Abstract
The ability to sense and respond to oxygen deprivation is required for survival; thus, understanding the mechanisms by which changes in oxygen are linked to cell viability and function is of great importance. Ion channels play a critical role in regulating cell function in a wide variety of biological processes, including neuronal transmission, control of ventilation, cardiac contractility, and control of vasomotor tone. Since the 1988 discovery of oxygen-sensitive potassium channels in chemoreceptors, the effect of hypoxia on an assortment of ion channels has been studied in an array of cell types. In this review, we describe the effects of both acute and sustained hypoxia (continuous and intermittent) on mammalian ion channels in several tissues, the mode of action, and their contribution to diverse cellular processes.
Keywords: oxygen deprivation, mammalian ion channels, oxygen homeostasis
sensing and appropriately responding to changes in O2 concentration is of paramount importance for the survival of all organisms. Over time, O2 homeostasis has evolved into a complex system to regulate O2 delivery and use. While the rapid response to acute reductions in O2 concentration typically results from processes that alter preexisting proteins, such as phosphorylation, stabilization, dimerization, or degradation, durable adaptation to prolonged hypoxic insult requires a coordinated response at the level of gene transcription. Since a deficit in O2 is an important component of various physiological processes and the pathogenesis of numerous diseases, understanding the mechanisms by which changes in O2 are linked to cell function is of great interest.
Ion channels play a critical role in regulating a wide variety of biological processes, including neuronal transmission; control of ventillation; contraction of cardiac, skeletal, and smooth muscle; transport of nutrients and ions across the epithelium; T-cell activation; and glucose metabolism and pancreatic β-cell insulin release. To date, over 300 types of ion channels, differing in their mode of activation (voltage-dependent or -independent, ligand-gated, mechanosensitive, pH) and their ionic permeability (K+, Ca2+, Cl−, Na+), have been identified. This heterogeneity in ion channel populations provides an astonishing array of variable responses within and between cell types. In 1988, a report demonstrating that rabbit carotid body glomus cells express an O2-sensitive potassium channel ushered in a new era of research exploring whether ion channel activity could be regulated in response to changes in O2 availability (131). Since that initial description of an O2-regulated ion channel, an abundance of work has been produced indicating that a variety of ion channels spread across the different ion channel families are O2 sensitive and exhibit changes in channel activity with acute hypoxia and alterations in channel quantity with prolonged hypoxic challenge. Although a great amount of work has been devoted to the study of hypoxia and ion channels in reptiles, amphibians, and fish, in this review, we will describe the effects of both acute and sustained hypoxia (continuous and intermittent) on mammalian ion channels in several tissues, the mode of action, and their contribution to various cellular processes. It should be noted that in many instances, controversies exist regarding whether the effects of hypoxia on ion channel function are direct or indirect and the exact mechanisms involved in these responses. Throughout this review, we have attempted to point out possible reasons for conflicting results, including factors such as duration of hypoxic exposure, cell type, experimental conditions, and developmental aspects, all of which can influence the magnitude of responses and may underlie differences in the responses that have been reported.
Continuous Hypoxia
There are a number of physiological and pathological conditions during which tissues are exposed to continuous hypoxia. This can happen for brief periods of time, such as during ischemia, or hypoxia can be for long duration, such as with chronic disease or residence at high altitude. In either case, cells are programmed to rapidly adjust to lowered O2 levels to maintain O2 delivery to critical organs such as the brain and heart. This is achieved, in large part, due to alterations in the activity and, when the hypoxic stimulus is sufficiently prolonged, expression of O2-sensitive ion channels (Table 1), which control a variety of cellular functions including secretion, contraction, excitability, and ultimately, survival.
Table 1.
Summary of effects of sustained hypoxia on ion channels
| Subtype | Effect | Subtype | Ref |
|---|---|---|---|
| Potassium Ion | |||
| KV | |||
| Chemoreceptors | ↓ | 92, 131, 134, 147, 253 | |
| Neurons | ↓ | KV2.1 | 104, 146 |
| KV1.2 | |||
| Heart | ↓ | 98 | |
| Pulmonary Vasculature | ↓ | KV1.2 | 6, 9, 94, 167, 176, 179, 268 |
| KV1.5 | |||
| KV2.1 | |||
| KCa | |||
| Chemoreceptors | ↓ | 169, 185, 252 | |
| Neurons | ↑ | 56, 202, 259 | |
| Systemic Vasculature | ↑ | 10, 71, 72 | |
| ↓ | 272 | ||
| KATP | |||
| Neurons | ↑ | 223 | |
| Heart | ↑ | 183 | |
| Systemic Vasculature | ↑ | 44, 46, 64, 91, 108, 113, 192, 215 | |
| KIR | |||
| Heart | ↑ | Kir2.1 | 188 |
| TASK | |||
| Chemoreceptors | ↓ | TASK-1 | 28 |
| Pulmonary vasculature | ↓ | TASK-1 | 157, 159 |
| hERG | |||
| Chemoreceptors | ↓ | 163 | |
| Sodium Ion | |||
| Voltage gated | |||
| Neurons | ↑ | 16, 184 | |
| Heart | ↑ | 84, 112, 242 | |
| Calcium Ion | |||
| VGCC | |||
| Chemoreceptors | ↑ | L-type | 29, 213 |
| ↓ | 147 | ||
| Neurons | ↑ | L-type | 35, 137, 218, 261 |
| N-type | |||
| Heart | ↓ | 58, 98, 99, 149 | |
| Systemic Vasculature | ↓ | L-type | 62, 63, 204, 225, 250 |
| Pulmonary Vasculature | ↑ | L-type | 42, 61, 130, 144, 225, 237, 246 |
| Nonselective Cation Channels | |||
| SOCC | |||
| Chemoreceptors | ↑ | 29 | |
| Neurons | ↑ | 35, 206 | |
| Pulmonary vasculature | ↑ | TRPC1,4,6 | 154, 237, 238 |
| Na+ permeable | |||
| Neurons | ↑ | 194, 206 | |
See text for definitions and more details.
Chemoreceptors
Acute hypoxia.
Breathing rate and compensatory cardiorespiratory adjustments to hypoxia are controlled, to a large extent, by chemoreceptors, which signal the respiratory center to increase breathing frequency and/or lung volume during inhalation. Chemoreceptors are classified as central, located in the medulla oblongata, or peripheral, localized to the carotid bodies and, in some animals, aortic bodies. Although the peripheral chemoreceptors exhibit sensitivity to pH and CO2 (60, 106, 121), the predominant response to changes in CO2 appear to be mediated by central chemoreceptors, since ventilatory responses to CO2 were still present in animals with denervation of peripheral chemoreceptors (88). In contrast, peripheral chemoreceptor denervation eliminates the ventilatory response to hypoxia, indicating a primary role for these chemoreceptors in hypoxia-stimulated breathing responses. Along with pulmonary neuroepithelial bodies in the lung, the aortic and carotid bodies contain neurosecretory cells that sense global O2 tension and, when Po2 falls below 50–60 mmHg, release neurotransmitters; however, peripheral chemoreceptors localized to the carotid bodies are the major factor controlling the ventilatory response to hypoxia (96). For over 50 years after the initial descriptions of chemoreceptors in the carotid body, investigators sought to identify the mechanism by which chemoreceptors sensed the hypoxic signal and transduced this into a change in respiration. It is now known that hypoxic-excitation of chemoreceptors is correlated with the presence of O2-sensitive ion channels. Indeed, the first reports of O2-regulated channels were in the carotid body (131). In that initial study, electrophysiological studies performed in carotid body type I (glomus) cells demonstrated that decreasing Po2 caused a marked, reversible reduction in K+ channel activity, whereas Na+ channels were unaffected (131). These initial findings were later confirmed (92, 134, 169), and similar results were described in airway neuroepithelial bodies (264).
Inhibition of K+ channels by hypoxia could exert a significant influence on carotid body intracellular calcium concentration ([Ca2+]i), and by extension, sensory output. A consequence of hypoxia-induced inhibition of K+ channel activity in glomus cells is reduced K+ efflux and depolarization (29, 252). Since it was clearly demonstrated that hypoxia caused an increase in [Ca2+]i and enhanced sensory activity in carotid bodies (29, 134, 147) because of influx through voltage-gated Ca2+ channels (VGCC) (29, 200) and that application of a step depolarizations to glomus cells lead to Ca2+ influx through VGCCs and neurotransmitter release (224), many investigators proposed that K+ channel-mediated depolarization would play an important role in mediating the carotid body hypoxic response by shifting membrane potential (Em) to a level where VGCC would be activated. Consistent with this hypothesis, preventing membrane depolarization during hypoxia markedly reduced the rise in [Ca2+]i (29). Moreover, initial studies appeared to rule out a direct excitatory effect of hypoxia on Ca2+ channels (92, 134, 147, 169). However, the effects of hypoxia on in vitro carotid body preparations are depressed when CO2/bicarbonate-free (i.e., HEPES-buffered) extracellular solution is utilized (105, 201), which causes a considerable alkaline shift in intracellular pH (30). In contrast to the studies in which HEPES-buffered solutions were used and no excitatory effect of hypoxia was observed (92, 134, 147, 169), in carotid bodies superfused with bicarbonate-based extracellular solution, hypoxia caused an increase in Ca2+ current (ICa) that was blocked by inhibitors of protein kinase C (PKC) (213), indicating that hypoxia could increase [Ca2+]i by phosphorylation-dependent activation of VGCCs. These results suggest that direct enhancement of VGCCs activation coupled with a K+ channel-mediated shift in Em could work in concert to produce Ca2+ influx.
The identity of the specific K+ channel subtype(s) responsible for chemoreceptor O2 sensing and the mechanism by which this occurs remains in some debate and may vary with species. For example, initial reports in the rabbit carotid body indicated that the O2-sensitive channels were voltage-dependent K+ (KV) channels (131); however, the fact that inhibitors of KV channels did not cause depolarization or increase [Ca2+]i in rat glomus cells suggested that these channels were not active at the resting Em (28, 257) and thus, could not be responsible for initiating depolarization in response to hypoxia. Later reports demonstrated that other K+ channel subtypes, including Ca2+-dependent K+ (KCa), human ether-a-go-go-related gene (hERG), a subtype of inwardly rectifying K+ channels, and twin pore acid-stimulated K+ (TASK) channel-like background currents can be modulated by O2 (28, 162, 169, 170, 252), and since application of inhibitors of TASK-1 (257) and hERG (162) channels caused significant depolarization of the resting Em and elevation of [Ca2+]i, these channels appeared to be excellent candidates for mediating hypoxia-induced depolarization. Consistent with this possibility, inhibitors of hERG and TASK-1 mimic the effects of hypoxia on glomus cells from rabbit (162) and rat (257), respectively, and genetic deletion of TASK-1, but not TASK-3, markedly attenuates the ventilatory response to hypoxia in mice (222). One explanation tying these various reports together is that initiation of depolarization by hypoxia occurs due to inhibition of K+ channels open at the resting Em (i.e., hERG or TASK channels), and that the inhibitory effects of hypoxia on KV and KCa channels, which normally open with a shift to more positive Em and/or elevations in [Ca2+]i, serves to limit their activity and maintain depolarization.
Interestingly, these various K+ channels have not always been shown to exhibit O2-dependent regulation, depending on the experimental conditions and the cell type used for heterologous expression (166), signifying complex regulation by hypoxia. Indeed, the mechanism by which K+ channels sense changes in O2 is unclear. In some studies, channels were found to retain their hypoxia responsiveness in excised membrane patches or recombinant expression systems, suggesting the possibility of direct regulation of the channel by O2 or that the O2 sensor is closely associated with the pore-forming α-subunits or regulatory β-subunits (110, 160, 166, 167, 185). In this case, such effects could include alteration of cysteine thiols, methionine, or oxidoreductase domains in channel proteins, where changing between oxi- and deoxi-conformations could impact channel gating due to direct allosteric interactions (133, 166). However, findings that the O2 sensitivity of some channel subtypes depended on the cell type and/or experimental conditions indicates that O2 sensitivity is not absolutely intrinsic to the ion channels themselves but may be modulated by the interaction between the O2-sensing signaling molecules and pore-forming subunits.
One possible signaling mechanism linking hypoxia to ion channel function that has received much attention is alterations in reactive oxygen species (ROS), O2-derived small molecules, including the O2 radicals superoxide and hydroxyl, ozone (O3), singlet O2, and hydrogen peroxide (H2O2). ROS avidly interact with a variety of molecules and play an important role in reversible regulatory processes. ROS are produced from a cascade of reactions that starts with the production of superoxide, generated by mitochondrial respiration, xanthine oxidase, uncoupled nitric oxide (NO) synthase, or via reduced nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase (NOX). Superoxide is highly reactive and rapidly dismutates to H2O2, either spontaneously or catalyzed by superoxide dismutase. Superoxide can also react with NO to form peroxynitrite, or the iron-catalyzed Fenton reaction leading to the generation of hydroxyl radicals. Of the mechanisms known to produce ROS, evidence suggests that during hypoxia, in many cell types the mitochondria and NOX appear to be the predominate sources.
Supporting a role for the proposal that ROS mediate the effects of hypoxia on carotid body function were studies reporting that application of exogenous oxidants modify K+ currents (IK) (3) and several ion channels contain residues that are susceptible to redox modification (6, 43). Since NOX is a major contributor to ROS production in a variety of cells and O2 supply determines the amount of H2O2 formed by NOX, it was proposed that hypoxia decreases H2O2 formation, leading to decreased open probability of K+ channels, depolarization, and transmitter release (3). Consistent with this hypothesis, application of exogenous H2O2 depressed chemoreceptor activity and diphenyleneiodonium, a purported inhibitor of NOX, augmented the basal sensory activity and blocked further augmentation by hypoxia (43). Similarly, ROS generation in glomus cells was reduced by the putative NOX inhibitor 4-(2-aminoethyl)-benzenesulfonyl fluoride (51, 87). However, neither of these inhibitors is specific for NOX, as diphenyleneiodonium can inhibit other oxidases and 4-(2-aminoethyl)-benzenesulfonyl fluoride is a general serine protease inhibitor. Investigators have also used transgenic approaches in an attempt to elucidate whether alterations in NOX-derived ROS production might contribute to K+ channel regulation during hypoxia. In mice deficient for the gp91catalytic subunit of the NOX2 isoform, O2 sensitivity of airway chemoreceptor cells was impaired (67), but loss of gp91 had no effect on the hypoxia responsiveness of carotid bodies (187) and did not alter glomus cell morphology or the O2 sensitivity of IK (86). However, there is evidence suggesting that NOX4, not NOX2, is the primary isoform in chemoreceptors (75). Expression of TASK-1 channels in human embryonic kidney (HEK) cells revealed not only that these currents were inhibited by hypoxia but also that NOX4 colocalized with TASK-1, and that depletion of endogenous NOX4 abolished the O2 sensitivity of the current (122). Whether this also occurs in chemoreceptors, or whether other NOX isoforms might be involved, is unknown.
Whereas modulation of K+ channels by ROS is an attractive hypothesis, several lines of evidence suggest that this may not occur in glomus cells. For example, some investigators found that neither oxidants nor reducing agents impacted carotid body function (4, 76), and O2-sensitive KCa channels of rat glomus cells respond to Po2 changes independently of redox modification (185). Also at odds with the hypothesis that a reduction in ROS inhibits K+ channels is the finding that acute hypoxia caused an increase ROS production in glomus cells (51, 87). Interestingly, whereas the hypoxia-induced increase in ROS was absent in cells from mice deficient for p47phox (87), these cells exhibited augmented hypoxia-induced alterations in [Ca2+]i and K+ channel activity, results that apparently rule out a functional role for increased ROS in the depolarization and elevation in [Ca2+]i (87).
The role of mitochondria has also been investigated. In many cell types, hypoxia alters the production of ROS from mitochondria, with labs reporting both decreased and increased mitochondrial ROS generation during hypoxia (for review, see Ref. 34). While it was reported that hypoxia caused mitochondrial depolarization in glomus cells (25) and the hypoxia responsiveness of intact glomus cells was reduced by rotenone, an inhibitor of complex I (158), other inhibitors of the mitochondrial electron transfer chain had no effect, suggesting that the action of rotenone may have been independent of its effects on the mitochondria. Moreover, the hypoxia-induced reduction of IK was maintained in airway chemoreceptor cells devoid of mitochondria or after mitochondrial inhibition (191).
Thus, whereas there is no doubt that hypoxia exerts an inhibitory effect on chemoreceptor K+ channels, the differences in reported results suggest that the exact mechanisms underlying this response remain to be completely defined and that there may be a combination of factors that contribute to hypoxia-induced inhibition of K+ channels in chemoreceptors.
Chronic hypoxia.
With chronic hypoxia (CH), carotid bodies exhibit marked hypertrophy due at least in part to glomus cell hyperplasia. CH also reduces IK amplitude (85, 90, 253) but increases the density of Na+ and Ca2+ channels in carotid body glomus cells (89, 211). Recently, detailed molecular biological and electrophysiological studies have shown that T-type (transient) VGCCs are upregulated by CH in the rat pheochromocytoma cell line (PC12), O2-responsive cells that release neurotransmitters and possibly in other tissues (48). Interestingly, although the inhibitory effect of hypoxia on whole cell IK was intact after CH, a specific deficiency of KCa channel activity was noted, leading to loss of depolarization in response to acute hypoxia (253), suggesting that some, but not all, of the O2-sensing machinery is impaired by CH.
Central Nervous System
The brain is exquisitely sensitive to hypoxia; induction of hypoxia or anoxia in glial cells and most neurons leads to cell death. Ischemic stroke, where tissue hypoxia is frequently a factor, is often accompanied by neuronal hyperexcitability, which further aggravates brain damage. A number of reports have detailed the effects of ischemia, low glucose, and hypoxia/anoxia on the brain (for review, see Ref. 35). In this review, we will focus only on literature in which the effects of hypoxia and/or anoxia were determined. In nerve cells, most investigators describe an initial hyperpolarization followed by severe depolarization and influx of calcium. The initial, transient hyperpolarization observed in response to hypoxia in hippocampal and dorsal vagal neurons is due to the opening of ATP-sensitive K+ (KATP) channels (223). KATP channels are inactive at normal cellular ATP levels, but as ATP is depleted during hypoxia, increased activity of these channels leads to K+ efflux and hyperpolarization, perhaps in an effort to protect the cells and minimize hypoxia-induced damage by reducing neuronal input (68, 101, 259). A few studies suggest that KCa channels, perhaps activated by release of Ca2+ from internal stores, may also participate in the initial hyperpolarization (56, 202, 259). However, sustained hypoxia/anoxia leads to depolarization in hippocampal (184) and hypoglossal (82) neurons. The mechanisms underlying this depolarization are likely to be complex and appear to involve a combination of factors including inhibition of KV channels and Na+ influx via nonselective cation channels (NSCC) or voltage-gated Na+ channels. For example, KV channels are potent suppressors of neuronal excitability; in particular the KV channel family member KV2.1 plays a pivotal role in the homeostasis, excitability, and survival of neurons, including hippocampal and cortical pyramidal neurons (20, 52, 141, 151, 164). Brief in vivo exposure to anoxia induces rapid, reversible dephosphorylation of KV2.1 in brain samples from the cortex and hippocampus due to overactivation of NMDA receptors by excess glutamate (104, 146). A caveat of these experiments is that hypoxia was induced by inhalation of 100% CO2, which could cofound the results; however, in cultured hippocampal neurons, chemical hypoxia, induced by a mixture of sodium azide and 2-deoxy-d-glucose, but not elevated CO2, reproduced Kv2.1 dephosphorylation (146). In these experiments, the dephosphorylation of KV2.1 was mediated by the activation of calcineurin secondary to intracellular Ca2+ release (146).
In addition to KV channels, other studies have shown that a large component of the hypoxia-induced depolarization was due to influx of Na+ (150, 184). Hypoxia/anoxia increased intracellular Na+ concentration ([Na+]i) in a variety of nerve cells, including those from the cortex (17, 65), hippocampus (184), substantia nigra (80), and hypoglossal cells (109). This increase is due in part to reduced activity of the Na+-K+-ATPase, likely as a result of lowered ATP levels (184). Voltage-gated Na+ channels are also present in neurons, and blockade of these channels attenuated depolarization and cell injury in response to anoxia/hypoxia (16, 184), suggesting that hypoxia activates these channels. Na+ influx may also occur through NSCCs, since Na+ influx induced by anoxia can be attenuated by blockade of NSCCs (194).
In vitro experiments almost universally demonstrate an elevation in [Ca2+]i during hypoxia (49, 137, 218, 259, 261), although several factors, including duration of hypoxia, cell type, experimental conditions, and age of the animals influence the magnitude of the response. In neurons, influx of calcium occurs primarily through VGCCs and Ca2+-permeable NSCCs, which include store-operated and receptor-operated channels (35). There is substantial evidence that hypoxia-induced activation of VGCCs contributes to the increase in [Ca2+]i, with most data suggesting that high-voltage (L- or N-type) rather than low-voltage-activated (T-type) VGCCs are responsible for Ca2+ influx (137, 218, 261). With respect to high-voltage VGCCs, both L-type (long-lasting) and N-type (neuronal) VGCC contribute to the increase [Ca2+]i during hypoxia/anoxia, with L-type channels exhibiting more sensitivity to hypoxia (137). While depolarization could certainly contribute to increased VGCC activity, the activation of VGCC and resultant increase in [Ca2+]i observed in hippocampal neurons during application of anoxic perfusate to rat brain slices was found to occur secondary to production of NO (218). Moreover, when L-type currents were examined in cultured hippocampal neurons, NO could directly modulate both the open probability and voltage-sensitivity of the channels (218), which the authors attributed to S-nitrosylation.
It is widely held that the increase in [Ca2+]i results in cell death; however, in dissociated, CA1 neurons cell injury and death in response to anoxia was not prevented by removal of extracellular Ca2+ and rather appeared to be dependent on Na+ influx (66, 254). Consistent with these studies, inhibiting voltage-gated Na+ channels attenuated depolarization and cell injury in response to anoxia/hypoxia (16, 184). In either case, it is clear that hypoxia has dramatic effects on the central nervous system that are mediated, to a large extent, by the activity of ion channels. Although most of these changes in ion channel function appear to be related to depletion of ATP, it appears that at least some of these effects could be attributable to O2-sensitive channels.
Similar to the effects reported in neurons, sustained hypoxia/anoxia also leads to depolarization (15) and increased [Ca2+]i (206) in glia. Little is known about the direct effects of acute hypoxia on K+ channels in glial cells, although in cultured astrocytes, acute hypoxic challenge leads to a ROS-dependent increase in KCa channel activity (260), rather than the decrease in activity that would be consistent with depolarization. Whether depolarization results from direct, active inhibition of other K+ channels or is due to passive inhibition of K+ channels due to increased extracellular K+, is unclear. The increase in [Ca2+]i in response to hypoxia appears to involve both influx of Ca2+ and release of Ca2+ from intracellular stores, with Ca2+ release initiating Ca2+-activated Ca2+ entry through NSCCs (206). Since many NSCCs are also Na+ permeable, it is possible that activation of NSCC by hypoxia could also contribute to depolarization.
Circulatory System
Heart.
The mammalian heart is an aerobic organ with one of the highest O2 consumption rates of any organ (8–15 ml O2·min−1·100 g tissue−1 at rest), requiring a constant supply of O2 to maintain essential cellular processes and sustain cardiac function and viability. Hypoxia can alter the cardiac action potential and lead to arrhythmia; thus, the myocardium has developed several defenses that allow these cells to rapidly adjust to changes in O2. Ion flux is critical to normal cardiac function, and there is significant evidence that hypoxia alters the function of ion channels and membrane transporters in cardiomyocytes.
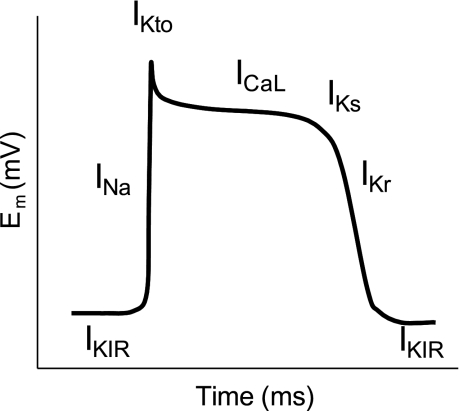
Resting Em in the heart is controlled primarily by K+ channels, in particular the current carried by inward rectifier K+ channels (IKIR). Action potentials are generated by depolarization reaching a threshold at which point voltage-gated Na+ channels open. Entry of Na+ through these channels generates a large inward current (INa) and rapid depolarization (Fig. 1), resulting in phase 0 of the cardiac action potential. The opening of Na+ channels is accompanied by the closing of IKIR, which shifts Em toward the equilibrium potential of Na+ ions. When the Em is sufficiently depolarized, L-type Ca2+ channels are activated, allowing Ca2+ entry. Repolarization occurs with activation of slow and fast transient outward K+ currents (IKto), and inactivation of Na+ channels. The late phase of the cardiac action potential results from activation of the rapid (IKr) and slow (IKs) components of the delayed rectifier channel as well as IKIR, resulting in inactivation of the L-type Ca2+ channels. Disturbances in ion channel conductance result in alterations in the shape and duration of the action potential that can lead to cardiac arrhythmias and sudden death.
Fig. 1.
Schematic representing the currents involved in the cardiac action potential. Baseline membrane potential (Em) is controlled primarily by current through inward rectifying K+ channels (IKIR). Opening of Na+ channels (INa) leads to rapid depolarization. Inactivation of Na+ channels along with the opening of K+ channels leads to a small downward deflection mediated by a transient outward current (Ito). The plateau of the action potential is maintained by the balance of an inward Ca2+ current through L-type channels (ICaL) and outward K+ current through slow delayed-rectifier KV channels (IKS). As Ca2+ channels close, the opening of rapid delayed-rectifier KV (IKr) and KIR channels contributes to rapid repolarization. As Em reaches the resting Em, IKs and IKr are inhibited.
Hypoxia has been shown to impact the activity of all of the channels involved in cardiac action potential generation. Acute hypoxia increases [Na+]i due to increased influx through late or persistent INa even though fast or transient INa are reduced in rat ventricular myocytes (84, 112, 242). With respect to maintaining Ca2+ homeostasis, L-type VGCCs provide the main entry pathway for Ca2+ into cardiomyocytes and are a major component of excitation-contraction coupling. Hypoxia/anoxia causes a rapid decrease in the basal current carried by L-type Ca2+ channels (ICa) (58, 98, 149). Interestingly, hypoxia reversibly reduces peak ICa without shifting the current-voltage relationship (99). In the rabbit heart, hypoxia-induced early AP shortening and speeding up of the late phase of repolarization are sensitive to low concentrations of Ba2+ (188), a specific blocker of IKIR, with Kir2.1 channels as the major isoform underlying IKIR in cardiac myocytes. The slow component of the delayed rectifier K+ channel (IKs) was also decreased during hypoxia, without affecting the rapid component (IKr) (98). All of these changes could contribute to generation of arrthymias or alterations in action potential duration.
Most evidence suggests that the effects of acute hypoxia on myocyte ion channels are due to a change in the phosphorylation state of the channel or in the redox status of the cell. Evidence for a direct effect on the channels includes reports that the reduction in VGCC activity occurs both in native cardiomyocytes and in HEK cells expressing a recombinant protein and requires a subset of residues in the cytoplasmic tail (58, 149), where phosphorylation of the channel by protein kinase A (PKA) augments the current and prevents inhibition by hypoxia (149). In addition to direct effects on the channels themselves, hypoxia may also alter the regulation of these channels via indirect mechanisms, such as changes in ROS. ROS levels change during hypoxia, although whether they increase or decrease in cardiomyocytes is still a matter of some debate and may vary due to a variety of factors, including age, localization within the cell, and source of ROS being measured. For example, hypoxia decreased ROS production from NADPH oxidase in adult cells (100, 241), resulting in repression of ICa (100). This is consistent with reports indicating that ROS can target L-type calcium channels on the sarcolemma and suppress ICa (81). In contrast, chick cardiomyocytes respond to hypoxia with an increase in ROS (54, 119), which could serve to increase [Ca2+]i by depressing the activity of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), SERCA2, a membrane calcium pump that has been shown to play a crucial role in cardiac Ca2+ handling and as a determinant of myocardial contractility (114). Increased generation of ROS can also alter the activity of cardiac Na+ channels, K+ channels, and ion exchangers, such as the Na+/Ca2+ exchanger (NCX) (74, 152).
With respect to prolonged or long-term exposure to hypoxia, changes in channel expression have been noted. KATP subunit expression in human myocardium is inversely correlated with venous Po2 and culturing ventricular myocytes under hypoxic conditions resulted in hypoxia-inducible factor 1 (HIF-1)-dependent upregulation of KATP channel expression (183).
Vasculature.
Most blood vessels respond to hypoxia, although the manner of response is dramatically different. With rare exceptions, systemic vessels dilate in an attempt to increase O2 delivery to tissues, while pulmonary vessels constrict to divert blood from poorly perfused areas to enhance ventilation-perfusion matching. Both mechanisms have been well-studied, with alterations in ion channel activity and/or expression playing a fundamental role in these responses.
SYSTEMIC VASCULATURE.
In the systemic circulation, vasodilation in response to hypoxia is largely due to a reduction in [Ca2+]i in the smooth muscle cells (SMCs), although the response may be modulated by changes in endothelial cell-derived relaxing and contracting factors, the production of which may also be Ca2+ dependent. Numerous studies have indicated that ion channels in the systemic vasculature, both Ca2+ permeable and Ca2+ impermeable, are regulated by hypoxia. Electrophysiological studies in vascular SMCs from systemic arteries demonstrated that hypoxia can exert a direct effect on VGCCs, with a reduction of Po2 rapidly and reversibly inhibiting ICa (63, 204, 225). The inhibition of L-type VGCCs has a direct functional effect as hypoxia-induced relaxation was absent in aortic rings that were pretreated with antagonists of L-type VGCCs (91). In isolated human coronary SMCs, low O2 tension decreased L-type calcium current and reduced [Ca2+]i (204). A role for decreased Ca2+ influx through VGCCs during hypoxia has also been demonstrated in smooth muscle from porcine coronary, rabbit cerebral, celiac, and femoral arteries and hamster cheek pouch arterioles (62, 63, 204, 250). The reductions in [Ca2+]i and relaxation observed in porcine coronary arteries, which were not dependent on changes in K+ channel activity or intracellular pH (168, 195), and the hypoxia-induced changes in [Ca2+]i observed in individual cells were not associated with depolarization (250), suggesting the possibility that hypoxia directly inhibited ICa. This was tested in several types of systemic smooth muscle, where hypoxia reversibly inhibited VGCC currents (62), even in voltage-clamped cells where changes in Em would not be a contributing factor (63), although in hamster cremaster arterial smooth muscle cells, hypoxia had no effect on L-type currents (40). While the reason underlying the difference in response in this study is not clear, the majority of data suggests that hypoxia can exert a direct influence on VGCCs.
In addition to direct modulation of Ca2+ channels, changes in Em, which is controlled primarily by K+ channels in vascular smooth muscle, can also influence the activity of Ca2+ entry pathways and [Ca2+]i. Consistently, hypoxia induced hyperpolarization of cerebral or coronary arterial muscle cells that results from the activation of KCa and/or KATP channels and accounts for the associated cerebral vasodilation (10, 44, 46, 71, 192). Hyperpolarization would serve to decrease Ca2+ influx by reducing the likelihood of VGCC activation. Toward this end, hypoxia has been shown to enhance the activity of KCa channels in cerebral arterial SMCs (71, 72). In an early study, the effect of hypoxia was observed in excised patches and was not associated with a change in [Ca2+]i in cerebral vascular SMCs from cat (71), suggesting that hypoxia had a direct effect on the channel. However, this was not confirmed in a later study in rat cerebral vascular SMCs (72), indicating that enhancement of KCa was not due to a direct effect of hypoxia on the channel but was mediated by a second messenger. It is unlikely that the KCa channels were activated by an increase in [Ca2+]i, since hypoxia has routinely been demonstrated to decrease Ca2+ levels in cerebral arterial (226) and other systemic smooth muscle. Since the effect of hypoxia on KCa channel activity was blocked by antioxidants, and hypoxia increased superoxide formation in these cells (72), it would appear that redox modulation of KCa channel activity plays a role. In contrast, a hypoxia-induced inhibition of KCa channel currents in cerebral arterial muscle has been reported (272). Studies have suggested that KCa channels located in proximity to sarcoplasmic reticulum (SR) Ca2+ stores are activated by Ca2+ sparks to promote hyperpolarization and relaxation of vascular SMCs (249). Where a reduction in KCa activity in response to hypoxia was reported, it was suggested to be related to reduced sensitivity and/or coupling of KCa channel to Ca2+ sparks, possibly in an attempt to limit hypoxic cerebrovascular dilation (272). Whether these effects of hypoxia on K+ channels truly has a physiological impact remains uncertain, as some studies have found that inhibitors of KCa channels blocked hypoxia induced relaxation (71), whereas other studies have demonstrated that inhibition of these channels had no effect (195).
The loss of ATP or elevation of adenosine by hypoxia has been implicated in eliciting arterial dilation through opening KATP channels (113). In coronary artery resistance arteries, hyperpolarization to hypoxia was blocked by KATP channel inhibitors (70). In some studies, hypoxia activated glibenclamide-sensitive K+ currents in amphotericin B-perforated cells of isolated porcine coronary SMCs (44) and hypoxia-induced relaxation was at least partially inhibited by KATP blockers in thoracic aorta (91) and cremaster (108), carotid (215), and cerebral (64) arteries. However, in other studies, hypoxia did not increase KATP current of voltage-clamped, amphotericin B-perforated cells (108, 182) and hypoxic relaxations were resistant to glibenclamide (204). Whether these variations in findings are due to species and/or vascular bed differences is unclear.
Whereas numerous studies have explored the effect of hypoxia on ion channel function in systemic smooth muscle, only a few studies have focused on endothelial cells. Hypoxia increases [Ca2+]i in aortic (11, 145) and human umbilical vein (5) endothelial cells, primarily through increased Ca2+ influx. Hypoxia has also been shown to increase IK, via a mechanism involving Ca2+/calmodulin-dependent protein kinase (CaMK), presumably secondary to the increase in [Ca2+]i (13). In this case, hyperpolarization would increase the driving force for Ca2+ entry, perhaps helping to maintain Ca2+ influx. An increase in [Ca2+]i could lead to increased production of vasodilators, such as NO and prostacylin, which could participate in hypoxic vasorelaxation.
PULMONARY VASCULATURE.
A large amount of attention has focused on understanding the role of ion channels in the pulmonary responses to both acute and chronic hypoxia. Whereas acute hypoxic pulmonary vasoconstriction (HPV) is believed to be a compensatory response aimed at matching ventilation with perfusion by shunting blood flow away from poorly oxygenated areas of the lung, prolonged global alveolar hypoxia can occur with residence at high altitude and with a variety of chronic lung diseases and results in an elevation in pulmonary arterial pressure that can eventually lead to right heart failure and death.
Acute hypoxia.
Since the first detailed reports of HPV emerged over half a century ago (231), much attention has focused on the mechanism. The use of isolated perfused lung preparations, where conditions are such that changes in pressure reflect alterations in pulmonary vascular resistance, demonstrated that decreasing O2 tension causes an immediate elevation of pulmonary arterial pressure (for review, please see Ref. 244). In more reduced preparations, such as isolated vessels or pulmonary arterial smooth muscle cells (PASMCs), an abundance of research has revealed that while endothelial cells can produce vasodilator and vasoconstrictor substances that modulate HPV, the sensor and effector mechanisms reside in the PASMCs (for review, please see Refs. 1 and 2). There is also considerable heterogeneity in the magnitude of the hypoxic contractile response between species and in pulmonary vascular smooth muscle from different locations within the lung, with smooth muscle from small diameter, distal pulmonary arteries exhibiting greatest contraction. Thus differences in the species and portion of the vascular tree from which the arteries and/or cells were derived could contribute significantly to variations in reported results.
Although hypoxia-induced increases in Ca2+ sensitivity of the contractile apparatus have been described, most investigators agree that an increase in [Ca2+]i in PASMCs is a key component underlying the contractile response to hypoxia (1, 132, 245, 248), although how hypoxia triggers this response is a matter of some debate. Numerous studies report that HPV and the increase in [Ca2+]i is due to Ca2+ influx, as both are inhibited by the absence of extracellular Ca2+ or blockade of Ca2+ channels (42, 130, 144, 154, 226, 237, 246). In PASMCs from small diameter arteries, VGCCs were found to be activated by lowering of Po2 (61, 225); however, it is unlikely that the majority of the Ca2+ influx is due to a direct effect of hypoxia on VGCCs. Rather evidence indicates that influx via VGCCs is secondary to depolarization. In PASMCs from adult animals, Em is primarily regulated by KV channels, and several labs have demonstrated that hypoxia inhibits IKV (6, 9, 167, 179, 268), with some investigators demonstrating that this was due to Ca2+ release from the SR (179, 180), whereas others suggested that this was due to redox modulation of the channel (6, 247), interactions with regulatory proteins, such as KVβ subunits (174) or phosphorylation by PKC-ζ (39).
Numerous attempts have been made to identify the exact KV family member that is O2 sensitive. Some studies suggested a prominent role for the KV1.5 subunit (8, 73); however, this was countered by data from non-PASMC expression systems indicating that expression of either KV1.5 or KV1.2 resulted in currents that were insensitive to hypoxia (160, 176) or only inhibited at very high Em (103). Interestingly, a hypoxia-sensitive IK did develop when KV1.2 and KV1.5 were coexpressed (103, 160) or when KV1.5 was expressed in PASMCs (176). These findings suggest that O2 sensitivity may be determined by the host cell (41, 133). The effects of hypoxia on KV currents were also inhibited by antibodies to KV2.1 (9, 94), which produces a current that was inhibited by hypoxia but only in a few cells in non-PASMC expression systems. In PASMCs, KV2.1 and KV9.3 coimmunoprecipitate (167) and while KV9.3 does not form functional channel as a homotertramer, coexpression of KV2.1 and KV9.3 produced a current that was inhibited by hypoxia (103, 167). Taken together, the most logical explanation for these findings is that the O2-sensitive KV channel in PASMCs is composed of heterotetramers of two or more KV channel subunits.
In at least one study, the effect of hypoxia on ICa was observed in the presence of KV channel inhibitors, suggesting that activation occurs by mechanisms not involving KV channels (61); however, the exact KV channel inhibitor used in this study was not described and leaves open the possibility that only a subset of KV channels were inhibited. Nonetheless, other K+ channels have also been implicated in HPV and could contribute to depolarization. Since KCa channels are not active at the resting Em in PASMCs from adult animals (7, 159, 199, 267), it is unlikely that hypoxia-induced inhibition of KCa channels contributes to depolarization. In contrast, a noninactivating, background K+ current has been characterized in PASMCs that is active at the resting Em and is inhibited by hypoxia (57, 157, 159). It is believed that these channels include TASK-1 subunits, since overexpression of these proteins results in a current with similar features (69, 157). Moreover, depletion of TASK-1 by small interfering RNA reduced this current and eliminated its sensitivity to hypoxia (157). The mechanism by which hypoxia inhibits TASK channels remains unclear.
Interestingly, measurements of [Ca2+]i in PASMCs isolated from distal pulmonary arteries revealed that the hypoxia-induced increase in [Ca2+]i was only partially inhibited by VGCC inhibitors but was completely abolished by antagonists of NSCCs (237). Some NSCCs are highly Ca2+ permeable; both store-operated and receptor-operated Ca2+ channels, which are likely composed of canonical transient receptor potential (TRPC) proteins, fall into this category and are expressed in PASMCs (135, 136, 236). Acute hypoxia increased Ca2+ influx through store-operated Ca2+ channels, which may be due to Ca2+ release from the SR (107) or phosphorylation of the channels by Rho kinase or myosin light chain kinase (238). In addition to being Ca2+ permeable, these channels can also conduct Na+ influx, allowing for the possibility that opening of these channels also contributes to depolarization and activation of VGCC. Since inhibitors of either VGCC or NSCC equally reduce HPV in isolated lungs (246), it is clear that both of these Ca2+ influx pathways contribute to the contractile response.
Chronic hypoxia.
Prolonged exposure to hypoxia causes depolarization of the resting Em in PASMCs (59, 97, 196, 198, 205) and reduces the activity and expression of KV channels (59, 97, 177, 196, 198, 235, 240) through a mechanism involving both activation of the transcription factor, HIF-1, and the endothelium-derived factor endothelin-1 (196, 251). The discovery that Em was depolarized in PASMCs following exposure to CH prompted the assertion that L-type channels would be activated and [Ca2+]i would be increased in these cells, leading to the enhanced PASMC contraction and proliferation characteristic of pulmonary hypertension. Indeed, CH elevates [Ca2+]i (127, 177, 197, 207, 239, 251), but while this can be reduced by L-type channel antagonists early on (251), VGCC blockers have no effect on [Ca2+]i or tone in pulmonary arterial smooth muscle from chronically hypoxic animals (127, 197, 239), indicating that VGCCs no longer play an important role. Instead, inhibitors of NSCCs reduced both [Ca2+]i and tone in pulmonary vascular smooth muscle from chronically hypoxic rats (127, 239). NSCCs are not activated by depolarization, but their activity may be influenced by phosphorylation (93, 156, 238). Increased abundance of TRPC protein was observed in pulmonary arterial smooth muscle from chronically hypoxic animals (127, 239), and in PASMCs exposed to prolonged hypoxia in vitro, through a HIF-1-dependent mechanism (239), leading to a sustained elevation in [Ca2+]i. Thus, while CH certainly serves to inhibit the activity and expression of KV channels, the role of depolarization is unclear. Instead, reducing K+ efflux may serve to increase intracellular [K+], which has been associated with reduced apoptosis.
Intermittent hypoxia.
Transient declines in tissue O2 supply, or intermittent hypoxia (IH), are frequently occurring events in human life with widespread implications on cell metabolism and the pathogenesis of various diseases. IH most often occurs during sleep, where repetitive occlusions of the upper airways is a hallmark of sleep apnea syndrome with estimated prevalence in the range of 5–15% of the general adult population (181, 263) and as much as 30–40% in obese subjects (227). In the case of sleep-disordered breathing, brief periods of airways collapse leads to rapid, repetitive O2 desaturations, with the most severe patients experiencing ≥30 hypoxic episodes per hour. A majority of premature infants also suffer from repeated apneas during sleep due to immaturity of central and peripheral breathing regulation (178, 212). Several laboratories have developed animal models designed to mimic the IH experienced during sleep apnea. These models typically expose animals to short durations (several seconds) of moderate hypoxia at a rate of 5–60 cycles/h, 8–12 h/day for several days to weeks.
Whereas IH due to repetitive cessation of breathing is perhaps the most well-described pathological condition, periods of IH can also occur in organs affected by atherosclerotic narrowing of arteries and might contribute to hypoxic preconditioning, which has been clearly conceptualized in heart (18, 19, 31) and suggested to occur in the brain (27, 193). In contrast to the short, rapid decreases on oxygenation that occur over long periods of time with sleep apnea, the IH of hypoxic preconditioning typically involves slower cycles of IH (on the order of several minutes to hours). Although both sustained hypoxia and IH are defined by limitation of O2 supplies, IH exposure is differentiated by limited duration and repeated occurrence in time, and thus, might trigger different cellular adaptive responses, which may also be tissue or cell-type specific (Table 2).
Table 2.
Summary of effects of intermittent hypoxia on ion channels
| Subtype | Effect of Hypoxia | Subtype | Ref |
|---|---|---|---|
| Potassium Ion | |||
| KCa | |||
| Neurons | ↓ | 219, 220 | |
| KATP | |||
| Neurons | ↓ | Kir6.1, 6.2 | 269 |
| ↑ | 14, 223, 258 | ||
| Heart | ↑ | 12, 274 | |
| Sodium Ion | |||
| Voltage dependent | |||
| Heart | ↑ | 153, 255, 274, 275 | |
| Neurons | ↑ Short | 78, 79 | |
| ↓ Long | |||
| PC12 | ↑ | 117, 233 | |
| Voltage-Gated Ca2+ Channels | |||
| VCGG | |||
| Heart | ↑ | L-type | 270 |
| L-type | |||
| PC12 | ↑ | N-type | 117, 233 |
| T-type | |||
| Systemic Vasculature | ↑ | L-type | 208 |
| Nonselective Cation Channels | |||
| SOCC | |||
| Systemic Vasculature | ↑ | 232 | |
See text for definitions and more details.
Chemoreceptors
Reflexes arising from the carotid bodies contribute to control of blood pressure, sympathetic activity, and control of breathing. In animal models, exposure to IH using a regimen reminiscent of sleep apnea augments the hypoxia-induced increase in sensory discharge from carotid bodies due to a mechanism requiring mitochondrial and NADPH-derived ROS and increased HIF-1 activity (171–173). Increased carotid body sensory activity was observed with as little as 16 h of IH exposure in neonates (173), and when the duration of IH was prolonged (10 days), the augmented hypoxia sensitivity of the carotid body was associated with elevated blood pressure in adult animals (172), suggesting that alterations in carotid body function during long-term IH could contribute to the cardiovascular comorbidities associated with sleep apnea. Although the sensory activity of the carotid body is modulated by ion channel activity, whether IH exposure leads to alterations in channel function or expression in the carotid body is currently unknown; however, given the effects of ROS on various channels and the known presence of O2-sensitive channels in carotid body, this is certainly a possibility.
Adrenal chromaffin cells, derived from adrenal medulla, and PC12 cells are commonly used O2-responsive models for investigation of neurotransmitter release. Exposure of PC12 cells to short-term exposure to IH (15–60 cycles) augments the release of neurotransmitters and neurotrophic factors in response to a subsequent acute hypoxic challenge by activation of voltage-gated Na+ channels, presumably leading to depolarization and elevation in [Ca2+]i through both Ca2+ influx from extracellular sources via N-type and L-type VGCCs as well as Ca2+ release from intracellular stores regulated by inositol trisphosphate (IP3) and ryanodine (Ry) receptors (IP3R and RyR, respectively)(117, 233). This response was not observed (117) or blunted (233) when cells were exposed to preconditioning with sustained hypoxia. Similar results were observed in neonatal animals exposed to 5 days of IH, where hypoxic challenge caused increase catecholamine release from freshly isolated medular chromaffin cells related to upregulation of T-type VGCC mRNA, increased ICa, and increased RyR mRNA expression (210). Surprisingly, the IH-induced potentiation of hypoxia-induced catecholamine release persisted into adulthood (209). The effect of longer duration IH exposure on hypoxia-induced neurotransmitter/catecholamine release in these cells, and whether a similar response occurs in the carotid body, is unclear.
Short-term IH exposure (up to 120 cycles) in PC12 cells resulted in the activation of HIF-1 due to accumulation of HIF-1α, the O2-dependent subunit of the HIF-1 transcriptional complex (266). The induction of HIF-1 was found to occur by a process initiated in response to NOX-dependent generation of ROS, which activated phospholipase C, producing the second messenger molecules IP3 and diacylglycerol (266). Generation of diacylglycerol results in the activation of PKC, which in turn leads to the phosphorylation and activation of the cellular target, mTOR (mammalian target of rapamycin), stimulating synthesis of HIF-1α (266). Additionally, IP3 acts on IP3 receptors to induce the release of intracellular Ca2+. The elevation in [Ca2+]i not only contributes to activation of PKC (266), but also activation of CaMK, leading to phosphorylation of a regulatory protein, p300, which promotes HIF-1α transcription (265). Finally, induction of ROS by IH diminished hydroxylation of HIF-1α, resulting in stabilization of HIF-1α protein (266). These coordinated effects of IH lead to increased production and activation, in parallel with decreased degradation, of HIF-1α. Whereas studies in other cells types clearly demonstrate that the expression of several ion channels is HIF-1 dependent, how IH-induced activation of HIF-1 might modulate channel activity and/or expression in this system has not yet been examined.
Central Nervous System
In the brain, it has been suggested that short periods of O2 deprivation lead to adaptive mechanisms that protect against subsequent longer ischemic events (27). In contrast, long-term IH can have detrimental effects on neuronal functioning (83, 123, 125). Despite the recognized impact of IH, there is a dearth of information regarding IH-mediated effects on ion channels in the brain and central nervous system, and factors specific for the brain, including different regional susceptibility to hypoxia (83, 140, 148) and development/maturation of the brain tissue of neonates, together with variability in duration and type of hypoxic stimulus needs to be taken into account when interpreting the limited data available.
Neurons located in the hippocampus are a frequently investigated neuronal cell type owing to their higher susceptibility to hypoxia-induced morphological and functional changes and relation to neurocognitive dysfunction during IH (83, 123, 125). For example, in newborn mice, 4 wk of exposure to IH decreased the excitability of hippocampal neurons, as determined by reduced action potential firing, lower voltage-dependent INa, and prolonged deactivation time constant of Na+ channels (78), although opposite results were observed following shorter (2 wk) exposure (79). In contrast to these functional changes in INa, IH decreased voltage-gated Na+ channel protein expression following a 2-wk exposure, whereas an increase in protein expression was observed after 4 wk of exposure (273). These observations suggest that the changes in INa induced by IH are likely due to mechanisms other than simply increasing channel numbers and point to an important interplay between neuronal maturational processes and IH.
KCa channels are widely expressed in brain tissue and, as noted earlier, are responsible for regulation of cellular excitability and neurotransmitter release. It has been shown recently that pharmacological inactivation of KCa channels leads to learning impairment (143), and deficits in learned behavior have also been described in rodents after 14 days of IH (243). It is perhaps not surprising then, that exposure of adult rat hippocampal neurons to IH for several days decreased the activity of KCa channels (219, 220). In this model, reduced expression of neuronal NO synthase, lower NO production, and limited hypoxia-induced elevations in [Ca2+]i were also described (219), all of which can contribute to decreased KCa channel activity. Additionally, increased ROS production following prolonged IH exposure (186, 219, 228) represents another possible pathway by which KCa channel function might be reduced (190, 220). These results suggest that early long-term IH exposure could have a detrimental impact on cognitive function.
Examination of neurons located in the nucleus of the solitary tract (NTS), which are involved in chemoreceptor-mediated regulation of breathing, revealed downregulation of KATP channel protein expression and limited hypoxia-induced hyperpolarization following 1 wk of IH exposure (269). These findings were interpreted as a counter-regulatory response that, even though possibly not beneficial for the cell, renders these neurons sensitive to chemoreceptor signaling under hypoxic conditions and enables the appropriate respiratory responses. This hypothesis is supported by the observation that NTS neurons derived from IH-exposed rats and obtaining afferentation from the carotid body showed higher excitatory response following glutamate receptor activation compared with normoxic animals (47). In contrast, when the severity of the hypoxia challenge was greater and the duration of IH exposure slightly longer, other investigators observed diminished postsynaptic currents in NTS neurons following IH, which was mediated by reduced presynaptic neurotransmitter release through inhibition of N-type VGCCs by dopamine (118).
Despite overall limited tolerance of neurons to hypoxia, certain regions of brain (dorsal vagal nucleus) show higher tolerance to hypoxia (14, 15, 83). In contrast to other neuronal populations, where ischemia rapidly leads to terminal depolarization (preceded only by short transient hyperpolarization), these hypoxia-tolerant cells are distinguished by prolonged activation (several minutes) of KATP channels. Such activation leads to hyperpolarization of the plasma membrane, decreased excitability, and lowered metabolic demands (14, 223, 258). Direct evidence for IH-induced activation of KATP channels in brain is lacking; however, events leading to neuronal KATP activation, including ROS production, NO-induced protein kinase G (PKG) signaling, mitochondrial KATP activation and activation of CaMK (32, 33, 115, 243) were observed to occur in brain following IH exposure (115, 243).
Wherease the effects of IH on brain KV channels has not been described, high-throughput analysis of the transcriptomic response to a single hypoxia/reoxygenation event identified KV channels as hypoxia responsive genes in non-astrocyte, non-neuron brain tissue, suggesting the possibility that these channels may be regulated by IH in at least some regions of the brain (23).
Circulatory System
Heart.
Research on the effects of IH in the heart follows two major lines: 1) investigation of adverse cardiac outcomes (coronary artery disease, heart failure, hypertension) associated with IH due to sleep-disordered breathing and 2) elucidating the mechanisms contributing to the protective effects of short, repetitive bouts of hypoxia, a phenomenon known as “hypoxic preconditioning.” Literature describing IH-induced changes in ion channel operation entirely represents the second category.
It is well established that ischemia-reperfusion (I/R)-induced damage to the myocardium can be diminished by preexposition of the heart to ischemic periods, the phenomenon known as “ischemic preconditioning” (45, 116). Although ischemic preconditioning involves reductions in the availability of both O2 and glucose, several reports suggest that the hypoxia is more important for protection (18, 19, 31). In contrast to the experimental paradigms of IH used to model sleep apnea, IH models of hypoxic preconditioning typically involve longer cycles of hypoxia (on the order of several minutes to several hours). In some cases, the duration of exposure to IH is very short (only a few hours), although most studies continued daily hypoxic exposures for 1–6 wk. The preventive effect of IH preconditioning on myocardial damage seems to be dose and time dependent, as short exposure (30 min) is not as efficient as longer exposure (1–4 h) and IH cycles where the O2 tension in the inspired air drops as far as 5% (compared with less severe regimes with a minimum FiO2 of 10%) might actually increase ischemic damage (19, 111). Several mechanisms have been suggested to participate in hypoxic preconditioning, including changes in the metabolic pathways, substrate utilization, mitochondrial metabolism, excitation-contraction coupling regulation, production of ROS, decreased apoptosis of cardiomyocytes, increased myocardial capillary density, and activation of protein kinase C (see Refs. 18, 24, and 165 for review). The subsequent paragraphs summarize the impact of IH exposure on ion channel function in the plasma and mitochondrial membranes and SR.
As described earlier in this review, upon depolarization, Ca2+ entry into cardiomyocytes occurs primarily via L-type VGCCs. The increase in [Ca2+]i is further facilitated by subsequent Ca2+-induced calcium release from the SR, a process tightly regulated by RyR localized on the SR membrane (175). [Ca2+]i is returned to basal values mainly by reduced influx due to inactivation of VGCCs, ATP-dependent uptake of Ca2+ back into SR by SERCA, and by efflux of Ca2+ across the plasma membrane via the NCX (128). Alterations in these Ca2+-handling pathways, leading to elevated baseline [Ca2+]i and intracellular Ca2+ overload, represents one of the main factors involved in mediating I/R myocardial damage and contributes to both decreased contractility and electric instability of cardiomyocytes (203). Several studies have demonstrated that the detrimental increases in [Ca2+]i, myocardial damage, and impaired cardiac contractility induced by I/R in rat cardiomyocytes were prevented by both short- (18) and long-term (50, 234, 255, 262) IH preexposure, with the most common experimental regimen consisting of 4–6 h of moderate hypoxia daily for 2–6 wk.
Given the well-documented role of Ca2+ overload in I/R injury, it might be expected that ischemia would enhance Ca2+ influx via ICa. Instead, ischemia causes a reduction in the transmembrane ICa carried by VGCCs, which, together with prolonged inactivation of the channel, contributes to electrical instability and impairs excitation-contraction coupling (124, 138). The effects of short-term IH on VGCC activity during I/R are unclear; however, long-term IH adaptation, which had no effect on basal ICa, lessened L-type VGCC inactivation and facilitated ICa during ischemic challenge (270), changes that might serve to increase electrical stability, improved myocardial contraction, and diminish cardiac electrical remodeling following an ischemic event (53).
Although Ca2+ influx via VGCC might be diminished during I/R, another mechanism that contributes to Ca2+ overload is impaired Ca2+ uptake into the SR mediated by decreased quantity and activity of SERCA (120, 216, 217). After ischemic injury, SERCA protein expression drops to ∼60% of its preischemic values. The decrease in SERCA expression can be ameliorated by 2 wk of adaptation to daily hypoxic exposure (6 h/day) (36). The activity of SERCA is inhibited by the SR membrane protein phospholamban. When phospholamban is phosphorylated on Thy17 by CaMK and on Ser16 by PKA and PKG, its inhibitory effects on SERCA are reduced (230). Ischemia and reperfusion decrease phospholamban phosphorylation, which contributes to diminished SERCA activity and increased [Ca2+]i (256). Preischemic IH adaptation for several weeks increased phospholamban phosphorylation at both sites and ameliorated SERCA dysfunction, resulting in beneficial effects on cardiac contractility and ICa (36, 256). The loss of IH-induced protection after blockade of PKA or CaMK activity (256) suggests that these kinases are critical for the IH-induced phosphorylation of phospholamban in this model. Consistent with this possibility, a rapid increase in PKA activity following hypoxia/reoxygenation was also documented in other cell types (26, 271).
Similar to SERCA, RyR protein expression (particularly RyR2) was decreased during I/R (36); however, other studies found no change in RyR protein following I/R (262). Nevertheless, IH adaptation reduced this response (36) and enhanced RyR activity (36, 234, 262). The mechanisms responsible for these effects have not been fully elucidated, but ROS-induced oxidation and S-glutathionylation of RyR (38, 55, 102, 210, 214) together with phosphorylation by PKA (262) have been suggested. Although an increase in RyR activity following IH exposure might theoretically be hypothesized to further aggravate ischemia-induced Ca2+ overload by increasing Ca2+ release into the cytosol and thus, be at odds with cardioprotection, preserved RyR activity is connected with higher amplitude ICa, shorter time to peak [Ca2+]i, effective excitation-contraction coupling, and preserved contractility of cardiomyocytes (36, 262).
[Ca2+]i results from the balance between Ca2+ influx, uptake, release, and efflux mechanisms. The main mechanism by which Ca2+ efflux occurs in cardiomyocytes is via NCX. I/R leads to decreased forward activity of NCX, or reversal of its function, with subsequent accumulation of [Ca2+]i, prolongation of action potentials, and risk of arrhythmias (139, 229). Based on measurements of Ca2+ transients in cardiomyocytes, long-term IH preconditioning had no effect on baseline (preischemic) NCX activity or protein quantity; however, IH diminished ischemia-induced prolongation of Ca2+ transients either induced by electric stimulation or by Ca2+ release from the SR (262). Furthermore, it has been shown recently that NCX mRNA is increased following IH exposure in mice hearts (37). NCX activity was not influenced by IH when cells were exposed to PKC inhibitors, whereas PKA and CaMK inhibitors had no effect (262). Besides these preconditioning-related effects, NCX plays crucial role in IH-induced development of left ventricular dilation and systolic dysfunction (37).
In addition to modulating Ca2+-handling pathways, the cardioprotective effects of IH have been independently attributed to mechanisms leading to opening of plasma and/or mitochondrial membrane KATP channels (153, 255, 274, 275). Adult and neonatal rats exhibited diminished frequency of ischemia-induced, KATP channel-dependent arrhythmias, smaller infarct size and better postischemic contractility recovery following IH exposure (12, 153, 161). Furthermore, effects of IH adaptation were abolished by administration of KATP channel blockers (glibenclamide, 5-hydroxydecanoate) while KATP channel openers diazoxide (mitochondrial-selective) and pinacidil (general) mimicked the beneficial effects of IH in control animals (12, 274). Putative mechanisms of cardioprotection mediated by KATP channel opening involve: 1) dissipation of the inner mitochondrial Em with subsequent inhibition of mitochondrial Ca2+ uptake and increased Ca2+ release, limiting mitochondrial Ca2+ overload (95); 2) generation of ROS (129, 142, 163); and 3) regulation of mitochondrial volume and respiratory chain activity (126).
The hypoxic preconditioning mediated by short-term IH exposure has also been demonstrated to be dependent on activation of HIF-1, as the effects of preconditioning were lost in mice with heterozygous deficiency for HIF-1α (31) or when HIF-1 activation was prevented (22). PKA and CaMK, which contribute to cardioprotection by IH preconditioning (256), have also been implicated in HIF-1 activation (221). Whether HIF-1 exerts its effects via modulation of ion channel expression and whether HIF-1 is involved in the protection adaption afforded by long-term IH remain to be determined.
Vasculature.
It has been well established that chronic IH exposure leads to the development of systemic and pulmonary hypertension. The mechanisms underlying these responses are unclear, and whether IH alters the activity or function of vascular ion channels has not been rigorously studied. The involvement of L-type VGCCs in IH-induced outcomes in the systemic circulation is indirectly supported by data demonstrating aggravated hypertension in spontaneously hypertensive rats following postnatal exposure to IH and prevention of this result by the L-type VGCC blocker, nifedipine (208). Moreover, indirect and uncomprehensive evidence suggests that exposure of a brain endothelial cell line to IH leads to activation of NSCCs with subsequent involvement of store-operated Ca2+ release mediated by IP3R and RyR (232). Whether this occurs in endothelial cells from other vascular beds or contributes to the development of hypertension is unknown. IH results in the activation of NF-κB, an oxidant-sensitive transcription factor, in the cardiovascular system and specifically, endothelial cells (77, 189), although whether this is responsible for changes in Ca2+ handling pathways is also unknown.
Prolonged exposure to IH results in the development of pulmonary hypertension that is typically less severe than the pulmonary hypertension that develops in response to sustained hypoxia. IH increased pulmonary expression of NOX, and IH-induced pulmonary hypertension was blunted in animals deficient for the NOX subunit gp91phox (155). Whether pulmonary hypertension in this model results from alterations in pulmonary vascular ion channel activity and/or expression has not been studied; however, as discussed above, HIF-1 regulates the expression of several ion channels known to participate in the development of pulmonary hypertension in response to sustained hypoxia, and significant cross-talk between HIF-1α, ROS and NF-κB activation has been described in pulmonary arterial smooth muscle during IH (21).
Conclusion
It is clear that hypoxia is a critical modulator of cell function and that many of the effects of O2 deprivation can be attributed to changes in ion channel activity and/or expression. Most of the reviewed literature is based on animal models and cell culture experiments, which together with differences in exposure methods (atmospheric pressure vs. hypobaric, duration and frequency, severity), contributes to heterogeneity of observed results as well as limits interpolation to other tissues or even species. Nonetheless, because of their wide ranging effects, ion channels are a frequent target in the search for new drugs. Better understanding of the exact ion channels impacted by hypoxia in each tissue and/or cell type, coupled with the development of more specific, tissue-targeted, less toxic channel inhibitors could have important therapeutic implications for a variety of diseases where sustained or intermittent hypoxia is a complicating factor.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-67191, HL-73859, and HL-99952. J. Polak was supported by a Fulbright-Masaryk Grant under the Fulbright International Educational Exchange Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Because of space restrictions, it was not possible to quote all of the excellent studies that have been published with respect to the research described in this review; our apologies to those whose studies were not cited.
REFERENCES
- 1. Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JP. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol 570: 53–58, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aaronson PI, Robertson TP, Ward JP. Endothelium-derived mediators and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 132: 107–120, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Acker H, Bolling B, Delpiano MA, Dufau E, Gorlach A, Holtermann G. The meaning of H2O2 generation in carotid body cells for PO2 chemoreception. J Auton Nerv Syst 41: 41–51, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Agapito MT, Sanz-Alfayate G, Gomez-Nino A, Gonzalez C, Obeso A. General redox environment and carotid body chemoreceptor function. Am J Physiol Cell Physiol 296: C620–C631, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Aono Y, Ariyoshi H, Sakon M, Ueda A, Tsuji Y, Kawasaki T, Monden M. Human umbilical vein endothelial cells (HUVECs) show Ca2+ mobilization as well as Ca2+ influx upon hypoxia. J Cell Biochem 78: 458–464, 2000 [PubMed] [Google Scholar]
- 6. Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res 73: 1100–1112, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Archer SL, Huang JM, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res 78: 431–442, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Archer SL, London B, Hampl V, Wu X, Nsair A, Puttagunta L, Hashimoto K, Waite RE, Michelakis ED. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J 15: 1801–1803, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 101: 2319–2330, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armstead WM. Role of activation of calcium-sensitive K+ channels in NO- and hypoxia-induced pial artery vasodilation. Am J Physiol Heart Circ Physiol 272: H1785–H1790, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Arnould T, Michiels C, Alexandre I, Remacle J. Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J Cell Physiol 152: 215–221, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Asemu G, Papousek F, Ostadal B, Kolar F. Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial KATP channel. J Mol Cell Cardiol 31: 1821–1831, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Balla Z, Hoch B, Karczewski P, Blasig IE. Calcium/calmodulin-dependent protein kinase IIdelta 2 and gamma isoforms regulate potassium currents of rat brain capillary endothelial cells under hypoxic conditions. J Biol Chem 277: 21306–21314, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Ballanyi K. Protective role of neuronal KATP channels in brain hypoxia. J Exp Biol 207: 3201–3212, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Ballanyi K, Doutheil J, Brockhaus J. Membrane potentials and microenvironment of rat dorsal vagal cells in vitro during energy depletion. J Physiol 495: 769–784, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience 126: 31–44, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol 62: 215–249, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Beguin PC, Belaidi E, Godin-Ribuot D, Levy P, Ribuot C. Intermittent hypoxia-induced delayed cardioprotection is mediated by PKC and triggered by p38 MAP kinase and Erk1/2. J Mol Cell Cardiol 42: 343–351, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Beguin PC, Joyeux-Faure M, Godin-Ribuot D, Levy P, Ribuot C. Acute intermittent hypoxia improves rat myocardium tolerance to ischemia. J Appl Physiol 99: 1064–1069, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Bekkers JM. Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol 525: 611–620, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belaiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, Kietzmann T, Gorlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell 18: 4691–4697, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belaidi E, Beguin PC, Levy P, Ribuot C, Godin-Ribuot D. Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am J Physiol Heart Circ Physiol 294: H901–H908, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem 277: 39728–39738, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Bernhardt WM, Warnecke C, Willam C, Tanaka T, Wiesener MS, Eckardt KU. Organ protection by hypoxia and hypoxia-inducible factors. Methods Enzymol 435: 221–245, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Biscoe TJ, Duchen MR, Eisner DA, O'Neill SC, Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol 416: 421–434, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bolon ML, Ouellette Y, Li F, Tyml K. Abrupt reoxygenation following hypoxia reduces electrical coupling between endothelial cells of wild-type but not connexin40 null mice in oxidant- and PKA-dependent manner. FASEB J 19: 1725–1727, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Brzecka A. Brain preconditioning and obstructive sleep apnea syndrome. Acta Neurobiol Exp (Warsz) 65: 213–220, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol 498: 649–662, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol 476: 423–428, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buckler KJ, Vaughan-Jones RD, Peers C, Nye PC. Intracellular pH and its regulation in isolated type I carotid body cells of the neonatal rat. J Physiol 436: 107–129, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Chai Y, Lin YF. Dual regulation of the ATP-sensitive potassium channel by activation of cGMP-dependent protein kinase. Pflügers Arch 456: 897–915, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Chai Y, Lin YF. Stimulation of neuronal KATP channels by cGMP-dependent protein kinase: involvement of ROS and 5-hydroxydecanoate-sensitive factors in signal transduction. Am J Physiol Cell Physiol 298: C875–C892, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med 42: 165–174, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog Neurobiol 90: 439–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen L, Lu XY, Li J, Fu JD, Zhou ZN, Yang HT. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am J Physiol Cell Physiol 290: C1221–C1229, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Chen L, Zhang J, Hu X, Philipson KD, Scharf SM. The Na+/Ca2+ exchanger-1 mediates left ventricular dysfunction in mice with chronic intermittent hypoxia. J Appl Physiol 109: 1675–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheong E, Tumbev V, Abramson J, Salama G, Stoyanovsky DA. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium 37: 87–96, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Cogolludo A, Moreno L, Frazziano G, Moral-Sanz J, Menendez C, Castaneda J, Gonzalez C, Villamor E, Perez-Vizcaino F. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res 82: 296–302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen KD, Jackson WF. Hypoxia inhibits contraction but not calcium channel currents or changes in intracellular calcium in arteriolar muscle cells. Microcirculation 10: 133–141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coppock EA, Martens JR, Tamkun MM. Molecular basis of hypoxia-induced pulmonary vasoconstriction: role of voltage-gated K+ channels. Am J Physiol Lung Cell Mol Physiol 281: L1–L12, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Cornfield DN, Stevens T, McMurtry IF, Abman SH, Rodman DM. Acute hypoxia causes membrane depolarization and calcium influx in fetal pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 266: L469–L475, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Cross AR, Henderson L, Jones OT, Delpiano MA, Hentschel J, Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J 272: 743–747, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dart C, Standen NB. Activation of ATP-dependent K+ channels by hypoxia in smooth muscle cells isolated from the pig coronary artery. J Physiol 483: 29–39, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Das M, Das DK. Molecular mechanism of preconditioning. IUBMB Life 60: 199–203, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science 247: 1341–1344, 1990 [DOI] [PubMed] [Google Scholar]
- 47. de Paula PM, Tolstykh G, Mifflin S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292: R2259–R2265, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Del Toro R, Levitsky KL, Lopez-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem 278: 22316–22324, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Diarra A, Sheldon C, Brett CL, Baimbridge KG, Church J. Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons. Neuroscience 93: 1003–1016, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Ding HL, Zhu HF, Dong JW, Zhu WZ, Zhou ZN. Intermittent hypoxia protects the rat heart against ischemia/reperfusion injury by activating protein kinase C. Life Sci 75: 2587–2603, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol 157: 45–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol 522: 19–31, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Du YM, Nathan RD. Simulated ischemia enhances L-type calcium current in pacemaker cells isolated from the rabbit sinoatrial node. Am J Physiol Heart Circ Physiol 293: H2986–H2994, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619–11624, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Eager KR, Dulhunty AF. Cardiac ryanodine receptor activity is altered by oxidizing reagents in either the luminal or cytoplasmic solution. J Membr Biol 167: 205–214, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Erdemli G, Xu YZ, Krnjevic K. Potassium conductance causing hyperpolarization of CA1 hippocampal neurons during hypoxia. J Neurophysiol 80: 2378–2390, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Evans AM, Osipenko ON, Gurney AM. Properties of a novel K+ current that is active at resting potential in rabbit pulmonary artery smooth muscle cells. J Physiol 496: 407–420, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fearon IM, Varadi G, Koch S, Isaacsohn I, Ball SG, Peers C. Splice variants reveal the region involved in oxygen sensing by recombinant human L-type Ca2+ channels. Circ Res 87: 537–539, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Fike CD, Kaplowitz MR, Zhang Y, Madden JA. Voltage-gated K+ channels at an early stage of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 291: L1169–L1176, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Forster HV, Martino P, Hodges M, Krause K, Bonis J, Davis S, Pan L. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity and of PaCO2 during eupneic breathing. Adv Exp Med Biol 605: 322–326, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Franco-Obregon A, Lopez-Barneo J. Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. J Physiol 491: 511–518, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Franco-Obregon A, Lopez-Barneo J. Low Po2 inhibits calcium channel activity in arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 271: H2290–H2299, 1996 [DOI] [PubMed] [Google Scholar]
- 63. Franco-Obregon A, Urena J, Lopez-Barneo J. Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proc Natl Acad Sci USA 92: 4715–4719, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fredricks KT, Liu Y, Rusch NJ, Lombard JH. Role of endothelium and arterial K+ channels in mediating hypoxic dilation of middle cerebral arteries. Am J Physiol Heart Circ Physiol 267: H580–H586, 1994 [DOI] [PubMed] [Google Scholar]
- 65. Friedman JE, Haddad GG. Anoxia induces an increase in intracellular sodium in rat central neurons in vitro. Brain Res 663: 329–334, 1994 [DOI] [PubMed] [Google Scholar]
- 66. Friedman JE, Haddad GG. Removal of extracellular sodium prevents anoxia-induced injury in freshly dissociated rat CA1 hippocampal neurons. Brain Res 641: 57–64, 1994 [DOI] [PubMed] [Google Scholar]
- 67. Fu XW, Wang D, Nurse CA, Dinauer MC, Cutz E. NADPH oxidase is an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild-type and oxidase-deficient mice. Proc Natl Acad Sci USA 97: 4374–4379, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fujimura N, Tanaka E, Yamamoto S, Shigemori M, Higashi H. Contribution of ATP-sensitive potassium channels to hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J Neurophysiol 77: 378–385, 1997 [DOI] [PubMed] [Google Scholar]
- 69. Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol 142: 192–202, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gauthier-Rein KM, Bizub DM, Lombard JH, Rusch NJ. Hypoxia-induced hyperpolarization is not associated with vasodilation of bovine coronary resistance arteries. Am J Physiol Heart Circ Physiol 272: H1462–H1469, 1997 [DOI] [PubMed] [Google Scholar]
- 71. Gebremedhin D, Bonnet P, Greene AS, England SK, Rusch NJ, Lombard JH, Harder DR. Hypoxia increases the activity of Ca2+-sensitive K+ channels in cat cerebral arterial muscle cell membranes. Pflügers Arch 428: 621–630, 1994 [DOI] [PubMed] [Google Scholar]
- 72. Gebremedhin D, Yamaura K, Harder DR. Role of 20-HETE in the hypoxia-induced activation of Ca2+-activated K+ channel currents in rat cerebral arterial muscle cells. Am J Physiol Heart Circ Physiol 294: H107–H120, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Gelband CH, Gelband H. Ca2+ release from intracellular stores is an initial step in hypoxic pulmonary vasoconstriction of rat pulmonary artery resistance vessels. Circulation 96: 3647–3654, 1997 [DOI] [PubMed] [Google Scholar]
- 74. Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol Heart Circ Physiol 271: H823–H833, 1996 [DOI] [PubMed] [Google Scholar]
- 75. Gonzalez C, Agapito MT, Rocher A, Gonzalez-Martin MC, Vega-Agapito V, Gomez-Nino A, Rigual R, Castaneda J, Obeso A. Chemoreception in the context of the general biology of ROS. Respir Physiol Neurobiol 157: 30–44, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Gonzalez C, Sanz-Alyayate G, Agapito MT, Obeso A. Effects of reducing agents on glutathione metabolism and the function of carotid body chemoreceptor cells. Biol Chem 385: 265–274, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun 343: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 78. Gu XQ, Haddad GG. Decreased neuronal excitability in hippocampal neurons of mice exposed to cyclic hypoxia. J Appl Physiol 91: 1245–1250, 2001 [DOI] [PubMed] [Google Scholar]
- 79. Gu XQ, Haddad GG. Maturation of neuronal excitability in hippocampal neurons of mice chronically exposed to cyclic hypoxia. Am J Physiol Cell Physiol 284: C1156–C1163, 2003 [DOI] [PubMed] [Google Scholar]
- 80. Guatteo E, Mercuri NB, Bernardi G, Knopfel T. Intracellular sodium and calcium homeostasis during hypoxia in dopamine neurons of rat substantia nigra pars compacta. J Neurophysiol 80: 2237–2243, 1998 [DOI] [PubMed] [Google Scholar]
- 81. Guerra L, Cerbai E, Gessi S, Borea PA, Mugelli A. The effect of oxygen free radicals on calcium current and dihydropyridine binding sites in guinea-pig ventricular myocytes. Br J Pharmacol 118: 1278–1284, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Haddad GG, Donnelly DF. O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. J Physiol 429: 411–428, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Haddad GG, Jiang C. O2 deprivation in the central nervous system: on mechanisms of neuronal response, differential sensitivity and injury. Prog Neurobiol 40: 277–318, 1993 [DOI] [PubMed] [Google Scholar]
- 84. Hammarstrom AK, Gage PW. Hypoxia and persistent sodium current. Eur Biophys J 31: 323–330, 2002 [DOI] [PubMed] [Google Scholar]
- 85. Hatton CJ, Carpenter E, Pepper DR, Kumar P, Peers C. Developmental changes in isolated rat type I carotid body cell K+ currents and their modulation by hypoxia. J Physiol 501: 49–58, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. He L, Chen J, Dinger B, Sanders K, Sundar K, Hoidal J, Fidone S. Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am J Physiol Cell Physiol 282: C27–C33, 2002 [DOI] [PubMed] [Google Scholar]
- 87. He L, Dinger B, Sanders K, Hoidal J, Obeso A, Stensaas L, Fidone S, Gonzalez C. Effect of p47phox gene deletion on ROS production and oxygen sensing in mouse carotid body chemoreceptor cells. Am J Physiol Lung Cell Mol Physiol 289: L916–L924, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Heeringa J, Berkenbosch A, de Goede J, Olievier CN. Relative contribution of central and peripheral chemoreceptors to the ventilatory response to CO2 during hyperoxia. Respir Physiol 37: 365–379, 1979 [DOI] [PubMed] [Google Scholar]
- 89. Hempleman SC. Increased calcium current in carotid body glomus cells following in vivo acclimatization to chronic hypoxia. J Neurophysiol 76: 1880–1886, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Hempleman SC. Sodium and potassium current in neonatal rat carotid body cells following chronic in vivo hypoxia. Brain Res 699: 42–50, 1995 [DOI] [PubMed] [Google Scholar]
- 91. Herrera GM, Walker BR. Involvement of L-type calcium channels in hypoxic relaxation of vascular smooth muscle. J Vasc Res 35: 265–273, 1998 [DOI] [PubMed] [Google Scholar]
- 92. Hescheler J, Delpiano MA, Acker H, Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res 486: 79–88, 1989 [DOI] [PubMed] [Google Scholar]
- 93. Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem 279: 18887–18894, 2004 [DOI] [PubMed] [Google Scholar]
- 94. Hogg DS, Kozlowski RZ. Hypoxia activates a background conductance in freshly isolated pulmonary arterial endothelial cells of the rat. FEBS Lett 522: 125–129, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol 519: 347–360, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Honda Y. Respiratory and circulatory activities in carotid body-resected humans. J Appl Physiol 73: 1–8, 1992 [DOI] [PubMed] [Google Scholar]
- 97. Hong Z, Weir EK, Nelson DP, Olschewski A. Subacute hypoxia decreases voltage-activated potassium channel expression and function in pulmonary artery myocytes. Am J Respir Cell Mol Biol 31: 337–343, 2004 [DOI] [PubMed] [Google Scholar]
- 98. Hool LC. Differential regulation of the slow and rapid components of guinea-pig cardiac delayed rectifier K+ channels by hypoxia. J Physiol 554: 743–754, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hool LC. Hypoxia increases the sensitivity of the L-type Ca2+ current to beta-adrenergic receptor stimulation via a C2 region-containing protein kinase C isoform. Circ Res 87: 1164–1171, 2000 [DOI] [PubMed] [Google Scholar]
- 100. Hool LC, Di Maria CA, Viola HM, Arthur PG. Role of NAD(P)H oxidase in the regulation of cardiac L-type Ca2+ channel function during acute hypoxia. Cardiovasc Res 67: 624–635, 2005 [DOI] [PubMed] [Google Scholar]
- 101. Huang L, Li W, Li B, Zou F. Activation of ATP-sensitive K channels protects hippocampal CA1 neurons from hypoxia by suppressing p53 expression. Neurosci Lett 398: 34–38, 2006 [DOI] [PubMed] [Google Scholar]
- 102. Huddleston AT, Tang W, Takeshima H, Hamilton SL, Klann E. Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J Neurophysiol 99: 1565–1571, 2008 [DOI] [PubMed] [Google Scholar]
- 103. Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K+ channels expressed in the pulmonary vasculature. Circ Res 85: 489–497, 1999 [DOI] [PubMed] [Google Scholar]
- 104. Ito T, Nuriya M, Yasui M. Regulation of Kv2.1 phosphorylation in an animal model of anoxia. Neurobiol Dis 38: 85–91, 2010 [DOI] [PubMed] [Google Scholar]
- 105. Iturriaga R, Lahiri S. Carotid body chemoreception in the absence and presence of CO2-HCO3. Brain Res 568: 253–260, 1991 [DOI] [PubMed] [Google Scholar]
- 106. Iturriaga R, Lahiri S, Mokashi A. Carbonic anhydrase and chemoreception in the cat carotid body. Am J Physiol Cell Physiol 261: C565–C573, 1991 [DOI] [PubMed] [Google Scholar]
- 107. Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 122: 21–30, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jackson WF. Hypoxia does not activate ATP-sensitive K+ channels in arteriolar muscle cells. Microcirculation 7: 137–145, 2000 [PMC free article] [PubMed] [Google Scholar]
- 109. Jiang C, Agulian S, Haddad GG. Cl− and Na+ homeostasis during anoxia in rat hypoglossal neurons: intracellular and extracellular in vitro studies. J Physiol 448: 697–708, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jiang C, Haddad GG. A direct mechanism for sensing low oxygen levels by central neurons. Proc Natl Acad Sci USA 91: 7198–7201, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Joyeux-Faure M, Stanke-Labesque F, Lefebvre B, Beguin P, Godin-Ribuot D, Ribuot C, Launois SH, Bessard G, Levy P. Chronic intermittent hypoxia increases infarction in the isolated rat heart. J Appl Physiol 98: 1691–1696, 2005 [DOI] [PubMed] [Google Scholar]
- 112. Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol 497: 337–347, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kalsner S. Hypoxic relaxation in functionally intact cattle coronary artery segments involves K+ ATP channels. J Pharmacol Exp Ther 275: 1219–1226, 1995 [PubMed] [Google Scholar]
- 114. Kaplan P, Babusikova E, Lehotsky J, Dobrota D. Free radical-induced protein modification and inhibition of Ca2+-ATPase of cardiac sarcoplasmic reticulum. Mol Cell Biochem 248: 41–47, 2003 [DOI] [PubMed] [Google Scholar]
- 115. Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14: 533–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kharbanda RK. Cardiac conditioning: a review of evolving strategies to reduce ischaemia-reperfusion injury. Heart 96: 1179–1186, 2010 [DOI] [PubMed] [Google Scholar]
- 117. Kim DK, Natarajan N, Prabhakar NR, Kumar GK. Facilitation of dopamine and acetylcholine release by intermittent hypoxia in PC12 cells: involvement of calcium and reactive oxygen species. J Appl Physiol 96: 1206–1215; discussion 1196, 2004 [DOI] [PubMed] [Google Scholar]
- 118. Kline DD, Hendricks G, Hermann G, Rogers RC, Kunze DL. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J Neurophysiol 101: 2270–2278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kulisz A, Chen N, Chandel NS, Shao Z, Schumacker PT. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am J Physiol Lung Cell Mol Physiol 282: L1324–L1329, 2002 [DOI] [PubMed] [Google Scholar]
- 120. Kumar S, Hall RJ, Mani AR, Moore KP, Camici PG, Rimoldi OE, Williams AJ, Macleod KT. Myocardial stunning is associated with impaired calcium uptake by sarcoplasmic reticulum. Biochem Biophys Res Commun 387: 77–82, 2009 [DOI] [PubMed] [Google Scholar]
- 121. Lahiri S, Forster RE., 2nd CO2/H+ sensing: peripheral and central chemoreception. Int J Biochem Cell Biol 35: 1413–1435, 2003 [DOI] [PubMed] [Google Scholar]
- 122. Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal 18: 499–507, 2006 [DOI] [PubMed] [Google Scholar]
- 123. Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis 17: 44–53, 2004 [DOI] [PubMed] [Google Scholar]
- 124. Liao P, Li G, Yu de J, Yong TF, Wang JJ, Wang J, Soong TW. Molecular alteration of Cav1.2 calcium channel in chronic myocardial infarction. Pflügers Arch 458: 701–711, 2009 [DOI] [PubMed] [Google Scholar]
- 125. Lim DC, Veasey SC. Neural injury in sleep apnea. Curr Neurol Neurosci Rep 10: 47–52, 2010 [DOI] [PubMed] [Google Scholar]
- 126. Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol 545: 961–974, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 128. Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca2+ ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther 10: 29–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu B, Zhu X, Chen CL, Hu K, Swartz HM, Chen YR, He G. Opening of the mitoKATP channel and decoupling of mitochondrial complex II and III contribute to the suppression of myocardial reperfusion hyperoxygenation. Mol Cell Biochem 337: 25–38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liu Q, Sham JS, Shimoda LA, Sylvester JT. Hypoxic constriction of porcine distal pulmonary arteries: endothelium and endothelin dependence. Am J Physiol Lung Cell Mol Physiol 280: L856–L865, 2001 [DOI] [PubMed] [Google Scholar]
- 131. Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science 241: 580–582, 1988 [DOI] [PubMed] [Google Scholar]
- 132. Lopez-Barneo J, Pardal R, Montoro RJ, Smani T, Garcia-Hirschfeld J, Urena J. K+ and Ca2+ channel activity and cytosolic [Ca2+] in oxygen-sensing tissues. Respir Physiol 115: 215–227, 1999 [DOI] [PubMed] [Google Scholar]
- 133. Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol 63: 259–287, 2001 [DOI] [PubMed] [Google Scholar]
- 134. Lopez-Lopez J, Gonzalez C, Urena J, Lopez-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol 93: 1001–1015, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L17–L25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lukyanetz EA, Shkryl VM, Kravchuk OV, Kostyuk PG. Action of hypoxia on different types of calcium channels in hippocampal neurons. Biochim Biophys Acta 1618: 33–38, 2003 [DOI] [PubMed] [Google Scholar]
- 138. Luqman N, Sung RJ, Wang CL, Kuo CT. Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol 119: 283–290, 2007 [DOI] [PubMed] [Google Scholar]
- 139. Lytton J. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406: 365–382, 2007 [DOI] [PubMed] [Google Scholar]
- 140. Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 166: 1382–1387, 2002 [DOI] [PubMed] [Google Scholar]
- 141. Malin SA, Nerbonne JM. Delayed rectifier K+ currents, IK, are encoded by Kv2 alpha-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci 22: 10094–10105, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Matejikova J, Kucharska J, Pinterova M, Pancza D, Ravingerova T. Protection against ischemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: involvement of mitochondrial KATP channels and reactive oxygen species. Physiol Res 58: 9–19, 2009 [DOI] [PubMed] [Google Scholar]
- 143. Matthews EA, Disterhoft JF. Blocking the BK channel impedes acquisition of trace eyeblink conditioning. Learn Mem 16: 106–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. McMurtry IF, Davidson AB, Reeves JT, Grover RF. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res 38: 99–104, 1976 [DOI] [PubMed] [Google Scholar]
- 145. Mertsch K, Grune T, Kunstmann S, Wiesner B, Ladhoff AM, Siems WG, Haseloff RF, Blasig IE. Protective effects of the thiophosphate amifostine (WR 2721) and a lazaroid (U83836E) on lipid peroxidation in endothelial cells during hypoxia/reoxygenation. Biochem Pharmacol 56: 945–954, 1998 [DOI] [PubMed] [Google Scholar]
- 146. Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci 25: 11184–11193, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Montoro RJ, Urena J, Fernandez-Chacon R, Alvarez de Toledo G, Lopez-Barneo J. Oxygen sensing by ion channels and chemotransduction in single glomus cells. J Gen Physiol 107: 133–143, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, Pardoe H, Pell GS, Abbott DF, Rochford PD, Jackson GD, Pierce RJ, O'Donoghue FJ, Corfield DR. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 65: 908–914, 2010 [DOI] [PubMed] [Google Scholar]
- 149. Movafagh S, Morad M. L-type calcium channel as a cardiac oxygen sensor. Ann NY Acad Sci 1188: 153–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Muller M, Somjen GG. Na+ dependence and the role of glutamate receptors and Na+ channels in ion fluxes during hypoxia of rat hippocampal slices. J Neurophysiol 84: 1869–1880, 2000 [DOI] [PubMed] [Google Scholar]
- 151. Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol 52: 821–828, 1997 [DOI] [PubMed] [Google Scholar]
- 152. Nakaya H, Takeda Y, Tohse N, Kanno M. Mechanism of the membrane depolarization induced by oxidative stress in guinea-pig ventricular cells. J Mol Cell Cardiol 24: 523–534, 1992 [DOI] [PubMed] [Google Scholar]
- 153. Neckar J, Szarszoi O, Koten L, Papousek F, Ost'adal B, Grover GJ, Kolar F. Effects of mitochondrial KATP modulators on cardioprotection induced by chronic high altitude hypoxia in rats. Cardiovasc Res 55: 567–575, 2002 [DOI] [PubMed] [Google Scholar]
- 154. Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol 563: 409–419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Odell AF, Scott JL, Van Helden DF. EGF induces tyrosine phosphorylation, membrane insertion and activation of transient receptor potential channel 4. J Biol Chem 280: 37974–37987, 2005 [DOI] [PubMed] [Google Scholar]
- 157. Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, Wilhelm J, Morty RE, Brau ME, Weir EK, Kwapiszewska G, Klepetko W, Seeger W, Olschewski H. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res 98: 1072–1080, 2006 [DOI] [PubMed] [Google Scholar]
- 158. Ortega-Saenz P, Pardal R, Garcia-Fernandez M, Lopez-Barneo J. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol 548: 789–800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Osipenko ON, Evans AM, Gurney AM. Regulation of the resting potential of rabbit pulmonary artery myocytes by a low threshold, O2-sensing potassium current. Br J Pharmacol 120: 1461–1470, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Osipenko ON, Tate RJ, Gurney AM. Potential role for Kv3.1b channels as oxygen sensors. Circ Res 86: 534–540, 2000 [DOI] [PubMed] [Google Scholar]
- 161. Ostadalova I, Ostadal B, Jarkovska D, Kolar F. Ischemic preconditioning in chronically hypoxic neonatal rat heart. Pediatr Res 52: 561–567, 2002 [DOI] [PubMed] [Google Scholar]
- 162. Overholt JL, Ficker E, Yang T, Shams H, Bright GR, Prabhakar NR. HERG-Like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body. J Neurophysiol 83: 1150–1157, 2000 [DOI] [PubMed] [Google Scholar]
- 163. Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 87: 460–466, 2000 [DOI] [PubMed] [Google Scholar]
- 164. Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci 23: 4798–4802, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Park AM, Nagase H, Kumar SV, Suzuki YJ. Effects of intermittent hypoxia on the heart. Antioxid Redox Signal 9: 723–729, 2007 [DOI] [PubMed] [Google Scholar]
- 166. Patel AJ, Honore E. Molecular physiology of oxygen-sensitive potassium channels. Eur Respir J 18: 221–227, 2001 [DOI] [PubMed] [Google Scholar]
- 167. Patel AJ, Lazdunski M, Honore E. KV2.1/KV9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J 16: 6615–6625, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pearce WJ, Ashwal S, Long DM, Cuevas J. Hypoxia inhibits calcium influx in rabbit basilar and carotid arteries. Am J Physiol Heart Circ Physiol 262: H106–H113, 1992 [DOI] [PubMed] [Google Scholar]
- 169. Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2+-activated K+ current. Neurosci Lett 119: 253–256, 1990 [DOI] [PubMed] [Google Scholar]
- 170. Peers C, Wyatt CN. The role of maxiK channels in carotid body chemotransduction. Respir Physiol Neurobiol 157: 75–82, 2007 [DOI] [PubMed] [Google Scholar]
- 171. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96: 1236–1242, 2004 [DOI] [PubMed] [Google Scholar]
- 173. Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol 97: 2020–2025, 2004 [DOI] [PubMed] [Google Scholar]
- 174. Perez-Garcia MT, Lopez-Lopez JR, Gonzalez C. Kvbeta1.2 subunit coexpression in HEK293 cells confers O2 sensitivity to kv4.2 but not to Shaker channels. J Gen Physiol 113: 897–907, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Petrovic MM, Vales K, Putnikovic B, Djulejic V, Mitrovic DM. Ryanodine receptors, voltage-gated calcium channels and their relationship with protein kinase A in the myocardium. Physiol Res 57: 141–149, 2008 [DOI] [PubMed] [Google Scholar]
- 176. Platoshyn O, Brevnova EE, Burg ED, Yu Y, Remillard CV, Yuan JX. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 290: C907–C916, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol 280: L801–L812, 2001 [DOI] [PubMed] [Google Scholar]
- 178. Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate 65: 211–219, 1994 [DOI] [PubMed] [Google Scholar]
- 179. Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res 77: 131–139, 1995 [DOI] [PubMed] [Google Scholar]
- 180. Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol Cell Physiol 262: C882–C890, 1992 [DOI] [PubMed] [Google Scholar]
- 181. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5: 136–143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Quayle JM, Turner MR, Burrell HE, Kamishima T. Effects of hypoxia, anoxia, and metabolic inhibitors on KATP channels in rat femoral artery myocytes. Am J Physiol Heart Circ Physiol 291: H71–H80, 2006 [DOI] [PubMed] [Google Scholar]
- 183. Raeis V, Philip-Couderc P, Roatti A, Habre W, Sierra J, Kalangos A, Beghetti M, Baertschi AJ. Central venous hypoxemia is a determinant of human atrial ATP-sensitive potassium channel expression: evidence for a novel hypoxia-inducible factor 1alpha-Forkhead box class O signaling pathway. Hypertension 55: 1186–1192, 2010 [DOI] [PubMed] [Google Scholar]
- 184. Raley-Susman KM, Kass IS, Cottrell JE, Newman RB, Chambers G, Wang J. Sodium influx blockade and hypoxic damage to CA1 pyramidal neurons in rat hippocampal slices. J Neurophysiol 86: 2715–2726, 2001 [DOI] [PubMed] [Google Scholar]
- 185. Riesco-Fagundo AM, Perez-Garcia MT, Gonzalez C, Lopez-Lopez JR. O2 modulates large-conductance Ca2+-dependent K+ channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res 89: 430–436, 2001 [DOI] [PubMed] [Google Scholar]
- 186. Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167: 1548–1553, 2003 [DOI] [PubMed] [Google Scholar]
- 187. Roy A, Rozanov C, Mokashi A, Daudu P, Al-mehdi AB, Shams H, Lahiri S. Mice lacking in gp91 phox subunit of NAD(P)H oxidase showed glomus cell [Ca2+]i and respiratory responses to hypoxia. Brain Res 872: 188–193, 2000 [DOI] [PubMed] [Google Scholar]
- 188. Ruiz-Petrich E, de Lorenzi F, Chartier D. Role of the inward rectifier IK1 in the myocardial response to hypoxia. Cardiovasc Res 25: 17–26, 1991 [DOI] [PubMed] [Google Scholar]
- 189. Ryan S, McNicholas WT, Taylor CT. A critical role for p38 map kinase in NF-kappaB signaling during intermittent hypoxia/reoxygenation. Biochem Biophys Res Commun 355: 728–733, 2007 [DOI] [PubMed] [Google Scholar]
- 190. Santarelli LC, Wassef R, Heinemann SH, Hoshi T. Three methionine residues located within the regulator of conductance for K+ (RCK) domains confer oxidative sensitivity to large-conductance Ca2+-activated K+ channels. J Physiol 571: 329–348, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Searle GJ, Hartness ME, Hoareau R, Peers C, Kemp PJ. Lack of contribution of mitochondrial electron transport to acute O2 sensing in model airway chemoreceptors. Biochem Biophys Res Commun 291: 332–337, 2002 [DOI] [PubMed] [Google Scholar]
- 192. Shankar V, Armstead WM. Opioids contribute to hypoxia-induced pial artery dilation through activation of ATP-sensitive K+ channels. Am J Physiol Heart Circ Physiol 269: H997–H1002, 1995 [DOI] [PubMed] [Google Scholar]
- 193. Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, Glass T, Ardizzone T, Bernaudin M. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx 1: 26–35, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sheldon C, Diarra A, Cheng YM, Church J. Sodium influx pathways during and after anoxia in rat hippocampal neurons. J Neurosci 24: 11057–11069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Shimizu S, Bowman PS, Thorne G, 3rd, Paul RJ. Effects of hypoxia on isometric force, intracellular Ca2+, pH, and energetics in porcine coronary artery. Circ Res 86: 862–870, 2000 [DOI] [PubMed] [Google Scholar]
- 196. Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 281: L202–L208, 2001 [DOI] [PubMed] [Google Scholar]
- 197. Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000 [DOI] [PubMed] [Google Scholar]
- 198. Shimoda LA, Sylvester JT, Sham JS. Chronic hypoxia alters effects of endothelin and angiotensin on K+ currents in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 277: L431–L439, 1999 [DOI] [PubMed] [Google Scholar]
- 199. Shimoda LA, Sylvester JT, Sham JS. Inhibition of voltage-gated K+ current in rat intrapulmonary arterial myocytes by endothelin-1. Am J Physiol Lung Cell Mol Physiol 274: L842–L853, 1998 [DOI] [PubMed] [Google Scholar]
- 200. Shirahata M, Fitzgerald RS. Dependency of hypoxic chemotransduction in cat carotid body on voltage-gated calcium channels. J Appl Physiol 71: 1062–1069, 1991 [DOI] [PubMed] [Google Scholar]
- 201. Shirahata M, Fitzgerald RS. The presence of CO2/HCO3− is essential for hypoxic chemotransduction in the in vivo perfused carotid body. Brain Res 545: 297–300, 1991 [DOI] [PubMed] [Google Scholar]
- 202. Silver IA, Erecinska M. Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J Gen Physiol 95: 837–866, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Sipido KR. Calcium overload, spontaneous calcium release, and ventricular arrhythmias. Heart Rhythm 3: 977–979, 2006 [DOI] [PubMed] [Google Scholar]
- 204. Smani T, Hernandez A, Urena J, Castellano AG, Franco-Obregon A, Ordonez A, Lopez-Barneo J. Reduction of Ca2+ channel activity by hypoxia in human and porcine coronary myocytes. Cardiovasc Res 53: 97–104, 2002 [DOI] [PubMed] [Google Scholar]
- 205. Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol Heart Circ Physiol 266: H365–H370, 1994 [DOI] [PubMed] [Google Scholar]
- 206. Smith IF, Boyle JP, Kang P, Rome S, Pearson HA, Peers C. Hypoxic regulation of Ca2+ signaling in cultured rat astrocytes. Glia 49: 153–157, 2005 [DOI] [PubMed] [Google Scholar]
- 207. Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104: 110–118, 2008 [DOI] [PubMed] [Google Scholar]
- 208. Soukhova-O'Hare GK, Ortines RV, Gu Y, Nozdrachev AD, Prabhu SD, Gozal D. Postnatal intermittent hypoxia and developmental programming of hypertension in spontaneously hypertensive rats: the role of reactive oxygen species and L-Ca2+ channels. Hypertension 52: 156–162, 2008 [DOI] [PubMed] [Google Scholar]
- 209. Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol 101: 2837–2846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci 30: 10763–10772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Stea A, Jackson A, Nurse CA. Hypoxia and N6,O2′-dibutyryladenosine 3′,5′-cyclic monophosphate, but not nerve growth factor, induce Na+ channels and hypertrophy in chromaffin-like arterial chemoreceptors. Proc Natl Acad Sci USA 89: 9469–9473, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Stokowski LA. A primer on apnea of prematurity. Adv Neonatal Care 5: 155–170, 2005 [DOI] [PubMed] [Google Scholar]
- 213. Summers BA, Overholt JL, Prabhakar NR. Augmentation of L-type calcium current by hypoxia in rabbit carotid body glomus cells: evidence for a PKC-sensitive pathway. J Neurophysiol 84: 1636–1644, 2000 [DOI] [PubMed] [Google Scholar]
- 214. Sun J, Xu L, Eu JP, Stamler JS, Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem 276: 15625–15630, 2001 [DOI] [PubMed] [Google Scholar]
- 215. Taguchi H, Faraci FM, Kitazono T, Heistad DD. Relaxation of the carotid artery to hypoxia is impaired in Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol 15: 1641–1645, 1995 [DOI] [PubMed] [Google Scholar]
- 216. Tang WH, Kravtsov GM, Sauert M, Tong XY, Hou XY, Wong TM, Chung SK, Man Chung SS. Polyol pathway impairs the function of SERCA and RyR in ischemic-reperfused rat hearts by increasing oxidative modifications of these proteins. J Mol Cell Cardiol 49: 58–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am J Physiol Heart Circ Physiol 277: H584–H594, 1999 [DOI] [PubMed] [Google Scholar]
- 218. Tjong YW, Jian K, Li M, Chen M, Gao TM, Fung ML. Elevated endogenous nitric oxide increases Ca2+ flux via L-type Ca2+ channels by S-nitrosylation in rat hippocampal neurons during severe hypoxia and in vitro ischemia. Free Radic Biol Med 42: 52–63, 2007 [DOI] [PubMed] [Google Scholar]
- 219. Tjong YW, Li M, Hung MW, Wang K, Fung ML. Nitric oxide deficit in chronic intermittent hypoxia impairs large conductance calcium-activated potassium channel activity in rat hippocampal neurons. Free Radic Biol Med 44: 547–557, 2008 [DOI] [PubMed] [Google Scholar]
- 220. Tjong YW, Li MF, Hung MW, Fung ML. Melatonin ameliorates hippocampal nitric oxide production and large conductance calcium-activated potassium channel activity in chronic intermittent hypoxia. J Pineal Res 44: 234–243, 2008 [DOI] [PubMed] [Google Scholar]
- 221. Toffoli S, Feron O, Raes M, Michiels C. Intermittent hypoxia changes HIF-1alpha phosphorylation pattern in endothelial cells: unravelling of a new PKA-dependent regulation of HIF-1alpha. Biochim Biophys Acta 1773: 1558–1571, 2007 [DOI] [PubMed] [Google Scholar]
- 222. Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci 28: 8844–8850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. J Physiol 487: 37–50, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Urena J, Fernandez-Chacon R, Benot AR, Alvarez de Toledo GA, Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci USA 91: 10208–10211, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Urena J, Franco-Obregon A, Lopez-Barneo J. Contrasting effects of hypoxia on cytosolic Ca2+ spikes in conduit and resistance myocytes of the rabbit pulmonary artery. J Physiol 496: 103–109, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Vadula MS, Kleinman JG, Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol Lung Cell Mol Physiol 265: L591–L597, 1993 [DOI] [PubMed] [Google Scholar]
- 227. Valencia-Flores M, Orea A, Castano VA, Resendiz M, Rosales M, Rebollar V, Santiago V, Gallegos J, Campos RM, Gonzalez J, Oseguera J, Garcia-Ramos G, Bliwise DL. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res 8: 262–269, 2000 [DOI] [PubMed] [Google Scholar]
- 228. Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201, 2004 [DOI] [PubMed] [Google Scholar]
- 229. Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res 100: 105–111, 2007 [DOI] [PubMed] [Google Scholar]
- 230. Vittone L, Mundina-Weilenmann C, Mattiazzi A. Phospholamban phosphorylation by CaMKII under pathophysiological conditions. Front Biosci 13: 5988–6005, 2008 [DOI] [PubMed] [Google Scholar]
- 231. von Euler U, Liljestrand G. Observations on the pulmonary arterial blood pressure of the cat. Acta Physiol Scand 12: 301–320, 1946 [Google Scholar]
- 232. Wang H, Ward N, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci 23: 1665–1670, 2006 [DOI] [PubMed] [Google Scholar]
- 233. Wang H, Yuan G, Prabhakar NR, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from PC12 cells in response to oxidative stress requires autocrine dopamine signaling. J Neurochem 96: 694–705, 2006 [DOI] [PubMed] [Google Scholar]
- 234. Wang H, Zhu HF, Xia R, Zhou ZN, Zhu PH. Effect of intermittent and continuous hypoxia on ryanodine receptors of rat heart. Life Sci 73: 2151–2160, 2003 [DOI] [PubMed] [Google Scholar]
- 235. Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest 100: 2347–2353, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 237. Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005 [DOI] [PubMed] [Google Scholar]
- 238. Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 293: L674–L685, 2007 [DOI] [PubMed] [Google Scholar]
- 239. Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 240. Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol 288: L1049–L1058, 2005 [DOI] [PubMed] [Google Scholar]
- 241. Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell 134: 279–290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wang W, Ma J, Zhang P, Luo A. Redox reaction modulates transient and persistent sodium current during hypoxia in guinea pig ventricular myocytes. Pflügers Arch 454: 461–475, 2007 [DOI] [PubMed] [Google Scholar]
- 243. Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174: 307–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Ward JP, Aaronson PI. Mechanisms of hypoxic pulmonary vasoconstriction: can anyone be right? Respir Physiol 115: 261–271, 1999 [DOI] [PubMed] [Google Scholar]
- 245. Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98: 404–414, 2005 [DOI] [PubMed] [Google Scholar]
- 246. Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 247. Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J 9: 183–189, 1995 [DOI] [PubMed] [Google Scholar]
- 248. Weir EK, Reeve HL, Peterson DA, Michelakis ED, Nelson DP, Archer SL. Pulmonary vasoconstriction, oxygen sensing, and the role of ion channels: Thomas A. Neff lecture. Chest 114: 17S-–22S., 1998 [DOI] [PubMed] [Google Scholar]
- 249. Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34: 211–229, 2003 [DOI] [PubMed] [Google Scholar]
- 250. Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca2+ channels. Am J Physiol Heart Circ Physiol 274: H2018–H2024, 1998 [DOI] [PubMed] [Google Scholar]
- 251. Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 294: L309–L318, 2008 [DOI] [PubMed] [Google Scholar]
- 252. Wyatt CN, Peers C. Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol 483: 559–565, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci USA 92: 295–299, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Xia Y, Zhao P, Xue J, Gu XQ, Sun X, Yao H, Haddad GG. Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J Neurophysiol 89: 229–236, 2003 [DOI] [PubMed] [Google Scholar]
- 255. Xie Y, Zhu WZ, Zhu Y, Chen L, Zhou ZN, Yang HT. Intermittent high altitude hypoxia protects the heart against lethal Ca2+ overload injury. Life Sci 76: 559–572, 2004 [DOI] [PubMed] [Google Scholar]
- 256. Xie Y, Zhu Y, Zhu WZ, Chen L, Zhou ZN, Yuan WJ, Yang HT. Role of dual-site phospholamban phosphorylation in intermittent hypoxia-induced cardioprotection against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 288: H2594–H2602, 2005 [DOI] [PubMed] [Google Scholar]
- 257. Xu F, Xu J, Tse FW, Tse A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol 290: C1592–C1598, 2006 [DOI] [PubMed] [Google Scholar]
- 258. Yamada K, Inagaki N. Neuroprotection by KATP channels. J Mol Cell Cardiol 38: 945–949, 2005 [DOI] [PubMed] [Google Scholar]
- 259. Yamamoto S, Tanaka E, Higashi H. Mediation by intracellular calcium-dependent signals of hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J Neurophysiol 77: 386–392, 1997 [DOI] [PubMed] [Google Scholar]
- 260. Yamaura K, Gebremedhin D, Zhang C, Narayanan J, Hoefert K, Jacobs ER, Koehler RC, Harder DR. Contribution of epoxyeicosatrienoic acids to the hypoxia-induced activation of Ca2+ -activated K+ channel current in cultured rat hippocampal astrocytes. Neuroscience 143: 703–716, 2006 [DOI] [PubMed] [Google Scholar]
- 261. Yao H, Haddad GG. Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium 36: 247–255, 2004 [DOI] [PubMed] [Google Scholar]
- 262. Yeung HM, Kravtsov GM, Ng KM, Wong TM, Fung ML. Chronic intermittent hypoxia alters Ca2+ handling in rat cardiomyocytes by augmented Na+/Ca2+ exchange and ryanodine receptor activities in ischemia-reperfusion. Am J Physiol Cell Physiol 292: C2046–C2056, 2007 [DOI] [PubMed] [Google Scholar]
- 263. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 264. Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature 365: 153–155, 1993 [DOI] [PubMed] [Google Scholar]
- 265. Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem 280: 4321–4328, 2005 [DOI] [PubMed] [Google Scholar]
- 266. Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res 77: 370–378, 1995 [DOI] [PubMed] [Google Scholar]
- 268. Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol Lung Cell Mol Physiol 264: L116–L123, 1993 [DOI] [PubMed] [Google Scholar]
- 269. Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1555–R1562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Zhang Y, Zhong N, Zhou ZN. Effects of chronic intermittent hypobaric hypoxia on the L-type calcium current in rat ventricular myocytes. High Alt Med Biol 11: 61–67, 2010 [DOI] [PubMed] [Google Scholar]
- 271. Zhang YL, Tavakoli H, Chachisvilis M. Apparent PKA activity responds to intermittent hypoxia in bone cells: a redox pathway? Am J Physiol Heart Circ Physiol 299: H225–H235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Zhao G, Adebiyi A, Xi Q, Jaggar JH. Hypoxia reduces KCa channel activity by inducing Ca2+ spark uncoupling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 292: C2122–C2128, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Zhao P, Xue J, Gu XQ, Haddad GG, Xia Y. Intermittent hypoxia modulates Na+ channel expression in developing mouse brain. Int J Dev Neurosci 23: 327–333, 2005 [DOI] [PubMed] [Google Scholar]
- 274. Zhu HF, Dong JW, Zhu WZ, Ding HL, Zhou ZN. ATP-dependent potassium channels involved in the cardiac protection induced by intermittent hypoxia against ischemia/reperfusion injury. Life Sci 73: 1275–1287, 2003 [DOI] [PubMed] [Google Scholar]
- 275. Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol 103: 1888–1893, 2007 [DOI] [PubMed] [Google Scholar]