The essence of this presentation is that under effective immunosuppression, there is a migration of sessile (passenger) leukocytes (prominently but not limited to dendritic cells) from the graft into ubiquitous recipient tissues, and replacement of these cells in the graft by similar leukocytes from the recipient. The consequence is a body wide engagement of donor and recipient cells followed, we believe, with variable donor and recipient-specific nonreactivity that may or may not require lifetime immunosuppression for stability1 (Fig. 1).
Fig. 1.

Current understanding of the graft and systemic chimerism that occurs after intestinal transplantation. Note the automatic production of mixed allogenic chimerism, providing the preexisting immunologic apparatus is not iatrogenically damaged on either donor or recipient side (see text). Evolution of this concept permitted successful clinical intestinal transplantation trials.11
Neglected Early Clues
The germ of this concept was stated ingenuously in the title of an article in 1963 which introduced the combined use of azathioprine and prednisone for renal transplantation— emphasizing, first, the ease with which kidney rejection could be reversed, and second, the collapse in time of antidonor immune reactivity.2 A characteristic clinical evolution in successful cases under azathioprine was a rejection, manifested by secondary functional failure of an initially well functioning allograft (fall in creatinine clearance and rise in BUN), reversal of these adverse events with augmented steroids, subsequent weaning of both steroids and azathioprine, and then a trouble-free survival lasting in the most successful cases for more than 30 years.
The observations of rejection reversal and an altered donor-recipient relation led in 1963 to development of an empiric therapeutic dogma upon which the specialty of whole organ transplantation is based. It calls for daily baseline treatment (originally azathioprine) plus trial and error intervention with the highly dose maneuverable adrenal cortical steroids (or later antilymphoid agents) to whatever level is required to maintain stable graft function. Increasingly potent new drugs with different sites of action (listed in Table 1) have been added through the years, but the therapeutic dogma has never changed.
Table 1.
Central Therapeutic Dogma
| Strategy | Baseline Agents | Sites of Action |
|---|---|---|
| 1. Baseline therapy with one or two drugs | 1. Azathioprine | DNA synthesis |
| 2. Secondary adjustments with steroids or antilymphoid agents | 2. Cyclophosphamide | DNA synthesis |
| 3. Case to case trial and potential error) of weaning | 3. Cyclosporine | IL-2 production |
| 4. FK506 | IL-2 production |
Although this treatment policy was successful, there was no explanation why. A clue was found in tuberculin, coccidiodin and other delayed hypersensitivity skin tests on these pioneer kidney recipients and their donors. Seventy-seven percent of the skin reactions that were positive in the donors but not the recipients crossed over to the previously negative recipients, along with the transplanted kidneys. When this did not occur (the other 23%), it meant that the kidney transplant had failed. Our immunologists Kirkpatrick and Wilson, speculated that the migration of the skin tests was “caused by adoptive transfer of donor cellular immunity by leukocytes in the renal graft vasculature and hilar lymphoid tissue.”3 Because the kidney was thought 30 years ago to be devoid of leukocytes, the credibility of the explanation was undermined.
Proof of Chimerism
After Kidney Transplantation
That Kirkpatrick and Wilson had been right was proved nearly 30 years later when five of the original Colorado patients who still bore their continuously functioning HLA mismatched kidneys, were restudied. In four of the cases, their volunteer donors were still alive and it was possible to show by mixed lymphocyte reaction (MLR) or cell mediated lymphocytotoxicity (CML) that there was donor-specific nonreactivity. Biopsies in the five patients were taken from the allograft and elsewhere. After immunostaining, viable donor cells that appeared to be dendritic cells were found in the lymph nodes and skin of all the recipients; the findings were confirmed with polymerase chain reaction (PCR).4
The liver and Other Extrarenal Organs
Other early clues about cell migration came from the second vital organ to be transplanted—the liver. In 1969, it was shown that the liver graft quickly became a composite with replacement of its Kupffer cells and other interstitial leukocytes by recipient cells5 — a change assumed for nearly 20 years to be a unique feature of the liver. However, in 1991, when it was seen by Murase6 and Iwaki7 that the transplanted intestine also was chimeric, it was realized that the changes must be generic with all organs. This soon was proved.
Finally in 1992, at the same time as the kidney recipient studies already cited, but in far greater detail, it was proved with cytostaining and PCR techniques that surviving donor cells were everywhere in the liver recipients.1,8–10 All 25 patients had donor cells in their tissues obtained by biopsy or at autopsy 2 to 23 years posttransplantation; 15 of the patients also were blood chimeras according to PCR results.
Augmentation of Chimerism
We have summarized elsewhere the evidence that the same phenomena occur with other organs.1,10 If these same events occur with all whole organ grafts but with quantitative differences, the heavy endowment of the liver with these migratory leukocytes would explain the well known relative ease of inducing its acceptance (sometimes without drugs), its ability to shield other concomitantly transplanted organs (hepatic tolerogenicity), and perhaps even its resistance to preformed antibodies.10 Other organs have similar although less tolerogenic potential. The smaller leukocyte substrate in the kidney, for example, would explain why it is harder for the renal allograft recipient to achieve drug-free donor-specific nonreactivity than for the liver patient.
At this meeting, strategies long advocated by Monaco and Thomas (and before them, Leslie Brent) have been discussed to elevate the underprivileged kidney and heart to the same level or beyond the advantage enjoyed by the liver by infusion of bone marrow. The logic of these strategies (Fig. 2) is reinforced by the realization that they are extensions or magnifications of a naturally occurring process of cell migration.
Fig. 2.
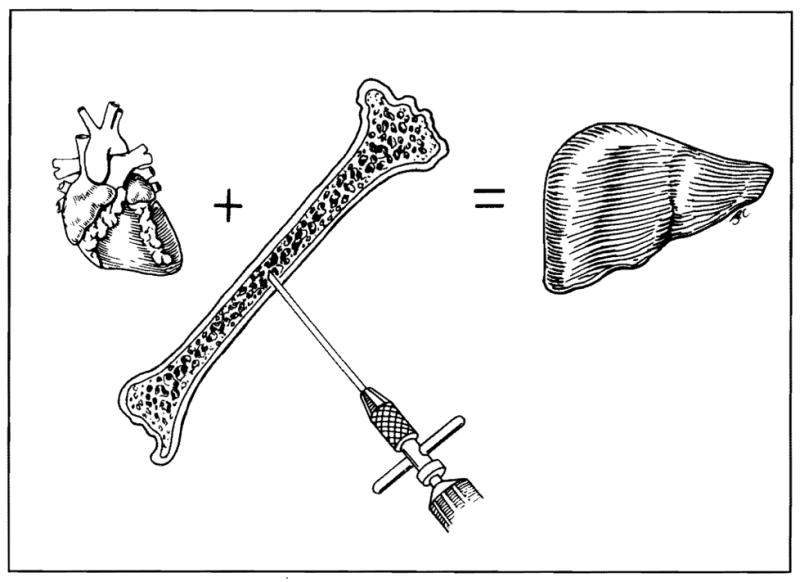
Strategies to bring “underprivileged” organs like the heart and kidney to the same level of tolerogenicity advantage as the liver, by infusing bone marrow cells or other immunocytes from the whole organ donor.
As a corollary, it may be suggested that observations about the time necessary to establish a drug-free chimeric state in liver recipients are predicted to be more or less applicable to the management of kidney, heart or other organ recipients given adjuvant bone marrow. It is clear that the instant tolerance achieved with mixed chimerism in mouse and other rodent systems is an illusion for outbred MHC mismatched large animals including humans. In human liver recipients, the chimeric state is dependent on immunosuppression for highly variable periods. So far, 22 liver recipients taken off therapy as early as two months postoperatively and as late as 11 years have had drug-free subsequent survival for as long as 14 years. We now suspect that the majority of liver recipients with a benign course exceeding five years can be weaned from drugs — one safety factor being the ability to effectively treat rejection, if it does occur, with FK 506. The addition of donor bone marrow to the liver transplant operation may shorten the interval of drug dependence but it will not eliminate it, even when a high level of mixed multilineage chimerism (exceeding 25% of circulating white cells) is produced. We already have shown that rejection or GVHD can be the price for premature drug weaning.
Cause and Effect Questions about Microchimerism
Metabolic Effects
Aside from their immunologic implications, the seemingly sparse peripheralized leukocytes following conventional organ transplantation can profoundly alter total body metabolism as shown in three liver transplant recipients who had metabolic storage diseases caused by pancellular enzyme deficiencies. In these patients, enzymes appeared to have been transported to the host cells by chimeric donor cells that were found by cytostaining or PCR in the heart, skin, lymph nodes, intestine, bone marrow or blood.9 In two patients with the branching enzyme deficiency of Type IV glycogen storage disease, the amylopectin characteristic of this disorder was absorbed from the heart and other tissues.
In the patient with Gaucher’s disease, chimerism was shown by immunostaining and PCR in the liver (the hepatocytes of which remained donor), and also in the recipient blood, bone marrow, skin, small bowel, and lymph nodes. The glucocerebroside deposits (Gaucher’s cells) in the lymph nodes of this patient diminished dramatically over two years.9
These pancellular storage disorders previously were thought to be correctable only with bone marrow transplantation, meaning that the liver engraftment is in essence a mini-bone marrow transplant. In turn, this implies a co-culture effect of a small number of chimeric donor cells on the contiguous overwhelming numbers of recipient cells. Important questions are thereby raised about the potential cell to cell effect of other molecules directly involved in immunologic processes including those involved in tolerance induction.
The Immunologic Interface
We have concluded from this recent information that for the last 30 years we have systematically produced mixed allogeneic chimerism in our whole organ recipients (of kidneys, livers, hearts, lungs, or intestines) without knowing it. In the process the coexisting immunocyte populations appear to come to view each other in a revised light. How this occurs is unknown but it seems clear as already discussed that the change is unpredictable and relatively slow under the immunosuppressive regimens in current use, even in the most successful cases. The nonreactivity that eventually evolves works both ways accounting for the eventual amelioration of whole organ rejection (host-versus-graft reaction, HVG) and also for the GVHD resistance of the whole organ recipient. Understanding this has been the single most important factor in our routine ability to transplant intestines or multiple viscera.11 The credibility of this explanation for the absence of GVHD after transplantation of leukocyte rich organs like the liver and intestine is enhanced by analogous much earlier observations of mixed chimerism in mouse bone marrow recipients conditioned by Slavin and Strober with total lymphoid irradiation12 — a procedure that leaves much of the host immune system intact—and in the classic mixed bone marrow transplant experiments of Ildstad and Sachs13 much discussed at this meeting.
In both directions (GVH and HVG), cellular interactions resulting in mutual natural immunosuppression (this has been called “exhaustive clonal differentiation” by Webb, Morris, and Sprent14) (Fig. 1) are envisioned as occurring on a sliding scale in which each further level of histoincompatibility provokes countervailing although not equal increases in initial response. If the acute storm can be weathered long enough to allow a rapprochement, as has been more and more possible under the protective umbrella of modern day immunosuppression, the anticipated HLA matching effect dwindles. We think this explains the poor correlation of tissue matching with outcome in whole organ cadaveric transplantation.
Failure of Mixed Chimerism
Having thus defined success, it will be prudent in closing to define failure. Failure connotes the inability to achieve acceptable mixed chimerism and most commonly signifies an imbalance despite immunosuppression that favors rejection. However, because an incipient GVH reaction is a necessary condition for success (this is our hypothesis), it is obvious that clinical GVHD is a theoretical possibility in every case no matter what the organ (Fig. 1). For example, we now realize that about 5% of all liver recipients have GVHD which in the past usually was attributed to an allergic reaction. This kind of GVHD usually can be controlled with increased or decreased immunosuppression, but the number of reports of a fatal outcome has steadily increased.
Relation of Cell Migration and Chimerism to Tolerance
Recent reviews have emphasized the inadequacy of thymic clonal deletion to explain acquired transplantation tolerance and have focused on postthymic mechanisms that include peripheral clonal deletion and anergy. However, we note here that all of the hypotheses to explain clonal “silencing”, whether or not these are called tolerance and are attributed to thymic or postthymic mechanisms, will be enriched by the discovery of the enduring intimacy of the chimeric graft and host immunologic systems.
However, the evidence of vitality and turnover of donor leukocytes in recipient tissues as long as three decades posttransplantation is particularly supportive of Coutinho15 who has defined acquired tolerance as a high (not anergic) level of sustained immune activity in communicating networks more complex than the idiotype systems originally postulated by Jerne.16 Suppressor and/or veto cells could be epiphenomena of this kind of activity; it is not too much to ask if these are altered dendritic cells.
Throughout the years, the testing of every genuinely potent immunosuppressant has been followed by excited claims of tolerance induction when drugs could be stopped after a brief induction period. The very simplicity of cell migration and chimerism as a common mechanism to explain these accomplishments no matter what the site of the drug action cloaked the existence of the chimeric phenomenon and delayed its discovery. With the clarifying reason of chimerism for the commonality of the end result, it should be possible to work backward using drugs with known sites of action to ask specific questions and test hypotheses about the true meaning of tolerance.
Conclusion
The cell migration concept creates a seamless single world for those transplanting bone marrow and those engrafting solid organs. Far from involving different mechanisms, these two seemingly disparate fields merely reflect different treatment dogmas. The bone marrow transplanters eliminate (or minimize) mutual cell engagement by recipient cytoablation and thus commit themselves to heavy reliance on HLA matching despite which there is a constant threat of GVHD. Solid organ transplanters encourage, or at least permit, the mutual cell engagement to occur, thereby liberating themselves from the restrictions of HLA matching and an overwhelming threat of GVHD. Knowing this, the disciplines reunite.
Acknowledgments
Aided by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson WEC, Kirkpatrick CH. Immunologic aspects of renal homotransplantation. In: Starzl TE, editor. Experience in Renal Transplantation. Philadelphia: WB Saunders Company; 1964. pp. 239–261. [Google Scholar]
- 4.Starzl TE, Demetris AJ, Trucco M. Chimerism and donor specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. doi: 10.1097/00007890-199306000-00012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashiwagi N, Porter KA, Penn I, et al. Studies of homograft sex and of gamma globulin phenotypes after orthotopic homotransplantation of the human liver. Surg Forum. 1969;20:374–376. [PMC free article] [PubMed] [Google Scholar]
- 6.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 7.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. 1991;337:818–819. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease and Type I Gaucher’s disease. New Engl J Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: The basis of graft acceptance. Hepatology. 1993:17. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 12.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: Long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 14.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 15.Coutinho A. Beyond clonal selection and network. Immunol Rev. 1989;110:63–87. doi: 10.1111/j.1600-065x.1989.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 16.Jerne NK. Idiotypic networks and other preconceived ideas. Immunol Rev. 1984;79:5–24. doi: 10.1111/j.1600-065x.1984.tb00484.x. [DOI] [PubMed] [Google Scholar]


