Abstract
Gap junction channels, which are made of connexins, are critical for intercellular communication, a function that may be disrupted in a variety of diseases. We studied the consequences of two cataract-associated mutations at adjacent positions at the first extracellular boundary in human connexin50 (Cx50), W45S and G46V. Both of these mutants formed gap junctional plaques when they were expressed in HeLa cells, suggesting that they trafficked to the plasma membrane properly. However, their functional properties differed. Dual two-microelectrode voltage-clamp studies showed that W45S did not form functional intercellular channels in paired Xenopus oocytes or hemichannel currents in single oocytes. When W45S was coexpressed with wild-type Cx50, the mutant acted as a dominant negative inhibitor of wild-type function. In contrast, G46V formed both functional gap junctional channels and hemichannels. G46V exhibited greatly enhanced currents compared with wild-type Cx50 in the presence of physiological calcium concentrations. This increase in hemichannel activity persisted when G46V was coexpressed with wild-type lens connexins, consistent with a dominant gain of hemichannel function for G46V. These data suggest that although these two mutations are in adjacent amino acids, they have very different effects on connexin function and cause disease by different mechanisms: W45S inhibits gap junctional channel function; G46V reduces cell viability by forming open hemichannels.
Keywords: connexin, gap junction, lens, intercellular communication
vertebrate gap junctional channels are formed of subunits from a multigene family of proteins called connexins (Cx), which has 21 human members (39). A gap junctional hemichannel, or connexon, is an oligomeric assembly of six connexins. Gap junctional channels are formed by docking of two gap junctional hemichannels from closely apposed cells via their extracellular loops. Undocked hemichannels may also be present within nonjunctional regions of the plasma membrane and can gate open under physiological and pathological conditions.
Mutations in connexins have been associated with a number of human genetic diseases including X-linked Charcot-Marie-Tooth disease (5, 27), congenital deafness (15), keratitis-ichthyosis-deafness (KID) syndrome (28), atrial fibrillation (12), and congenital cataracts (26). Congenital cataracts have been associated with mutations of the two connexins found in lens fiber cells, Cx50 and Cx46. Most of the cataract-associated mutants that have been studied act as “loss of function” mutants when expressed by themselves due to impaired trafficking to the plasma membrane (1, 2, 4, 7, 20, 24, 25, 33, 40).
The amino acid sequence at the interface between the first transmembrane domain (TM1) and the first extracellular loop (E1) of connexins is thought to play an important role in channel permeability, gating and regulation, and in interhemichannel interactions (16, 18, 22, 32, 34, 37). We recently published an initial characterization of a cataract-associated Cx50 mutant in this region, Cx50G46V (G46V), in which we show that expression of G46V in HeLa cells decreases cell growth and viability, most likely due to its “gain-of-hemichannel” function (21). In the present study, we have extended our examination of the physiological properties of G46V, and we have examined the cellular and physiological behavior of another cataract-associated Cx50 mutation that results in a single amino acid substitution of an adjacent residue at the TM1/E1 boundary, Cx50W45S (W45S) (36).
The data presented below show that W45S and G46V differ substantially in their physiological properties and their effects on wild-type Cx46 and Cx50 channel function.
MATERIALS AND METHODS
Generation of Cx50 constructs.
Wild-type Cx50 and Cx50G46V were previously subcloned into pSP64TII (for RNA transcription and Xenopus oocyte expression) and pcDNA 3.1/Hygro(+) (for expression in transfected mammalian cells) (2, 10, 21). DNA encoding W45S was obtained by polymerase chain reaction using oligonucleotides encoding the nucleotide substitution, Phusion DNA polymerase (New England BioLabs, Ipswich, MA), and plasmid templates containing wild-type Cx50 in pSP64TII and pcDNA 3.1/Hygro(+). Wild-type Cx50-GFP and G46V-GFP were obtained by appending the Emerald variant (Invitrogen Life Technologies, Carlsbad, CA) of green fluorescent protein (GFP) to the COOH-terminus of wild-type Cx50 and G46V. The coding regions of all amplified constructs were fully sequenced at the Cancer Research Center DNA Sequencing Facility of the University of Chicago (Chicago, IL) to confirm the absence of additional unwanted mutations.
Immunofluorescence.
Parental HeLa cells were transiently transfected with wild-type Cx50 or W45S in pcDNA3.1/Hygro(+) (Invitrogen Life Technologies), and HeLa cells stably transfected to inducibly express G46V were induced by treatment with 1 μM ponasterone A. Forty-eight hours later, cells were rinsed with phosphate-buffered saline (PBS), fixed for 15 min in 4% paraformaldehyde at room temperature, and permeabilized with 1% Triton X-100 in PBS. Immunofluorescence was carried out using affinity-purified rabbit polyclonal anti-Cx50 antibodies and Cy2-conjugated goat anti-rabbit IgG antibodies (Jackson ImmunoResearch, West Grove, PA) as previously described (4).
Immunoblotting.
Preparations enriched in plasma membranes from Xenopus oocytes were made according to White et al. (38) and Gupta et al. (13). Briefly, four control Xenopus oocytes or oocytes injected with cRNAS encoding wild-type Cx50 (Cx50), W45S, or G46V were homogenized in 5 mM Tris·HCl, 1 mM EDTA, 1 mM EGTA, and 2 mM PMSF, pH 8.0. The homogenates were centrifuged at 3,000 g for 5 min at 4°C. The supernatants were collected and centrifuged at 100,000 g for 1 h at 4°C. The supernatants were discarded and the pellets were resuspended in Laemmli sample buffer. Immunoblotting was performed using affinity-purified rabbit polyclonal anti-Cx50 antibodies (3) and horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies (Jackson ImmunoResearch). Detection of secondary antibody binding was performed by enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) as previously described (4).
Expression of connexins in Xenopus oocytes.
Connexin cRNAs were synthesized using the mMessage mMachine in vitro transcription kit (Ambion, Austin, TX) according to the manufacturer's instructions. The amount of cRNA was quantitated by measuring the absorbance at 260 nm.
Adult female Xenopus laevis frogs were anesthetized with tricaine, and a partial ovariectomy was performed in accordance with protocols approved by the Rosalind Franklin University Animal Care and Use Committee. The oocytes were manually defolliculated after treating them with collagenase IA (Worthington Biochemical, Lakewood, NJ). Stage V and VI oocytes were selected and pressure injected using a Nanoject variable microinjection apparatus (model no. 3-000-203, Drummond Scientific, Broomal, PA) with 36.8 nl of 0.5–600 ng/μl of connexin cRNA and 5 ng/36.8 nl of oligonucleotides antisense to mRNA for Xenopus Cx38 as previously described (9). The oocytes were incubated overnight at 18°C in L-15 medium (Invitrogen Life Technologies) containing 2 mM CaCl2 and 2 mM CoCl2 before electrophysiological experiments were performed.
Electrophysiological measurements.
For measurement of gap junctional coupling, connexin cRNA-injected oocytes were devitellinized and paired as previously described (8). Double two-microelectrode voltage-clamp experiments were performed using Geneclamp 500 and an Axoclamp 2A voltage-clamp amplifier (Axon Instruments, Union City, CA). The microelectrodes were filled with 3 M KCl and had a resistance between 0.1 and 0.6 MΩ. To prevent electrode leakage, the tips of the electrodes were backfilled with 1% agar in 3 M KCl. For simple measurements of gap junctional coupling, both cells of a pair were held initially at −40 mV, and voltage-clamp steps were applied to one cell while the second cell was held at −40 mV. Under these conditions, the change in current measured in the second cell would be equal to the junctional current (Ij), but of opposite polarity. The junctional conductance (gj) was calculated as (gj = Ij/Vj), where Vj (transjunctional voltage) = Vcell 2 − Vcell 1.
For measurements of hemichannel currents in single oocytes, a series of voltage-clamp steps were applied between −70 and +30 mV in increments of 10 mV from a holding potential of −80 mV. The standard external bath solution contained (in mM) 88 NaCl, 1 KCl, 2.4 NaHCO3, 1 MgCl2, and 15 HEPES, pH 7.6 to which different concentrations of calcium were added.
Pulse generation and data acquisition were performed using a PC computer equipped with PCLAMP6 software and a TL-1 acquisition system (Axon Instruments). Currents were filtered at 20–50 Hz and digitized using PCLAMP6 software and a Digidata 1200 (Axon Instruments). All experiments were performed at room temperature (20–22°C).
For single-channel measurements, the oocyte vitelline membrane was removed and the channels were studied with the cell-attached patch-clamp technique. All measurements were performed at room temperature. Pipettes were pulled using a Flaming/Brown micropipette puller (model P-87; Sutter Instruments, Novato, CA). The patch pipettes had resistances of 2–5 MΩ when filled with standard internal solution containing (in mM) 140 KCl, 1 EGTA, 0.5 CaCl2, 1 MgCl2, and 5 HEPES, pH 7.6. The bath chamber contained the same solution as the pipettes. Single-channel currents were recorded in the cell-attached patch configuration using an Axopatch 200B amplifier (Axon Instruments). Signals were filtered at 1 kHz and digitized with a Digidata 1322A analog/digital converter (Axon Instruments) at 10 kHz using pClamp 9.2 (Axon Instruments). Single-channel current-voltage (I-V) curves were determined using 200-ms voltage-clamp ramps between −100 mV and 100 mV. The I-V curves of the main open state were constructed by subtracting a segmented average trace of the baseline current from a single current trace or a segmented average trace of the current when the channel was in the main open state.
Confocal microscopy.
Oocytes expressing GFP-tagged connexins were placed on collagen-coated glass-bottom culture dishes (MatTek, Ashland, MA), and the vegetal hemispheres were observed using a confocal laser scanning system (VisiTech Infinity 3, Sunderland, UK) based on an Olympus IX71 inverted microscope with a ×20 objective. GFP was excited at 488 nm by an argon laser, and fluorescent emission was collected at wavelengths above 500 nm with a long pass barrier filter. Acquisition conditions were kept the same for all samples. The average intensity of connexins localized to the oocyte plasma membrane was quantified using a region of interest (ROI) defining a portion of the ring-like signal at the boundary of the oocytes, and subtracting the fluorescence intensity measured using a similar ROI in the extracellular space.
Oocyte viability experiments.
Oocyte viability was assessed by injecting oocytes with 6.9 ng of Cx46, Cx50, or G46V cRNA and incubating them in modified Barth's solution (MBS) containing 1 mM Ca2+ for 1, 24, 48, and 72 h at 18°C. In the case of the oocytes coinjected with a 1:1 mixture of Cx46 and G46V cRNA, the total amount of cRNA injected into each oocyte was 6.9 ng. For the oocytes coinjected with a 1:1 mixture of Cx50 and G46V cRNA, the total amount of cRNA injected into each oocyte was 13.8 ng. Cells were scored (dead or alive) by evaluating their appearances for evidence of cellular blebbing, irregularities in the border between animal and vegetal pole, and discoloration of the animal pole.
RESULTS
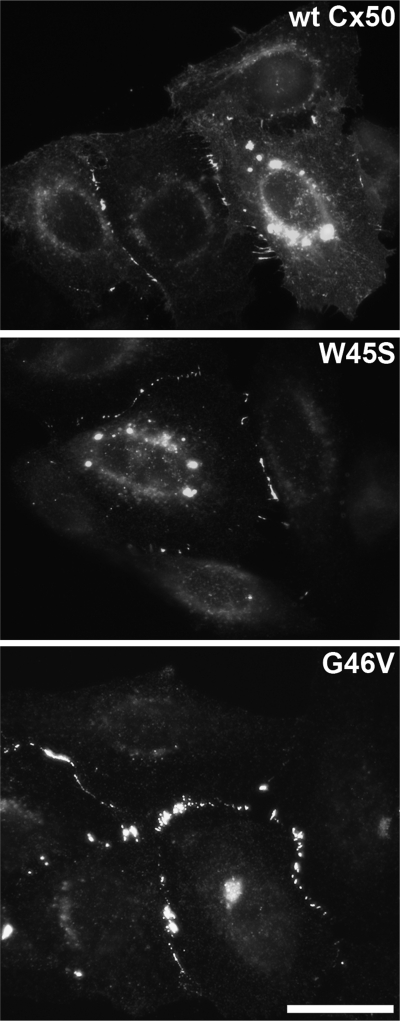
The capacity of W45S and G46V to form gap junctional plaques was assessed by immunofluorescence microscopy of HeLa cells transfected with wild-type Cx50, W45S, or G46V. Similar to wild-type Cx50, W45S and G46V localized at appositional membranes, where they formed gap junctional plaques, and in the perinuclear region, probably the Golgi compartment (Fig. 1).
Fig. 1.
Wild-type -and mutant connexin50 (Cx50) form gap junction plaques. Photomicrographs show the distribution of Cx50 immunoreactivity in HeLa cells expressing wild-type Cx50 (WT Cx50), W45S, or G46V. Scale bar, 31 μm.
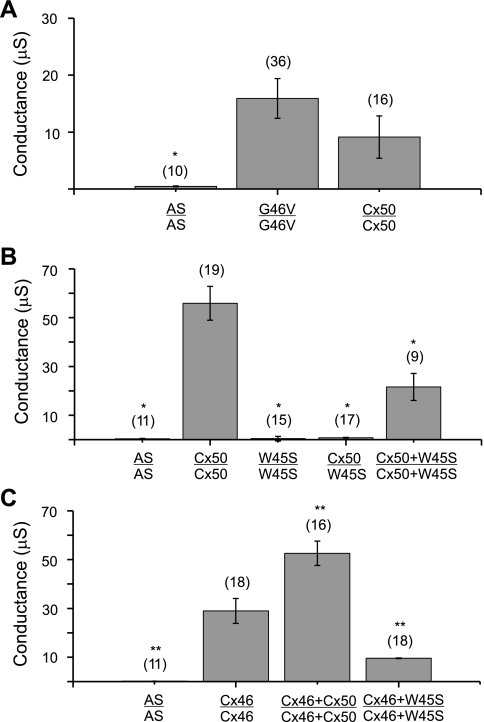
The ability of W45S and G46V to form functional gap junctional channels was tested by double two-electrode voltage clamp in pairs of Xenopus oocytes injected with the corresponding cRNAs. We have previously shown that pairs of oocytes expressing G46V exhibit similar levels of gap junctional conductance to oocytes expressing wild-type Cx50 (21). We confirmed these results in the current study (Fig. 2A). While homotypic oocyte pairs expressing G46V or wild-type Cx50 were well coupled, oocytes injected with cRNA for W45S failed to induce the formation of functional gap junctional channels when paired homotypically (Fig. 2B). Furthermore, heterotypic pairing of oocytes expressing wild-type Cx50 with oocytes expressing W45S did not induce significant coupling (Fig. 2B).
Fig. 2.
G46V and W45S differ in their abilities to form intercellular channels. A–C: bar graphs showing the junctional conductances (means ± SE) in pairs of Xenopus oocytes expressing wild-type Cx50, G46V, W45S, Cx46, or combinations of these connexins as determined by double whole cell voltage clamp. All oocytes were injected with antisense oligonucleotides (AS) to block endogenous Cx38 junctional currents. The number of pairs is indicated in parentheses. *P < 0.001 compared with wild-type Cx50-injected oocyte pairs; **P < 0.001 compared with Cx46-injected oocyte pairs.
To determine whether coexpression of W45S affected the junctional conductance produced by wild-type lens connexins, we studied pairs of oocytes coinjected with equal amounts of W45S and wild-type Cx50 or wild-type Cx46 cRNA. Coexpression of W45S decreased the junctional conductance induced by wild-type Cx50 and wild-type Cx46 by 61% and 66%, respectively (Fig. 2, B and C). These results suggest that the W45S mutant inhibits both wild-type Cx50 and wild-type Cx46 in a dominant negative manner.
To examine the effects of the G46V and W45S mutations on hemichannel activity, we measured hemichannel currents in oocytes injected with W45S, G46V, or wild-type Cx50 under conditions previously shown to enhance hemichannel activity of mouse Cx50 (i.e., KCl solution containing zero added calcium, pH 8.0) (3, 30). Both G46V and wild-type Cx50 cRNA-injected oocytes developed hemichannel currents (Table 1). Consistent with our previous observations (21), the hemichannel currents in G46V cRNA-injected oocytes were much larger than those in wild-type Cx50 cRNA-injected oocytes when the oocytes were injected with similar amounts of cRNA (data not shown). We attempted to reduce the functional expression of G46V hemichannels by injecting oocytes with much smaller amounts of G46V cRNA; even when oocytes were injected with ∼800-fold lower amounts of G46V than wild-type Cx50 cRNA, the G46V-induced currents were still significantly larger than those induced by wild-type Cx50 (Table 1). In contrast, oocytes injected with W45S cRNA did not develop detectable hemichannel currents (Table 1). The amplitudes of the currents in oocytes injected with W45S cRNA were significantly smaller (P < 0.007 by Student's t-test) than the currents in antisense-treated control oocytes, suggesting that W45S partially blocked residual endogenous hemichannels.
Table 1.
Hemichannel currents induced by wild-type and mutant Cx50
| cRNA Injected | cRNA Amount, ng | Hemichannel Current, nA | n |
|---|---|---|---|
| None | 0 | 136.50 ± 6.64 | 4 |
| WT Cx50 | 400 | 390.88 ± 45.90 | 17 |
| W45S | 400 | 81.57 ± 11.21 | 7 |
| G46V | 0.5 | 998.10 ± 270.21* | 12 |
Values are means ± SE; n, no. of oocytes. Hemichannel currents were measured at +30 mV in single Xenopus oocytes.
P < 0.001 compared with wild-type connexin50 (WT Cx50) cRNA-injected cells by Student's t-test.
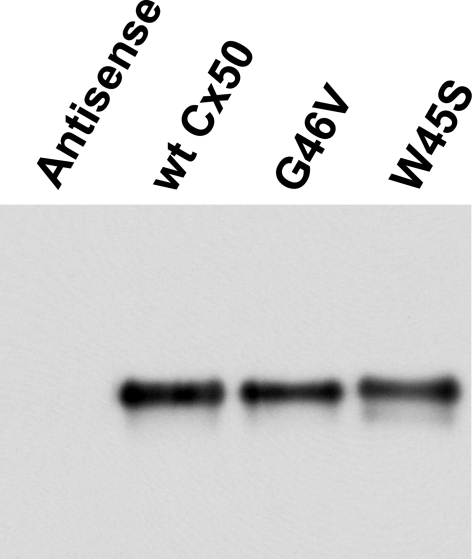
Possible explanations for the increased hemichannel currents induced by the G46V mutation include higher levels of protein, an augmentation of the number of Cx50 hemichannels at the cell surface, a larger single channel conductance, or an increase in channel open probability. To test for any differences in protein levels of the wild-type and mutant connexin proteins, we performed immunoblot analyses of membrane-enriched samples prepared from oocytes injected with equal amounts of the cRNAs encoding wild-type Cx50, W45S, or G46V. Samples from oocytes injected with wild-type Cx50 or either mutant cRNA exhibited a predominant band with an electrophoretic mobility of ∼60 kDa (Fig. 3). No immunoreactive bands were detected in antisense-injected control oocytes (Fig. 3). The mean intensities of the bands corresponding to wild-type Cx50, G46V, and W45S were consistently similar, indicating that levels of wild-type and mutant Cx50 were comparable.
Fig. 3.
Levels of wild-type Cx50, G46V, and W45S are similar in cRNA-injected oocytes. Immunoblot shows the levels of wild-type and mutant Cx50 in samples enriched in plasma membranes prepared from control Xenopus oocytes or oocytes injected with cRNAS encoding wild-type Cx50, G46V, or W45S. All oocytes were injected with antisense Cx38 oligonucleotides.
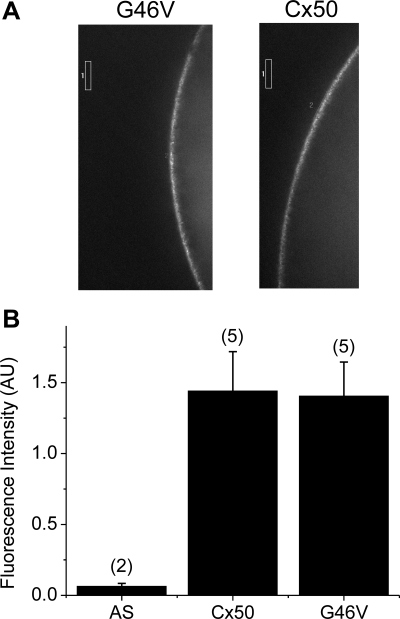
We next examined the possibility that the dramatic difference in the magnitudes of wild-type Cx50 and G46V hemichannel currents might reflect dissimilarities in their abundances in the oocyte plasma membranes by comparing the fluorescence intensities in oocytes injected with GFP-tagged versions of these proteins. The fluorescence intensity in the cell membrane of oocytes injected with G46V-GFP cRNA appeared similar to that of oocytes injected with wild-type Cx50-GFP cRNA (Fig. 4A). Figure 4B summarizes the results of quantification of these experiments; there was no significant difference in surface fluorescence intensity between the two tagged proteins. Consistent with the behavior of the untagged connexins, the G46V-GFP cRNA-injected oocytes developed much larger hemichannel currents than wild-type Cx50-GFP cRNA-injected oocytes (data not shown).
Fig. 4.
Mutation of glycine-46 to valine does not alter levels of Cx50 in the plasma membrane of oocytes. A: photomicrographs showing the fluorescence intensity in oocytes injected with wild-type Cx50-green fluorescent protein (GFP) or G46V-GFP cRNA. B: histogram comparing the average fluorescence intensity in the cell membrane of oocytes injected with wild-type Cx50-GFP or G46V-GFP cRNA. The boxes indicate the regions of interest used to quantify fluorescence intensity. The number of cells is indicated in parentheses. AU, arbitrary units.
To explore the possibility that the increased hemichannel currents produced by the G46V mutation were due to alterations of Cx50 single-channel properties, we studied these properties in oocytes using the cell-attached patch-clamp technique. Both wild-type Cx50 and G46V channels were predominantly in the open state at 0 mV and closed to a long-lasting substate after application of a voltage-clamp step to +40 mV. When the potential was changed to −40 mV, the channels reopened and remained in the fully open state for the duration of the pulse as illustrated in Fig. 5. Wild-type and mutant Cx50 hemichannels also showed similar open channel I-V curves. The slope conductances at −40 mV were 652 ± 53 pS and 670.5 ± 10.5 pS for wild-type Cx50 (n = 2) and G46V (n = 2), respectively.
Fig. 5.
Wild-type and mutant Cx50 hemichannels have similar single-channel properties. A and B: single-channel current traces for wild-type Cx50 (A) and G46V (B) in the cell-attached mode. Voltage-clamp steps of ± 40 mV were applied from a holding potential of 0 mV. C and D: open channel current-voltage (I-V) curves for wild-type Cx50 (C) and G46V (D) obtained by application of a 200-ms voltage ramp between −100 mV and +100 mV to cell-attached patches containing only one active channel. Voltage-clamp ramp data were corrected for leakage currents as described in materials and methods.
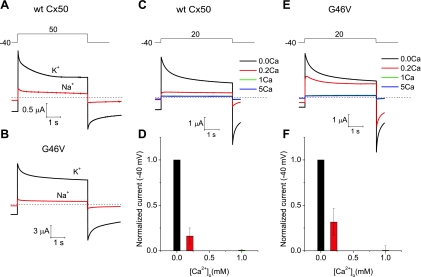
We also considered the possibility that the G to V missense mutation at position 46 might alter the regulation of Cx50 channels. Replacement of extracellular potassium with sodium while maintaining extracellular calcium constant resulted in a large reduction in the magnitude of both wild-type Cx50- and G46V-induced currents (Fig. 6, A and B); thus, these currents appeared to be similarly regulated by external monovalent cations. Like wild-type Cx50, the G46V-induced currents were inhibited by raising the extracellular calcium concentration ([Ca2+]o) (Fig. 6, C–F). Both wild-type Cx50 and G46V were blocked to a similar extent in 1 mM [Ca2+]o. Thus, the G46V mutant did not appear to exhibit a significantly altered sensitivity to extracellular calcium.
Fig. 6.
Wild-type and mutant Cx50 hemichannel currents are regulated by monovalent and divalent cations. A and B: membrane currents recorded in oocytes expressing wild-type Cx50 (A) and G46V (B) during repetitive voltage-clamp steps to 50 mV from a holding potential of −40 mV. The oocytes were initially bathed in 89 mM KCl containing zero added extracellular Ca2+ concentration ([Ca2+]o; black trace). Equimolar replacement of K+ with Na+ in the continued presence of zero added [Ca2+]o caused a large reduction in the amplitude of the hemichannel current (red trace). C: example of membrane currents recorded from a wild-type Cx50-expressing oocyte at different [Ca2+]o. The oocyte was sequentially exposed to 89 mM KCl containing Ca2+ concentrations of 0, 0.2, 1, and 5 mM. D: bar graph summarizing the concentration-response data for Ca2+ inhibition of wild-type Cx50 hemichannel currents in external K+. The current amplitudes at each Ca2+ concentration were normalized to the responses obtained in 0 mM [Ca2+]o. Data are presented as means ± SE (n = 7). E: example of membrane currents recorded from a G46V-expressing oocyte at different [Ca2+]o. The oocyte was sequentially exposed to 89 mM KCl containing Ca2+ concentrations of 0, 0.2, 1, and 5 mM. F: bar graph summarizing the concentration-response data for Ca2+ inhibition of G46V hemichannel currents in external K+. The current amplitudes at each Ca2+ concentration were normalized to the responses obtained in 0 mM [Ca2+]o. Data are means ± SE (n = 6). To reduce the size of the G46V hemichannel currents to levels comparable to those observed in wild-type Cx50-expressing oocytes, we injected oocytes with ∼800-fold lower amounts of G46V than wild-type Cx50 cRNA.
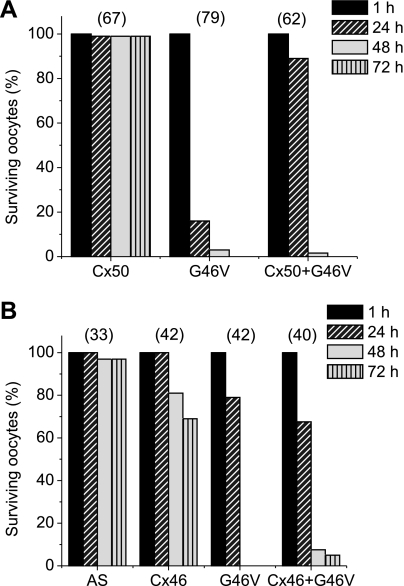
To gain further insight regarding the impact of the G46V mutation in the lens, we examined the effect of coexpressing G46V with other lens connexins on oocyte survival. The results obtained from several oocyte batches are presented in Fig. 7, A and B. Nearly all control oocytes and oocytes expressing wild-type Cx50 remained healthy when incubated in MBS containing 1 mM [Ca2+]o and 1 mM [Mg2+]o for at least 72 h, the longest time point studied (Fig. 7A). Oocytes expressing Cx46 exhibited 100% survival 24 h postinjection, but signs of cellular degeneration were observed at longer times after Cx46 cRNA injection; oocyte viability decreased to 81% and 69% after 48 and 72 h postinjection, respectively (Fig. 7B). Oocytes expressing G46V deteriorated even more rapidly than Cx46-injected oocytes, although the exact time course of deterioration differed among different batches of oocytes (Fig. 7, A and B). In all experiments, >80% of G46V-expressing oocytes showed degenerative changes by 48 h after cRNA injection (viability was <20%). Furthermore, oocytes coinjected with G46V cRNA and wild-type Cx50 or Cx46 cRNA deteriorated to approximately the same extent as oocytes injected with G46V cRNA alone 48–72 h after cRNA injection (Fig. 7, A and B).
Fig. 7.
Coexpression of G46V mutant with wild-type lens connexins decreases oocyte viability. Graphs represent the number of surviving oocytes in media containing 1 mM Ca2+ at different times following injection of connexin cRNA. A: oocytes were injected with Cx50, G46V, or a mixture of equal amounts of Cx50 and G46V (Cx50 + G46V) cRNAs, or no cRNA (AS) and incubated for 1, 24, 48, or 72 h at 18°C. The total amount of cRNA injected was 6.6 ng/oocyte. B: oocytes were injected with wild-type Cx46, G46V, or a mixture of equal amounts of wild-type Cx46 and G46V (Cx46 + G46V) cRNAs, or no cRNA (AS) and incubated for 1, 24, 48, or 72 h at 18°C. The total amount of cRNA injected was 6.9 ng/oocyte, except in the case of Cx50 + G46V where it was 13.8 ng/oocyte. The number of oocytes injected with each type of cRNA is indicated in parentheses.
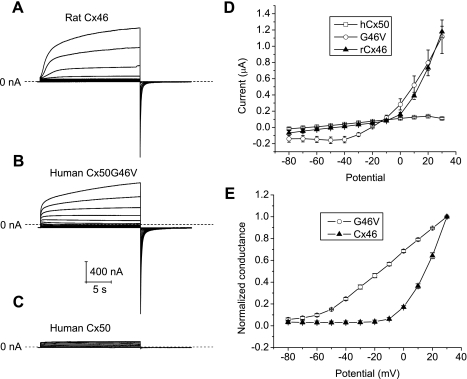
To determine whether the differences in oocyte survival could be attributed to differences in the magnitude or voltage gating properties of the hemichannel currents produced by G46V and wild-type connexins, the biophysical properties of macroscopic hemichannel currents were investigated in single oocytes expressing Cx46, wild-type Cx50, or G46V (in NaCl solutions containing 0.7 mM [Ca2+]o and 1 mM [Mg2+]o). Both G46V and Cx46 cRNA-injected oocytes developed time-dependent currents that activated on depolarization and had a reversal potential near −10 mV (Fig. 8, A and B). No hemichannel currents greater than those detected in antisense-injected oocytes were observed in oocytes injected with wild-type Cx50 alone (Fig. 8, C and D). G46V hemichannel currents could be distinguished from the Cx46 hemichannel currents by their faster time course of activation and slower time course of deactivation. In addition, the G46V hemichannels activated at more negative voltages than Cx46. The voltage dependence of activation of Cx46 and G46V hemichannels was quantified by plotting the amplitude of the initial tail current at −80 mV as a function of test potential. Cx46 hemichannels had a threshold for activation of approximately −10 mV while G46V hemichannels had a threshold for activation of approximately −80 mV. Thus, while G46V hemichannels exhibited a substantial open probability at hyperpolarized potentials, Cx46 hemichannels were mostly closed at potentials negative to −10 mV (Fig. 8, D and E).
Fig. 8.
Membrane currents from hemichannels composed of Cx46 and G46V exhibit distinct voltage-dependent properties. A–C: representative families of current traces recorded from single oocytes injected with similar amounts of cRNA for Cx46 (A), G46V (B), or wild-type Cx50 (C) in NaCl solution containing 0.7 mM [Ca2+]o and 1 mM [Mg2+]o. The oocytes were held at −80 mV between pulse sequences. Voltage-clamp steps were applied to voltages between −80 mV and 30 mV in increments of 10 mV. The data were not corrected for leakage current. Dashed line represents zero current. D: quasi-steady-state I-V relationships for Cx46 (▲, n = 3), G46V (○, n = 4), and wild-type Cx50 (□, n = 5) were determined by measuring the current at the end of the test pulse and plotting it as a function of pulse potential. hCx50, human Cx50; rCx46, rat Cx46. E: isochronal conductance-voltage relationships for Cx46 (▲, n = 3) and G46V (○, n = 4). Initial amplitude of tail currents were measured at −80 mV after 20-s pulses to different potentials and then normalized to the amplitude of the tail current after a voltage-clamp pulse to +30 mV.
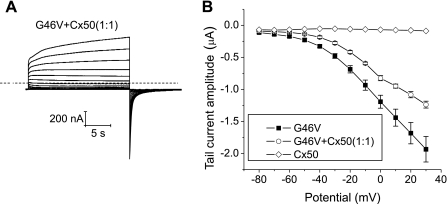
The magnitudes and voltage dependent gating of hemichannel currents were also studied in single oocytes expressing G46V in combination with wild-type Cx50. Coinjection of G46V with wild-type Cx50 cRNA at a ratio of 1:1 induced hemichannel currents whose voltage dependence of activation resembled those of G46V (Fig. 9). The magnitudes of the heteromeric hemichannel currents were slightly smaller than those of the homomeric G46V hemichannel currents.
Fig. 9.
G46V has a dominant behavior when mixed with wild-type Cx50. A: representative family of current traces recorded from single oocytes coinjected with cRNA for G46V and wild-type Cx50 in NaCl solution containing 0.7 mM [Ca2+]o and 1 mM [Mg2+]o. Voltage-clamp steps were applied to voltages between −80 mV and +30 mV in 10-mV increments from a holding potential of −80 mV. No leakage current correction was applied. Dashed line represents zero current. B: tail I-V relationships for G46V (■, n = 5), Cx50 (◊, n = 1), or G46V + Cx50 (1:1, ○, n = 4). Initial amplitudes of tail currents were measured at −80 mV after 20-s pulses to different potentials and plotted as a function of pulse potential. The total amount of cRNA injected was 0.147 ng/oocyte, except in the case of Cx50 + G46V where it was 0.294 ng/oocyte.
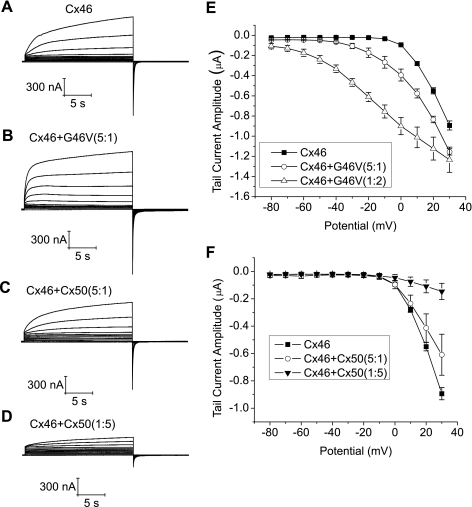
The effect of mixing of wild-type or mutant Cx50 with Cx46 on hemichannel function was examined by coinjecting oocytes with different ratios of wild-type or mutant Cx50 cRNA to Cx46 cRNA while keeping the amount of injected Cx46 cRNA constant (Fig. 10). The main effect of coexpressing wild-type Cx50 with Cx46 was to reduce the magnitude of the hemichannel currents. This effect was more pronounced at higher ratios of wild-type Cx50:wild-type Cx46 (Fig. 10, C and D). Coexpression of wild-type Cx50 with Cx46 at ratios of 1:5 and 5:1 also caused a slowing of the time course of both activation and deactivation of hemichannel currents but did not shift the threshold for Cx46 hemichannel activation. In contrast, when G46V cRNA was coinjected with Cx46 cRNA at a ratio of 1:5 (to obtain an average of one mutant subunit per hemichannel), there was a small shift in the threshold for hemichannel activation to more negative potentials, an acceleration in the time course of hemichannel current activation and a slowing in the time course of hemichannel current deactivation, but the magnitude of the currents did not change significantly. Increasing the ratio of G46V to Cx46 cRNA caused a progressively larger negative shift in the threshold of activation and increased the magnitude of the tail currents. These findings demonstrate that although both wild-type Cx50 and G46V can interact with Cx46 to form heteromeric hemichannels, the identity of the amino acid residue at position 46 can have differential effects on hemichannel gating.
Fig. 10.
Mixing of mutant Cx50 but not wild-type Cx50 with Cx46 results in increased hemichannel opening at negative potentials. A–D: membrane currents recorded in response to voltage-clamp steps from −80 mV to +30 mV in 10-mV increments from a holding potential of −80 mV. Equal amounts of Cx46 cRNA were injected into each oocyte. Oocytes were bathed in NaCl solution containing 0.7 mM [Ca2+]o and 1 mM [Mg2+]o. E: tail I-V relationships for Cx46 (■, n = 3), Cx46 + G46V (5:1, ○, n = 3), and Cx46 + G46V (1:2, ▵, n = 3). Initial amplitudes of tail currents were measured at − 80 mV after 20-s pulses to different potentials and plotted as a function of pulse potential. F: tail I-V relationships for Cx46 (■, n = 3), Cx46 + Cx50 (5:1, ○, n = 3), and Cx46 + Cx50 (1:5, ▼, n = 3). Initial amplitudes of tail currents were measured at −80 mV after 20-s pulses to different potentials and plotted as a function of pulse potential.
DISCUSSION
In this study, we investigated the functional and cellular properties of two congenital cataract-associated mutations that alter amino acids located at the TM1/E1 boundary of Cx50, W45S, and G46V. Both of these mutants formed gap junctional plaques when they were expressed in transfected HeLa cells, suggesting that they were able to oligomerize and traffic to the plasma membrane properly. However, the functional behavior of the two mutants differed.
We expressed W45S and G46V in Xenopus oocytes, because this is an excellent system to study the channel properties of proteins that oligomerize and traffic to the plasma membrane properly like these mutants. Usually, the functional properties of connexin channels in Xenopus oocytes closely parallel their properties in mammalian cells. In the Xenopus oocyte expression system, levels of the expressed connexin can be manipulated by injecting different amounts of cRNA and the consequences of mixing of wild-type and mutant connexins can be evaluated by coinjecting different ratios of their cRNAs. Potential disadvantages of this system may include altered connexin behavior due to the lower temperature in which oocytes are maintained and possible differences in posttranslational modifications.
W45S did not form functional intercellular channels or hemichannels. Moreover, it acted as a dominant negative inhibitor of wild-type Cx50 and Cx46 gap junctional channels. The tryptophan at position 45 is highly conserved within the connexin family. Indeed, 14 of the 21 human connexins contain a tryptophan at this residue; the only substitutions are other aromatic residues, Y and F (even in other species). The 3.5 Å resolution crystal structure of Cx26 shows that the tryptophan at the corresponding position is packed within the hydrophobic environment of the four helix bundle and is involved in interprotomer interactions that stabilize hexamer structures (18). Thus, it is not surprising that introduction of a polar residue, as in the W45S mutation, would result in loss of channel function. Our results may be generalizable to other connexins. Replacement of the corresponding residue with S or C in Cx26, with S in Cx46, and with L in Cx32 is associated with deafness, cataracts, and X-linked Charcot-Marie-Tooth disease, respectively (5, 6, 17). Consistent with our findings that W45S acted as a dominant negative inhibitor of coexpressed wild-type Cx50, the deafness and cataracts due to the substitutions in Cx26 and Cx46 are inherited as dominant traits. The functional consequences of substitution at this position have only been studied for Cx46 and Cx26; replacement with cysteines resulted in loss of channel function in both cases (16, 19). Similar to our findings for W45S, the cysteine-substituted Cx26 formed gap junctional plaques (19).
In contrast, the results presented here and in our previous study (21) showed that the G46V mutant is functional. Indeed, it shows increased hemichannel function. G46 might have been expected to be more tolerant of amino acid substitutions than W45, because it is predicted to be a pore lining amino acid based on the crystal structure of Cx26 (18). Although the corresponding residue is a glycine in 9 of the 21 human connexins, other amino acids (including D, N, E, Q, S, Y, and K) are present at the corresponding position in other connexins, suggesting that a variety of polar or charged amino acids can be tolerated in some contexts. However, replacement of this glycine with glutamate in Cx26 has been associated with deafness and KID syndrome (14, 23) and in Cx30 with Erythrokeratodermia variabilis (The Connexin-deafness homepage, Mutations databases, http://davinci.crg.es/deafness/index.php).
In our previous publication, we showed that expression of G46V led to cell death (21). The results of our current study suggest that cells expressing G46V exhibit greatly enhanced hemichannel activity compared with wild-type Cx50 at normal membrane potentials. This enhanced hemichannel activity would tend to depolarize the cells and increase entry/exit of metabolites and ions, thus explaining the observed decrease in cell viability. The increase in hemichannel activity of G46V did not appear to be the result of altered sensitivity to factors previously shown to regulate Cx50 [i.e., external calcium (3), monovalent cations (30), [pH]o (3)], nor could it be explained by increased total connexin levels or amounts of connexin at the plasma membrane. However, previous studies using cysteine substitution have shown that G46 lies in a region of the pore that narrows during hemichannel closure by external calcium and hyperpolarization, suggesting that G46 is involved in Cx50 hemichannel gating (27).
Our data are also consistent with a dominant gain of hemichannel function for G46V, which rapidly killed Xenopus oocytes even when coexpressed with wild-type lens connexins. Thus, the cellular toxicity due to the mutant allele is a dominant trait that is not ameliorated by the presence of either wild-type Cx50 or Cx46. Interestingly, heteromeric hemichannels formed by G46V and Cx46 had clearly different voltage gating properties compared with heteromeric channels formed by wild-type Cx50 and Cx46. Coexpression of G46V with wild-type Cx46 had a deleterious effect on oocyte survival and correlated with an increase in hemichannel activity at negative potentials. In contrast, coexpression of wild-type Cx50 with wild-type Cx46 appeared to have a protective effect (data not shown) and was associated with a diminution of hemichannel activity. These results indicate that the interactions of wild-type Cx50 or G46V with wild-type Cx46 result in formation of hemichannels with different behaviors.
We can explain the effects of the G46V mutation on Cx50 behavior using the following simple kinetic scheme:
where A, B, and C are closed states and O is an open state. According to this model, nearly all of wild-type Cx50 channels reside in A, where A is a very long lived closed state, even in the presence of low [Ca2+]o. The main effect of the G46V mutation is to destabilize A either by slowing entry into A or by increasing exit from A. This will increase the number of channels in B that are available for opening following application of a depolarizing step or reduction of calcium. The behavior of the heteromeric channels can also be explained using this simple kinetic scheme by assuming that their behavior is intermediate between that of the wild-type Cx50 (or Cx46) channels and the G46V mutant channels and depends on the ratio of mutant to wild-type subunits. Channels containing five wild-type and one mutant subunit would be expected to exhibit behavior that more closely resembles that of wild-type Cx50 (or Cx46). In contrast, channels containing five mutant subunits and one wild-type subunit would be expected to behave like G46V.
Comparison of the functional behavior of the G46V mutation to the behavior of a disease-associated form of Cx26 with a missense mutation at the corresponding position (Cx26G45E) shows a number of similarities as well as some differences (11, 29, 31). Like G46V, Cx26G45E formed both gap junctional channels and hemichannels. Furthermore, the single-channel conductance, voltage-gating properties, and calcium sensitivity of the Cx26G45E hemichannels were similar to wild type. There are conflicting data about the effect of the G45E mutation on the size of the macroscopic Cx26 hemichannel current. While Gerido et al. (11) reported significantly larger currents for Cx26G45E compared with wild-type Cx26, Sánchez et al. (29) did not. Sánchez et al. (29) also reported that Cx26G45E channels exhibited altered permeability to calcium. Our results more closely resemble those of Gerido et al. (11). We were unable to test whether G46V hemichannels showed altered permeability to calcium because of the extremely small size of wild-type Cx50 currents in the presence of physiological calcium concentrations.
Many of the cataracts that develop in individuals with mutations in the GJA8 gene have been described as nuclear, sometimes with a pulverulent appearance. The cataracts in members of the family with the W45S mutation have a fanlike opacity that tends to hide the deeper structure of the opacity (36). In contrast, the individual carrying the G46V mutation had a total cataract (21). The different effects of W45S and G46V on channel function may contribute to the difference in cataract phenotypes observed in people carrying the mutations. Since W45S acts as a dominant negative inhibitor of coexpressed wild-type Cx50 or Cx46, it would be expected to decrease gap junctional coupling between lens fiber cells. In contrast, it is likely that increased hemichannel function is the primary cause of cataracts in people carrying the G46V mutation. Sustained, high hemichannel activity would be expected to cause alterations in ionic homeostasis, loss of plasma membrane potential, and depletion of vital metabolites such as ATP and glutathione, leading to cell death. However, the relationships between Cx50 mutant genotype and cataract phenotype must be labeled as speculative. Indeed, the same Cx50 mutant, Cx50P88Q, has been associated with cataracts of different phenotypes in families of different ethnic backgrounds (2, 35).
In summary, our data suggest that mutations at adjacent residues at the TM1/E1 boundary lead to differential effects on hemichannel and gap junction channel function, and that these mutations may differentially affect the function of wild-type connexins.
GRANTS
This work was supported by National Institutes of Health Grants RO1 EY-10589 (to L. Ebihara) and R01 EY-08368 (to E. C. Beyer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jing Li for excellent technical assistance.
REFERENCES
- 1. Arora A, Minogue PJ, Liu X, Addison PK, Russel-Eggitt I, Webster AR, Hunt DM, Ebihara L, Beyer EC, Berthoud VM, Moore AT. A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J Med Genet 45: 155–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet 43: e2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys J 82: 2016–2031, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berthoud VM, Minogue PJ, Guo J, Williamson EK, Xu X, Ebihara L, Beyer EC. Loss of function and impaired degradation of a cataract-associated mutant connexin50. Eur J Cell Biol 82: 209–221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bone LJ, Deschenes SM, Balice-Gordon RJ, Fischbeck KH, Scherer SS. Connexin32 and X-linked Charcot-Marie-Tooth disease. Neurobiol Dis 4: 221–230, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Denoyelle F, Lina-Granade G, Plauchu H, Bruzzone R, Chaib H, Levi-Acobas F, Weil D, Petit C. Connexin 26 gene linked to a dominant deafness. Nature 393: 319–320, 1998 [DOI] [PubMed] [Google Scholar]
- 7. DeRosa AM, Xia CH, Gong X, White TW. The cataract-inducing S50P mutation in Cx50 dominantly alters the channel gating of wild-type lens connexins. J Cell Sci 120: 4107–4116, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Ebihara L. Expression of gap junction proteins in Xenopus oocyte pairs. In: Ion Channels, edited by Rudy B, Iverson LE.San Diego, CA: Academic, 1992, p. 376–380 [DOI] [PubMed] [Google Scholar]
- 9. Ebihara L. Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys J 71: 742–748, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ebihara L, Berthoud VM, Beyer EC. Distinct behavior of connexin56 and connexin46 gap junctional channels can be predicted from the behavior of their hemi-gap-junctional channels. Biophys J 68: 1796–1803, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol 293: C337–C345, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AF, Tesson F, Klein GJ YR, Skanes AC, Guiraudon GM, Ebihara L, Ebihara L, Bai D. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med 354: 2677–2688, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gupta VK, Berthoud VM, Atal N, Jarillo JA, Barrio LC, Beyer EC. Bovine connexin44, a lens gap junction protein: molecular cloning, immunological characterization, and functional expression. Invest Ophthalmol Vis Sci 35: 3747–3758, 1994 [PubMed] [Google Scholar]
- 14. Janecke AR, Hennies HC, Gunther B, Gansl G, Smolle J, Messmer EM, Utermann G, Rittinger O. GJB2 mutations in keratitis-ichthyosis-deafness syndrome including its fatal form. Am J Med Genet 133A: 128–131, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387: 80–83, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J Gen Physiol 122: 389–405, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma ZW, Zheng JQ, Li J, Li XR, Tang X, Yuan XY, Zhang XM, Sun HM. Two novel mutations of connexin genes in Chinese families with autosomal dominant congenital nuclear cataract. Br J Ophthalmol 89: 1535–1537, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458: 597–602, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Martin PE, Coleman SL, Casalotti SO, Forge A, Evans WH. Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal heriditary deafness. Hum Mol Genet 8: 2369–2376, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Minogue PJ, Liu X, Ebihara L, Beyer EC, Berthoud VM. An aberrant sequence in a connexin46 mutant underlies congenital cataracts. J Biol Chem 280: 40788–40795, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minogue PJ, Tong JJ, Arora A, Russell-Eggitt I, Hunt DM, Moore AT, Ebihara L, Beyer EC, Berthoud VM. A mutant connexin50 with enhanced hemichannel function leads to cell death. Invest Ophthalmol Vis Sci 50: 5837–5845, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol 114: 339–364, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohtsuka A, Yuge I, Kimura S, Namba A, Abe S, Van LL, Van CG, Usami S. GJB2 deafness gene shows a specific spectrum of mutations in Japan, including a frequent founder mutation. Hum Genet 112: 329–333, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Pal JD, Berthoud VM, Beyer EC, Mackay D, Shiels A, Ebihara L. Molecular mechanism underlying a Cx50-linked congenital cataract. Am J Physiol Cell Physiol 276: C1443–C1446, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol Cell Physiol 279: C596–C602, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT. Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol 49: 300–315, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Ressot C, Bruzzone R. Connexin channels in Schwann cells and the development of the X-linked form of Charcot-Marie-Tooth disease. Brain Res Brain Res Rev 32: 192–202, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet 70: 1341–1348, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J Gen Physiol 136: 47–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srinivas M, Calderon DP, Kronengold J, Verselis VK. Regulation of connexin hemichannels by monovalent cations. J Gen Physiol 127: 67–75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stong BC, Chang Q, Ahmad S, Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope 116: 2205–2210, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Tang Q, Dowd TL, Verselis VK, Bargiello TA. Conformational changes in a pore-forming region underlie voltage-dependent “loop gating” of an unapposed connexin hemichannel. J Gen Physiol 133: 555–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas BC, Minogue PJ, Valiunas V, Kanaporis G, Brink PR, Berthoud VM, Beyer EC. Cataracts are caused by alterations of a critical N-terminal positive charge in connexin50. Invest Ophthalmol Vis Sci 49: 2549–2556, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tong JJ, Ebihara L. Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45.6 gap-junctional hemichannels. Biophys J 91: 2142–2154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vanita V, Singh JR, Singh D, Varon R, Sperling K. A mutation in GJA8 (p.P88Q) is associated with “balloon-like” cataract with Y-sutural opacities in a family of Indian origin. Mol Vis 14: 1171–1175, 2008 [PMC free article] [PubMed] [Google Scholar]
- 36. Vanita V, Singh JR, Singh D, Varon R, Sperling K. A novel mutation in GJA8 associated with jellyfish-like cataract in a family of Indian origin. Mol Vis 14: 323–326, 2008 [PMC free article] [PubMed] [Google Scholar]
- 37. Verselis VK, Trelles MP, Rubinos C, Bargiello TA, Srinivas M. Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J Biol Chem 284: 4484–4493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell 361: 711–720, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383: 725–737, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Xu X, Ebihara L. Characterization of a mouse Cx50 mutation associated with the No2 mouse cataract. Invest Ophthalmol Vis Sci 40: 1844–1850, 1999 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.