Summary
Foreign DNA activates the innate immune response through Toll-like receptor 9 (TLR9). In this issue of Immunity, Park et al (2011). present evidence that granulin is a cofactor for TLR9 activation, delivering CpG-oligodeoxynucleotides to TLR9 in endolysosomes.
A far cry from the simple saccules drawn in textbooks, the endolysosomes of phagocytic cells are complex organelles within which many biochemical transformations occur, some of them essential for normal immune function. Only recently has the role of the endolysosome as a sensory organelle been appreciated, and much work still needs to be done to fully understand its operation as such.
The DNA sensor Toll-like receptor 9 (TLR9) traffics from the endoplasmic reticulum (ER) to endolysosomal compartments where it encounters ligand and activates innate immune responses (Blasius and Beutler, 2010). UNC93B, a twelve transmembrane spanning protein, associates with TLR9 and is required for the transport of TLR9 from the ER to endolysosomes. ER chaperones Gp96 and PRAT4A also play roles in TLR9 trafficking. Once in endolysosomes, TLR9 is proteolytically cleaved within its ectodomain to generate a signaling-competent receptor capable of recruiting MyD88 and initiating downstream signaling. Multiple proteases have been implicated in TLR9 cleavage, an event that requires proper endolysosomal acidification.
Different proteins are needed to set up the endolysosomal DNA sensing system in different cell types. In addition to the aforementioned proteins, in plasmacytoid dendritic cells lysosomal sorting proteins of the AP-3, BLOC-1, and BLOC-2 complexes, and the twelve transmembrane spanning peptide-proton symporter channel Slc15a4, are additionally required to permit TLR9 signaling, leading both to inflammatory cytokine production and to the abundant type I interferon production for which these cells are specialized (Blasius et al., 2010). TLR7 signaling depends on the same set of proteins, and the same modified endolysosomal compartment.
To this collection of molecules and events essential for TLR9 signaling, Park et al. contribute information concerning the initiation of signaling by TLR9 (Park et al., 2011). They present evidence that the cysteine-rich protein granulin functions to deliver CpG oligodeoxynucleotides (CpG-ODN) to TLR9 in endolysosomal compartments. Granulin interacts with both TLR9 and CpG-ODN in pull down assays from RAW264.7 macrophages. It is constitutively secreted by wild type bone marrow-derived macrophages (BMDM) and dendritic cells (BMDC), and its presence in the extracellular milieu is required for proinflammatory cytokine production induced specifically by CpG-ODN, but not poly I:C or imiquimod (TLR3 and TLR7 ligands, respectively). Ironically, the importance of granulin to TLR9 activation may have gone unnoticed (and in fact granulin was proposed to be anti-inflammatory) (Yin et al., 2010) because of its property as a secreted protein, which causes its presence in serum. As a result, granulin-deficient BMDM cultured in serum-containing media respond normally to stimulation with CpG-ODN. When cultured in serum-free media, granulin-deficient BMDM have a greatly reduced response to CpG-ODN, a defect that is rescued by addition of purified granulin. Thus, the role of granulin in promoting TLR9 activation is cell-extrinsic.
The idea that granulin serves to deliver CpG-ODN to TLR9 is supported by the finding that fluorescently labeled CpG-ODN internalized by RAW264.7 macrophages colocalized with fluorescently-tagged granulin expressed heterologously. The association of biotin-CpG with the cleaved form of TLR9 in pull down assays from granulin-deficient cells was greatly enhanced by the addition of purified granulin, suggesting that granulin is a cofactor for CpG-mediated TLR9 activation. More importantly, 75% fewer granulin-deficient BMDM (in serum-free culture) internalized CpG-ODN compared to wild type BMDM; supplying exogenous granulin to the cells restored their ability to acquire CpG-ODN. Paradoxically, this internalized CpG-ODN failed to colocalize with a marker of endolysosomal compartments. Although not tested in this report, granulin itself likely enters endolysosomes to interact transiently with TLR9 before being rapidly degraded by lysosomal proteases. In support of this hypothesis, copurification of granulin and TLR9 from RAW264.7 macrophages was found to require treatment with z-FA-fmk, a cysteine protease inhibitor specific for cathepsins.
What is granulin? Granulin was initially identified during experiments to purify peptides of the innate immune response from extracts of human leukocytes, including granulocytes; it was independently found as a modulator of cell proliferation from kidney epithelial cells (Bateman and Bennett, 2009). Granulin is now known to have widespread effects throughout the body, participating in embryogenesis, wound repair, oncogenesis, inflammation, and the maintenance of neuronal survival. Heterozygous mutations in the human granulin gene, GRN, cause tau-negative ubiquitin-positive frontotemporal lobar degeneration (FTLDU) due to haploinsufficiency (Bateman and Bennett, 2009). As a result of neuronal death in the frontotemporal cortex, FTLDU patients experience progressive changes in behavior, personality, and cognitive and language ability, sometimes accompanied by Parkinsonism. The mechanism by which loss of function mutations in GRN cause neurodegeneration remains to be fully elucidated, a particularly challenging task because of the lack of a Grn-deficient rodent model that phenocopies the human disease in either neurodegeneration or behavioral abnormalities.
The name granulin most commonly refers to each of the 6 kDa peptides derived by proteolysis of a precursor designated progranulin. A 593-amino acid secreted protein, progranulin is cleaved by elastase (Zhu et al., 2002) into seven peptides, each of which encodes a signature motif bearing twelve cysteine residues fully crosslinked to form six disulfide bridges. Both progranulin and granulin peptides possess biological activity (Tolkatchev et al., 2008). In the case of TLR9 activation, proteolytic processing of granulin is required for TLR9 signaling via an effect independent of TLR9 cleavage. Different granulins exert distinct effects on cancer cells (Tolkatchev et al., 2008), but whether one or more specific granulin peptides mediate the effect on TLR9 signaling was not tested. (Note that the authors do not distinguish between progranulin and granulin peptides, referring generally to ‘granulin,’ which could encompass either or both the precursor and the processed peptides).
In advancing understanding of TLR biology, the findings of Park et al. naturally also raise basic questions about the precise role of granulin in TLR9-dependent innate immune activation. First, does granulin show specificity in binding to different types of nucleic acids? Does granulin bind to single-stranded nucleic acids in preference to double-stranded, or to DNA in preference to RNA? Because granulin appears to be required specifically for TLR9 signaling, it might be presumed that it binds only to DNA, but this remains to be determined. Determining the affinity of granulin for CpG-ODN or other TLR9 ligands may also shed light on the mechanism of granulin function. Moreover, whether and how the functions of granulin intersect with those of other putative CpG delivery proteins, such as HMGB proteins (Ivanov et al., 2007), should be explored.
Another unanswered question is how granulin, with or without bound CpG-ODN, gains access to TLR9 in endolysosomes. The glycoprotein receptor sortilin, a mediator of mannose 6-phosphate receptor-independent delivery of soluble lysosomal proteins to lysosomes and a transmembrane receptor for selected neuropeptides, was recently identified as the major neuronal receptor for progranulin (Hu et al., 2010). Using COS-7 cells, sortilin was shown to associate with and direct extracellularly applied progranulin to lysosomes (Hu et al., 2010). Elevated levels of progranulin exist in brain lysates from sortilin-deficient mice, and neurodegeneration is not observed in these animals. It is clear that the contributions of progranulin, granulin, and sortilin to neuronal maintenance are complex, and likely involve both genetic and environmental modifiers that affect disease outcome. In macrophages, sortilin traffics from the Golgi to phagosomes, and its expression is upregulated in response to infection of macrophages with mycobacteria (Wahe et al., 2010). Thus, an intriguing hypothesis is that sortilin serves as the receptor for CpG-bound progranulin or granulin, transporting it to lysosomes where cleaved, signaling-competent TLR9 is present.
Finally, the questions of whether granulin is required in vivo to deliver viral or bacterial DNA to TLR9, and whether granulin-deficient mice are therefore generally or in certain cases hypersusceptible to infection must be answered. The authors showed that granulin-deficient mice failed to induce wild type levels of serum TNF-α and IL-6 upon intraperitoneal injection of CpG-ODN, and note that in a previous study granulin-deficient mice were unable to rapidly clear an infection of Listeria monocytogenes and displayed elevated bacterial burdens in the spleen, liver, and brain (Yin et al., 2010). However, these mice also experienced an excessive and prolonged inflammatory response to infection, and exhibited age-dependent hyperactivation of microglia and astrocytes, both of which suggest a state of exaggerated inflammation that at present is difficult to reconcile with a requirement for granulin in innate immune responses and particularly proinflammatory cytokine production. Survival during certain viral infections (notably infection by mouse cytomegalovirus and other herpesviruses) is strongly dependent on TLR9 signaling, and may be taken as a sensitive indicator of TLR9 function. The conclusion that granulin is a molecule of central importance in signaling viral invasion thus awaits further testing.
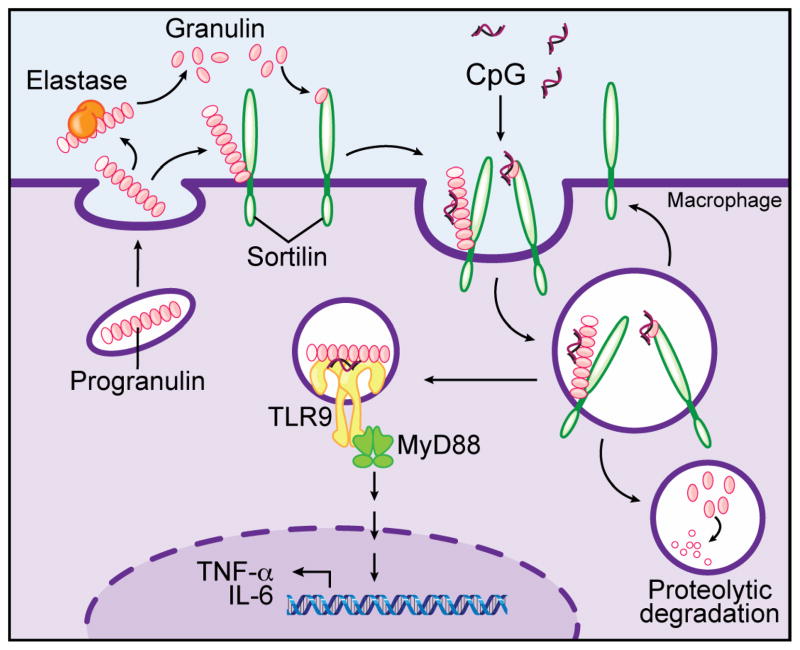
Taken together, the available data suggest a hypothetical model in which secreted granulin encounters and binds to CpG-ODN, either extracellularly or (more likely) in phagosomes (Figure 1). Sortilin may function as the receptor for granulin, constitutively transporting it to endolysosomes. When bound to CpG-ODN or perhaps other TLR9 ligands, granulin may serve to concentrate ligand for optimal efficiency of receptor activation, as well as act as a cofactor for TLR9 binding and activation. Future studies will undoubtedly clarify the role of granulin in TLR9 signaling.
Figure 1. Hypothetical model for granulin function in TLR9 signaling in macrophages.
Progranulin is constitutively secreted into the extracellular space, where it is cleaved by elastase. Whether this cleavage is signal-dependent is unknown. When present, CpG-ODN are bound by progranulin and/or granulin; the mode of interaction is unknown. The lysosomal sorting protein sortilin may act as the granulin receptor (as it does in neurons), bringing progranulin or granulin and bound CpG-ODN into the cell to endolysosomal compartments containing TLR9. Progranulin or granulin facilitates the interaction of CpG-ODN with TLR9, permitting the efficient initiation of MyD88-dependent signaling leading to proinflammatory cytokine production. Once in endolysosomes, CpG-bound and free progranulin or granulin are rapidly degraded, possibly by cathepsins, and sortilin may be recycled to the cell surface.
Acknowledgments
We thank Diantha La Vine for preparation of the illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–1254. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak Syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B, Buti B, Lee S, Matsuwaki T, Spooner E, Brinkmann MM, Nishihara M, Ploegh HL. Granulin is a novel soluble cofactor for Toll-like receptor 9 signaling. Immunity. 2011;xx:xxx–xxx. doi: 10.1016/j.immuni.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, Bennett HP, Bateman A, Ni F. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 2008;17:711–724. doi: 10.1110/ps.073295308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahe A, Kasmapour B, Schmaderer C, Liebl D, Sandhoff K, Nykjaer A, Griffiths G, Gutierrez MG. Golgi-to-phagosome transport of acid sphingomyelinase and prosaposin is mediated by sortilin. J Cell Sci. 2010;123:2502–2511. doi: 10.1242/jcs.067686. [DOI] [PubMed] [Google Scholar]
- Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, Nathan C, Ding A. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]