Abstract
Objectives. SLE is a systemic autoimmune disease with an annual incidence of 3.8 per 100 000. Several pathogenic mechanisms are believed to be operating in SLE, including an impaired clearance of apoptotic cells, activation of the type I IFN pathway and generation of autoimmune leucocytes. Growth arrest-specific protein 6 (Gas6) and its receptor Axl are known to regulate inflammation and may be implicated in lupus pathogenesis. We have recently developed immunological methods to quantify the vitamin-K-dependent protein Gas6 and its soluble receptor sAxl in human plasma, which we have used to investigate the role of Gas6 and soluble Axl in SLE.
Methods. We have investigated the relation between the plasma concentrations of Gas6 and sAxl and disease activity and specific symptoms in 96 SLE patients.
Results. Gas6 and sAxl concentrations correlated with SLEDAI (r = 0.48, P < 0.001 and r = 0.39, P < 0.001, respectively). Furthermore, concentrations of Gas6 and sAxl correlated with ESR and CRP and inversely with haemoglobin levels. Gas6 and sAxl concentrations were significantly higher in patients with anti-DNA antibodies, leucopenia and GN.
Conclusion. The plasma concentrations of Gas6 and sAxl vary with disease activity in SLE, in particular GN, and may have a role in lupus pathogenesis. Furthermore, Gas6 and sAxl may be of use as biomarkers of disease activity.
Keywords: Systemic lupus erythematosus, Growth arrest-specific protein 6, Axl, Receptor tyrosine kinase, Vitamin K, Inflammation, Autoimmunity
Introduction
SLE is a systemic autoimmune disease with complex clinical manifestations affecting 27.7 of 100 000 of the population, according to a study performed in Birmingham, UK: a large city with a broad ethnic mix [1]. The disease is characterized by the presence of ANAs [2], deficient clearance of apoptotic debris [3] and over-activation of the type I IFN genes [4]. Peripheral blood mononuclear cells from SLE patients have an increased expression of IFN-regulated genes [5].
It has been shown that pre-incubation with Growth arrest-specific protein 6 (Gas6) can decrease the secretion of pro-inflammatory cytokines of dendritic cells [6]. Gas6 up-regulates suppressor of cytokine signalling-1 (SOCS1), decreasing the response to inflammatory stimuli [7], implicating Gas6 as a potential regulator of SLE pathogenesis.
Gas6 was originally found as a protein produced by growth arrested fibroblasts [8]. It is structurally related to the anticoagulant protein S, sharing its domain organization and 44% of amino acid identity. While protein S has been shown to be an important regulator of the coagulation cascade, Gas6 has not, so far, been shown to influence coagulation in humans. However, according to studies of Gas6−/− mice, Gas6 is suggested to stimulate platelet-dependent primary haemostasis reactions [9]. Gas6 has been found to be present in human circulation at low levels (0.16–0.28 nM) [10]. It reacts as an acute-phase reactant and is increased during sepsis [11, 12] and pancreatitis [13].
Gas6 binds to the Axl tyrosine kinase receptor [14], which results in activation of the PI3K [15] and ERK kinase pathways and increased proliferation and survival [16]. The Axl receptor tyrosine kinase was originally found as an over-expressed gene in leukaemia cells, but is expressed in a wide variety of cells [17]. High Axl expression is a marker of negative prognosis for glioma [18] and kidney cancer [19].
We have recently shown that a soluble form of Axl, consisting of the extracellular part of the protein, is present in normal human serum at sub-nanomolar concentrations (0.3–0.9 nM) [20]. The soluble Axl (sAxl) is bound to Gas6 in serum, which suggests circulating Gas6 to be inactive because sAxl binding to Gas6 has been shown to inhibit Gas6-mediated activation of cell-bound receptors in vitro [21] and in vivo [22]. In this study, we show that Gas6 and sAxl concentrations correlate to disease activity in SLE, particularly in cases with lupus nephritis, indicating that both Gas6 and Axl may have a role in the disease.
Materials and methods
Patients
The 96 SLE patients included in the study were recruited from 1985 to 2005 at the Department of Rheumatology, Lund University, Lund, Sweden. There were 11 men and 85 women in the study, all fulfilling four or more ACR classification criteria [23]. The mean disease duration at the time of the study was 10 years and the mean age at SLE diagnosis was 35 years. Disease activity was evaluated using SLEDAI-2K [24], and DNA antibodies were detected with Crithidia luciliae IF test. EDTA plasma were collected and stored in aliquots at −80°C until tested.
Evaluation time points were selected with the objective of including a wide range of manifestations and SLEDAI scores, also including nine patients without evidence of active disease. The ACR criteria fulfilled are summarized in Table 1. The different manifestations of disease that were present at the time of blood sampling are summarized in Table 2. The mean SLEDAI score was 8.7 (range 0–32). Out of the 96 patients, 69 were treated with glucocorticoids, 49 were treated with anti-malarial drugs, 20 with AZA, 13 with warfarin, 5 with ciclosporin, 3 with CYC, 3 with immunoglobulin, 2 with MMF and 1 with tacrolimus. As warfarin affects Gas6, we considered the possibility of excluding warfarin-treated patients from the study. However, the warfarin-treated patients did not have SLEDAI scores significantly different from the other treated patients, and the correlations reported were valid even when excluding the warfarin-treated patients. Therefore, these patients were included in the study. All the patients gave written consent to the study, which was approved by the Regional Ethical Review Board in Lund.
Table 1.
Demographic data and distribution of ACR classification criteria for the patients involved in the study (n = 96)
| Patient demographics | |
|---|---|
| Number of female patients | 85 |
| Number of male patients | 11 |
| Age at diagnosis, mean, years | 35 |
| Age range, years | 14–85 |
| Mean years affected | 10 |
| Criteria | Positive, % (n = 96) |
|---|---|
| Malar rash | 57 |
| Discoid LE | 31 |
| Photosensitivity | 67 |
| Oral ulcers | 29 |
| Arthritis | 75 |
| Serositis | 54 |
| GN | 24 |
| Seizures, psychosis | 10 |
| Haematology | 52 |
| Immunology | 71 |
| ANA | 100 |
Table 2.
Clinical manifestations as defined by SLEDAI scoring system at time point of active disease
| SLEDAI manifestation | Positive, % (n = 96) |
|---|---|
| Seizure | 1 |
| Visual | 4 |
| Cranial neuropathy | 1 |
| Lupus headache | 1 |
| Cerebrovascular insult | 3 |
| Vasculitis | 14 |
| Arthritis | 24 |
| Myositis | 2 |
| Cylinderuria | 17 |
| Haemoglobinuria | 20 |
| Proteinuria | 23 |
| Pyuria | 0 |
| Rash | 34 |
| Alopecia | 8 |
| Oral ulcers | 4 |
| Pleuritis | 7 |
| Pericarditis | 5 |
| Complement | 45 |
| Anti-DNA antibodies | 33 |
| Fever | 11 |
| Thrombocytopenia | 6 |
| Leucopenia | 17 |
| Other | 0 |
ELISAs
The ELISAs for Gas6 [10] and sAxl [20] have been described earlier. In short, maxisorp plates (Dako, Glostrup, Denmark) were coated with a polyclonal antibody to respective antigen before blocking with 3% fish gelatin in 50 mM Tris, 150 mM NaCl, pH 7.5 [Tris-buffered saline (TBS)] and 0.1% Tween 20. The plates were washed 5-fold with TBS with 0.1% Tween 20 between every step. The plasma samples were diluted 10-fold in the blocking buffer but with the addition of 10% rabbit serum to minimize problems with autoreactive antibodies in the samples. The standard curves were made by serial dilution of known amounts of recombinant protein and negative controls by using buffer alone. Biotinylated rabbit antibodies were used to detect the proteins together with avidin-biotinylated enzyme complex/horseradish peroxidase (ABC/HRP) complexes (Dako). The signals were visualized with ortophenylenediamine and hydrogen peroxide before stopping the colour reaction with addition of 1 M sulphuric acid. The absorbance was determined in an automated plate reader at 490 nm.
Statistics
SPSS 17 (IBM, New York, NY, USA) was used for the multivariate analyses [analysis of variance (ANOVA) and multiple regression] and Graphpad 4.0 (Graphpad Software, La Jolla, CA, USA) was used for all other statistical analysis. Correlations were evaluated with Spearman’s rank correlation test. Comparisons between groups were performed using the Mann–Whitney U-test. When comparing pairs of samples, the Wilcoxon signed rank test was used. As there is no consensual way to correct for multiple testing with dependent variables, P < 0.01 was considered statistically significant to reduce the risk of accepting false hypotheses. The results of the initial exploratory analysis using this significance level (Tables 3 and 4) were further analysed by multivariate analyses, using methods mentioned above.
Table 3.
Correlations between Gas6 and sAxl concentrations and biochemical analytes and age
| Parameter | Spearman’s r | P-value |
|---|---|---|
| Gas6 correlations | ||
| SLEDAI | 0.48 | <0.0001 |
| Haemoglobin | −0.48 | <0.0001 |
| Leucocytes | −0.39 | <0.0001 |
| Thrombocytes | −0.07 | 0.4867 |
| Creatinine | −0.03 | 0.7838 |
| Sedimentation rate | 0.44 | <0.0001 |
| CRP | 0.30 | 0.0038 |
| C1q | −0.13 | 0.2150 |
| C3 | −0.26 | 0.0110 |
| C4 | −0.07 | 0.4828 |
| Age | −0.09 | 0.3576 |
| sAxl | 0.57 | <0.0001 |
| sAxl correlations | ||
| SLEDAI | 0.39 | <0.0001 |
| Haemoglobin | −0.51 | <0.0001 |
| Leucocytes | −0.15 | 0.1419 |
| Thrombocytes | −0.09 | 0.3814 |
| Creatinine | −0.00 | 0.9772 |
| Sedimentation rate | 0.48 | <0.0001 |
| CRP | 0.32 | 0.0025 |
| C1q | −0.12 | 0.2473 |
| C3 | −0.20 | 0.0555 |
| C4 | −0.03 | 0.7651 |
| Age | −0.18 | 0.0849 |
The correlations were estimated using the Spearman’s rank correlation test. The highly significant correlations (SLEDAI, haemoglobin, leucocytes, sedimentation rate, CRP and sAxl) were analysed by multiple regression.
Table 4.
Gas6 and sAxl concentrations in patients in relation to manifestations seen in SLE
| Symptom | Positive, n | Negative, median (range) | Positive, median (range) | P-value |
|---|---|---|---|---|
| n = 96 | Gas6, nM | |||
| Anti-DNA antibodies | 31 | 0.16 (0.09–0.43) | 0.22 (0.07–0.47) | 0.0004 |
| Arthritis | 23 | 0.17 (0.09–0.43) | 0.16 (0.07–0.47) | 0.2055 |
| Fever | 11 | 0.17 (0.07–0.47) | 0.22 (0.12–0.31) | 0.3033 |
| GN | 24 | 0.16 (0.07–0.47) | 0.26 (0.09–0.37) | <0.0001 |
| Leucopenia | 16 | 0.16 (0.07–0.43) | 0.27 (0.13–0.47) | 0.0003 |
| Rash | 33 | 0.16 (0.07–0.47) | 0.19 (0.11–0.37) | 0.0068 |
| Serositis | 9 | 0.17 (0.07–0.47) | 0.18 (0.12–0.31) | 0.5631 |
| Thrombocytopenia | 6 | 0.17 (0.07–0.43) | 0.16 (0.13–0.47) | 0.8145 |
| Vasculitis | 15 | 0.17 (0.09–0.47) | 0.23 (0.07–0.34) | 0.0406 |
| n = 94 | sAxl (nM) | |||
| Anti-DNA antibodies | 30 | 0.72 (0.38−2.34) | 0.97 (0.53−3.01) | 0.0004 |
| Arthritis | 22 | 0.82 (0.38–3.01) | 0.67 (0.44–2.34) | 0.0495 |
| Fever | 9 | 0.74 (0.38–3.01) | 0.83 (0.47–1.18) | 0.4959 |
| GN | 24 | 0.68 (0.38–2.34) | 1.12 (0.60–3.01) | <0.0001 |
| Leucopenia | 15 | 0.73 (0.38–3.01) | 0.94 (0.63–1.59) | 0.0025 |
| Rash | 31 | 0.73 (0.38–3.01) | 0.82 (0.55–2.02) | 0.2552 |
| Serositis | 9 | 0.74 (0.38–3.01) | 0.98 (0.55–1.50) | 0.0516 |
| Thrombocytopenia | 6 | 0.75 (0.38–3.01) | 0.78 (0.61–1.15) | 0.7278 |
| Vasculitis | 14 | 0.74 (0.38–3.01) | 0.99 (0.45–2.02) | 0.0501 |
Differences in Gas6 and sAxl levels were evaluated with the Mann–Whitney U-test. The highly significant variables were analysed further with ANOVA.
Results
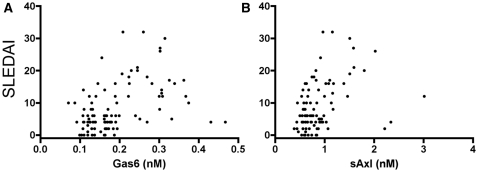
All patients with low SLEDAI score (<3) had Gas6 concentration below the upper normal level, and 18 of 20 patients with low SLEDAI had sAxl within the normal range determined in healthy controls, as described [10, 20]. However, we noticed a statistically significant correlation between SLEDAI score and concentrations of Gas6 (P < 0.0001, r = 0.48, n = 96) and sAxl (P = 0.0001, r = 0.39, n = 94) (Fig. 1A and B).
Fig. 1.
Correlation of the SLEDAI with Gas6 and sAxl concentrations in plasma. Each symbol represents one person. (A) The correlation between SLEDAI and Gas6 (r = 0.48, P < 0.0001, n = 96). (B) The correlation between SLEDAI and sAxl (r = 0.39, P = 0.0001, n = 94). The correlations were evaluated with Spearman’s non-parametric test.
Gas6 and sAxl correlated strongly to each other (r = 0.57) and both were positively correlating to sedimentation rate (r = 0.44 and r = 0.48) and CRP (r = 0.30 and r = 0.32), and inversely to haemoglobin (r = −0.48 and r = −0.51). Gas6 correlated inversely with leucocyte count (r = −0.39). There was a trend of a negative correlation between Gas6 and C3—and a similar trend for sAxl—but there were no correlations to creatinine clearance, C4, C1q or age (Table 3). The parameters giving the significant correlations of Table 3 were subjected to multiple regression analysis. When Gas6 was used as dependent variable, sAxl was the only highly significant independent variable (P < 0.0003).
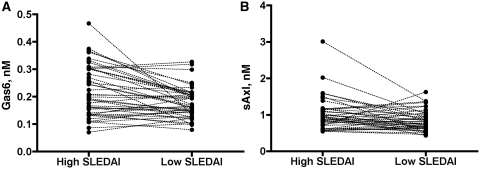
In 45 patients, samples from two time points, one with higher and one with lower disease activity, were available. Median and range of SLEDAI was 10 (3–32) and 0 (0–12) in the high and low SLEDAI group, respectively. The mean Gas6 concentration was 0.21 nM for the high SLEDAI group as compared with 0.16 nM for low SLEDAI group (P = 0.0003, n = 45). For sAxl, the corresponding concentrations were 0.92 and 0.81 nM, respectively (P = 0.027, n = 42) (Fig. 2A and B).
Fig. 2.
Concentrations of Gas6 (A) and sAxl (B) in the same patient at high and low SLEDAI scores. Each patient is represented by one dot in each column, the dots between columns being interconnected by a line. The level of significance is P = 0.0003 for Gas6 and P = 0.0271 for sAxl.
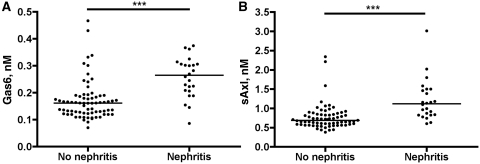
We evaluated the correlation between Gas6 and sAxl concentrations and active SLE manifestations. High Gas6 and sAxl were observed in the 24 patients with GN (P < 0.001 for both, Fig. 3A and B); in 21 patients, a biopsy was taken and in 18 of the patients, World Health Organization classes 3 or 4 were found. High Gas6 was also observed when anti-DNA antibodies, leucopenia or rash was present, and there was a trend of higher Gas6 in patients with vasculitis. Higher sAxl concentrations could be observed when anti-DNA antibodies or leucopenia was present. There was a trend to higher sAxl in patients with serositis and vasculitis and lower sAxl in patients with arthritis (Table 4). The significant variables in Table 4 were analysed with ANOVA. In the ANOVA analysis of Gas6, GN (P = 0.0013) and leucopenia (P = 0.0059) were the only independent factors. In the sAxl ANOVA analysis, only GN remained independent (P = 0.0009).
Fig. 3.
Distribution of Gas6 (A) and sAxl (B) concentrations in patients without and with GN (70 and 24, respectively). Both are significant using the Mann–Whitney U-test, P < 0.001 as indicated by the triple asterisks.
Discussion
We have determined the Gas6 and sAxl concentrations in a number of SLE patients in relation to disease phenotype and activity. Both Gas6 and sAxl correlate with the overall disease activity measured by SLEDAI-2K and increase during periods of higher disease intensity. Gas6 and sAxl correlate with sedimentation rate and CRP, indicating a role linked to inflammation. Gas6 and sAxl plasma concentrations are higher in patients with active GN, but Gas6 or sAxl did not correlate to creatinin, C1q or C4. Recently, Suh et al. [25] reported on a study in which Gas6 was measured in patients with SLE. In agreement with our results, they found the concentrations of Gas6 in SLE patients to be similar to those of controls. They found slightly higher Gas6 levels in a small number of patients with neurological disease but apart from that found no association between disease activity and Gas6 levels. Our study includes twice as many patients as that of Suh et al. [25] and contains patients with a wide variation in symptoms, which may contribute explanation to the difference in results.
Interestingly, Gas6 and Axl have been implicated to be important in experimental GN (Gas6−/− mice are protected) [22], as well as diabetic and nephrotoxic nephritis [26, 27]. Whether Gas6 has a role in the pathogenesis of SLE-associated GN remains to be determined. Gas6 signalling can have several effects, related to both the initiation of inflammation and the regulation of established inflammation. Gas6 has been shown to decrease the levels of secreted cytokines by dendritic cells [7], and warfarin treatment or Axl-Fc can decrease the symptoms of GN in mice [22].
In conclusion, this study suggests that both Gas6 and sAxl concentrations correlate with the SLE disease activity and are increased in patients with anti-DNA antibodies, leucopenia and GN. Gas6 and sAxl may be useful additions to the panel of biomarkers of SLE disease activity.

Acknowledgements
We thank Mr Jan-Åke Nilsson for valuable help with the statistical analysis.
Funding: This study was supported by grants from the Swedish government funds for clinical research (ALF), funds from the University hospital in Lund and Malmö, Swedish Research Council (grant #07143), The Wallenberg Foundation, Österlund’s Foundation, the Swedish Research Council (2008-2201), the Medical Faculty at Lund University, The Crafoord Foundation, Greta and Johan Kock’s Foundation, King Gustaf V’s 80th Birthday Foundation, Lund University Hospital, the Swedish Rheumatism Association, Swedish Society of Medicine and the Foundation of the National Board of Health and Welfare. Funding to pay the Open Access publication charges for this article was provided by the Swedish Research Council.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth. Arthritis Rheum. 1995;38:551–8. doi: 10.1002/art.1780380415. [DOI] [PubMed] [Google Scholar]
- 2.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology. 2007;46:1052–6. doi: 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 3.Munoz LE, Gaipl US, Franz S, et al. SLE–a disease of clearance deficiency? Rheumatology. 2005;44:1101–7. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 4.Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr Opin Rheumatol. 2009;21:471–7. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- 5.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87:869–75. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 7.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (Gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–85. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelillo-Scherrer A, de Frutos P, Aparicio C, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–21. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 10.Balogh I, Hafizi S, Stenhoff J, Hansson K, Dahlback B. Analysis of Gas6 in human platelets and plasma. Arterioscler, Thromb, Vasc Biol. 2005;25:1280–6. doi: 10.1161/01.ATV.0000163845.07146.48. [DOI] [PubMed] [Google Scholar]
- 11.Borgel D, Clauser S, Bornstain C, et al. Elevated growth-arrest-specific protein 6 plasma levels in patients with severe sepsis. Crit Care Med. 2006;34:219–22. doi: 10.1097/01.ccm.0000195014.56254.8a. [DOI] [PubMed] [Google Scholar]
- 12.Gibot S, Massin F, Cravoisy A, et al. Growth arrest-specific protein 6 plasma concentrations during septic shock. Crit Care. 2007;11:R8. doi: 10.1186/cc5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uehara S, Handa H, Gotoh K, Tomita H, Sennshuu M. Plasma concentrations of growth arrest-specific protein 6 and protein S in patients with acute pancreatitis. J Gastroenterol Hepatol. 2009;24:1567–73. doi: 10.1111/j.1440-1746.2009.05875.x. [DOI] [PubMed] [Google Scholar]
- 14.Stitt TN, Conn G, Gore M, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–70. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 15.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–53. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenhoff J, Dahlback B, Hafizi S. Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth of cardiac fibroblasts. Biochem Biophys Res Commun. 2004;319:871–8. doi: 10.1016/j.bbrc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 17.O'Bryan JP, Frye RA, Cogswell PC, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–31. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vajkoczy P, Knyazev P, Kunkel A, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci USA. 2006;103:5799–804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson A, Martuszewska D, Johansson M, et al. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009;15:4742–9. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- 20.Ekman C, Site DF, Gottsater A, Lindblad B, Dahlback B. Plasma concentrations of growth arrest specific protein 6 and the soluble form of its tyrosine kinase receptor Axl as markers of large abdominal aortic aneurysms. Clin Biochem. 2010;43:110–4. doi: 10.1016/j.clinbiochem.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Park IK, Giovenzana C, Hughes TL, Yu J, Trotta R, Caligiuri MA. The Axl/Gas6 pathway is required for optimal cytokine signaling during human natural killer cell development. Blood. 2009;113:2470–7. doi: 10.1182/blood-2008-05-157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagita M, Arai H, Ishii K, et al. Gas6 regulates mesangial cell proliferation through Axl in experimental glomerulonephritis. Am J Pathol. 2001;158:1423–32. doi: 10.1016/S0002-9440(10)64093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 24.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 25.Suh CH, Hilliard B, Li S, Merrill JT, Cohen PL. TAM receptor ligands in lupus: Protein S but not Gas6 levels reflect disease activity in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R146. doi: 10.1186/ar3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai K, Arai H, Yanagita M, et al. Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J Biol Chem. 2003;278:18229–34. doi: 10.1074/jbc.M213266200. [DOI] [PubMed] [Google Scholar]
- 27.Yanagita M, Ishimoto Y, Arai H, et al. Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. J Clin Invest. 2002;110:239–46. doi: 10.1172/JCI14861. [DOI] [PMC free article] [PubMed] [Google Scholar]