Abstract
Elevated intraocular pressure is the main risk factor in primary open-angle glaucoma, involving an increased resistance to aqueous humor outflow in the juxtacanalicular region of the conventional outflow pathway which includes the trabecular meshwork (TM) and the inner wall of Schlemm's canal (SC). Previously, sphingosine-1-phosphate (S1P) was shown to decrease outflow facility in porcine and human eyes, thus increasing outflow resistance and intraocular pressure. Owing to S1P's known effect of increasing barrier function in endothelial cells and the robust expression of the S1P1 receptor on the inner wall of SC, we hypothesized that S1P1 receptor activation promotes junction formation and decreases outflow facility. The effects of subtype-specific S1P receptor compounds were tested in human and porcine whole-eye perfusions and human primary cultures of SC and TM cells to determine the receptor responsible for S1P effects on outflow resistance. The S1P1-specific agonist SEW2871 failed to both mimic S1P effects in paired human eye perfusions, as well as increase myosin light chain (MLC) phosphorylation in cell culture, a prominent outcome in S1P-treated SC and TM cells. In contrast, the S1P2 antagonist JTE-013, but not the S1P1 or S1P1,3 antagonists, blocked the S1P-promoted increase in MLC phosphorylation. Moreover, JTE-013 prevented S1P-induced decrease in outflow facility in perfused human eyes (P < 0.05, n = 6 pairs). Similarly, porcine eyes perfused with JTE-013 + S1P did not differ from eyes with JTE-013 alone (P = 0.53, n = 3). These results demonstrate that S1P2, and not S1P1 or S1P3, receptor activation increases conventional outflow resistance and is a potential target to regulate intraocular pressure.
Keywords: aqueous humor, bioactive lipid, glaucoma, intraocular pressure, myosin light chain
elevated intraocular pressure (IOP), a major risk-factor for glaucoma, is due to an increased resistance to aqueous humor outflow in the pressure-responsive, conventional pathway. The majority of resistance to outflow is attributed to the juxtacanalicular connective tissue (JCT) region of the trabecular meshwork (TM) and the inner wall of Schlemm's canal (SC) (7, 8, 11, 17). To enter the SC lumen and return to venous circulation, aqueous humor flows through the JCT, a 2- to 20-μm-thick region adjacent to SC that is composed of a loose arrangement of extracellular matrix interspersed with TM cells that are attached to SC cells and the basal lamina (6). Aqueous humor then crosses the inner wall of SC, a continuous endothelial monolayer. It is important to determine how these tissues regulate resistance to find new mechanisms to lower IOP in those with glaucoma.
The endogenous bioactive lipid sphingosine-1-phosphate (S1P) decreases outflow facility, thereby increasing resistance, in both porcine and human eyes (12, 21). Originally believed to be an intracellular signaling molecule, S1P is now known to be involved in receptor-mediated signaling following the identification of S1P as the ligand for the EDG1 receptor (S1P1) (10). Of the five S1P receptors (S1P1–5) that have been identified, the S1P1–3 receptors are ubiquitously expressed, while S1P4 is limited to lymphoid and S1P5 to brain and skin tissue (16). The regulation of cytoskeletal dynamics is a main downstream effect of S1P receptor activation (2). For example, in cultured TM cells, S1P induces Rho activation, thus promoting myosin light chain (MLC) phosphorylation and increased stress fiber formation (12). In SC cells, S1P increases MLC phosphorylation while also promoting a cortical rearrangement of the actomyosin system (24). The cortical actomyosin assembly in SC cells is similar to the S1P effect observed in vascular endothelial cells, an effect mediated by the S1P1 receptor to promote endothelial barrier function (26).
The S1P receptor subtype(s) that is responsible for mediating the decrease in outflow facility by S1P represents a viable pharmacological target to modulate outflow resistance and reduce IOP. Therefore, the aim of this study was to identify S1P receptor(s) that mediate effects on outflow facility by testing the pharmacology of receptor-selective compounds in the porcine and human whole-eye perfusion models, as well as in human primary cultures of SC and TM cells. Despite similarities between SC and vascular blood endothelia (14) and the robust expression of the S1P1 receptor in SC cells (21), the S1P1-specific agonist SEW2871 (19) failed to decrease outflow facility in the human whole-eye perfusion and promote MLC phosphorylation in both SC and TM cells. Additionally, the antagonists W146 (S1P1-specific) (20) and VPC23019 (S1P1,3-specific) (1) failed to block the S1P increase in MLC phosphorylation. However, the S1P2-specific antagonist JTE-013 (13) blocked the S1P decrease of outflow facility in both porcine and human whole-eye perfusions, as well as the S1P-promoted MLC phosphorylation in SC and TM cells.
MATERIALS AND METHODS
Reagents.
All chemicals and reagents used were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. S1P was dissolved by adding to methanol and boiled. Methanol was then evaporated with a stream of nitrogen gas. The subsequent thin film of S1P was solubilized in phosphate-buffered saline (PBS) containing 4 mg/ml fatty acid-free bovine serum albumin. W146 and VPC23019 were acquired from Avanti Polar Lipids (Alabaster, AL) while SEW2871 and JTE-013 were acquired from Cayman Chemical (Ann Arbor, MI).
Eye tissue.
Enucleated human donor eyes were obtained from the National Disease Research Interchange (Philadelphia, PA), Sun Health Research Institute (Sun City, AZ), and Life Legacy Foundation (Tucson, AZ). The enucleated human eyes were free of any known ocular disease and were stored in a moist chamber at 4°C until use. Enucleated porcine eyes were obtained from the University of Arizona Meat Sciences Laboratory.
Cell culture.
Human SC cells were isolated from donor eyes using a cannulation technique and characterized and cultured as previously described (22). Human TM cells were isolated using a blunt dissection technique, followed by extracellular matrix digestion. Characterization and culturing were performed as previously described (23). Both cell types were maintained in Dulbecco's modified Eagle's medium (low glucose) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (0.1 mg/ml), and glutamine (0.29 mg/ml). The SC cell strains used were SC51, SC53, SC55/56, SC57, and SC58 that were derived from donors of ages 66, 44, 29, 78, and 34 yr, respectively. The TM cell strains used were TM83, TM86, TM88, TM90, and TM 93 that were derived from donors of ages 54 yr, 3 mo, 25 yr, 4 mo, and 35 yr, respectively. At least two different cell strains (passages 3–7) were tested in each type of experiment and were chosen on the basis of strain availability at the time of the experiments.
Human umbilical vein endothelial cells (HUVEC-2, BD Biosciences, Bedford, MA) were maintained in medium 199 (Invitrogen) supplemented with 15% fetal bovine serum, heparin sodium salt (90 μg/ml), endothelial mitogen (0.1 mg/ml, Biomedical Technologies, Stoughton, MA), penicillin (100 U/ml), streptomycin (0.1 mg/ml), and glutamine (0.29 mg/ml).
Immunoblot analyses.
Immunoblot analyses were performed as previously described (24). Nitrocellulose membranes were blocked with 5% milk for 1 h, then incubated overnight at 4°C with rabbit IgGs against phospho (Thr18/Ser19)-MLC (1:1,000 dilution, Cell Signaling, Beverly, MA), phospho (Ser473)-Akt (1:2,000 dilution, D9E, Cell Signaling), or pan-Akt (1:1,000 dilution, C67E7, Cell Signaling). Secondary antibodies used were horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgGs (40 ng/ml, Jackson Immunoresearch Laboratories, West Grove, PA) for 1 h at room temperature. Membranes were incubated with either Amersham ECL Advance (GE Healthcare) or HyGLO (Denville Scientific, Metuchen, NJ) chemiluminescence reagents and exposed to X-ray film (Genesee Scientific, San Diego, CA). Membranes were reprobed with ascites fluid containing mouse monoclonal IgG against β-actin (1:10,000 dilution, Sigma-Aldrich,) for a loading control. Protein signals were captured digitally, and densitometry was performed using GeneSnap and GeneTools software (Syngene, Frederick, MD).
Human whole-eye perfusion.
Whole-eye perfusions were performed according to the system described by Ethier et al. (3). Upon enucleation, eyes were stored in a moist chamber at 4°C until the beginning of the experiment. All human eyes were perfused within 30 h from time of death (TOD). At the beginning of an experiment, eyes were placed in PBS at 34°C and a needle was inserted through the cornea and placed in the posterior chamber, behind the iris. The eyes were then perfused with Dulbecco's PBS containing 5.5 mM glucose (DBG) prefiltered through a syringe-driven filter unit with 0.22-μm pores (Millipore). Human eyes were perfused at a constant pressure of 8 mmHg (equivalent to 15 mmHg in vivo) for 60–90 min to establish a baseline outflow facility (stable flow for at least 30 min). Following baseline perfusion, the contents of the anterior chamber were exchanged with DBG containing either experimental compounds or control. Following exchanges, perfusions at 8 mmHg were resumed and new baselines were reached (defined as reasonably stable flow for at least 30 min before the end of the perfusion). Normalized facilities were then calculated, dividing the new baseline following exchange by the original baseline facility. Additionally, the percent net facility change between paired eyes was calculated by subtracting the percent facility change from baseline in the contralateral control eye from the percent facility change in the experimental eye.
Porcine whole-eye perfusion.
Porcine eyes were enucleated immediately following TOD. The eyes were received within 2 h of TOD and used in experiments within 12 h of TOD. The porcine eyes were perfused using the same procedure as the human eye perfusions but were perfused at a constant pressure of 15 mmHg as has been done previously (12). The porcine eye perfusions were used to test the effects of antagonist ± S1P. After a stable baseline, eyes were exchanged with antagonist and perfused for an additional 1 h to expose the outflow tissue to the antagonist and determine whether facility changes occur with antagonist alone. The eyes were then exchanged a second time with antagonist alone or antagonist + S1P, and then perfused for an additional 2 h. Separate experiments were also done to test S1P's effect in porcine eyes without antagonists. Control eyes received media alone in both exchanges, while experimental eyes received media first, then S1P in the second exchange.
Statistical analyses.
Results for human whole-eye perfusions were analyzed using a two-tailed, paired Student's t-test. Porcine whole-eye perfusions were analyzed using a two-tailed, unpaired Student's t-test with equal variance. Cell culture experiments were analyzed using a one-way ANOVA with Dunnett's post hoc test. Results were considered significant when P < 0.05. Sigmoidal dose-response curves were generated in Sigmaplot.
RESULTS
S1P1 receptor activation alone does not mimic S1P effects on human outflow tissue.
We used two complementary approaches, outflow facility in whole-eye perfusions and MLC phosphorylation in cultured cells, to determine whether the S1P1 receptor is responsible for S1P effects on outflow resistance in human eyes. In human whole-eye perfusions with SEW2871, the mean donor age of perfused eyes was 82.7 yr (range 77–87 yr) and the mean TOD to start of perfusion was 17.5 h (range 9.5–29.5 h) (Table 1). Unlike the 36 ± 20% decrease in outflow facility previously observed in S1P-treated eyes (21), 5 μM SEW2871-treated eyes displayed no differences in outflow facility compared with contralateral control eyes up to 1 h following anterior chamber exchanges (−4.3 ± 14.0% net facility change, n = 3). The normalized facility baselines following exchanges in control and SEW2871-treated eyes were 1.02 ± 0.14 and 0.98 ± 0.14, respectively (means ± SD, P = 0.62).
Table 1.
Donor information and summary of results from human whole-eye perfusions
| Outflow Facility, μl·min−1·mmHg−1 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Donor Tissue ID | Age, yr | Sex | TOD-TOE, h | TOD-TOP, h | Stable Baseline | New Baseline | % Facility Change | % Net Facility Change |
| SEW2871 | ||||||||
| 63E | 77 | F | 4.5 | 9.5 | 0.220 | 0.246 | 11.8 | 10.1 |
| 64C | 0.119 | 0.121 | 1.7 | |||||
| 67C | 84 | F | 3 | 29.5 | 0.192 | 0.170 | −11.5 | −5.2 |
| 68E | 0.240 | 0.200 | −16.7 | |||||
| 69C | 87 | M | 2 | 13.5 | 0.101 | 0.118 | 16.8 | −17.8 |
| 70E | 0.196 | 0.194 | −1.0 | |||||
| Mean | 82.7 | 2F/1M | 3.2 | 17.5 | ||||
| Control | 0.137 ± 0.048 | 0.136 ± 0.029 | 2.4 ± 14.2 | −4.3 ± 14.0 | ||||
| Experimental (5 μM SEW2871) | 0.219 ± 0.022 | 0.213 ± 0.028 | −2.0 ± 14.3 | |||||
| JTE-013 + S1P | ||||||||
| 87C | 85 | M | 2.6 | 12.5 | 0.164 | 0.121 | −26.2 | 23.8 |
| 88E | 0.168 | 0.164 | −2.4 | |||||
| 89E | 82 | M | 2.4 | 23 | 0.200 | 0.218 | 9.0 | 34.6 |
| 90C | 0.227 | 0.169 | −25.6 | |||||
| 91C | 80 | F | 9 | 22.5 | 0.239 | 0.192 | −19.7 | −2.9 |
| 92E | 0.235 | 0.182 | −22.6 | |||||
| 101E | 62 | M | 4 | 15 | 0.106 | 0.173 | 63.2 | 80.6 |
| 102C | 0.242 | 0.200 | −17.4 | |||||
| 103C | 89 | M | Unkn | 15 | 0.272 | 0.225 | −17.3 | 22.4 |
| 104E | 0.255 | 0.268 | 5.1 | |||||
| 117C | 84 | F | 2 | 10 | 0.186 | 0.144 | −22.6 | 27.3 |
| 118E | 0.190 | 0.199 | 4.7 | |||||
| Mean | 80.3 | 2F/4M | 4.0 | 16.3 | ||||
| Control (5 μM S1P) | 0.222 ± 0.040 | 0.175 ± 0.038 | −21.4 ± 4.0 | 31.0 ± 27.4 | ||||
| Experimental (5 μM JTE-013 + 5 μM S1P) | 0.192 ± 0.053 | 0.201 ± 0.038 | 9.5 ± 28.6 | |||||
Control and experimental values are means ± SD. Bold text and values indicate treatment groups. ID, identification; C, control; E, experimental; TOD, time of death; TOE, time of enucleation; TOP, time of perfusion; unkn, unknown, F, female; M, male; S1P, sphingosine-1-phosphate.
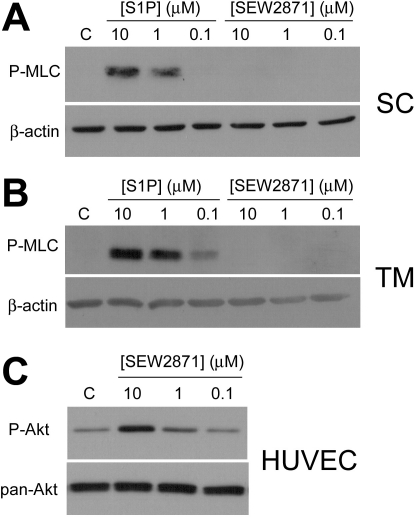
We next treated SC and TM cells with SEW2871 to determine whether S1P1 participates in the S1P-promoted increase in MLC phosphorylation, a prominent and reliable effect in both cell types (12, 24). Following a 2 h serum starvation, SEW2871 (10, 1, and 0.1 μM) did not induce MLC phosphorylation in both SC (Fig. 1A) and TM cells (Fig. 1B), thus indicating that S1P1 activation alone does not promote MLC phosphorylation. As a control, SEW2871 activity was verified with Akt phosphorylation in HUVEC (Fig. 1C), an effect mediated by S1P1 (19).
Fig. 1.
Sphingosine-1-phosphate 1 (S1P1) receptor activation does not promote myosin light chain (MLC) phosphorylation in outflow cells. A and B: Schlemm's canal (SC; A) and trabecular meshwork (TM cells; B) were treated for 5 min with S1P and the S1P1-specific agonist SEW2871 (SEW) at concentrations of 10, 1 and 0.1 μM, and then probed for MLC phosphorylation (P-MLC). β-Actin was used as a loading control. Blots are representative of five experiments using two cell strains each for both cell types. C: SEW2871 activity was confirmed through Akt phosphorylation (P-Akt) in human umbilical vein endothelial cells (HUVEC) following 5 min SEW2871 treatments at concentrations of 10, 1, and 0.1 μM. Blots were also probed for pan-Akt as a loading control. Blots are representative of four experiments. C, control.
Blockade of S1P2 activation prevents S1P-promoted MLC phosphorylation in SC and TM cells.
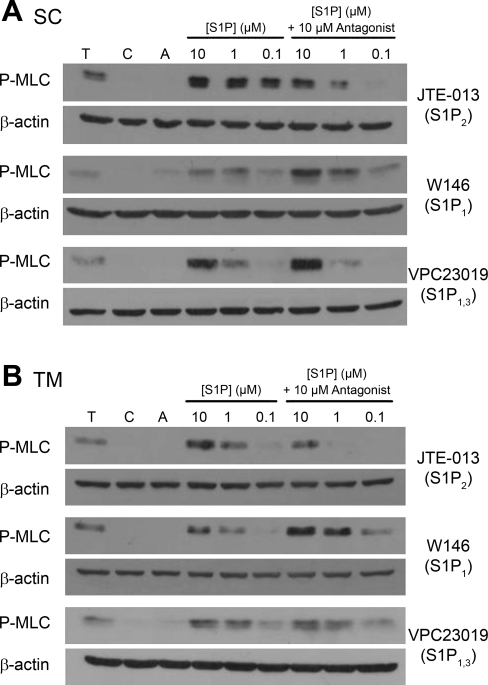
Since S1P1 alone did not appear responsible for S1P effects on outflow resistance, we screened three different S1P receptor-selective antagonists to test their effectiveness in blocking the S1P-promoted MLC phosphorylation in both SC and TM cells. Following 2 h serum starvation, SC and TM cells were pretreated (30 min) with S1P receptor antagonists before S1P treatment (5 min). In the presence of 10 μM JTE-013 (S1P2 antagonist), MLC phosphorylation levels following S1P treatments of 10, 1, and 0.1 μM were reduced compared with S1P alone in both SC (Fig. 2A) and TM cells (Fig. 2B). In agreement with the inability of SEW2871 to promote MLC phosphorylation (Fig. 1), the S1P1 antagonist W146, as well as the S1P1,3 antagonist VPC23019, failed to block MLC phosphorylation by S1P.
Fig. 2.
Effect of a single concentration of S1P receptor-specific antagonist on MLC phosphorylation in the presence of increasing concentrations of S1P. Cells were treated with 1 U/ml thrombin (T) and S1P at concentrations of 10, 1, and 0.1 μM for 5 min and probed for MLC phosphorylation. S1P effects in the presence of antagonists were tested in additional cells pretreated with 10 μM of JTE-013 (S1P2), W146 (S1P1), or VPC23019 (S1P1,3) for 30 min. SC (A) and TM (B) blots are representative of five different experiments using three cell strains for each cell type. β-Actin was used as a loading control.
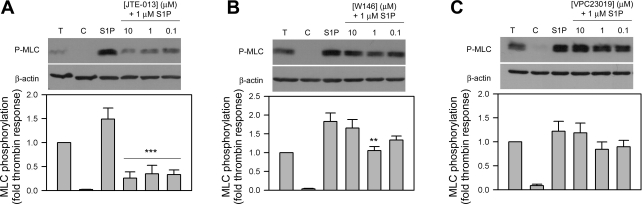
Because of possible nonspecific interactions of antagonist, we also performed the reciprocal experiments where antagonist concentrations were varied in the presence of a single concentration of S1P (1 μM). The 1 μM S1P-promoted MLC phosphorylation in SC cells was significantly reduced when SC cells were pretreated with all three doses of JTE-013 (10–0.1 μM, P < 0.001) (Fig. 3A). Interestingly, inclusion of 1 μM W146 reduced MLC phosphorylation by S1P (P < 0.01), although similar responses were not observed with a higher 10 μM dose (Fig. 3B). No changes in MLC phosphorylation were observed with the inclusion of VPC23019 in SC cells (Fig. 3C).
Fig. 3.
Effect of increasing antagonist concentration on S1P-mediated MLC phosphorylation in SC cells. Cells were treated with 1 U/ml thrombin and 1 μM S1P for 5 min and probed for MLC phosphorylation. S1P effects in the presence of the antagonists JTE-013 (A, S1P2 antagonist), W146 (B, S1P1 antagonist), or VPC23019 (C, S1P1,3 antagonist) at concentrations of 10, 1, and 0.1 μM were compared. Blots are representative of six different experiments using two SC cell strains. Changes to the S1P-promoted MLC phosphorylation response (compared through the fold thrombin response) with the inclusion of antagonists are represented in the histograms beneath the correlating blots. β-Actin was used as a loading control. Data are presented as means ± SE and are compared with S1P control response (**P < 0.01, ***P < 0.001).
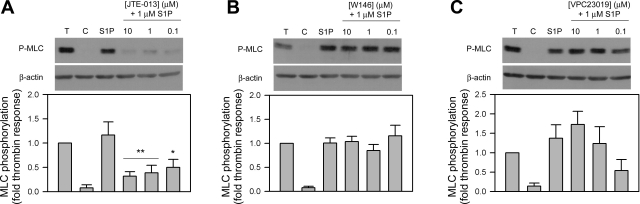
When tested in TM cells, S1P-promoted MLC phosphorylation was also significantly reduced by JTE-013 (10 and 1 μM JTE, P < 0.01; 0.1 μM JTE, P < 0.05) (Fig. 4A). The MLC phosphorylation responses by S1P were reduced with JTE-013 by 73% (10 μM), 67% (1 μM), and 57% (0.1 μM). By comparison, SC cells displayed a more robust blocking of MLC phosphorylation at all three doses of JTE-013 by reducing the S1P response by 83% (10 μM), 77% (1 μM), and 78% (0.1 μM). No differences in phosphorylation status were observed with the inclusion of W146 and VPC23019 in TM cells (Fig. 4, B and C).
Fig. 4.
Effect of increasing antagonist concentration on S1P-mediated MLC phosphorylation in TM cells. Cells were treated with 1 U/ml thrombin and 1 μM S1P for 5 min and probed for MLC phosphorylation. S1P effects in the presence of the antagonists JTE-013 (A, S1P2 antagonist), W146 (B, S1P1 antagonist), or VPC23019 (C, S1P1,3 antagonist) at concentrations of 10, 1, and 0.1 μM were compared. Blots are representative of six different experiments using two TM cell strains. Changes to the S1P-promoted MLC phosphorylation response (compared through the fold thrombin response) with the inclusion of antagonists are represented in the histograms beneath the correlating blots. β-Actin was used as a loading control. Data are presented as means ± SE and are compared with S1P control response (*P < 0.05, **P < 0.01).
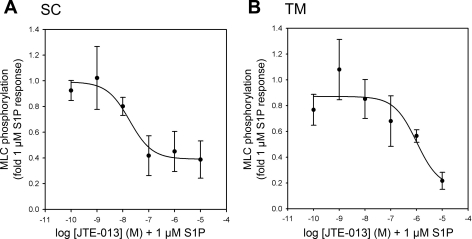
Because of similar levels of inhibition by JTE-013 at concentrations initially tested in both SC (Fig. 3A) and TM cells (Fig. 4A), we assessed JTE-013 effects over a wider range of concentrations (10−5 to 10−10 M). We observed that pretreatment of cells with JTE-013 produced half-maximal inhibitory concentration (IC50) values of 18.3 nM in SC (Fig. 5A) and 1.0 μM in TM cells (Fig. 5B) treated with 1 μM S1P.
Fig. 5.
Dose-dependent inhibition of S1P-mediated MLC phosphorylation by JTE-013 in human outflow cells. The effects of preincubation of JTE-013 (30 min) at concentrations ranging from 10−5 to 10−10 M on MLC phosphorylation in SC and TM cells treated with 1 μM S1P for 5 min were tested. Analysis of S1P-promoted MLC phosphorylation in the presence of JTE-013 at increasing concentrations demonstrated IC50 values for SC (A) and TM (B) cells of 18.3 nM and 1.0 μM, respectively. Data are displayed as means ± SE from combined independent experiments (n = 4).
Blockade of S1P2 prevents the S1P-induced decrease of outflow facility in perfused porcine whole-eyes.
Because of the inability of the S1P1 agonist SEW2871 to mimic the S1P effects in the whole-eye perfusion (decrease facility) and cell culture models (MLC phosphorylation), the focus was shifted to S1P2 and the antagonist JTE-013 due to its blocking of S1P-promoted MLC phosphorylation in our cell models. Thus, JTE-013 was included in the whole-eye perfusion model to determine whether blocking the S1P2 receptor would prevent the S1P-induced decrease in outflow facility. In initial studies, we used porcine eyes first to test the antagonist before use in human eyes due to the similar decrease in outflow facility observed between both species (12, 21), in addition to the availability and freshness of porcine eyes compared with human eyes.
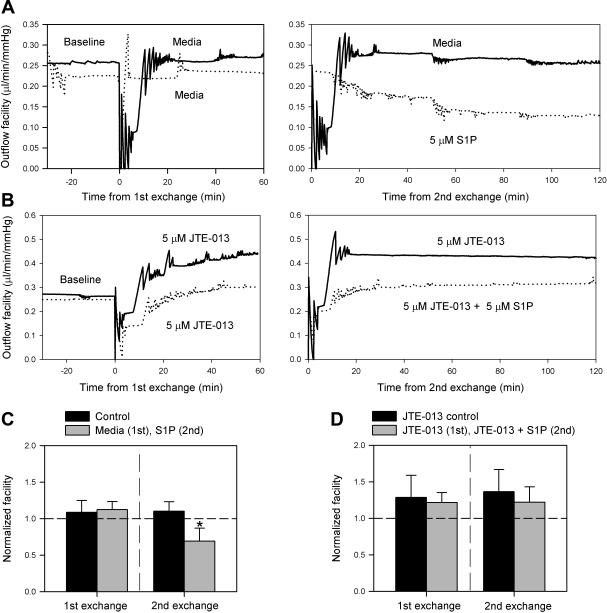
We started with a double exchange perfusion experiment. The aim of the first exchange was to determine whether antagonist alone affects outflow while the aim of the second exchange was to compare the inclusion of S1P with antagonist versus antagonist alone. Parallel, control experiments using this experimental setup were also performed (media alone for both exchanges) and experimental eyes (media in first exchange, 5 μM S1P in second exchange), reproducing the S1P-mediated decrease in facility without antagonist (Fig. 6A). Following verification of the S1P response in the control experiments, experiments with the inclusion of antagonist were conducted to compare JTE-013 control eyes (5 μM JTE-013 for both exchanges) with experimental eyes (5 μM JTE-013 for first exchange, 5 μM JTE-013 + 5 μM S1P for second exchange). The inclusion of antagonist, JTE-013, clearly blocks the S1P-mediated decrease in outflow facility (Fig. 6B).
Fig. 6.
Blockade of S1P2 prevents the S1P-mediated decrease in outflow facility in porcine eyes. Following a baseline perfusion, porcine eyes received two exchanges with subsequent perfusions over the course of an experiment. The first exchange was used to pretreat the outflow tissue with the S1P2 antagonist JTE-013 while the second exchange was used to compare eyes treated with JTE-013 alone, or in addition to 5 μM S1P (n = 3). Separate control experiments using media in the first exchange, and in the second exchange, comparing media against 5 μM S1P (n = 3), were also done. Examples of outflow facility traces for experiments comparing media alone (−) to S1P (. . . .) (A), and comparing JTE-013 (−) to JTE-013 + S1P (. . . .) (B) are shown. Average normalized facilities following anterior chamber exchanges are also shown (C and D). Control and experimental perfusion data were compared following each exchange and are presented as means ± SD (*P < 0.05).
In analyzing the control experiments without antagonist (Fig. 6C), normalized facilities following the first exchange and 1 h perfusion for the media control and experimental groups were 1.09 ± 0.16 and 1.12 ± 0.11, respectively (means ± SD, P = 0.76). Following the second exchange and subsequent 2 h perfusion, experimental eyes with S1P displayed a typical decrease in normalized facility of 0.69 ± 0.18 compared with 1.10 ± 0.13 in the control group with media alone (means ± SD, P = 0.03, n = 3) (Fig. 6C).
In the experiments with the inclusion of S1P2 antagonist (Fig. 6D), normalized facilities following the first exchange and perfusion for the JTE-013 control and experimental groups were 1.28 ± 0.30 and 1.21 ± 0.14, respectively (means ± SD, P = 0.74, n = 3). Following the second exchange and subsequent perfusion, normalized facilities for the experimental eyes with JTE-013 + S1P and JTE-013 control eyes remained comparable at 1.22 ± 0.21 and 1.36 ± 0.30, respectively (means ± SD, P = 0.53) (Fig. 6D). Thus, inclusion of JTE-013 in porcine whole-eye perfusions prevented the S1P-induced decrease in outflow facility.
In investigating the effect of antagonist alone, it was observed that normalized facilities following the first exchange for all eyes receiving 5 μM JTE-013 (1.25 ± 0.21) displayed a trend toward increasing outflow facility compared with all eyes receiving media alone (1.11 ± 0.13) (means ± SD, P = 0.19, n = 6).
S1P2 is responsible for the S1P-induced decrease of outflow facility in perfused human whole-eyes.
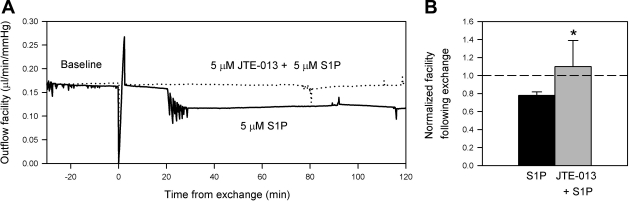
Since JTE-013 blocked the S1P-induced decrease in outflow facility in porcine eyes, the antagonist was next used in human eye perfusions (Fig. 7). The mean donor age for perfused eyes was 80.3 yr (range 62–89 yr) and the mean TOD to start of perfusion was 16.3 h (range 10–23 h) (Table 1). Following an initial baseline perfusion, control eyes were exchanged with media containing 5 μM S1P while the contralateral experimental eyes were exchanged with media containing 5 μM JTE-013 + 5 μM S1P. Because of limitations in the freshness of human tissue post mortem, JTE-013 and S1P were given together in one exchange. After 2 h of perfusion following the exchange, JTE-013 + S1P eyes and S1P control eyes displayed normalized facilities of 1.10 ± 0.29 and 0.78 ± 0.04, respectively (means ± SD, P < 0.05, n = 6) (Fig. 7B). The percent facility change in JTE-013 + S1P and S1P control eyes were 9.5 ± 28.6% and −21.4 ± 4.0% (mean % facility change ± SD, P < 0.05, n = 6). The net facility difference with the addition of JTE-013 was 31.0 ± 27.4%. Interestingly, the inclusion of JTE-013 in human eyes increased outflow facility above baseline despite the presence of S1P. Even though the antagonist appeared not to have an effect in one eye pair (91C, 92E), JTE-013 still significantly blocked the S1P decrease of outflow facility in human eyes.
Fig. 7.
Outflow facility effects of S1P2 receptor blockade in S1P-perfused human eyes. Following a baseline perfusion in paired human eyes, the contents of the anterior chambers of the experimental eyes were exchanged with media containing 5 μM JTE-013 + 5 μM S1P, while the contralateral S1P control eyes received 5 μM S1P alone. Examples of outflow facility traces for experiments comparing S1P control (−) to JTE-013 + S1P experimental (. . . .) eyes are shown (A); paired eyes were from an 85 yr-old donor (87C, 88E) with a net facility increase of 23.8% with the inclusion of JTE-013, compared with S1P control. Average normalized facilities following anterior chamber exchanges are shown (B). Data are presented as means ± SD (*P < 0.05).
DISCUSSION
We originally hypothesized that the S1P1 receptor was responsible for the S1P-mediated decrease in outflow facility due to receptor expression patterns in the outflow pathway and predicted mechanisms of action, but surprisingly, activation of the S1P1 receptor alone did not have an effect on outflow facility, nor did it have an effect on MLC phosphorylation in the cell culture models. Moreover, the use of a S1P1 or S1P1,3-selective antagonist failed to block S1P-mediated MLC phosphorylation. Rather, blocking the S1P2 receptor prevented S1P-promoted MLC phosphorylation in cultured SC and TM cells. Thus, our focus shifted from the S1P1 to the S1P2 receptor, where blockade of S1P2 effectively prevented the entire S1P-mediated decrease in outflow facility in human and porcine whole-eye perfusions. These results suggest that controlling S1P2 receptor activation in the conventional outflow pathway may provide a new pharmacological target to reduce ocular hypertension in patients with, or are at risk for primary open-angle glaucoma.
In this study, the effects observed with JTE-013 on outflow facility in both porcine and human eyes demonstrate S1P2 to be a dominant receptor subtype in the outflow tissue that may be responsible in mediating S1P's effect of increasing outflow resistance. JTE-013 blocked the S1P effect in human eyes despite not pretreating the outflow tissue with antagonist as was done in the porcine experiments. The pretreatment procedure was omitted due to donor age and length of time from time of death (TOD) to start of perfusion. We have found empirically that tissue responsiveness decreases with increasing time between TOD and experimental treatment, as well as with tissue from older donors. Despite the conservative approach, the human perfusion experiments clearly demonstrate S1P2 to be the key receptor in the S1P-mediated effects in organ culture.
Three commercially available antagonists were tested to block S1P-promoted MLC phosphorylation, a Rho/Rho-kinase-mediated effect observed robustly and reliably in both SC and TM cells in this study (12, 24). The S1P2 antagonist JTE-013 significantly reduced MLC phosphorylation by S1P in both SC and TM cell culture models, in agreement with previous findings that S1P2 receptor activation increases MLC phosphorylation in a Rho-dependent manner (5, 28). JTE-013 is a selective S1P2 antagonist with IC50 values for human S1P1 and S1P2 receptors at >10 μM and ∼17 nM, respectively. For the S1P3 receptor, only 4.2% inhibition of S1P binding was observed at 10 μM JTE-013 (13). However, recent evidence has suggested that JTE-013 may not be highly selective to the S1P2 receptor at higher concentrations in rodents (18). Therefore, we used two different experimental setups in cell culture treatments using the S1P receptor antagonists. First, we pretreated with a constant 10 μM antagonist concentration, followed by S1P treatments of 10, 1, and 0.1 μM. Second, rather than varying the S1P concentration, we varied the antagonist pretreatment concentrations (10, 1, and 0.1 μM) and followed with a constant 1 μM S1P treatment. Both experimental setups demonstrated JTE-013 to block the S1P-promoted MLC phosphorylation in both SC and TM cells, supporting S1P2 as the receptor involved in S1P-promoted MLC phosphorylation.
The S1P2 receptor stimulates the Rho/Rho-kinase pathway, thus increasing MLC phosphorylation by inhibiting MLC phosphatase (5, 28). Activation of the Rho pathway in the outflow tissue has been shown to increase outflow resistance (27) with the contractile state of TM cells through MLC phosphorylation being the key regulating event (12, 15). Thus, S1P may promote increased TM cell contractility, thereby constricting the passageways through the extracellular matrix in the JCT region of the TM. Meanwhile, cell-cell and cell-matrix junctions in conjunction with a sustained contractile state are vital to SC cells due to the large variation in trans-endothelial pressure experienced by the inner wall SC cells. Thus, an S1P-mediated increase in Rho-dependent MLC phosphorylation in SC cells at the cell cortex in conjunction with cortical actin formation (24) may promote endothelial barrier enhancement with an increase in cell-cell and cell-matrix connections, therefore raising outflow resistance. These events in the TM and along the inner wall of SC, whether working individually or in conjunction with one another, may prove to be the mechanism in the S1P-mediated increase in outflow resistance.
Because of the exposure of the conventional outflow pathway to a wide range of flow and pressure, the S1P receptor system may play a role in accommodating and responding to changing conditions in the outflow tissue. Under normal conditions, basal activation of the S1P receptor system may support normal cell-cell and cell-matrix interactions with moderate actomyosin tone for both SC and TM cells. In the case of high outflow, the spaces between TM cells and the extracellular matrix in the JCT are expanded, as well as the sub-endothelial space of the inner wall. To help prevent detachment of SC at elevated IOP, an increase in S1P release by outflow cells and subsequent saturation of the S1P receptors may promote an increase in actomyosin tone via the S1P2/Rho/Rho-kinase pathway to further increase stiffness to maintain the outflow structure. In support of outflow cells increasing S1P release under high outflow conditions, vascular cells have been shown to increase S1P release when under shear stress (25). Accordingly, the shear stress experienced by the SC cells at elevated IOPs is predicted to be comparable to the shear stress experienced by arterial vascular endothelial cells (4).
Determining the localization and expression levels of the enzymes involved in S1P formation and breakdown within the outflow pathway may provide insight into the mechanism of how the S1P receptor system may modulate outflow resistance. Sphingosine kinases phosphorylate sphingosine to form S1P while S1P lyase and the S1P phosphatases break down S1P (9). In the outflow pathway, the cells of the TM are ideally situated to be a source of S1P, potentially releasing S1P downstream and activating S1P receptors on fellow outflow cells, thus modulating actomyosin assembly/disassembly.
In conclusion, the S1P2 receptor may prove to be a valuable target to reduce outflow resistance and IOP in glaucoma patients. S1P2 preferentially promotes a contractile response in the cells of the outflow pathway, thus antagonizing S1P2 provides a receptor-based target to increase aqueous outflow. In support of a potential S1P2-specific antagonist to decrease outflow resistance in glaucoma patients, our perfusions with JTE-013 alone displayed a trend toward increasing outflow facility in porcine eyes while human eyes treated with JTE-013 + S1P displayed an increase of outflow facility over baseline. Therefore, future studies using long-term perfusions with either JTE-013, or another, more efficacious S1P2 antagonist are needed to verify S1P2 as a potential glaucoma therapeutic.
GRANTS
Research was supported by the National Eye Institute (EY17007) and the Research to Prevent Blindness Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Kristin Perkumas and Emely Hoffman for providing Schlemm's canal and trabecular meshwork cells, respectively. The authors also thank the Lion's Club for assistance in transporting human tissue.
REFERENCES
- 1. Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem 280: 9833–9841, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Donati C, Bruni P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: implications in its biological response. Biochim Biophys Acta 1758: 2037–2048, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Ethier CR, Ajersch P, Pirog R. An improved ocular perfusion system. Curr Eye Res 12: 765–770, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Ethier CR, Read AT, Chan D. Biomechanics of Schlemm's canal endothelial cells: influence on F-actin architecture. Biophys J 87: 2828–2837, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonda K, Okamoto H, Takuwa N, Yatomi Y, Okazaki H, Sakurai T, Kimura S, Sillard R, Harii K, Takuwa Y. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem J 337: 67–75, 1999 [PMC free article] [PubMed] [Google Scholar]
- 6. Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microsc Res Tech 33: 336–367, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol 69: 783–801, 1963 [DOI] [PubMed] [Google Scholar]
- 8. Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci 33: 1670–1675, 1992 [PubMed] [Google Scholar]
- 9. Le Stunff H, Milstien S, Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem 92: 882–899, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552–1555, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res 54: 879–883, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Mettu PS, Deng PF, Misra UK, Gawdi G, Epstein DL, Rao PV. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest Ophthalmol Vis Sci 45: 2263–2271, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Osada M, Yatomi Y, Ohmori T, Hosogaya S, Ozaki Y. Modulation of sphingosine 1-phosphate/EDG signaling by tumor necrosis factor-alpha in vascular endothelial cells. Thromb Res 108: 169–174, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Ramos RF, Hoying JB, Witte MH, Daniel Stamer W. Schlemm's canal endothelia, lymphatic, or blood vasculature? J Glaucoma 16: 391–405, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res 80: 197–206, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 78: 743–768, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res 8: 1233–1240, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol 153: 140–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839–13848, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2: 434–441, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Stamer WD, Read AT, Sumida GM, Ethier CR. Sphingosine-1-phosphate effects on the inner wall of Schlemm's canal and outflow facility in perfused human eyes. Exp Eye Res 89: 980–988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci 39: 1804–1812, 1998 [PubMed] [Google Scholar]
- 23. Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res 14: 611–617, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Sumida GM, Stamer WD. Sphingosine-1-phosphate enhancement of cortical actomyosin organization in cultured human Schlemm's canal endothelial cell monolayers. Invest Ophthalmol Vis Sci 51: 6633–6638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102: 669–676, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 77: 39–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol 295: C1057–C1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol 286: C1130–C1138, 2004 [DOI] [PubMed] [Google Scholar]