity. Reprod Biol Endocrinol. 2005;3:44–55. doi: 10.1186/1477-7827-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi D, Dyck MK, Uwiera RRE, Russel JC, Proctor SD, Vine DF. A unique rodent model of cardiometabolic risk associatd with the metabolic syndrome and polycystic ovary syndrome. Endocrinol. 2009;150:4425–36. doi: 10.1210/en.2008-1612. [DOI] [PubMed] [Google Scholar]
- 14.Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinol. 2007;148:3781–91. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 15.Fortepiani LA, Zhang H, Racusen LC, Roberts LJ, II, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in SHR. Hypertension. 2003;41:460–3. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- 16.Mannerås L, Cajander S, Lönn M, Stener -Victorin E. Acupuncture and exercise restore adipose tissue expression of sympathetic markers and improve ovarian morphology in rats with dihydrotestosterone-induced PCOS. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1124–R31. doi: 10.1152/ajpregu.90947.2008. [DOI] [PubMed] [Google Scholar]
- 17.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex Differences in Angiotensin II-induced Hypertension: Impact of high sodium intake. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 18.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, et al. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–82. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 19.Reckelhoff JF, Kellum J. Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:1895–902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- 20.Fortepiani LA, Reckelhoff JF. Increasing oxidative stress with molsidomine increases blood pressure in SHR, but not WKY. Am J Physiol Regul Integr Comp Physiol. 2005;289:R763–70. doi: 10.1152/ajpregu.00526.2004. [DOI] [PubMed] [Google Scholar]
- 21.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Reg Integ Comp Physiol. 2006;291:383–90. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
- 22.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, et al. Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol. 2004;15:2050–6. doi: 10.1097/01.ASN.0000133023.89251.01. [DOI] [PubMed] [Google Scholar]
- 23.Hall JE, Guyton AC, Brands MJ. Control of sodium excretion and arterial pressure by intrarenal mechanisms and the renin-angiotensin system. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management, Second Edition. New York: Raven Press; 1995. pp. 1451–75. [Google Scholar]
- 24.Hall JE, Brands MW, Henegar JR. Mechanisms of hypertension and kidney disease in obesity. Ann NY Acad Sci. 1999;892:91–107. doi: 10.1111/j.1749-6632.1999.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A. Is there a rationale for angiotensin blockade in the management of obesity hypertension? Hypertension. 2004;44:12–9. doi: 10.1161/01.HYP.0000132568.71409.a2. [DOI] [PubMed] [Google Scholar]
- 26.Baba T, Endo T, Honnma H, Kitajima Y, Hayashi T, Ikeda H, et al. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod. 2006;22:1011–6. doi: 10.1093/humrep/del474. [DOI] [PubMed] [Google Scholar]
- 27.Hall JE. Pathophysiology of obesity hypertension. Curr Hypertens Rep. 2000;2:139–47. doi: 10.1007/s11906-000-0073-4. [DOI] [PubMed] [Google Scholar]
- 28.Sverrisdottir YB, Mogren T, Kataoka J, Jansen PO, Stener-Victorin E. Is PCOS associated with high sympathetic nerve activity and size at birth? Am J Phys Endocrinol Metab. 2008;294:E542–E7. doi: 10.1152/ajpendo.00725.2007. [DOI] [PubMed] [Google Scholar]
- 29.Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009;37:74–90. doi: 10.1159/000176046. [DOI] [PubMed] [Google Scholar]
- 30.Allan C, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–32. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Mao J, Zhang Q, Shi YF. Testosterone replacement therapy improves insulin sensitivity and decreases high sensitivity C-reactive protein levels in hypogonadotropic hypogonadal young male patients. Chin Med J. 2009;122:2846–50. [PubMed] [Google Scholar]
- 32.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 33.Giltay EJ, Haider A, Saad F, Gooren LJ. C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia. 2008;40:398–400. doi: 10.1111/j.1439-0272.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 34.Tarkun I, Arslan BC, Canturk Z, Turemen E, Sahin T, Duman C. Endothelial dysfunction in young women with PCOS: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89:5592–6. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 35.Hermsdorff HH, Zulet MA, Puchau B, Martinez JA. Central adiposity rather than total adiposity measurements are specifically involved in the inflammatory status from healthy young adults. Inflammation. 2010 doi: 10.1007/s10753-010-9219-y. In press. [DOI] [PubMed] [Google Scholar]
- 36.Elks CM, Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr Hypertens Rep. 2010;12:99–104. doi: 10.1007/s11906-010-0096-4. [DOI] [PubMed] [Google Scholar]
- 37.Rizvi A. Hypertension, Obesity, and Inflammation: The complex designs of a deadly trio. Meta Syndr Relat Disord. 2010 doi: 10.1089/met.2009.0116. In press. [DOI] [PubMed] [Google Scholar]
- 38.Guo Z, Wang S, Jiao Q, Xu M, Xu Z. Soluble TNFR II/IgG1 Fc fusion protein treatment in the LPS-mediated septic shock of rats. Biomed Pharmacotherap. 2009;63:537–42. doi: 10.1016/j.biopha.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Ding J, Song D, Ye X, Liu SF. A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenssis of septic shock and septic vascular dysfunction. J Immunol. 2009;183:4031–8. doi: 10.4049/jimmunol.0900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaMarca BD, Chandler DL, Grubbs L, Bain J, McLemore GR, Granger JP, et al. Role of sex steroids in modulating tumor necrosis factor alpha-induced changes in vascular function and blood pressure. Am J Hypertens. 2007;20:1216–21. doi: 10.1016/j.amjhyper.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaMarca BD, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–71. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensterle M, Janez A, Vrtovec B, Meden-Vrtovec H, Pfeifer M, Prezelj J, et al. Decreased androgen levels and improved menstrual pattern after angiotensin II receptor antagonist telmisartan treatment in four hypertensive patients with PCOS. Croat Med J. 2007;48:864–70. doi: 10.3325/cmj.2007.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diamanti-Kandarakis E, Economou FN, Livadas S, Tantalaki E, Piperi C, Papavassiliou AG, et al. Hyperreninemia characterizing women with PCOS improves after metformin therapy. Kid Blood Press Res. 2009;32:24–31. doi: 10.1159/000201791. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y-F, Naftilan AJ, Oparil S. Androgen-Dependent Angiotensinogen and Renin Messenger RNA Expression in Hypertensive Rats. Hypertension. 1992;19:456–63. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 45.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen Regulation of Rat Renal Angiotensinogen Messenger RNA Expression. J Clin Invest. 1989;83:1941–5. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickenig G, Strehlow K, Baumer AT, Baudler S, Wassmann S, Sauer H, et al. Negative feedback regulation of reactive oxygen species on AT1 receptor gene expression. Br J Pharmacol. 2000;131:795–803. doi: 10.1038/sj.bjp.0703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172:1174–83. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, et al. Estrogen Modulates AT1 Receptor Gene Expression in Vitro and in Vivo. Circ. 1998;97:2197–201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 49.Song J, Kost CK, Martin DS. Androgens augment renal vascular responses to Ang II in New Zealand genetically hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1608–R15. doi: 10.1152/ajpregu.00364.2005. [DOI] [PubMed] [Google Scholar]
- 50.Rajagopalan K, Kurz S, Munzel T, Tarpey M, Freeman B, Griendling K, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarez E, Rodiño-Janeiro BK, Ucieda-Somoza R, González-Juanatey JR. Pravastatin counteracts angiotensin II-induced upregulation and activation of NADPH oxidase at plasma membrane of human endothelial cells. J Cardiovasc Pharmacol. 2010;55:203–12. doi: 10.1097/FJC.0b013e3181ce5f5a. [DOI] [PubMed] [Google Scholar]
- 52.Meng S, Cason G, Gannon A, Racusen L, Manning R. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–52. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 53.Yanes LL, Romero DG, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, et al. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the RAS, and oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–R33. doi: 10.1152/ajpregu.00530.2004. [DOI] [PubMed] [Google Scholar]
- 54.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharm Physiol. 2007;34:938–45. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]


