Abstract
While acute tissue injury potently induces endogenous danger signal expression, the role of these molecules in chronic wound healing and lymphedema is undefined. The purpose of this study was to determine the spatial and temporal expression patterns of the endogenous danger signals high-mobility group box 1 (HMGB1) and heat shock protein (HSP)70 during wound healing and chronic lymphatic fluid stasis. In a surgical mouse tail model of tissue injury and lymphedema, HMGB1 and HSP70 expression occurred along a spatial gradient relative to the site of injury, with peak expression at the wound and greater than twofold reduced expression within 5 mm (P < 0.05). Expression primarily occurred in cells native to injured tissue. In particular, HMGB1 was highly expressed by lymphatic endothelial cells (>40% positivity; twofold increase in chronic inflammation, P < 0.001). We found similar findings using a peritoneal inflammation model. Interestingly, upregulation of HMGB1 (2.2-fold), HSP70 (1.4-fold), and nuclear factor (NF)-κβ activation persisted at least 6 wk postoperatively only in lymphedematous tissues. Similarly, we found upregulation of endogenous danger signals in soft tissue of the arm after axillary lymphadenectomy in a mouse model and in matched biopsy samples obtained from patients with secondary lymphedema comparing normal to lymphedematous arms (2.4-fold increased HMGB1, 1.9-fold increased HSP70; P < 0.01). Finally, HMGB1 blockade significantly reduced inflammatory lymphangiogenesis within inflamed draining lymph nodes (35% reduction, P < 0.01). In conclusion, HMGB1 and HSP70 are expressed along spatial gradients and upregulated in chronic lymphatic fluid stasis. Furthermore, acute expression of endogenous danger signals may play a role in inflammatory lymphangiogenesis.
Keywords: high-mobility group box 1, heat shock protein 70, inflammation
inflammation after tissue injury is a critical component of wound repair. Inflammatory cells migrate to the wound and promote tissue regeneration by removing cellular debris, killing and phagocytosing invading pathogens, and producing cytokines that promote angiogenesis, collagen production, cellular migration, and wound epithelialization. Activation of the innate immune system is critical for these responses, as depletion of macrophages is known to significantly inhibit wound repair and regeneration (15, 29). Although it is clear that inflammatory cell migration and innate immune system activation are critical for wound repair, the cellular mechanisms that regulate these responses have not been completely elucidated. Previous studies have focused on production of local cytokines by degranulating platelets (26) and activation of immune responses by invading pathogens (1); however, these mechanisms fail to provide a rationale for inflammation during wound healing responses in platelet-deficient animals or in sterile wounds (25, 43). Therefore, recent studies have investigated the role of locally produced factors released as a result of tissue injury in the regulation of inflammation, wound repair, and tissue remodeling (6, 39).
The hypothesis that locally produced tissue factors can regulate inflammatory responses is supported by studies of traumatic injury (23), ischemia-reperfusion (19, 53), and autoimmunity (40), demonstrating that expression of endogenous molecular signals can promote activation of innate immune responses and sterile inflammation (52). These endogenous mediators of inflammation, termed endogenous danger signals, include high-mobility group box 1 (HMGB1), S100A8, heat shock protein 70 (HSP70), and a variety of others. These molecules are ubiquitously expressed, normal cell constituents released by damaged cells (46) or secreted by activated immune cells (12, 47, 54). Similar to pathogen-associated molecular patterns, endogenous danger signals activate pattern recognition receptors including toll-like receptors (TLRs) to initiate innate immune responses (6). The importance of these pathways to wound repair is illustrated by studies conducted on mice deficient in MyD88, a downstream effector of TLRs, demonstrating abnormal dermal wound healing as a result of dysregulated angiogenesis and macrophage function (24). In addition, recent studies have demonstrated that HMGB1 expression is decreased during impaired wound healing in diabetic mice and that exogenous application of this molecule can promote wound repair in these (49) and other circumstances by increasing cellular migration and proliferation (8, 33, 35). However, the precise spatial and temporal patterns of HMGB1 expression during wound healing are less well characterized. Furthermore, the cellular sources of endogenous danger signal expression during wound repair are inadequately described.
Similar to chronic wounds, sustained inflammatory responses are known to occur pathologically in lymphedema, and recent studies suggest that this response is an important regulator of lymphatic dysfunction and soft tissue fibrosis (5). In addition, microarray analysis of lymphedematous tissues has demonstrated that lymphedema is a potent inducer of gene expression for several endogenous danger signals, suggesting that these molecules contribute to the inflammatory reaction associated with lymphedema (50). No previous studies, however, have determined the expression patterns of these molecules in lymphedematous tissues or the role they play in the regulation of inflammatory lymphangiogenesis, thus limiting our knowledge of the potential complex roles of endogenous danger signals in the regulation of inflammation and lymphangiogenesis.
In the current study, we determined the spatial and temporal patterns of the endogenous danger signals HMGB1 and HSP70 using surgical mouse models. During wound healing, we found that the primary sources of these molecules are a variety of native cell types including fibroblasts, adipocytes, and endothelial cells. Interestingly, we show that expression of HMGB1 is upregulated in lymphatic endothelial cells (LECs) in response to acute and chronic inflammation. In addition to wound healing, HMGB1 and HSP70 expression was upregulated by lymphatic fluid stasis both in the mouse tail model and in a mouse axillary dissection model. Similarly, HMGB1 and HSP70 expression was increased in lymphedematous tissues harvested from patients with secondary lymphedema compared with the normal contralateral limb. Finally, we show that the blockade of HMGB1 function attenuates inflammatory lymphangiogenesis. Taken together, our data suggest that the expression of endogenous danger signals is increased during wound repair and in association with lymphatic fluid stasis. Expression of HMGB1 in response to inflammation may play a role in regulating lymphangiogenesis.
METHODS
Lymphedema Models
Mouse tail model of wound healing and lymphedema.
Tail incision or excision was performed in 8- to 10-wk-old female C57BL/6 (The Jackson Laboratory, Bar Harbor, ME) mice using previously described techniques (3, 9). Briefly, for incisional healing, a circumferential incision of the mouse tail was made 20 mm from the base of the tail with care taken not to disrupt the deep lymphatic system or the lateral tail veins. For excisional wound healing, a 2-mm wide circumferential portion of skin was excised 20 mm from the base of the tail, thereby disrupting the superficial lymphatic system. In addition, the deep collecting lymphatics adjacent to the paired lateral tail veins were disrupted using a dissecting microscope (Stereozoom SZ-4; Leica, Wetzlar, Germany). Tegaderm dressings were applied circumferentially and removed after 5 days. We and others (7, 9) have shown that tail excision leads to sustained tail edema for >6 wk, whereas tail incision results in minimal edema and rapid wound repair by ∼14 days postoperatively (50). Animals (n = 7–10 per group per time point) were euthanized after 1, 2, 3, or 6 wk postoperatively, and tail tissue was harvested for tissue sectioning and protein extraction.
Axillary dissections.
C57BL/6 female mice (8–10 wk old; n = 8) were injected in the right front paw with 10 μl 1% Evans blue dye (Sigma-Aldrich, St. Louis, MO) and anesthetized (80 mg/kg ketamine and 12 mg/kg xylazine). An incision was made in the right axilla and carried down through clavipectoral fascia to the axillary fat pad and axillary lymph nodes. Blue-stained nodes and any associated axillary lymph nodes were removed along with the axillary fat pad. The same amount of Evans blue dye was injected in the contralateral paw and an identical incision was made in the contralateral (left) axilla, and but no lymphadenectomy was carried out. Micro-CT scans (MicroCAT II, ImTek, Berlin, Germany) of front limbs were performed at 3 wk postoperatively, and bilateral arm area calculations were determined by analysis of transverse CT slices using ASIPro (Concorde Micro-systems, Knoxville, TN) and IDL Imaging Software (Research Systems). Tissue was harvested (n = 5) from the upper and lower portion of the limb and protein pooled for Western blot analysis of HSP70 and HMGB1 expression. All animal experiments followed regulations set forth by the Institutional Animal Care and Use Committee and Resource Animal Research Center at Memorial Sloan Kettering Cancer Center.
Human chronic lymphedema.
Six patients with chronic grade II or grade III lymphedema of an upper extremity secondary to axillary dissection underwent 5-mm punch biopsies of the lymphedematous limb and the exact same location of the contralateral normal limb as previously described (4). Matched punch biopsy sections were paraffin embedded and sectioned for further histological study. All patients identified gave informed consent and all protocols for the use of human subjects were approved by the Stanford University IRB Committee.
Lymphangiogenesis Models
Chronic peritonitis model.
C57BL/6 mice 8–12 wk of age (n = 6 per group) were administered lipopolysaccharide (LPS, 50 mg, Sigma-Aldrich) or phosphate-buffered saline (PBS) injections intraperitoneally daily for 2 wk. Mice were euthanized and diaphragms were excised and embedded in OCT media (Tissue-TEK, Torrence, CA). Fresh frozen tissue sections (5 μm) were prepared with transverse cuts through diaphragms for histological staining.
Inflammatory lymph node lymphangiogenesis.
C57BL/6 mice 8–12 wk of age (n = 7 per group) were administered 20 μl of a 1:1 mixture of complete freund's adjuvant (CFA, Sigma):ovalbumin (OVA, Sigma; 2 mg/ml) in the hindlimb paw. PBS (20 μl) was administered to the hindlimb paw of the contralateral limb. To inhibit HMGB1 function, animals in the experimental group received intraperitoneal injections of glycyrrhizin (GLZ, 10 mg every other day, Calbiochem, San Diego, CA) starting 24 h before paw injection, while control animals received intraperitoneal injections of PBS according to the same schedule (10). GLZ is small molecule inhibitor previously described to directly bind and inhibit chemotactic and mitogenic activity of HMGB1 (14, 30). A 100 mg/ml GLZ solution was prepared in a 50 mM NaOH and brought to pH 7.4 according to previously published methods (10). After a 5-day treatment period, animals were euthanized, and popliteal lymph nodes were harvested and embedded for fresh frozen section preparation and histological staining.
Tail Volume Measurement
Tail diameters were measured by two blinded investigators at 10-mm intervals from the distal wound edge using a digital caliper at weekly intervals in each animal, and tail volumes were calculated using a truncated cone formula according to previously published methods (9). Pre- and postoperative volumes were compared in the same animal to determine percent change in volume over time.
Histology
For sectioning, tail specimens prepared from sagittal sections containing the wound bed were fixed in 4% paraformaldehyde, decalcified in Immunocal reagent (Tallman Chemical, Tallman, NY), paraffin-embedded, and prepared in 5-μm sections for histological analysis.
Immunohistochemistry (IHC) was performed on paraffin-embedded mouse tail and human tissue sections to analyze endogenous danger signal expression patterns. Primary antibodies were used against HMGB1 and HSP70 (Abcam, Cambridge, MA), and staining was performed according to our previously published techniques (27). Briefly, permanent sections were deparaffinized, rehydrated, and permeabilized with 0.5% Triton X-100 (Sigma), followed by antigen retrieval in boiling 10 mM citric acid. Endogenous peroxidase activity was quenched with 0.6% hydrogen peroxide in methanol, nonspecific binding was blocked with 20% normal serum, and sections were incubated overnight with primary antibody against HMGB1 or HSP70. Biotinylated secondary antibody and avidin-peroxidase complex were applied (Vectastain ABC kit, Vector Laboratories, Burlingame, CA), and 3,3′ diaminobenzidine tetrahydrochloride (Dako, Carpinteria, CA) was used for antibody-antigen binding detection. Slides were counterstained with Harris hematoxylin (Dako), dehydrated, mounted, and analyzed using brightfield microscopy (Axioscope 40, Carl Zeiss, Germany), and images were captured using a Mirax slide scanner (Carl Zeiss). For mouse tail sections, two independent, blinded reviewers counted the number of positively stained cells in at least six to seven high-powered fields (hpf, ×40) per tissue section (n = 5 animals per group) at fixed intervals (1 or 5 mm) proximal and distal to the center of the wound bed. For human sections, two independent reviewers counted positively stained cells in 10–15 hpf per section; counts were expressed as a fraction of the total number of cells per hpf (percent positive cells). Sections from both mouse and human tissue were analyzed by a blinded pathologist.
Immunofluorescence staining of fresh frozen tissues including diaphragms and draining lymph nodes was performed. Briefly, slides were air dried, fixed in acetone (Sigma), and permeabilized with Triton-X. Slides were blocked with 20% normal serum and incubated sequentially with primary antibody against HMGB1, lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), or podoplanin (all from Abcam), followed by tetramethyl rhodamine isothiocyanate (TRITC) and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies, respectively (Invitrogen, Carlsbad, CA). Immunofluorescence was imaged using fluorescence microscopy (Akioskope, Carl Zeiss) and AxioVision imaging software (Carl Zeiss). Immunofluorescence staining of tail tissue was performed similarly with hydration and dehydration steps included. For HMGB1/podoplanin colocalization, HMGB1-podoplanin double-positive cells were counted by two independent, blinded reviewers and counts expressed as a percentage of HMGB1+ LECs per hpf. For vessel density calculations, the number of LYVE-1+ vessels per hpf was determined by blinded reviewers and expressed per area of lymph node tissue, according to previously established techniques (21).
Western Blot Analysis
After the mice were euthanized, 5-mm tissue sections were harvested at 10-mm intervals along the tail for protein extraction (n = 3–5 animals per group per time point). Tissue was snap frozen in liquid nitrogen and homogenized, and total cellular protein fractions were extracted using T-PER extraction reagent (ThermoFisher Scientific, Rockford, IL) according to the manufacturer's instructions. Alternatively, for analysis of NF-κβ, a downstream regulator of HMGB1, we isolated both the cytoplasmic and nuclear (activated) fractions using N-PER extraction reagent (ThermoFisher Scientific). Protein was quantified using the Bradford technique (Bio-Rad, Hercules, CA), pooled (n = 3–5 samples pooled per tail section), and analyzed by Western blot analysis. Briefly, 20 μg of protein were subject to 10% SDS-polyacrylamide gel electrophoresis (1× Tris-glycine-0.1% SDS buffer) followed by protein transfer to polyvinylidene fluoride membrane (Bioexpress, Kaysville, UT) for 2 h at 4°C in 1× Tris-glycine-20% methanol buffer. Membranes were blocked using 5% nonfat milk in 1× Tris-buffered saline Tween20 solution.
Primary antibodies were used against HMGB1, HSP70, and NF-κβ (all from Abcam) followed by membrane incubation with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Antigen-antibody binding was detected by ECL Plus Western Blotting Detection System (GE Healthcare, Little Chalfont, UK). Equal loading was confirmed by detection of β-tubulin (Santa Cruz), β-actin (Abcam), or TATA binding protein (TATA BP), a transcription factor that binds to a DNA sequence called TATA box and serves as a nuclear loading control (Abcam). All experiments were performed in at least triplicate. Quantification of Western blots was performed using ImageJ analysis (software available at http://rsweb.nih.gov/ij/), and relative signal densities compared as previously described (4).
Statistical Analysis
Multigroup comparisons involving more than one factor (i.e., two time points) were compared using a two-way ANOVA with a Bonferroni posttest analysis. Multigroup comparison involving one factor was performed using one-way ANOVA with the Tukey-Kramer post hoc test. Student's t-test was used for analyzing differences between two groups. For clinical lymphedema samples, a matched Student's t-test was used comparing differences from matched normal and lymphedema samples for each patient. Data are presented as means ± SD unless otherwise noted.
RESULTS
Endogenous Danger Signals are Expressed Along a Spatial Gradient Relative to the Site of Tissue Injury
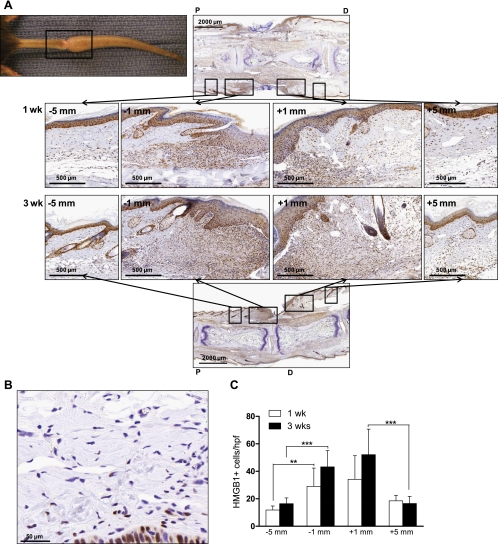
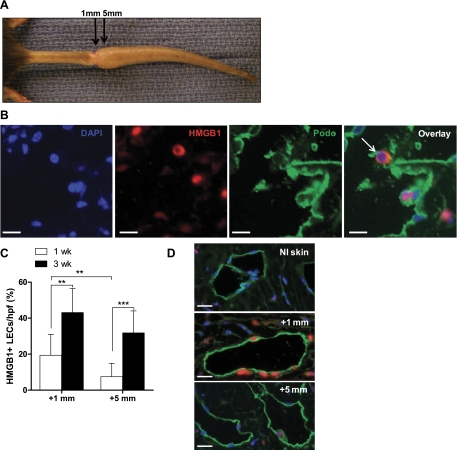
One week after tail skin excision, immunohistochemical staining revealed peak HMGB1 expression at the site of tissue injury and the presence of a spatial gradient of HMGB1 expression relative to the site of injury (Fig. 1A). Peak HMGB1 expression occurred within 1 mm of the center of injury (29.0 ± 13.4 cells/hpf, −1 mm, 34.2 ± 17.2 cells/hpf, +1 mm, Fig. 1C) with a twofold reduction in cellular expression at more proximal locations (within 5 mm, Fig. 1C, P < 0.01) and similar though nonsignificant reduction distally (P = 0.055). This spatial expression gradient persisted 3 wk postoperatively (Fig. 1A, bottom) with peak expression occurring within 1 mm of the injury and decreasing in more distally located tissues (52.1 ± 18.6 cells/hpf, +1 mm, 16.6 ± 5.2 cells/hpf, +5 mm, P < 0.001, Fig. 1C). Similarly, the number of HMGB1-positive cells/hpf decreased in regions located 5-mm proximal (2.6-fold reduction) and distal (3.2-fold reduction) to the center of the wound (Fig. 1C, P < 0.001), demonstrating clear gradients of HMGB1 expression in relation to the wound. The differences between regions immediately proximal and immediately distal to the wound were not statistically significant. Similarly, although the mean number of positive cells was higher in the region immediately adjacent to the wound at 3 wk compared with those from 1 wk, these differences did not reach statistical significance. Noninjured tail skin of control animals demonstrated minimal cellular HMGB1 expression, primarily located within the nucleus of dermal fibroblasts and keratinocytes (Fig. 1B). Immunohistochemical analysis of injured tails additionally revealed nuclear-to-cytoplasmic redistribution of HMGB1 compared with noninjured controls. This was evidenced primarily in fibroblasts and keratinocytes of the dermis (Fig. 2, A and B) in accordance with known mechanisms of HMGB1 activation including cytoplasmic translocation from the nucleus (13, 55).
Fig. 1.
High-mobility group box 1 (HMGB1) expression occurs along a spatial gradient relative to the site of tissue injury. A: gross representation of mouse tail wound (top left). The indicated (black boxed) region was sectioned sagitally at 1 or 3 wk postoperatively and subjected to immunohistochemical staining for HMGB1. Representative ×3 and ×10 images are displayed across the region of the wound. ×10 images represent the regions proximal and distal to the wound (5 or 1 mm on either side). B: representative immunohistochemistry (IHC) for HMGB1 performed on noninjured dermis of the mouse tail. C: number of positively stained cells were counted at 5 or 1 mm proximal (−5 mm, −1 mm) or distal (+1 mm, +5 mm) to the wound as indicated for both postoperative time points. Bars represent the mean number of positive cells per high power field (hpf) ± SD, **P < 0.01, ***P < 0.001.
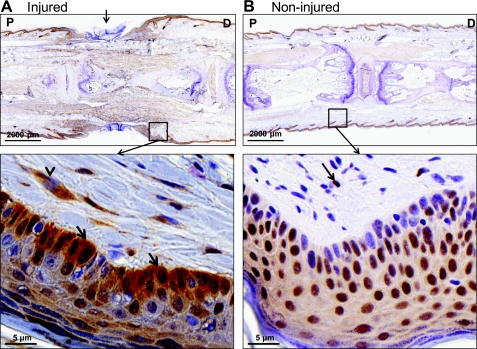
Fig. 2.
HMGB1 translocates from the nucleus to the cytoplasm following tissue injury. A: representative IHC (×3, top) for HMGB1 across the site of injury (arrow) at 3 wk postoperatively. HMGB1 staining of dermis and epidermis is represented below (×20); arrowheads indicate cytoplasmic staining (brown) in fibroblasts; arrows indicate cytoplasmic staining in keratinocytes. B: IHC for HMGB1 of noninjured mouse tail (×3, top); arrows indicate nuclear staining of fibroblasts and keratinocytes.
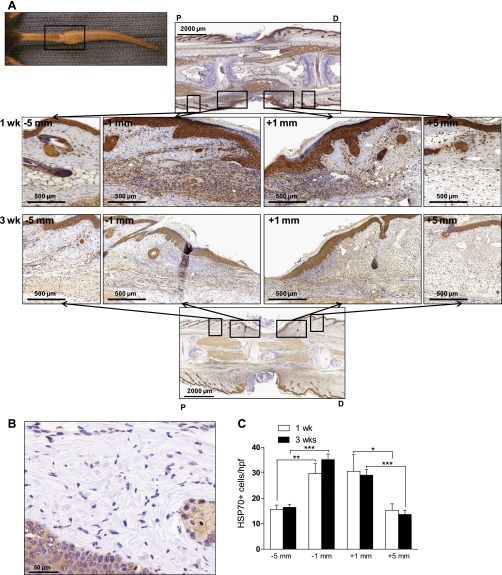
HSP70, a chaperone protein released in response to cellular stress (32), demonstrated an expression pattern similar to that of HMGB1 in response to tissue injury. By IHC, peak cytoplasmic HSP70 expression occurred immediately proximal and distal (within 1 mm) to the center of tissue injury at postoperative week 1, and a spatial gradient of expression was present relative to the site of injury (Fig. 3A). Similar to HMGB1, the number of positively stained cells decreased approximately twofold at more proximal and distal locations (within 5 mm, Fig. 3C, P < 0.01). This trend persisted at 3 wk postoperatively as cell counts were largely unchanged (for example, 30.7 ± 6.4 HSP70+ cells/hpf at 1 wk vs. 29.1 ± 2.2 cells/hpf at 3 wk, P > 0.05, 1 mm distal to the wound, Fig. 3C). In contrast, noninjured skin from control animals had limited dermal and epidermal expression of HSP70 (Fig. 3C). There was no statistically significant difference in regions immediately proximal or immediately distal to the wound. Together, these data demonstrate that tissue injury induces a local gradient of endogenous danger signal expression relative to the site of injury, and this expression persists into subacute phases of wound healing, at least 3 postoperative weeks.
Fig. 3.
Heat shock protein 70 (HSP70) expression occurs along a spatial gradient relative to the site of tissue injury. A: gross representation of mouse tail wound (top left). The indicated (black boxed) region was sectioned sagitally at 1 or 3 wk postoperatively and subjected to immunohistochemical staining for HSP70. Representative ×3 and ×10 images are displayed across the region of the wound. ×10 images represent the regions proximal and distal to the wound (5 or 1 mm on either side). B: representative IHC for HSP70 performed on noninjured dermis of the mouse tail. C: number of positively stained cells were counted at 5 or 1 mm proximal (−5 mm, −1 mm) or distal (+1 mm, +5 mm) to the wound as indicated for both postoperative time points. Bars represent the mean number of positive cells per hpf ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
Endogenous Danger Signal Expression is Induced in Cells Native to Injured Tissue
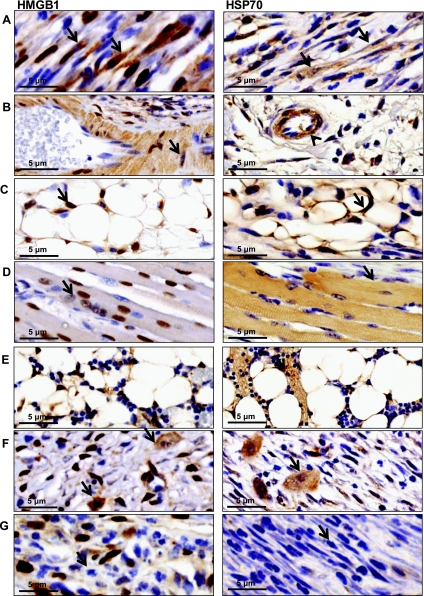
We next sought to determine the cellular origins of endogenous danger signal expression after tissue injury. Analysis of immunohistochemical staining distal to the site of injury in mouse tail sections harvested 3 wk postoperatively revealed that cells endogenous to injured tissue including fibroblasts, endothelial cells, smooth muscle cells of vascular endothelium, and adipocytes (Fig. 4, A–C) demonstrated positive intracellular staining for both markers compared with nonintervened controls. Nuclear-to-cytoplasmic redistribution of HMGB1 was apparent in fibroblasts and myofibroblasts (Fig. 4A, left), again suggesting activation of this molecule. Skeletal muscle in proximity to the site of injury was positive for HMGB1 and HSP70 (Fig. 4D). Expression of both markers was upregulated by postoperative week 3 (Fig. 4F) in macrophages but failed to show uniform upregulation in this population by week 1. This finding was supported by positive staining within the bone marrow for both markers at week 3 (Fig. 4E). Infiltrating neutrophils and lymphocytes were negative for both markers at each time point (Fig. 4G). These findings suggest that endogenous danger signal expression in injured tissues occurs primarily in intact cells native to the site of tissue injury with expression in inflammatory cells at later time points.
Fig. 4.
Endogenous danger signal expression is induced in cells native to injured tissue. Representative high-power views (×100) of immunohistochemical staining for HMGB1 (left) or HSP70 (right) obtained 3–5 mm distal to tail wounds at 3 wk postoperatively. Positive cellular staining for both markers was present in fibroblasts (arrow) (A), vascular endothelium (arrowhead) (B) including smooth muscle cells of vascular endothelium (arrow), and adipocytes (C) (arrow). Skeletal muscles cells in close proximity to the wound stained positively for HMGB1 but not HSP70 (D). Bone marrow (E) and macrophages (F) stained positively for HSP70 and HMGB1 at postoperative week 3. Infiltrating lymphocytes and neutrophils remained negative (G). N = 5 slides were analyzed per group by a blinded pathologist and independent reviewer.
Tissue Injury Induces Endogenous Danger Signal Expression by Lymphatic Endothelial Cells
Interestingly, immunohistochemical staining demonstrated expression of HMGB1 in numerous LECs in the tail sections. Double immunofluorescence for HMGB1 and podoplanin, an LEC marker, localized HMGB1 (red, TRITC) to LECs (green, FITC) in tail sections harvested at 1 and 3 wk postoperatively (Fig. 5, A and B). The distribution of HMGB1 occurred in a nuclear-to-cytoplasmic pattern (overlay, Fig. 5B). More than 40% of the LECS in the region within 1 mm of the wound stained positively for HMGB1 (Fig. 5C) at 3 wk. Similar to other resident cell types, HMGB1 expression occurred in a gradient relative to the site of tissue injury. A higher proportion of LECs stained positively for HMGB1 immediately distal (within 1 mm) to the site of injury with a 2.4-fold and 1.4-fold reduction in expression within 5 mm of the wound at postoperative weeks 1 and 3, respectively (P < 0.001 at 1 wk, not significant at 3 wk, Fig. 5, C and D). Furthermore, LEC expression of HMGB1 increased from 1 to 3 wk in both tissue regions, with a 2.2-fold increase in expression in the immediately distal region (Fig. 5C, P < 0.01) and a 3.9-fold increase 5 mm from the site of injury (Fig. 5C, P < 0.001). Similar to expression of HMGB1 in other native cells, the expression of this molecule by LECs correlated with proximity to the wound, and there was no difference in sections immediately proximal or immediately distal to the wound (not shown).
Fig. 5.
Tissue injury induces endogenous danger signal expression by lymphatic endothelial cells. A: gross representation of the injured mouse tail at 3 wks postoperatively; double immunofluorescence for HMGB1 and podoplanin was performed on longitudinal sections and analyzed immediately distal (within 1 mm) to the wound or 5 mm distal to the wound as indicated. B: DAPI (blue), HMGB1 [tetramethyl rhodamine isothiocyanate (TRITC), red], and podoplanin [fluorescein isothiocyanate (FITC), green] single stains and image overlays are displayed. Arrows indicate HMGB1-podoplanin double-positive cells. C: counts of positively stained cells were performed at 1 or 5 mm distal to the center of the wound. Mean percentage of HMGB1+ LECs per hpf is displayed. Bars represent means ± SD, **P < 0.01, ***P < 0.001. D: representative lymphatic vessel staining for HMGB1 within normal skin, 1 mm distal to tail wounds, or 5 mm distal to the wound.
Endogenous Danger Signal Expression is Upregulated in Sustained Lymphatic Fluid Stasis
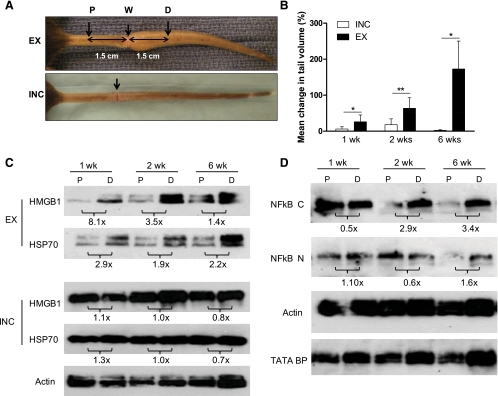
Recent microarray analysis of gene activation in lymphedematous tissue has suggested that endogenous danger signal expression is upregulated in the setting of chronic lymphedema (50). Based on these findings, we hypothesized that lymphatic fluid stasis in addition to wound healing may induce endogenous danger signal expression. To test this hypothesis, we performed tail excisions to remove the superficial and deep lymphatic system and compared tissue from the proximal nonlymphedematous region of the tail (1.5 cm proximal to the skin excision site, P, Fig. 6A) to tissues exposed to sustained lymphatic fluid stasis 1.5 cm distal to the wound (D, Fig. 6A). We chose these regions because they are equidistant from the wound and, more importantly, well away from the zone of inflammation associated with the wound itself. For example, the regions examined in these experiments are nearly 15-fold further from the wound than those examined in Figs. 1–5. In addition, to further control for the effects of wound healing alone, we performed tail incisions in which the skin was incised but deep lymphatics were uninjured; tissues from the same regions of the tail (P and D; Fig. 6A) were then harvested.
Fig. 6.
Endogenous danger signal expression is upregulated in sustained lymphatic fluid stasis. A: representative gross images of tails 3 wk postoperatively following tail skin excision and lymphatic disruption (EX, top) or circumferential incision without lymphatic disruption (INC, bottom). Protein from tail tissues was harvested 1.5 cm proximal (P) or distal (D) to the wound (W). B: mean percentage change in tail volume at 1, 2, and 6 wk postoperatively (from preoperative baseline) are displayed; bars represent means ± SD. *P < 0.05, **P < 0.01. C: Western blot analysis of pooled protein samples (n = 5) from the indicated locations (P, D) for HMGB1, HSP70, or actin at 1, 2, and 6 wk performed for both EX and INC groups. Protein from 1, 2, and 6 wk time points were run simultaneously. ImageJ analysis comparing band density at P and D and normalized to actin are displayed as fold change below blots. D: cytoplasmic and nuclear protein fractions isolated from the same tissue regions were subjected to Western blot analysis for NF-κβ (NF-κβ C and NF-κβ N, respectively), actin, or TATA BP nuclear loading control at 1, 2, and 6 wk. ImageJ analysis demonstrating fold change from P to D and normalized to loading control is displayed below blots. All blots were run in at least triplicate and fold change in band density represents an average of at least 3 ImageJ analyses.
As expected, tail excision resulted in significant tail volume increases over time demonstrating a 26% increase at 1 wk, 64% increase by 2 wk, and 172.9% increase after 6 wk. In contrast, animals that underwent tail incision developed only minimal edema postoperatively, peaking at 18% by 2 wk and virtually no change by 6 wk (Fig. 6B). The differences in tail volumes between excision- and incision-treated animals were statistically significant at all time points (P < 0.05).
At all time points examined, comparison of HMGB1 and HSP70 expression in P and D tail regions revealed increased expression of these molecules in the distal portions of the tail exposed to lymphatic fluid stasis (Fig. 6C). Even 6 wk after surgery, HMGB1 expression was increased 1.4-fold and HSP70 expression was increased 2.2-fold in the distal tissues relative to the proximal regions of the tail. Furthermore, the expression of these molecules in the distal, lymphedematous segment of the tail increased progressively over time, such that the expression of both HMGB1 and HSP70 was markedly higher (2.2-fold and 1.8-fold, respectively) in distal tail tissues harvested 6 wk after surgery compared with those harvested 1 wk postoperatively. In contrast, expression of HMBG1 and HSP70 in incision-treated animals demonstrated minimal differences when comparing proximal to distal segments of the tail (Fig. 6C) and little difference as a function of time postoperatively. Taken together, these findings strongly suggest that expression of HMBG1 and HSP70 is regulated by gradients of lymphatic fluid stasis.
Cytoplasmic expression of NF-κβ, a downstream effector activated by both HMGB1 and HSP70 in proximal and distal tail tissues harvested from excision-treated animals, demonstrated a progressive increase over time in the D segment (3.4-fold increase over P expression by 6 wk, Fig. 6D). Nuclear NF-κβ expression paralleled these findings, although we noted a smaller relative fold-change in overall expression comparing proximal to distal tissues 6 wk following surgery (1.6-fold increase over P, Fig. 6D). These findings correlate with the expression patterns of HMGB1 and HSP70 over time in tissues exposed to sustained lymphedema and suggest that the pathways downstream from endogenous danger signals are activated by sustained lymphatic fluid stasis.
Endogenous Danger Signal Expression is Upregulated After Axillary Lymph Node Dissection
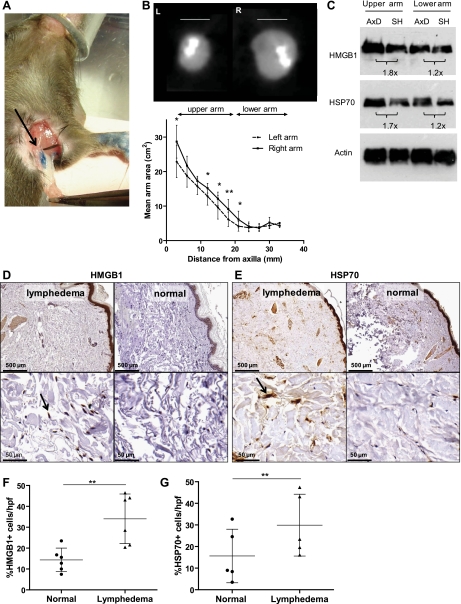
In an effort to further differentiate the effects of wound healing from lymphatic fluid stasis on the expression of endogenous danger signals, we modified a mouse axillary lymph node dissection model (51) and determined the expression of HMGB1 and HSP70 in various regions of the arm comparing animals that underwent lymph node dissection with those that had axillary incision only (sham operation; Fig. 7A). Three weeks after surgery, we found that axillary dissection resulted in a modest but statistically significant increase in limb volume in the upper portions of the limb when analyzed by MicroCT (P < 0.05; Fig. 7B). This finding is not surprising since lymph node dissection does not cause lymphedema acutely either in animals or in the clinical setting (38). Western blot analysis of tissues obtained from the upper portions of the arm revealed a 1.8-fold increase in HMGB1 and 1.7-fold increase in HSP70 expression in animals that underwent lymph node dissection compared with sham-operated controls (Fig. 7C). Changes in the lower arm mirrored the changes in limb volume, although were more subtle, resulting in a 1.2-fold increase in expression of both HMGB1 and HSP70 after lymph node dissection. These findings suggest that even modest increases in lymphatic fluid stasis promote the expression of endogenous danger signals and that the expression of these molecules follows gradients of lymphatic fluid stasis.
Fig. 7.
Endogenous danger signal expression is upregulated after axillary lymph node dissection. A: gross image of axillary lymph nodes (arrow) and axillary fat pad resected following distal injection of blue dye in paw. B: axillary dissection (AxD) was performed in the right axilla and sham operation (SH) in the left axilla (n = 8); arm areas were determined from consecutive transverse CT slices as represented. Areas were determined every 3 mm starting 5 mm from the axilla and graphed according to distance distal to the axilla; bars represent means ± SD, *P < 0.05, **P < 0.01. C: Western blot analysis of HMGB1, HSP70, and actin expression from tissues of upper and lower arms (AxD, Axillary dissection; SH, sham). ImageJ analysis of fold-change in band density is displayed below blots. All blots were performed in at least triplicate. D and E: representative HMGB1 and HSP70 immunohistochemical staining of matched human punch biopsy specimen obtained from lymphedematous (left) and nonlymphedematous (right) contralateral limbs (n = 6); representative low (×5, top) and high power (×50, bottom) views are displayed. Arrow indicates positive cell. F and G: percentage of HMGB1+ or HSP70+ cells per hpf is displayed; bars represent means ± SD, **P < 0.01.
To translate our mouse model findings and to determine the effects of chronic lymphedema on the expression of endogenous danger signals, we compared the expression of HMGB1 and HSP70 in matched samples obtained from normal and lymphedematous arms of six patients with grade II or III lymphedema secondary to axillary lymph node dissection using immunohistochemistry. When comparing the normal and lymphedematous limbs of the same patient, we found clear differences in the number of HMGB1 and HSP70-positive cells/hpf (Fig. 7, D and E). To control for differences in cellularity between normal and chronically inflamed lymphedema samples, we calculated the percentage of cells that stained positive for HMGB1 or HSP70 as a function of all cells present in each high-powered field. This analysis revealed a more than twofold (34.0 ± 11.9% vs. 14.4 ± 5.6%, Fig. 7F) increase in the number of HMGB1+ cells (P < 0.01). Similarly, the percentage of HSP70+ cells in lymphedematous tissues was increased almost twofold (29.2 ± 14.4% vs. 15.6 ± 12.4%; P < 0.01, Fig. 7G). Analysis of the cellular origins of HMGB1 and HSP70 in these sections demonstrated similar findings to those from our mouse tail model, including increased staining in native fibroblasts, endothelial cells, epithelium, adipocytes, and sweat glands (∼50% positivity; not shown). Lymphocyte populations largely stained negative for both markers. Most prominent staining included dermal fibroblasts for HSP70. Together, these findings further support the conclusion that the expression of endogenous dangers signals is increased by lymphatic fluid stasis and is chronically upregulated in lymphedematous tissues.
HMGB1 Blockade Attenuates Inflammatory Lymphangiogenesis in Draining Lymph Nodes
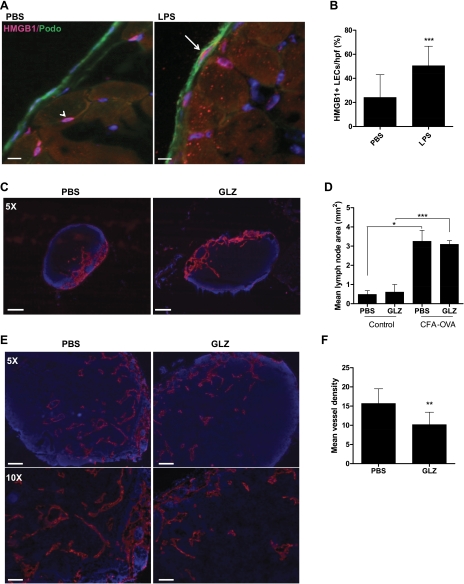
To determine whether inflammation independent of wound healing activates endogenous danger signal expression, we used a model of inflammatory lymphangiogenesis in which mice were administered intraperitoneal injections of LPS daily for 2 wk (n = 6 per group) to induce chronic peritonitis and inflammatory lymphangiogenesis on the peritoneal diaphragm surface (21). LPS induced a moderate though not significant increase in the number of HMGB1-positive cells (see online supplemental Fig. S1 at the AJP-Cell Physiol website). Interestingly, however, LPS treatment resulted in a greater than twofold increase in the number of LECs that stained positive for HMGB1 (50.5 ± 16.2% vs. 24.2 ± 18.9%; P < 0.001; Fig. 8, A and B), suggesting that inflammation upregulates the expression of HMGB1 in LECs.
Fig. 8.
HMGB1 blockade attenuates inflammatory lymphangiogenesis in draining lymph nodes. A: HMGB1 (pink) and podoplanin (green) double immunofluorescence of mouse diaphragms (n = 6 per group) after 2 wk daily administration of PBS or LPS. Arrow indicates costained LEC. Note presence of positively stained cells in PBS-treated animals not colocalized with podoplanin (arrowhead). Scale bars represent 10 μm. B: HMGB1/podoplanin double-positive cells on the peritoneal surface of the diaphragm. Bars represent means ± SD, **P < 0.01, ***P < 0.001. C: lymphatic vessel endothelial hylaluronan (LYVE)-1 stains of popliteal nodes from animals treated with PBS or glycyrrhizin (GLZ). Scale bars represent 100 μm. D: mean lymph node area (mm2) for control or complete freund's adjuvant (CFA)-ovalbumin (OVA)-treated animals treated with PBS or GLZ (n = 6). Bars represent means ± SD, *P < 0.05, ***P < 0.001. E: LYVE-1 staining in lymph nodes harvested from animals treated with CFA-OVA and PBS (left) or GLZ (right). Scale bars represent 200 μm (top) and 100 μm (bottom). F: lymphatic vessel density in animals treated with CFA-OVA and PBS or GLZ. Bars represent means ± SD, **P < 0.01.
Finally, in an effort to determine the functional effects of HMGB1 activation in LECs during inflammation, we used a lymph node lymphangiogenesis model and studied the effects of systemic GLZ, a well-characterized inhibitor of HMGB1 function (30). We used the lymph node model since we found that peritoneal lymphangiogenesis using LPS was associated with some toxicity due to the fact that LPS was administered frequently (i.e., daily for 2 wk). GLZ treatment without CFA-OVA had no significant effect on lymph node lymphangiogenesis or inflammation (Fig. 8, C and D). Treatment with CFA-OVA resulted in marked increases in lymph node size and inflammation either with or without GLZ (Fig. 8D). Interestingly, analysis of lymphatic vessel density demonstrated that blockade of HMGB1 with GLZ significantly decreased CFA-OVA-induced lymphangiogenesis (35% reduction in mean vessel density, P < 0.01, Fig. 8, E and F) suggesting that HMGB1 contributes to the regulation of inflammatory lymphangiogenesis.
DISCUSSION
Endogenous danger signals mediate activation and recruitment of cells of the innate immune system to modulate wound healing and tissue repair (48). To characterize the role of these molecules in the local wound environment, we determined the temporal and spatial patterns of local endogenous danger signal expression following acute tissue injury. Prior studies have demonstrated that the release of endogenous danger signals occurs early although not immediately following sterile tissue injury (20, 23). HMGB1 has since been denoted as a late mediator of inflammation given that serum HMGB1 levels are minimally detectable at 8 h and plateau after 16–32 h following endotoxin exposure (54). Wound healing studies additionally have shown that normal and diabetic mice injured by 4-mm skin punch biopsies demonstrate peak HMGB1 expression at 3 days postinjury (49), and further studies report persistence of these signals at postoperative day 4 following epidermal injury (45). However, few studies have investigated the precise temporal expression of these molecules beyond the early phases of tissue injury.
The results of our study show that local endogenous danger signal expression occurs along a spatial gradient relative to the site of wounding and persist beyond immediate phases of injury. In fact, in our mouse tail model of tissue injury, both local HSP70 and HMGB1 tissue expression were sustained after 3 postoperative weeks. In addition to wound healing, the expression of HMGB1 and HSP70 was elevated in lymphedematous tissues located well away from the zone of injury. Marked upregulation of both molecules was sustained at even 6 wk postoperatively in tissues exposed to persistent edema compared with nonedematous proximal tissues or compared with sham-operated tails. In support of this, HMGB1 and HSP70 was upregulated and correlated with axillary lymphadenectomy in a mouse model. Even more importantly, we found that the expression of endogenous danger signals was increased in tissue obtained from clinical lymphedema samples compared with normal tissues. Finally, our findings are supported by the fact that both cytoplasmic and nuclear expression of NF-κβ, a downstream effector of HMGB1 (34), persisted and were elevated even 6 wk after surgery, suggesting downstream activation of pathways regulated by HMGB1 (17, 22, 36) within lymphedematous tissues. Together, these findings suggest that HMGB1 and HSP70 expression are regulated not only by wound healing but by gradients of interstitial fluid stasis.
Whereas endogenous danger signals are known to undergo extracellular secretion following tissue injury and serve as proinflammatory mediators (2, 54), the precise cellular origins of these molecules have not been determined in the setting of chronic wounds. Previous studies have shown that HMGB1 is actively secreted by monocytes (12), macrophages (42, 54), NK cells, and mature myeloid dendritic cells (47). In addition, this molecule is passively released from necrotic cells (46). In the current study, we show that increased expression of HMGB1 and HSP70 occurs in native cell types including fibroblasts, muscle and nerve cells, adipocytes, and LECs both acutely and even as long as 3 wk after wounding. In addition, we found that expression of these factors in macrophages occurred primarily in the subacute phases of wound healing (i.e., 3 wk postoperatively). Thus it is possible that initial release of endogenous danger signals from necrotic cells in the wound as well as active production of these molecules by native and infiltrating inflammatory cells contribute to ongoing inflammation and initiation of reparative cascades. This hypothesis is supported by the findings of Hamada et al. (16) in models of pulmonary fibrosis demonstrating HMGB1 expression in bronchiolar epithelial cells in the early phases of bleomycin injury followed by expression in infiltrating inflammatory cells and epithelial cells of fibrotic lesions at later time periods (7–14 days after bleomycin instillation). This hypothesis is further supported by studies demonstrating that, in addition to upregulation in myeloid-derived cell populations, a variety of nonmyeloid-derived cell types including vascular smooth muscle cells (18, 41), endothelial cells (31), and neurons (11) express and secrete HMGB1 in response to inflammatory stimuli.
Interestingly, we noted that LECs expressed HMGB1 in response to inflammation. This finding is important since recent studies have demonstrated that LECs express multiple TLRs and that activation of these receptors can induce macrophage recruitment and inflammatory lymphangiogenesis (37). Thus, similar to bacterial or viral byproducts, activation of endogenous danger signals by LECs in response to wounding or interstitial fluid stasis may directly activate TLRs located on LECs to initiate cellular interactions with infiltrating inflammatory cells. In support of this concept, we observed that blockade of HMGB1 with GLZ, a naturally occurring inhibitor of HMGB1, led to significant reductions in inflammatory lymphangiogenesis within draining lymph nodes stimulated by CFA-OVA. This finding provides evidence that HMGB1 may play a modulatory role in inflammatory lymphangiogenesis; however, further studies will be required to elucidate the mechanism of this process.
In conclusion, we have shown that the endogenous danger signals, HMGB1 and HSP70, are expressed along a spatial gradient relative to the site of tissue injury that persists during subacute phases of wound healing. Interstitial fluid accumulation and chronic lymphatic fluid stasis led to sustained expression of these molecules within lymphedematous tissue from animal models and matched human biopsy specimens. Cells native to injured tissue, including LECs, upregulate these molecules in response to tissue injury or inflammation. Given the well-defined role of HMGB1 as a chemoattractant for inflammatory cells (44, 54) and activating signal for cells of the innate immune system (28, 54), the persistence of this molecule in the wound microenvironment suggests its presence may be an inciting event promoting inflammatory responses associated with chronic lymphedema.
GRANTS
Sources of funding for this work are gratefully acknowledged and include an National Institutes of Health T32 grant, Plastic Surgery Education Foundation National Endowment Grant, Plastic Surgery Education Foundation Research Fellowship Grant, and the Sloan-Kettering Institute Department of Surgery.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Akira S, Uematsu S, Takeuchi O.Pathogen recognition and innate immunity.Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Andersson U, Tracey KJ.HMGB1 in sepsis.Scand J Infect Dis 35: 577–584, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Avraham T, Clavin NW, Daluvoy SV, Fernandez J, Soares MA, Cordeiro AP, Mehrara BJ.Fibrosis is a key inhibitor of lymphatic regeneration.Plast Reconstr Surg 124: 438–450, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ.Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair.Am J Pathol 177: 3202–3214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bianchi ME.DAMPs, PAMPs and alarmins: all we need to know about danger.J Leukoc Biol 81: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Boardman KC, Swartz MA.Interstitial flow as a guide for lymphangiogenesis.Circ Res 92: 801–808, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S.High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells.Circ Res 100: 204–212, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ.TGF-β1 is a negative regulator of lymphatic regeneration during wound repair.Am J Physiol Heart Circ Physiol 295: H2113–H2127, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG.HMGB1 mediates endogenous TLR2 activation and brain tumor regression.PLoS Med 6: e10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A.High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo.J Neurochem 103: 590–603, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A.The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway.EMBO Rep 3: 995–1001, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gauley J, Pisetsky DS.The translocation of HMGB1 during cell activation and cell death.Autoimmunity 42: 299–301, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Girard JP.A direct inhibitor of HMGB1 cytokine.Chem Biol 14: 345–347, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S.A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes.Am J Pathol 175: 132–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, Yamada S, Kuwano K, Nakanishi Y.The role of high mobility group box1 in pulmonary fibrosis.Am J Respir Cell Mol Biol 39: 440–447, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system.J Biol Chem 270: 25752–25761, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, Maruyama I.HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques.Cardiovasc Pathol 16: 136–143, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, Demarco RA, Lotze MT, Fink MP, Geller DA, Billiar TR.Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury.J Immunol 176: 7154–7158, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR.Early events in the recognition of danger signals after tissue injury.J Leukoc Biol 83: 546–552, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY.Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution.Blood 113: 5650–5659, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE.RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages.Scand J Immunol 61: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y, Billiar TR.Systemic inflammation and remote organ injury following trauma require HMGB1.Am J Physiol Regul Integr Comp Physiol 293: R1538–R1544, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ.Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors.Am J Pathol 171: 1774–1788, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matzinger P.The danger model: a renewed sense of self.Science 296: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Mazzucco L, Borzini P, Gope R.Platelet-derived factors involved in tissue repair-from signal to function.Transfus Med Rev 24: 218–234 [DOI] [PubMed] [Google Scholar]
- 27. Mehrara BJ, Mackool RJ, McCarthy JG, Gittes GK, Longaker MT.Immunolocalization of basic fibroblast growth factor and fibroblast growth factor receptor-1 and receptor-2 in rat cranial sutures.Plast Reconstr Surg 102: 1805–1817, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N.High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization.J Immunol 173: 307–313, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mirza R, DiPietro LA, Koh TJ.Selective and specific macrophage ablation is detrimental to wound healing in mice.Am J Pathol 175: 2454–2462, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME.Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities.Chem Biol 14: 431–441, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Mullins GE, Sunden-Cullberg J, Johansson AS, Rouhiainen A, Erlandsson-Harris H, Yang H, Tracey KJ, Rauvala H, Palmblad J, Andersson J, Treutiger CJ.Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1.Scand J Immunol 60: 566–573, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Osterloh A, Breloer M.Heat shock proteins: linking danger and pathogen recognition.Med Microbiol Immunol 197: 1–8, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Palumbo R, Bianchi ME.High mobility group box 1 protein, a cue for stem cell recruitment.Biochem Pharmacol 68: 1165–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME.Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation.J Cell Biol 179: 33–40, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME.Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation.J Cell Biol 164: 441–449, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E.Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein.J Biol Chem 279: 7370–7377, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Pegu A, Qin S, Fallert Junecko BA, Nisato RE, Pepper MS, Reinhart TA.Human lymphatic endothelial cells express multiple functional TLRs.J Immunol 180: 3399–3405, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Petrek JA, Heelan MC.Incidence of breast carcinoma-related lymphedema.Cancer 83: 2776–2781, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Piccinini AM, Midwood KS.DAMPening inflammation by modulating TLR signalling.Mediators Inflamm 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pisetsky DS, Erlandsson-Harris H, Andersson U.High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease.Arthritis Res Ther 10: 209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi ME.Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein.FASEB J 20: 2565–2566, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H.Role of HMGB1 in apoptosis-mediated sepsis lethality.J Exp Med 203: 1637–1642, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rock KL, Latz E, Ontiveros F, Kono H.The sterile inflammatory response.Annu Rev Immunol 28: 321–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepantalo M, Carpen O, Parkkinen J, Rauvala H.Regulation of monocyte migration by amphoterin (HMGB1).Blood 104: 1174–1182, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Roupe KM, Nybo M, Sjobring U, Alberius P, Schmidtchen A, Sorensen OE.Injury is a major inducer of epidermal innate immune responses during wound healing.J Invest Dermatol 130: 1167–1177 [DOI] [PubMed] [Google Scholar]
- 46. Scaffidi P, Misteli T, Bianchi ME.Release of chromatin protein HMGB1 by necrotic cells triggers inflammation.Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Semino C, Ceccarelli J, Lotti LV, Torrisi MR, Angelini G, Rubartelli A.The maturation potential of NK cell clones toward autologous dendritic cells correlates with HMGB1 secretion.J Leukoc Biol 81: 92–99, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ.HMGB1 and RAGE in inflammation and cancer.Annu Rev Immunol 28: 367–388, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Straino S, Di Carlo A, Mangoni A, De Mori R, Guerra L, Maurelli R, Panacchia L, Di Giacomo F, Palumbo R, Di Campli C, Uccioli L, Biglioli P, Bianchi ME, Capogrossi MC, Germani A.High-mobility group box 1 protein in human and murine skin: involvement in wound healing.J Invest Dermatol 128: 1545–1553, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Tabibiazar R, Cheung L, Han J, Swanson J, Beilhack A, An A, Dadras SS, Rockson N, Joshi S, Wagner R, Rockson SG.Inflammatory manifestations of experimental lymphatic insufficiency.PLoS Med 3: e254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K.Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation.Nat Med 13: 1458–1466, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Tang AH, Brunn GJ, Cascalho M, Platt JL.Pivotal advance: endogenous pathway to SIRS, sepsis, and related conditions.J Leukoc Biol 82: 282–285, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR.Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells.J Immunol 175: 7661–7668, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ.HMG-1 as a late mediator of endotoxin lethality in mice.Science 285: 248–251, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Youn JH, Shin JS.Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion.J Immunol 177: 7889–7897, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.