Abstract
Enhanced vascular arginase activity impairs endothelium-dependent vasorelaxation by decreasing l-arginine availability to endothelial nitric oxide (NO) synthase, thereby reducing NO production. Elevated angiotensin II (ANG II) is a key component of endothelial dysfunction in many cardiovascular diseases and has been linked to elevated arginase activity. We determined signaling mechanisms by which ANG II increases endothelial arginase function. Results show that ANG II (0.1 μM, 24 h) elevates arginase activity and arginase I expression in bovine aortic endothelial cells (BAECs) and decreases NO production. These effects are prevented by the arginase inhibitor BEC (100 μM). Blockade of ANG II AT1 receptors or transfection with small interfering RNA (siRNA) for Gα12 and Gα13 also prevents ANG II-induced elevation of arginase activity, but siRNA for Gαq does not. ANG II also elevates active RhoA levels and induces phosphorylation of p38 MAPK. Inhibitors of RhoA activation (simvastatin, 0.1 μM) or Rho kinase (ROCK) (Y-27632, 10 μM; H1152, 0.5 μM) block both ANG II-induced elevation of arginase activity and phosphorylation of p38 MAPK. Furthermore, pretreatment of BAECs with p38 inhibitor SB-202190 (2 μM) or transfection with p38 MAPK siRNA prevents ANG II-induced increased arginase activity/expression and maintains NO production. Additionally, inhibitors of p38 MAPK (SB-203580, 5 μg·kg−1·day−1) or arginase (ABH, 8 mg·kg−1·day−1) or arginase gene knockout in mice prevents ANG II-induced vascular endothelial dysfunction and associated enhancement of arginase. These results indicate that ANG II increases endothelial arginase activity/expression through Gα12/13 G proteins coupled to AT1 receptors and subsequent activation of RhoA/ROCK/p38 MAPK pathways leading to endothelial dysfunction.
Keywords: endothelial nitric oxide synthase dysfunction
arginase is a hydrolytic enzyme responsible for conversion of l-arginine to urea and l-ornithine (13, 46). Arginase can reciprocally regulate nitric oxide (NO) production in endothelial cells by competing with nitric oxide synthase (NOS) for the substrate l-arginine (2, 3, 33). Two distinct isoforms of arginase have been identified (43). Arginase I is mainly expressed in the liver as part of the urea cycle, while arginase II is expressed in extrahepatic tissues, particularly in the kidney. Both isoforms of arginase are expressed in vascular endothelial and smooth muscle cells (27). We have reported a role for arginase I in coronary vascular dysfunction in streptozocin-diabetic rats and also shown that high glucose increases arginase activity in bovine coronary endothelial cells via a RhoA/Rho kinase (ROCK) mechanism (33).
Vascular complications of diabetes also involve elevated tissue levels of angiotensin II (ANG II) (23). Conventional therapies to treat vascular complications in diabetes include the use of angiotensin-converting enzyme inhibitors and ANG II AT1 receptor blockers. However, even though beneficial outcomes have been obtained with these agents, micro- and macrovascular complications are still linked to a substantially elevated morbidity and mortality among diabetic patients (37).
Activation of the renin-angiotensin system is associated with many more cardiovascular pathologies such as systemic hypertension, cardiac hypertrophy, atherosclerosis, and glomerulosclerosis (30). Most of the actions of ANG II in endothelial cells are known to be associated with endothelial NOS (eNOS) dysfunction/uncoupling, which lead to decreased levels of NO and increased superoxide production (34). Impairment of NOS function has important implications for vascular tone, inflammation, procoagulation factors, and unregulated growth of smooth muscle cells.
Elevated arginase activity has been also associated with systemic hypertension. Inhibition of arginase has been reported to decrease blood pressure and improve vascular function of resistance vessels in adult hypertensive rats (2, 13). These findings thus suggest a central role for arginase in diseases in which vascular dysfunction is linked to elevated levels of angiotensin II (ANG II).
We and others have found that inflammatory cytokines, reactive oxygen species (ROS), thrombin, LPS, and activation of the RhoA/ROCK pathway can elevate arginase expression and activity (16, 26, 33, 48). RhoA/ROCK has also been indicated as an upstream regulator of mitogen-activated protein kinase (MAPK) family members such as p38 MAPK (22). p38 MAPK has been shown to have a central role in cardiovascular dysfunction (11, 44) and to increase arginase I expression in macrophages (7).
Given the importance of endothelial arginase in causing eNOS dysfunction, and the link of arginase with vascular diseases associated with elevated levels of ANG II, we sought to define the signaling pathway by which ANG II enhances arginase activity/expression in endothelial cells and impairs vascular endothelial function.
MATERIALS AND METHODS
Cell culture and treatment.
Bovine aortic endothelial cells (BAECs) (Cell Applications, San Diego, CA) were cultured in endothelial growth medium (Cell Applications) and maintained in a humidified atmosphere at 37°C and 5% CO2. Before the start of the experiments, cells were adapted to grow in medium 199 (M199) supplemented with 50 μM l-arginine (Invitrogen, Carlsbad, CA) to match the normal plasma l-arginine concentration, which ranges from 40 to 100 μM (32). In addition, the medium was also supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% l-glutamine. When cells reached 80% confluency, they were then serum starved overnight in M199 supplemented with 50 μM l-arginine, 1% l-glutamine, 1% penicillin/streptomycin, and 0.2% FBS. This medium was used under all experimental conditions and will be indicated as M199 for simplicity. Cells were then subjected to treatments with different inhibitors, as follows: arginase inhibitor, (S)-(2 boronoethyl)-l-cysteine (BEC, 100 μM); blocker of RhoA activation, simvastatin (0.1 μM); ROCK inhibitors, (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide, 2HCl (Y-27632, 10 μM) and (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine, 2HCl (H1152, 0.5 μM); p38 MAPK inhibitor, SB-202190 (2 μM) (EMD Biosciences, San Diego, CA); AT1 receptor blocker, telmisartan (1 μM ); or AT2 receptor blocker, PD123319 (1 μM) (Sigma Aldrich, St. Louis, MO), for 2 h before the addition of ANG II (0.1 μM, for different time points) (Sigma Aldrich) or with anisomycin (0.1 μM) (EMD Biosciences). All experiments were performed with cells from passage 3–7.
Arginase activity.
Arginase activity was measured using a colorimetric determination of urea production from l-arginine as described previously (10). Cells were lysed or frozen mouse aortas were homogenized by pulverization with 1:4 wt/vol of Tris buffer (50 mM Tris·HCl, 0.1 mM EDTA and EGTA, pH 7.5) containing protease inhibitors (Sigma, St. Louis, MO). These mixtures were subjected to three freeze-thaw cycles and then were centrifuged for 10 min at 14,000 rpm. The supernatants of soluble proteins were used for arginase activity assay.
In brief, 25 μl of supernatant was heated with MnCl2 (10 mM) for 10 min at 56°C to activate arginase. The mixture was then incubated with 50 μl l-arginine (0.5 M, pH 9.7) for 1 h at 37°C to hydrolyze the l-arginine. The hydrolysis reaction was stopped with acid, and the mixture was then heated at 100°C with 25 μl α-isonitrosopropiophenone (9% α-ISPF in EtOH) for 45 min. The samples were kept in the dark at room temperature for 10 min, and absorbance was then measured at 540 nm.
Nitric oxide measurement.
To measure NO, nitrite (NO2), the stable breakdown product of NO in the cell-conditioned medium, was analyzed using NO-specific chemiluminescence. After cells were treated, medium was replaced with fresh M199 for 30 min, and medium aliquots were then collected for basal reading. Cells were then exposed to the calcium ionophore ionomycin (Sigma Aldrich) (1 μM) for 30 min and medium samples were collected.
In brief, samples containing NO2 were injected in glacial acetic acid containing sodium iodide. NO2 is quantitatively reduced to NO under these conditions, which can be quantified by a chemiluminescence detector after reaction with ozone in a NO analyzer (Sievers, Boulder, CO). The amount of NO generated is calculated as the difference in basal and ionomycin-stimulated NO levels.
siRNA transfection.
BAECs were transfected with siRNA targeting Gα12, Gα13, or Gαq subunits of the G protein-coupled receptor family (Ambion, Austin, TX) or p38 MAPK (Cell Signaling, Boston, MA) using siPORT Amine (Ambion), according to the manufacturer's instructions. Scrambled siRNA (nontargeting siRNA) served as control to validate the specificity of the siRNAs. In brief, cells were transfected with 50 nM targeting or nontargeting siRNA for 48 h. Specific mRNA depletion was analyzed by RT-PCR or Western blotting. To evaluate the effects of siRNA transfection on arginase activity, transfected cells were incubated with ANG II (0.1 μM, 24 h) and arginase activity was determined as described earlier.
RT-PCR analysis of expression of mRNA transcripts for Gα12, Gα13, and Gαq.
The presence of specific mRNA transcripts for the Gα12, Gα13, and Gαq in BAECs was evaluated by RT-PCR. The PCR primers obtained (Invitrogen) were as follows: 5′-TCGACAACATCCTCAAGGGCTCAA-3′, 5′-ATACAGAATGCCTATGACCGGCGT-3′, and 5′-GGACAGGAGAGAGTGGCAAG-3′ (forward primers); and 5′-AGTGCTTCTTGATGCTCACGGTCT-3′, 5′-TGCACCTTCTCCTCAAGCAAGTCT-3′, and 5′-TGGGATCTTGAGTGTGTCCA-3′ (reverse primers), respectively. Total RNA was prepared from BAECs (TRIzol; Invitrogen-Gibco). RT-PCR was performed with optimal conditions specific for individual primer pairs (41).
Western blot analysis.
Cells were lysed or frozen mouse aortas were pulverized in Ripa buffer (Upstate, Temecula, CA) containing protease and phosphatase inhibitors (Sigma). Cell lysates or aorta homogenates were centrifuged for 10 min at 14,000 rpm, and supernatants of soluble protein were collected for Western blot analysis. Protein estimation was carried out in supernatants using protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein were loaded, separated by electrophoresis using 10% SDS-PAGE gels, and transferred into nitrocellulose membranes. The blots were blocked using 5% bovine serum albumin (Sigma), incubated with their respective primary antibodies [anti-arginase I (BD Biosciences, San Diego, CA), anti-arginase II (Santa Cruz Biotechnology, Santa Cruz, CA), anti-RhoA (Abcam, Cambridge, MA), anti-phospho-p38 and total p38 MAPK (Cell Signaling), and anti-actin (Sigma)], followed by the respective secondary antibodies. Signals were detected using chemiluminescence. To quantify the resultant blots, individual band intensities were measured (arbitrary units) and ratios of protein to actin were calculated per sample using ImageJ software (National Institutes of Health, Bethesda, MD).
Measurement of active RhoA.
RhoA activation was determined in confluent BAECs after exposure to ANG II (0.1 μM) for different periods of time, using two different methods: 1) detection of active membrane-bound Rho after cell fractionation and 2) affinity precipitation assay of whole cell lysate incorporating the Rho binding domain (RBD) of rhotekin, which binds only the active GTP-Rho (Upstate). For membrane protein isolation (1), cells were incubated for different time points with ANG II (0.1 μM) and then lysed in extraction buffer containing Tris·HCl (100 mM), EDTA (1 mM), EGTA (1 mM), and protease and phosphatase inhibitor cocktail (Sigma Aldrich). After centrifugation at 100,000 g for 20 min at 4°C, the cytosolic fraction was collected in the supernatant and the pellet was solubilized in 1% Triton X-100 extraction buffer to obtain the membrane fraction. Equal amounts of protein were loaded for Western blot analysis. For affinity precipitation assay (2), BAECs exposed to ANG II (24 h) were scraped in lysis buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, containing protease and phosphatase inhibitor cocktail) at 4°C. Whole cell lysates were incubated with rhotekin-RBD-conjugated agarose beads for 45 min at 4°C and washed three times with lysis buffer. Agarose beads were boiled in 2× Laemmli reducing sample buffer containing 50 mM dithiothreitol (DTT) to release active Rho. Samples were resolved on a 12.5% polyacrylamide gel followed by immunoblotting with RhoA antibody (Upstate Cell Signaling Solutions, Temecula, CA).
Animals and treatments.
Protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia. Experiments were performed with C57BL/6J wild-type (WT) mice and mice partially deficient in arginase I as well as completely deficient in arginase II (AI+/−AII−/−). Arginase I homozygous knockout mice (AI−/−) do not survive beyond 2 wk and therefore were not used for this study. The C57BL/6J AI+/−AII−/− mice developed by Cederbaum and colleagues (12) and O'Brien and colleagues (35) were provided by Dr. Steven Cederbaum with permission of Dr. William O'Brien. Mice were subjected to a subcutaneous infusion of either ANG II (42 μg·kg−1·h−1) or saline via osmotic minipumps (Alzet) for a period of 2 wk. Minipumps were implanted subcutaneously in the midscapular region of mice anesthetized by an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Three groups of ANG II-infused WT mice were treated either with an inhibitor for p38 MAPK, SB-203580 (5 μg·kg−1·day−1 ip), or with an inhibitor for arginase, 2(S)-amino-6-boronohexonic acid (ABH; 8 mg·kg−1·day−1, in drinking water) 2 days before minipump implantation, or with hydralazine (80 mg·kg−1·day−1, in drinking water) as a blood pressure control treatment.
Blood pressure measurement.
Tail-cuff systolic arterial pressure was determined by the noninvasive plethysmography tail-cuff at 0, 1, and 2 wk in all groups. The tail of each relaxed and acclimated mouse was inserted through the appropriate tail-cuff attached to the pressure monitoring system. A mouse underwent 10 preliminary cycles, for which data were not recorded. Another 10 measurement cycles were recorded. The mean of the last four recordings, among which there was not more than a 10-mmHg difference, was accepted as the mean systolic blood pressure (SBP).
Tissue harvest.
After 2 wk of treatments, mice were deeply anesthetized with a mixture of ketamine and xylazine. Blood was collected with heparinized syringes by cardiac puncture for plasma separation and storage. Hearts were quickly excised for euthanasia, and aortas were immediately harvested for vascular function and assays.
Vascular function studies.
After tissues were harvested, mouse aortas were placed immediately in ice-cold Krebs-Henseleit buffer, cleaned, and cut into 2- to 3-mm segments. Aortic rings were mounted in an oxygenated wire myograph chamber (Danish Myo Technology). Tissues were allowed to equilibrate at a resting tension of 5 mN for 1 h with buffer changes. Following phenylephrine (1 μM) precontraction, relaxation curves were performed using progressive doses of the endothelium-independent vasorelaxant sodium nitroprusside (SNP) and the endothelium-dependent vasorelaxant acetylcholine (ACh). Changes in tension were measured by force transducer. A 1 h equilibration was performed between subsequent relaxation curves. Vasorelaxation responses were calculated as the percentage of phenylephrine-induced contraction.
Statistical analysis.
Data are given as means ± SE. Statistical analysis was performed by one-way analysis of variance with the Tukey post test. In some experiments, statistical differences were determined by Student's t-test. Experiments were performed three to five times. All statistical analysis was performed with GraphPad Prism version 4.03 (San Diego, CA). Results were considered significant when P < 0.05.
RESULTS
Effect of ANG II on arginase activity and expression and NO production in endothelial cells.
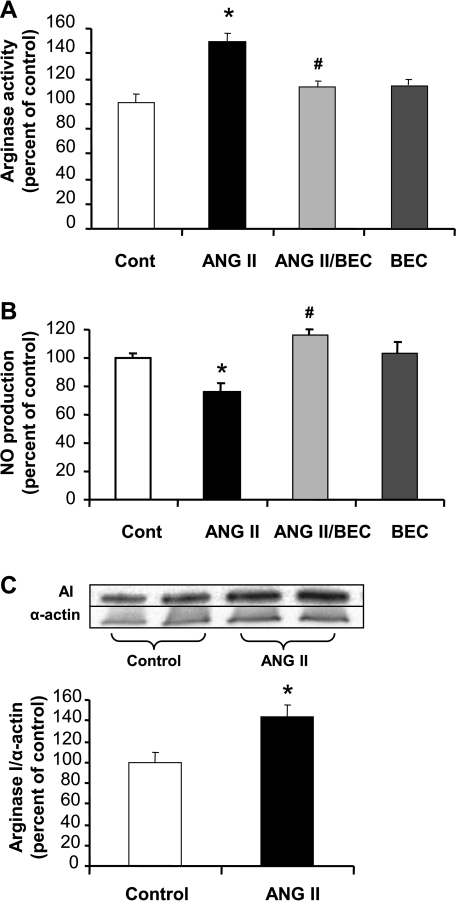
Treatment of BAECs with ANG II (0.1 μM, 24 h) produced a 49% increase (P < 0.05) in arginase activity (Fig. 1A), which was accompanied by a 25% decrease in NO production (P < 0.05) in response to the calcium-dependent eNOS agonist ionomycin (1 μM) (Fig. 1B). Both of these effects were prevented by pretreatment (2 h) with an arginase inhibitor, BEC (100 μM).
Fig. 1.
Effect of angiotensin II (ANG II) on arginase activity/expression and nitric oxide (NO) production in bovine aortic endothelial cells (BAECs). A: ANG II (0.1 μM) increased arginase activity in BAECs after exposure for 24 h. Pretreatment with (S)-(2 boronoethyl)-l-cysteine (BEC) for 2 h (100 μM) prevented this increase. Cont, control. B: ANG II (0.1 μM) decreased ionomycin-stimulated NO production in BAECs after exposure for 24 h, and 2 h pretreatment with BEC (100 μM) prevented this decrease. C: ANG II (0.1 μM) increased arginase I (AI) expression in BAECs after exposure for 24 h. Values are expressed as means ± SE from five independent experiments carried out in triplicate. *P < 0.05 vs. control; #P < 0.05 vs. ANG II.
ANG II also increased arginase I protein expression by 45% (P < 0.05) as shown by Western blot analysis (Fig. 1C). Arginase II protein levels were not altered with exposure to ANG II (data not shown). These data suggest that arginase I is responsible for the increase in arginase activity and is involved in the reduced NO production.
Role of AT1 G protein-coupled receptor and Gα12, Gα13, and Gαq subunits in ANG II-induced increase in arginase activity in endothelial cells.
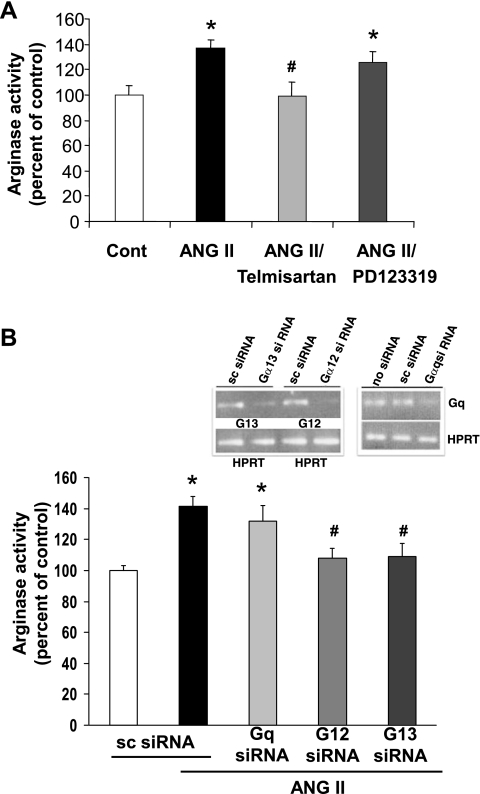
To identify the ANG II receptor involved in the elevation of arginase activity, BAECs were pretreated with blockers of either the AT1 (telmisartan, 1 μM) or the AT2 (PD123319, 1 μM) receptors for 2 h before the addition of ANG II (0.1 μM, 24 h). Blockade of the AT1 receptor, but not the AT2 receptor, prevented the ANG II-induced increase in arginase activity (Fig. 2A). Stimulation by ANG II is known to activate various heterotrimeric G proteins coupled to its AT1 receptor, which in turn activate various signaling cascades. To determine the role of Gα12, Gα13, and Gαq in ANG II-induced upregulation of arginase, BAECs were transfected with G protein-specific silencing RNA. Knockdown efficiency of specified G proteins was ∼80% as determined by real-time RT-PCR (Fig. 2B, inset). Depletion of either Gα12 or Gα13 with specific siRNAs significantly attenuated the ANG II-induced elevation of arginase activity (Fig. 2B), which confirms involvement of both Gα12 and Gα13 subunits. However, depletion of Gαq, via its siRNA, did not significantly affect the ANG II-induced elevation of arginase activity. The control siRNA with scrambled (sc) sequence failed to attenuate the ANG II-induced increase in arginase activity, thus demonstrating the sequence-specific depletion effect.
Fig. 2.
Effect of AT1, AT2 blockers and small interfering RNA (siRNA) transfection of Gα12, Gα13, or Gαq on ANG II-induced elevation of arginase activity in BAECs. A: elevated arginase activity of BAECs exposed to ANG II (0.1 μM, 24 h) is blocked by 2 h pretreatment of AT1 blocker telmisartan (1 μM), but not with AT2 blocker PD123319 (1 μM). Values are expressed as means ± SE from three independent experiments carried out in triplicate. *P < 0.05 vs. control; #P < 0.05 vs. ANG II. B: ANG II-induced increases in arginase activity are prevented in BAECs transfected with siRNA for Gα12 or Gα13, but not in cells transfected with scrambled (sc) or Gαq siRNA. Inset: specific siRNA depletes native RNA, but sc siRNA does not. HPRT, hypoxanthine–guanine phosphoribosyltransferase, as a normalization factor. Values are expressed as means ± SE from three independent experiments carried out in triplicate. *P < 0.05 vs. sc siRNA; #P < 0.05 vs. ANG II/sc.
Role of RhoA/ROCK in ANG II-induced increase in arginase activity and decrease in NO production in endothelial cells.
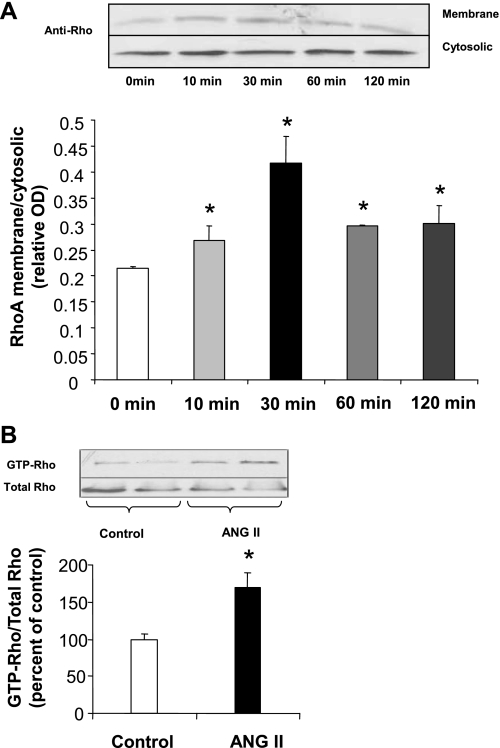
It has previously been shown that ANG II signals RhoA activation through AT1 receptor-coupled G proteins in vascular smooth muscle cells (4). Thus, we investigated whether ANG II induces activation of RhoA in our cell model and whether this pathway is associated with elevation of arginase activity in BAECs. Exposure of BAECs to ANG II caused a time-dependent increase in translocation of the active RhoA protein which was evident by 10 min and peaked at 30 min (Fig. 3A). In BAECs treated with ANG II for 24 h, there was also a 70% elevation of GTP-RhoA/total RhoA levels (P < 0.05) (Fig. 3B).
Fig. 3.
Activation of RhoA by ANG II. A: BAECs exposed to ANG II (0.1 μM) for 10 to 120 min show a time-dependent increase in membrane translocation (activation) of RhoA that peaks at 30 min of treatment. OD, optical density. B: BAECs exposed to ANG II (0.1 μM) for 24 h show an increase in the ratio of GTP-bound RhoA to total RhoA as compared with untreated controls. Values are expressed as means ± SE from three independent experiments carried out in duplicate. *P < 0.05 vs. control.
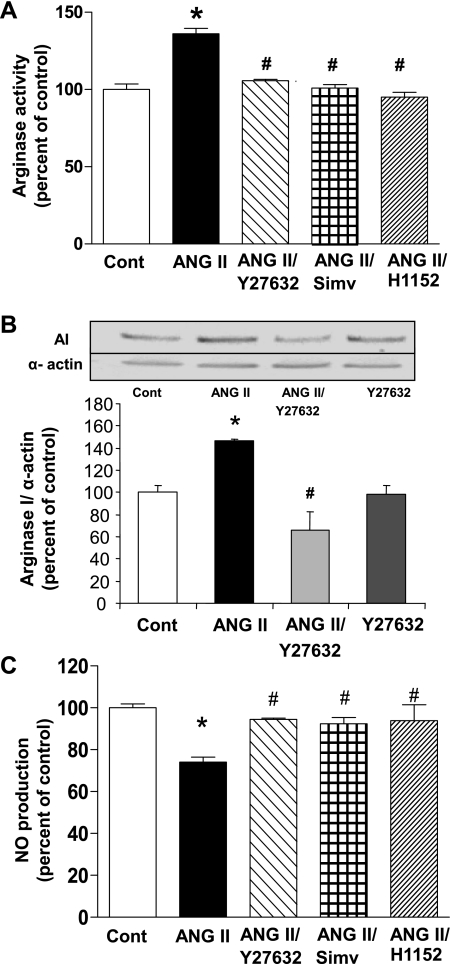
We determined whether inhibition of either RhoA or ROCK prevents ANG II-induced elevation of arginase activity. Pretreatment of BAECs with the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor, simvastatin (0.1 μM) that prevents the activation of RhoA, or with the ROCK inhibitor, Y-27632 (10 μM) prevented elevation of arginase activity (Fig. 4A) and protein expression of arginase I (Fig. 4B). Another more selective ROCK inhibitor, H1152 (0.5 μM) also prevented ANG II induced activation of arginase (Fig. 4A).
Fig. 4.
Effect of RhoA/Rho kinase (ROCK) inhibition on ANG II induced-elevation of arginase activity and expression and decrease in NO production. A: pretreatment of BAECs with Y-27632 (10 μM), H1152 (0.5 μM), or simvastatin (Simv; 0.1 μM) for 2 h prevented the increase of arginase activity induced by ANG II (0.1 μM, 24 h). B: pretreatment of BAECs for 2 h with Y-27632 (10 μM) prevented the increase of AI expression induced by ANG II (0.1 μM, 24 h). C: pretreatment of BAECs with Y-27632 (10 μM), simvastatin (0.1 μM), or H1152 (0.5 μM) for 2 h prevented the decrease in ionomycin-induced NO production caused by exposure to ANG II (0.1 μM, 24 h). Values are expressed as means ± SE from four independent experiments carried out in triplicate. *P < 0.05 vs. control; #P < 0.05 vs. ANG II.
As stated before, exposure of BAECs to ANG II for 24 h diminishes NO in response to the calcium-dependent eNOS activator ionomycin (1 μM). This effect of ANG II was prevented by simvastatin (0.1 μM), Y-27632 (10 μM), or H1152 (0.5 μM), confirming the role of RhoA/ROCK and arginase in ANG II-induced eNOS dysfunction under our experimental conditions (Fig. 4C).
Involvement of RhoA/ROCK pathway in ANG II-induced activation of p38 MAPK in endothelial cells.
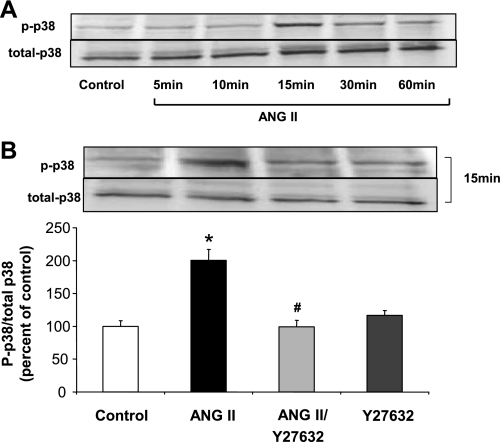
We next examined the potential role of p38 MAPK as a downstream target of the ANG II/RhoA/ROCK pathway. Western blot determination of phospho-p38 MAPK and total p38 MAPK in whole BAEC lysates showed a time-dependent increase in phosphorylation which did not become evident until 15 min of exposure to ANG II (Fig. 5A). Pretreatment with the ROCK inhibitor (Y-27632, 10 μM) prevented this effect (Fig. 5B), indicating that the RhoA/ROCK pathway is upstream to p38 MAPK and regulates its phosphorylation.
Fig. 5.
Activation of p38 MAPK by ANG II. A: BAECs exposed to ANG II (0.1 μM) for 5 to 60 min show a time-dependent increase in phospho-p38 MAPK expression first evident at 15 min. B: pretreatment of BAEC with Y-27632 (10 μM) for 2 h blocked the phosphorylation of p38 MAPK caused by ANG II (0.1 μM, 15 min). Values are expressed as means ± SE of the ratio of phospho-p38 MAPK to total p38 MAPK from three independent experiments carried out in duplicate. *P < 0.05 vs. control; #P < 0.05 vs. ANG II.
Role of p38 MAPK signaling in arginase activity/expression in endothelial cells.
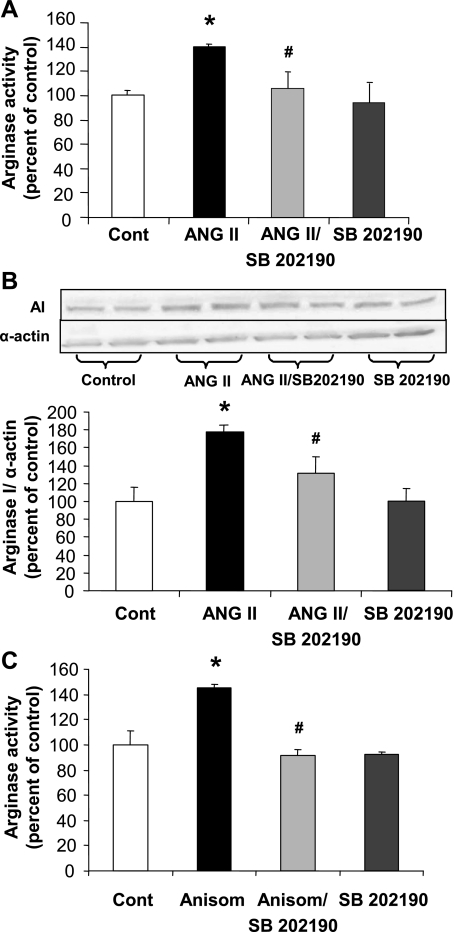
Pretreatment of BAECs with the p38 MAPK inhibitor SB-202190 (2 μM) prevented ANG II-induced elevation of arginase activity (Fig. 6A) and expression (Fig. 6B). Anisomycin, an antibiotic that is a known activator of p38 MAPK (45), was also tested. Exposure of cells to anisomycin (0.1 μM, 24 h) caused an increase in arginase activity of 45% that was blocked by SB-202190 pretreatment (Fig. 6C).
Fig. 6.
Role of p38 MAPK pathway in arginase activity and expression. A and B: pretreatment of BAECs for 2 h with p38 MAPK inhibitor SB-202190 (2 μM) prevented the increase of arginase activity (A) and the elevation of AI expression (B) in response to ANG II exposure (0.1 μM, 24 h). C: BAECs treated with an activator of p38 MAPK, anisomycin (Anisom, 0.1 μM, 24 h), showed an elevated arginase activity, which was prevented by pretreatment with SB-202190 (2 μM, 2 h). Values are expressed as means ± SE from four independent experiments carried out in triplicate. *P < 0.05 vs. control; #P < 0.05 vs. ANG II.
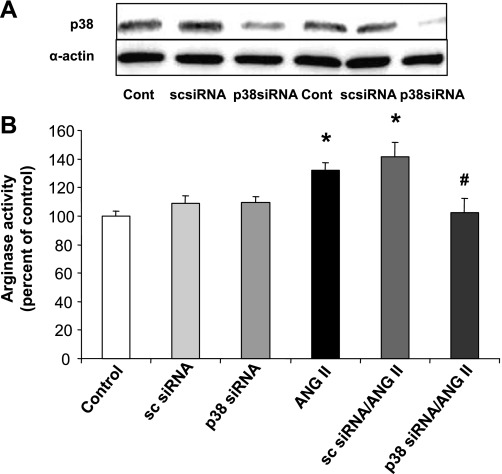
To further confirm the specific role of p38 MAPK in the ANG II-induced increase in arginase activity, we transfected BAECs with siRNA for p38 MAPK (50 nM). This treatment markedly reduced p38 MAPK protein levels (Fig. 7A) and completely blocked the ANG II-induced increase in arginase activity, indicating that p38 MAPK is integrally involved in elevation of arginase activity (Fig. 7B). Scrambled siRNA transfection did not alter the increase in arginase activity in response to ANG II.
Fig. 7.
Effect of p38 MAPK transfection on arginase activity. A: transfection of BAECs with siRNA for p38 MAPK (50 nM) for 48 h decreased total p38 MAPK protein in BAECs; however, sc siRNA (50 nM) transfection did not alter p38 MAPK expression. B: elevation of arginase activity by exposure of BAECs to ANG II (0.1 μM, 24 h) was prevented by transfection of cells with p38 MAPK siRNA. Transfection with sc siRNA did not prevent this response to ANG II. Values are expressed as means ± SE from three independent experiments carried out in triplicate. *P < 0.05 vs. control; #P < 0.05 vs. ANG II.
Role of p38 MAPK signaling in NO production in endothelial cells.
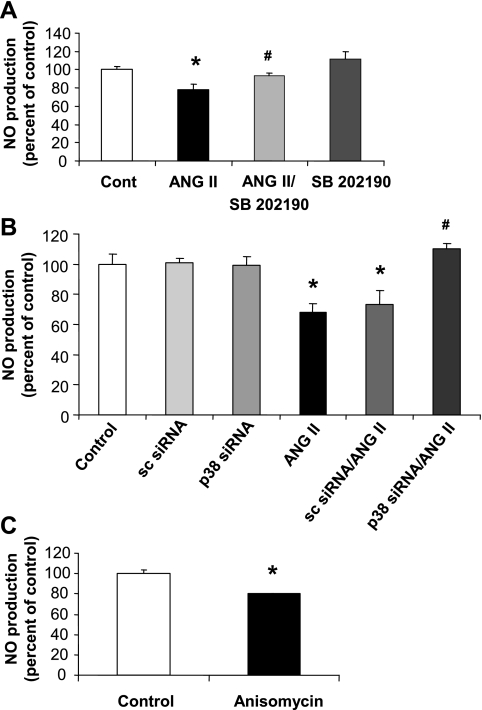
To examine the impact of the ANG II/p38 MAPK/arginase pathway on NO production, we pretreated BAECs with the p38 MAPK inhibitor SB-202190 (2 μM) and determined the effects of ANG II on NO production in response to the calcium-dependent eNOS activator, ionomycin (1 μM). The study showed that blocking p38 MAPK activation largely prevented the ANG II-induced decrease in NO formation (Fig. 8A). Additionally, transfection of BAECs with siRNA to p38 MAPK, but not control scrambled siRNA, prevented the reduction in NO production in response to ionomycin in the ANG II-treated cells (Fig. 8B). In contrast, activation of p38 MAPK with anisomycin (0.1 μM, 24 h) caused a decrease (21%) in NO production (Fig. 8C).
Fig. 8.
Effect of p38 MAPK pathway on NO production. A: pretreatment of BAECs for 2 h with p38 MAPK inhibitor SB-202190 (2 μM) prevented the decrease in ionomycin-induced NO production caused by ANG II (0.1 μM, 24 h). B: transfection of BAECs with p38 MAPK siRNA prevented the decrease in NO production caused by exposure to ANG II, but this response was not blocked in sc siRNA-transfected cells. C: BAECs treated with the p38 MAPK activator anisomycin (0.1 μM, 24 h) showed a decrease in NO production. (Error bar with anisomycin treatment is very small and does not appear clearly in the image.) Values are expressed as means ± SE from three independent experiments carried out in triplicate. *P < 0.05 vs. control; #P < 0.05 vs. ANG II.
Effect of ANG II infusion and treatments on SBP.
Tail-cuff SBP was elevated by ANG II infusion in mice after both 7 and 14 days when compared with saline-infused mice (132.7 ± 2.2 and 140.9 ± 3.0 mmHg vs. 108.3 ± 4.8 and 105.6 ± 3.1 mmHg, respectively) (Table 1). SBP levels were significantly blunted at both 7 and 14 days in ANG II-infused mice by treatment with the arginase inhibitor ABH (113.5 ± 3.3 and 117.7 ± 4.9 mmHg) or the p38 inhibitor SB-203580 (122.7 ± 3.4 and 121.6 ± 2.0 mmHg). The p38 inhibitor had no effect on SBP in control mice after 7 or 14 days of treatment (101.6 ± 2.5 and 106 ± 5.5 mmHg). Elevated SBP observed in mice after ANG II infusion was completely prevented by treatment with hydralazine at both 7 (98.7 ± 4.5 mmHg) and 14 (101.7 ± 3.3 mmHg) days. There was no difference in SBP among the groups before minipump implantation, with an average of 101.3 ± 4.8 mmHg.
Table 1.
Effect of ANG II infusion and treatments on tail-cuff systolic blood pressure
| Systolic Blood Pressure, mmHg |
||
|---|---|---|
| After 7 Days of Treatment | After 14 Days of Treatment | |
| Wild-type mice | ||
| Control (saline infusion) (8) | 108.3 ± 4.8 | 105.6 ± 3.1 |
| ANG II (12) | 132.7 ± 2.2* | 140.9 ± 3.0*† |
| ANG II + SB203580 (12) | 122.7 ± 3.4*‡ | 121.6 ± 2.0*‡ |
| SB-203580 (8) | 101.6 ± 2.5 | 106.8 ± 5.5 |
| ANG II + hydralazine (6) | 98.7 ± 4.5 | 101.7 ± 3.3 |
| ANG II + ABH (6) | 113.5 ± 3.3*‡ | 117.7 ± 4.9*‡ |
| AI+/−AII−/−knockout mice | ||
| Control (saline infusion) (6) | 110.2 ± 4.5 | 110.7 ± 2.9 |
| ANG II (6) | 118.3 ± 5.7* | 122.2 ± 3.9*† |
Values are means ± SE; n , no. of animals tested (in parentheses). Systolic blood pressure was measured in wild-type mice after 7 and 14 days of ANG II infusion with or without treatments and in AI+/− AII−/−-knockout mice after 7 and 14 days of ANG II infusion.
P < 0.05 vs. respective control group;
P < 0.05 vs. ANG II after 7 days;
P < 0.05 vs. respective ANG II group.
In AI+/−AII−/− knockout mice, SBP at 7 and 14 days of saline infusion was 110.2 ± 4.5 and 110.7 ± 2.9 mmHg, respectively. Infusion of ANG II caused a modest elevation of SBP after both 7 (118.3 ± 5.7 mmHg) and 14 (122.2 ± 3.9 mmHg) days compared with WT mice (Table 1). Before minipump implantation, both saline- and ANG II-infused groups showed no difference in SBP with an average of 109 ± 3.6 mmHg, which was similar to WT mice.
Effect of arginase inhibition or its genetic deletion on vascular endothelial dysfunction.
To determine the role of arginase in ANG II-induced endothelial dysfunction in vivo, we performed studies using aortas isolated from ANG II-infused or saline-infused WT mice with or without treatment with the arginase inhibitor ABH, or aortas isolated from mice partially deficient in arginase I and completely deficient in arginase II (AI+/−AII−/−). We examined vasorelaxation responses to the endothelium-dependent vasodilator ACh and the endothelium-independent vasodilator SNP.
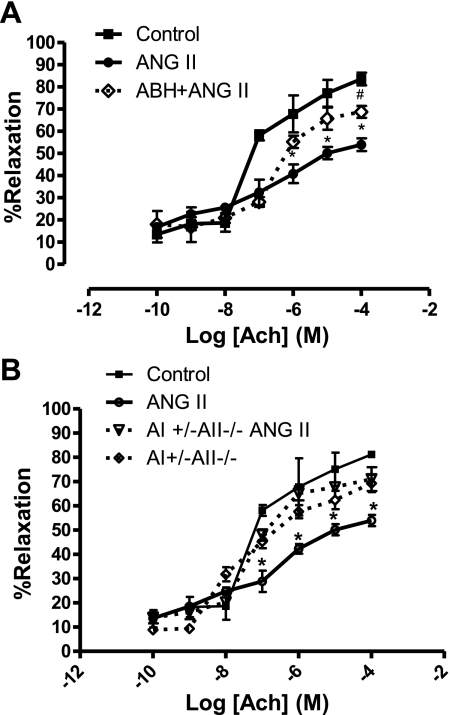
ANG II induced an impairment of vasorelaxation response to ACh in WT mice (maximum relaxation of 53.9 ± 5.7% vs. 81.8 ± 1.7% in control mice) (Fig. 9A). ABH partially prevented ANG II-induced impaired vasorelaxation with a maximum relaxation of 69.4 ± 5.3%. Moreover, a maximum relaxation of 71.1 ± 5.9% was observed in the AI+/−AII−/− knockout group treated with ANG II (Fig. 9B), which was not significantly different from the WT or the AI+/−AII−/− knockout control groups (81.8 ± 1.7% and 69.3 ± 3.6%, respectively). Thus, blocking activation of or knocking out arginase corrected the vascular dysfunction associated with ANG II in WT mice. Aortic relaxation responses to SNP were not different among the groups (data not shown).
Fig. 9.
Dose-response relaxation curves for endothelium-dependent vasorelaxant acetylcholine (ACh) in phenylephrine (1 μM)-preconstricted aortas from wild-type (WT) and AI+/−AII−/− knockout mice. A: from WT mice: solid black line with square symbols indicates responses in control mice; solid gray line with circles indicates responses in ANG II-infused mice; dashed black line with diamond symbols indicates responses in ANG II-infused mice treated with ABH. n = 6 in each group; *P < 0.05 vs. control, #P < 0.05 vs. ANG II. B: from AI+/−AII−/− knockout mice: solid black line with square symbols indicates responses in saline-infused control mice; solid gray line with circles indicates responses in ANG II-infused mice; dashed black line with diamond symbols indicates responses in saline-infused control AI+/−AII−/− knockout mice; dashed gray line with open triangle symbols indicates responses in ANG II-infused AI+/−AII−/− knockout mice. n = 6; *P < 0.05 vs. control.
Effect of ANG II on aortic arginase activity and expression in wild-type and AI+/−AII−/− knockout mice.
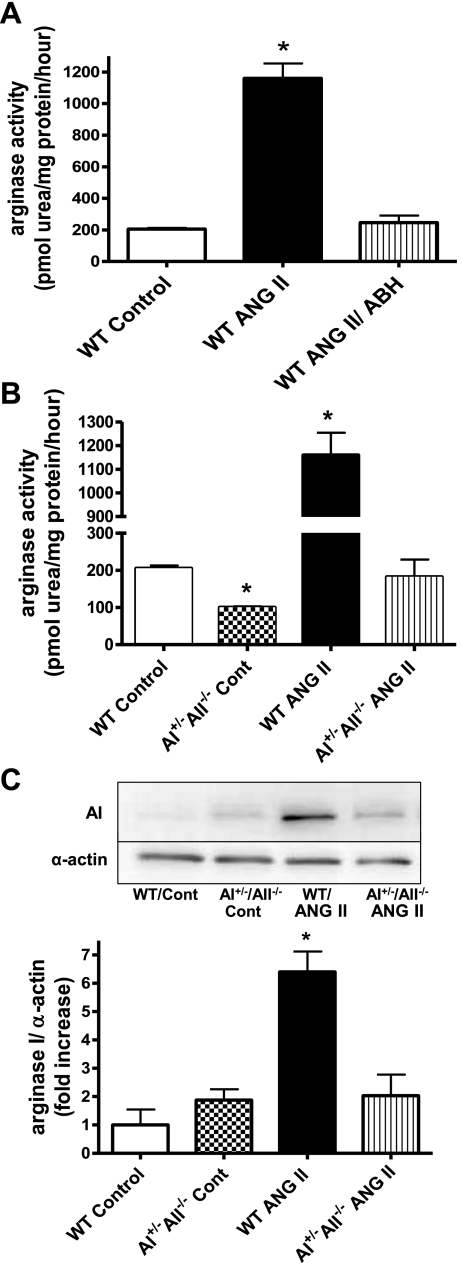
Infusion of ANG II in WT mice resulted in a significant elevation of arginase activity (1,170 ± 93 pmol urea·mg protein−1·h−1) in aortic vascular tissue when compared with control mice (205.7 ± 12.6 pmol urea·mg protein−1·h−1) (Fig. 10A). However, treatment with the arginase inhibitor ABH in ANG II-infused WT mice resulted in arginase activity levels similar to control group (243 ± 47 pmol urea·mg protein−1·h−1). Furthermore, treatment of ANG II-infused mice with the antihypertensive vasodilator hydralazine normalized systemic blood pressure (Table 1) but did not affect aortic arginase activity (1,133 ± 87 pmol urea·mg protein−1·h−1), suggesting that the protective action of the ABH treatment is not secondary to an effect on SBP.
Fig. 10.
Effect of ANG II infusion on aortic arginase activity and expression in WT and AI+/− AII−/− knockout mice. A: arginase activity (in pmol urea·mg protein−1·h−1) in aorta from saline- or ANG II-infused WT mice with or without treatment with the arginase inhibitor ABH. B: arginase activity (in pmol urea·mg protein−1·h−1) in aorta of WT mice vs. AI+/−AII−/− knockout mice with and without ANG II infusion. C: AI expression in WT and AI+/−AII−/− knockout mouse aorta with or without ANG II infusion. Values were corrected for α-actin and are expressed as fold increase over control. n = 6 for all groups; *P < 0.05 vs. control.
In addition, ANG II infusion in AI+/−AII−/− mice failed to elevate aortic arginase activity when compared with ANG II-infused WT mice (Fig. 10B). Arginase activity in AI+/−AII−/− control mice was ∼50% of that in the WT controls. These results correlate with elevated arginase I expression in ANG II-infused WT mice (6.5-fold over control WT) which was not observed in AI+/−AII−/− knockout mice infused with ANG II or saline (Fig. 10C).
Effect of p38 inhibition on ANG II-induced vascular endothelial dysfunction.
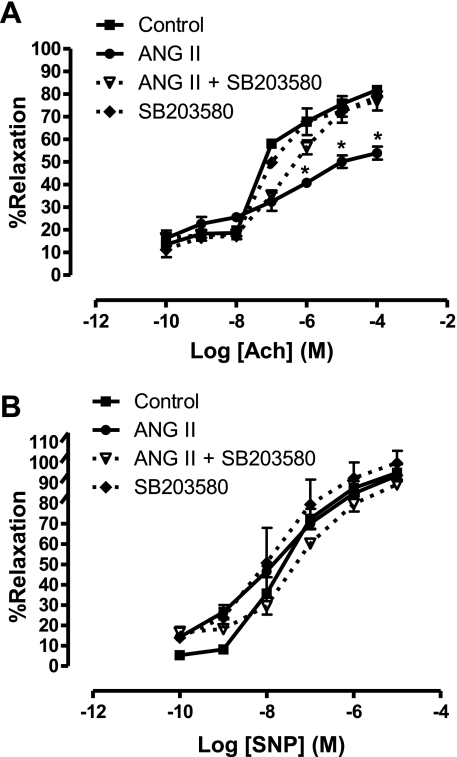
The impaired endothelium-dependent relaxation induced by ANG II infusion in WT mice (maximum relaxation 53.9 ± 5.7% vs. 81.8 ± 1.7% for control mice) was prevented by treatment with SB-203580 (maximum relaxation 76.9 ± 8.3%) (Fig. 11A). Treatment with SB-203580 in control WT mice had no significant effect on endothelium-dependent vasorelaxation responses compared with untreated controls. Aortic responses to the endothelium-independent vasodilator SNP were not significantly different among the groups (Fig. 11B).
Fig. 11.
Endothelium-dependent and -independent vascular relaxation in ANG II-infused mice with or without p38 MAPK inhibition. A: dose-response relaxation curves for endothelium-dependent vasorelaxant ACh in phenylephrine (1 μM)-preconstricted aorta. B: dose-response relaxation curves for endothelium-independent vasorelaxant sodium nitroprusside (SNP) in phenylephrine (1 μM)-preconstricted aorta. Solid black line with square symbols indicates responses in saline-infused control mice; solid gray line with circles indicates responses in ANG II-infused mice; dashed black line with open triangle symbols indicates responses in ANG II-infused mice treated with the p38 inhibitor SB-203580; dashed black line with diamond symbols indicates responses in the saline-infused control mice treated with SB-203580. n = 6 in each group, *P < 0.05 vs. control.
Effect of ANG II and SB-203580 on aortic arginase activity/expression and p38 MAPK phosphorylation.
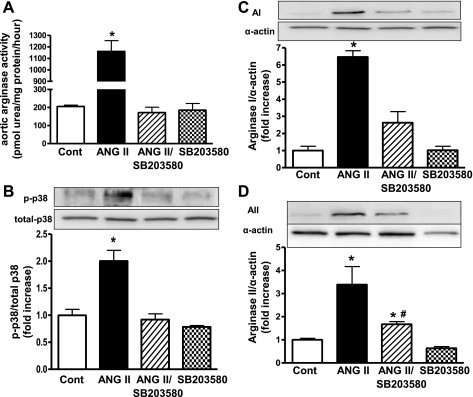
Aortas from WT mice infused with ANG II had elevated arginase activity (5.6-fold) (Fig. 12A) and phosphorylation of p38 MAPK (2-fold) (Fig. 12B) compared with control mice. These effects were prevented by treatment with a selective p38 MAPK inhibitor, SB-203580. Furthermore, expression of arginase I (Fig. 12C) and II (Fig. 12D) was increased in aortas of ANG II-infused mice by 6.5- and 3.4-fold, respectively, over control. ANG II-treated mice receiving SB-203580 had no significant elevation of arginase I or II expression when compared with control mice.
Fig. 12.
Effect of ANG II infusion and SB-203580 on mouse aortic arginase activity/expression and p38 MAPK phosphorylation. A: arginase activity (in pmol urea·mg protein−1·h−1) in mouse aorta tissue. B: expression of phosphorylated p38 MAPK in mouse aorta. Values were corrected for total p38 MAPK levels and are expressed as fold increase over control. C and D: AI (C) and AII (D) expression in mouse aorta. Values were corrected for α-actin and are expressed as fold increase over control. n = 6 for all groups. Key: control (saline-infused), open bars; ANG II-infused, black bars; ANG II-infused treated with SB-203580, striped bars; saline-infused treated with SB-203580, checkered bars. *P < 0.05 vs. control; #P < 0.05 vs. ANG II.
DISCUSSION
Our study demonstrates for the first time that elevation of arginase expression and activity significantly contributes to ANG II-induced endothelial dysfunction both in cells and in a mouse model. Exposure of cultured endothelial cells to ANG II reduces NO production in response to a calcium ionophore. This suppression can be prevented by pretreatment with the arginase inhibitor BEC, indicating a role of elevated arginase activity in this effect. The ANG II-induced alterations in arginase activity/expression and NO formation are blocked by pretreatment of the cells with inhibitors of RhoA/ROCK or p38 MAPK, indicating the involvement of these signaling mediators in the pathological process. These data confirm previous reports by us and others on the role of arginase in restricting NO production (3, 33).
There is growing evidence and recognition that arginase is a key participant in vascular disease and a prime target for therapeutic interventions. Elevated arginase activity and/or expression is involved in vascular endothelial dysfunction in a number of disease states, such as hypertension, diabetes, atherosclerosis, ischemia-reperfusion injury, and inflammation (13, 14, 33, 47, 48). Despite an array of pathologic conditions associated with elevated arginase function, organs such as the liver require arginase for physiologic function. Complete knockout of the arginase I gene in mice is lethal by 2 wk of age because of hyperammonemia via disruption of the hepatic urea cycle (17). Blockade of such essential functions could limit the clinical usefulness of arginase inhibitors to control endothelial arginase activity. However, identifying signaling pathways that lead to enhanced arginase activity in endothelial cells can provide more specific targets to control/limit arginase activity in pathologic conditions, without disrupting upstream pathways essential for normal organ functions.
Arginase has been linked to hypertensive models (2, 8, 13) associated with elevated levels of ANG II. ANG II is an important biologically active component of the renin-angiotensin system and acts through two receptor subtypes, the AT1 and AT2 receptors (9). Most of the pathophysiological effects of ANG II, including endothelial dysfunction and processes leading to atherothrombosis, are mediated by the AT1 receptor subtype (15, 38). Given the importance of these pathological processes, we sought to define the signaling pathway by which ANG II enhances arginase activity/expression in endothelial cells and in vascular tissue of ANG II hypertensive mice.
We have found that ANG II induces eNOS dysfunction by elevating arginase activity in endothelial cells through the AT1 receptor, since AT1 receptor blocker telmisartan, but not the AT2 blocker PD123319, prevents ANG II-induced elevation of arginase activity. AT1 is predominantly expressed in cardiovascular tissues and kidney and is a member of the seven transmembrane-spanning G protein-coupled receptor family (30), from which many signaling cascades begin. Gαq is an important signaling molecule of ANG II in smooth muscle cells (18). However, AT1 receptors are also reported to couple to the Gα12/13 family of G proteins (21). We examined the role of these G proteins in ANG II-induced arginase activation in endothelial cells. Our results indicate major involvement of the heterotrimeric Gα protein subunits G12 and 13 but not Gq, since siRNA knockdown of Gα12 or 13 fully prevented the ANG II-induced increase in arginase activity while Gαq knockdown did not. These results indicate that both Gα12 and 13 subunits are required for the cascade of events leading to enhanced arginase activity.
The Gα12/13 family has been strongly linked to activation of the RhoA pathway in other cell types (25). We and others have previously shown the involvement of the small GTPase RhoA and its target Rho kinase (ROCK) in elevation of arginase activity caused by high glucose (33), thrombin (26), or cytokines (16). Angiotensin II is known to activate NADPH oxidase and enhance ROS production (5). ROS has also been reported to activate RhoA/ROCK and arginase and to uncouple NOS (6, 39).
Our present data demonstrate the involvement of RhoA/ROCK pathway in ANG II-induced elevation of arginase activity and expression in endothelial cells. ANG II treatment activates RhoA in a time-dependent manner. Furthermore, we have found that blocking the RhoA/ROCK pathway by either simvastatin, a blocker of RhoA activation, or by Y-27632, an inhibitor of ROCK, prevented the increased arginase activity and expression, and decreased NO production resulting from exposure to ANG II. In addition, a more selective inhibitor of ROCK, H1152, prevented the increase in arginase activity and the decrease in NO production. These results provide another mechanism by which RhoA/ROCK affects NO production, because RhoA/ROCK has previously been shown to suppress eNOS expression and function (36).
RhoA also has been reported to be an upstream regulator of mitogen-activated protein kinase (MAPK) family members, such as p38 MAPK (22, 31). In this regard, ANG II and ROS are reported to increase MAPK activation, specifically p38 MAPK, in endothelial cells (11). Our findings agree with these results since we show that exposure to ANG II causes a time-dependent increase in p38 activation that is blocked by pretreatment with ROCK inhibitor. Thus, our data strongly suggest that p38 MAPK activation is downstream from RhoA/ROCK signaling in endothelial cells treated with ANG II.
Activated p38 MAPK is reported to be associated with increased arginase activity and expression in macrophages (7). Furthermore, in rat aortic endothelial cells, p38 MAPK inhibition has been reported to prevent thrombin-induced arginase I upregulation (49). Our present results indicate the involvement of p38 MAPK in increased arginase activity and expression in endothelial cells exposed to ANG II. Pretreatment of endothelial cells with a p38 inhibitor, SB-202190, prevented ANG II-induced elevation of arginase activity/expression and reduced NO production. To further examine upstream involvement of p38 MAPK in arginase upregulation, we treated the cells with anisomycin, an antibiotic that is a known stimulator of p38 MAPK (45), and found that it caused an increase in arginase activity and a decrease in NO production. Additionally, inhibition of p38 MAPK protein synthesis with siRNA also prevented ANG II-induced increase in arginase activity. Collectively, these results show an important role of p38 MAPK in arginase activation and upregulation by ANG II and the role it has on NO production.
p38 MAPK is considered a potential therapeutic target in several animal models (1, 42) and human diseases (20, 24). Additionally, p38 MAPK inhibition has been shown to ameliorate ANG II-induced target organ damage (29). Also, inhibition of p38 MAPK is reported to correct nitrergic neurovascular dysfunction in corpus cavernosum of diabetic mice (28), and the arginase-induced corpus cavernosum dysfunction that we recently observed in ANG II-treated mice (40).
Our experiments in mice have also indicated that inhibition of arginase and p38 MAPK can prevent ANG II-induced vascular dysfunction. Our data indicated that ANG II infusion in mice causes an elevation in aortic arginase activity and expression and an increase in p38 MAPK phosphorylation. These effects were associated with impairment of vascular endothelium function and an increase in systolic blood pressure. Treating the ANG II-infused mice with inhibitors of either p38 MAPK or arginase prevented vascular dysfunction and partially attenuated the increase in blood pressure. Mice infused with ANG II exhibited greater protein levels of both arginase I (6.5-fold) and II (3.4-fold), rather than elevation of only arginase I in BAECs exposed to ANG II. The difference may be related to species and/or tissue. Further study is needed to understand involvement of the isozymes.
Involvement of arginases is also evident from our findings in AI+/−AII−/− mice, which were protected from the vascular dysfunction and increase in blood pressure caused by ANG II. Our findings support a possible role of arginase activation in endothelial dysfunction as reported by us and others. Elevated arginase activity has been also associated with systemic hypertension. Inhibition of arginase has been reported to decrease blood pressure and improve vascular function of resistance vessels in adult hypertensive rats (2, 13). Together with previous studies, our findings thus suggest a central role for arginase in diseases in which vascular dysfunction and hypertension are linked to elevated levels of ANG II. In this study we showed that hydralazine treatment prevented the elevation of blood pressure induced by ANG II without having an effect on arginase activity. This indicates that lowering of blood pressure with p38 MAPK or arginase inhibitors is due to decreasing arginase activity and correcting vascular endothelial dysfunction.
Our results indicate that Rho kinase-mediated p38 MAPK activation is a critical step in the elevation of arginase activity and protein expression in endothelial cells and that p38 activation by ANG II is an important signaling component to enhanced arginase in mouse aorta. Thus, targeting p38 MAPK might be a therapeutic point for preventing vascular endothelial dysfunction associated with elevated arginase activity. However, it must be noted that p38 MAPK has been reported to reduce ANG II-mediated vascular smooth muscle cell hyperplasia by negatively regulating ANG II-induced ERK1/2 activity and DNA synthesis in human coronary artery smooth muscle cells (19). Thus, the long-term use of p38 inhibition for treatment of cardiovascular disease is in question. Therefore, further evaluation of events downstream of p38 activation leading to arginase upregulation in endothelial and other cell types is needed, including investigation of these downstream events as suitable targets to prevent endothelial arginase upregulation and counteract arginase-induced endothelial dysfunction in arginase-associated vascular diseases.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL70215 (to R. W. Caldwell), EY11766 (to R. B. Caldwell and R. W. Caldwell), and EY04618 (to R. B. Caldwell), by NIH T32 Training Grant HL076146 and American Heart Association Predoctoral Fellowship 10PRE4440000 (for A. Shatanawi), and by a Juvenile Diabetes Research Foundation-Innovative Grant 5-2009-468 (to M. J. Romero).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Bruce Tomczuk of Corridor Pharmaceuticals, Inc., for the gift of the arginase inhibitor ABH, Dr. Jennifer C. Sullivan at Georgia Health Sciences University for providing the angiotensin II receptor blockers, Dr. Edward W. Inscho at Georgia Health Sciences University for access to the plethysmography blood pressure recording machine, and Dr. Steven Cederbaum of University of California, Los Angeles, for providing us with the arginase-knockout mice.
REFERENCES
- 1. Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther 279: 1453–1461, 1996 [PubMed] [Google Scholar]
- 2. Bagnost T, Berthelot A, Bouhaddi M, Laurant P, Andre C, Guillaume Y, Demougeot C. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-l-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. J Hypertens 26: 1110–1118, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bregeon J, Loirand G, Pacaud P, Rolli-Derkinderen M. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am J Physiol Cell Physiol 297: C1062–C1070, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Cai H, Li ZM, Dikalov S, Holland SM, Hwang JN, Jo H, Dudley SC, Harrison DG. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin. J Biol Chem 277: 48311–48317, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chandra S, Romero MJ, Shatanawi A, Caldwell RB, Caldwell RW. Peroxynitrite and hydrogen peroxide increase arginase activity through the RhoA/Rho kinase (RAK) pathway (Abstract). FASEB J 24: 959.–4., 2010 [Google Scholar]
- 7. Chang CI, Zoghi B, Liao JC, Kuo L. The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J Immunol 165: 2134–2141, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Chen GF, Wagner L, Sasser JM, Zharikov S, Moningka NC, Baylis C. Effects of angiotensin type 1 receptor blockade on arginine and ADMA synthesis and metabolic pathways in fawn-hooded hypertensive rats. Nephrol Dial Transplant 25: 3518–3525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL, Timmermans PBMWM. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun 165: 196–203, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 174: 231–235, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Costanzo A, Moretti F, Burgio VL, Bravi C, Guido F, Levrero M, Puri PL. Endothelial activation by angiotensin II through NFkappaB and p38 pathways: involvement of NFkappaB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J Cell Physiol 195: 402–410, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Deignan JL, Livesay JC, Yoo PK, Goodman SI, O'Brien WE, Iyer RK, Cederbaum SD, Grody WW. Ornithine deficiency in the arginase double knockout mouse. Mol Genet Metab 89: 87–96, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens 23: 971–978, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, Chilian WM, Zhang C. TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 27: 1269–1275, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension 35: 501–506, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, Andrekopoulos C, Kalyanaraman B, Otterson MF, Rafiee P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 292: G1323–G1336, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Iyer RK, Yoo PK, Kern RM, Rozengurt N, Tsoa R, O'Brien WE, Yu H, Grody WW, Cederbaum SD. Mouse model for human arginase deficiency. Mol Cell Biol 22: 4491–4498, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ju H, Zhao S, Tappia PS, Panagia V, Dixon IM. Expression of Gq alpha and PLC-beta in scar and border tissue in heart failure due to myocardial infarction. Circulation 97: 892–899, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Kintscher U, Bruemmer D, Blaschke F, Unger T, Law RE. p38 MAP kinase negatively regulates angiotensin II-mediated effects on cell cycle molecules in human coronary smooth muscle cells. Biochem Biophys Res Commun 305: 552–556, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology 47: 185–201, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Macrez N, Morel JL, Kalkbrenner F, Viard P, Schultz G, Mironneau J. A betagamma dimer derived from G13 transduces the angiotensin AT1 receptor signal to stimulation of Ca2+ channels in rat portal vein myocytes. J Biol Chem 272: 23180–23185, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Marinissen MJ, Chiariello M, Tanos T, Bernard O, Narumiya S, Gutkind JS. The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell 14: 29–41, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Marrero MB, Fulton D, Stepp D, Stern DM. Angiotensin II-induced signaling pathways in diabetes. Curr Diabetes Rev 1: 197–202, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Mayer RJ, Callahan JF. p38 MAP kinase inhibitors: a future therapy for inflammatory diseases. Drug Discov Today Ther Strateg 3: 49–54, 2006 [Google Scholar]
- 25. Meyer BH, Freuler F, Guerini D, Siehler S. Reversible translocation of p115-RhoGEF by G(12/13)-coupled receptors. J Cell Biochem 104: 1660–1670, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation 110: 3708–3714, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr 22: 87–105, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Nangle MR, Cotter MA, Cameron NE. Correction of nitrergic neurovascular dysfunction in diabetic mouse corpus cavernosum by p38 mitogen-activated protein kinase inhibition. Int J Impot Res 18: 258–263, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Park JK, Fischer R, Dechend R, Shagdarsuren E, Gapeljuk A, Wellner M, Meiners S, Gratze P, Al-Saadi N, Feldt S, Fiebeler A, Madwed JB, Schirdewan A, Haller H, Luft FC, Muller DN. p38 mitogen-activated protein kinase inhibition ameliorates angiotensin II-induced target organ damage. Hypertension 49: 481–489, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes–new concepts. Nephrol Dial Transplant 23: 3047–3049, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodrigues-Diez R, Carvajal-Gonzalez G, Sanchez-Lopez E, Rodriguez-Vita J, Rodrigues Diez R, Selgas R, Ortiz A, Egido J, Mezzano S, Ruiz-Ortega M. Pharmacological modulation of epithelial mesenchymal transition caused by angiotensin II. Role of ROCK and MAPK pathways. Pharm Res 25: 2447–2461, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev 24: 275–290, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102: 95–102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satoh M, Fujimoto S, Arakawa S, Yada T, Namikoshi T, Haruna Y, Horike H, Sasaki T, Kashihara N. Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. Nephrol Dial Transplant 23: 3806–3813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi O, Morris SM, Jr, Zoghbi H, Porter CW, O'Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol 21: 811–813, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiga N, Hirano K, Hirano M, Nishimura J, Nawata H, Kanaide H. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ Res 96: 1014–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the UK: a cohort study using the general practice research database. Diabetes Care 29: 798–804, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Szabo C, Pacher P, Zsengeller Z, Vaslin A, Komjati K, Benko R, Chen M, Mabley JG, Kollai M. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med 10: 28–35, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thengchaisri N, Hein TW, Wang W, Xu X, Li ZB, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 26: 2035–2042, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Toque HA, Romero MJ, Tostes RC, Shatanawi A, Iddings J, Carneir Z, Inscho E, Webb RC, Caldwell RB, Caldwell RW. Decrease of arginase activity by p38 mitogen-activated protein kinase inhibition improves corpora cavernosal relaxation in angiotensin-II mice. J Sex Med 7: 3857–3867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Umapathy NS, Li W, Mysona BA, Smith SB, Ganapathy V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Muller cells. Invest Ophthalmol Vis Sci 46: 3980–3987, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Underwood DC, Osborn RR, Kotzer CJ, Adams JL, Lee JC, Webb EF, Carpenter DC, Bochnowicz S, Thomas HC, Hay DW, Griswold DE. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J Pharmacol Exp Ther 293: 281–288, 2000 [PubMed] [Google Scholar]
- 43. Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD. Cloning and characterization of the human type II arginase gene. Genomics 38: 118–123, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 147: 2518–2525, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Xiong W, Kojic LZ, Zhang L, Prasad SS, Douglas R, Wang Y, Cynader MS. Anisomycin activates p38 MAP kinase to induce LTD in mouse primary visual cortex. Brain Res 1085: 68–76, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Yang L, Lewis CM, Chandrasekharan UM, Kinney CM, Dicorleto PE, Kashyap VS. Arginase activity is increased by thrombin: a mechanism for endothelial dysfunction in arterial thrombosis. J Am Coll Surg 203: 817–826, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Yang Z, Ming XF. Endothelial arginase: a new target in atherosclerosis. Curr Hypertense Rep 8: 54–59, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Zhang W, Baban B, Rojas M, Tofigh S, Virmani SK, Patel C, Behzadian MA, Romero MJ, Caldwell RW, Caldwell RB. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am J Pathol 175: 891–902, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu W, Chandrasekharan UM, Bandyopadhyay S, Morris SM, Jr, DiCorleto PE, Kashyap VS. Thrombin induces endothelial arginase through AP-1 activation. Am J Physiol Cell Physiol 298: C952–C960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]