Abstract
Purpose
Pancreatic cancer (PC) is one of the deadliest of all tumors. Previously, we were the first to show that Thymoquinone (TQ) derived from black seed (Nigella sativa) oil has anti-tumor activity against PC. However, the concentration of TQ required was considered to be high to show this efficacy. Therefore, novel analogs of TQ with lower IC50 are highly desirable.
Methods
We have synthesized a series of 27 new analogs of TQ by modifications at the carbonyl sites or the benzenoid sites using single pot synthesis and tested their biological activity in PC cells.
Results
Among these compounds, TQ-2G, TQ-4A1 and TQ-5A1 (patent pending) were found to be more potent than TQ in terms of inhibition of cell growth, induction of apoptosis and modulation of transcription factor-NF-κB. We also found that our novel analogs were able to sensitize gemcitabine and oxaliplatin-induced apoptosis in MiaPaCa-2 (gemcitabine resistant) PC cells, which was associated with down-regulation of Bcl-2, Bcl-xL, survivin, XIAP, COX-2 and the associated Prostaglandin E2.
Conclusion
From our results, we conclude that three of our novel TQ analogs warrant further investigation against PC, especially in combination with conventional chemotherapeutic agents.
Keywords: apoptosis, pancreatic cancer, thymoquinone, thymoquinone analogs
INTRODUCTION
Pancreatic cancer (PC), the fourth leading cause of cancer death in the United States, accounts for an estimated 37,680 new cases and 34,290 deaths in 2009 (1). This dismal outcome in survival of patients diagnosed with PC is in part due to lack of early detection, the indolent nature of disease progression and perceived de novo and acquired chemo-resistance to conventional cytotoxic agents (2–5). Additionally, the aggressiveness of PC could in part be due to enrichment of cancer stem cells (CSCs) during therapy and because of the fact that CSCs are highly resistant to conventional therapeutics and thus may contribute to poor therapeutic outcome. Therefore, to improve the survival outcome of patients diagnosed with PC without undesirable side effects normally associated with current therapies, novel approaches must be devised for this deadly disease. To that end, we have previously shown the therapeutic and chemopreventive potential of the “natural dietary agent” Thymoquinone (TQ) derived from black seed (Nigella sativa) oil (6).
Nigella sativa is a spice that grows in the Mediterranean region and in Western Asian countries, including India, Pakistan and Afghanistan. In folklore medicine, the seed is reportedly associated with diverse therapeutic benefits in bronchial asthma, dysentery, headache, gastrointestinal problems, eczema, hypertension and obesity (7). The bioactive compound derived from Nigella sativa oil is TQ, which has been shown to exhibit anti-tumor activities, including anti-proliferative and pro-apoptotic effects on cell lines derived from breast, colon, ovary, larynx, lung, myeloblastic leukemia and osteosarcoma (8–13). It has also inhibited hormone refractory prostate cancer by targeting androgen receptor and transcription factor E2F (14). Mechanistically, as proposed by us and others, TQ reportedly induces apoptosis in tumor cells by suppressing NF-κB, Akt activation and extracellular signal-regulated kinase signaling pathways, and also inhibits tumor angiogenesis (6,15,16). Although a number of crucial genes are altered in PC, several important molecules stand out, including the COX family of proteins, which play critical roles in the initiation of cancer and acquisition of resistance to chemotherapeutics in human PC (17–19).
It is believed that PC arises from abnormal tissues or lesions in the pancreas known as pancreatic intra-epithelial neoplasias (PanINs); by stalling the growth of PanINs, we hope to slow the development of, or prevent, PC. COX-2 is an enzyme that is up-regulated by growth factor and inflammatory cytokine-mediated activation of NF-κB in PC (20–23). Further studies on breast, colon, and PC have also shown that COX-2 plays a key role in aggressive tumor growth and metastases (24–29). Furthermore, we have confirmed that down-regulation of the “master transcription factor,” NF-κB, by natural agents such as TQ could lead to the sensitization of PC cells to cytotoxic conventional therapeutic agents, especially gemcitabine (6); however, we reasoned that the concentration used for this study may not be feasible for human patients. We therefore hypothesized that custom chemical synthesis of novel analogs of TQ with lower IC50 and with proven biological activity would be a step forward toward the development of novel therapeutic agents for the treatment of PC. Here, we present the results of our novel studies and document for the first time the synthesis of novel analogs of TQ with lower IC50 and with better biological activity. These compounds were initially evaluated for their ability to inhibit cell viability and induction of apoptosis at concentrations that are lower than the parental TQ in a panel of PC cell lines with different molecular signatures, and further tested their ability to sensitize gemcitabine-resistant PC cell line (MiaPaCa-2) to enhanced killing by gemcitabine. Moreover, we evaluated one of these promising analogs in a murine model for testing its toxicity to normal cells.
MATERIALS AND METHODS
Cell Culture and Reagents
The human PC cell lines-MiaPaCa-2, BxPC-3, AsPC-1and HPAC were obtained from American Type Culture Collection (Manassas, VA). Colo 357 and L3.6pl cells were obtained from Dr. Paul Chaio at MD Anderson Cancer Center (Houston, TX). The cell lines were maintained in continuous exponential growth in Dulbecco modified Eagle’s medium (DMEM, Life Technologies, Inc., Gaithesburg, MD) supplemented with 10% FBS, 100 units/ml penicillin and 10 mg/ml streptomycin in a humidified incubator containing 5% CO2 in air at 37°C.
Antibodies were obtained from the following commercial sources: anti-mouse Bcl-2 and Bcl-xL antibody from Abcam (Cambridge, MA), anti-PARP antibody from Biomol Research (Plymouth, PA), Caspase-3 antibody from Cell Signaling (Beverly, MA), anti-XIAP and anti-survivin from (RandD Systems) and anti-β-actin from Sigma Chemical Co. (St. Louis, MO). TQ (Sigma Chemicals, Ontario, Canada) and the investigated analogs were dissolved in DMSO to make 20 mM stock solution. Oxaliplatin and gemcitabine were obtained from the pharmacy of our institute.
Combinatorial Screening and Synthesis of TQ Analogs
For building a small combinatorial library of TQ analogs, we used a simple yet selective one-pot synthesis of 2, 5-bis (alkyl/aryl amino) and mercaptan 1, 4-benzoquinones as shown in Fig. 1. The reaction was found to be exceptionally selective and lead to only 2, 5-bis (alkyl/aryl-amino) 1,4-benzoquinones of the corresponding amine. We have used single-pot synthesis that induces minimal changes, as well as retains most of the original biological property of parent TQ. From the reaction mixture, the products were filtered and recrystallised. For quinone building blocks, we chose simple TQ, p-benzoquinone (TQ-2A) and 2,6-di-t-butyl benzoquinone (TQ3). By reacting compound from the quinone building block with the amines (A) to (I), we were able to generate the combinatorial matrix of 27 TQ analogs. Among these, 11 derivatives were initially screened for cell viability in the MiaPaCa-2 cell line, and three of these analogs (TQ-2G, TQ-4A1 and TQ-5A1) were found to be more potent than the parental TQ at equimolar concentrations (10 μM). Table I summarizes the chemical structure, acronyms used to cite these compounds, MW and IC50 values. Other building blocks, such as tetrachlorobenzoquinone, were also combined with amine (A) to (I) but did not yield stable compounds (data not shown) and thus were not further investigated.
Fig. 1.
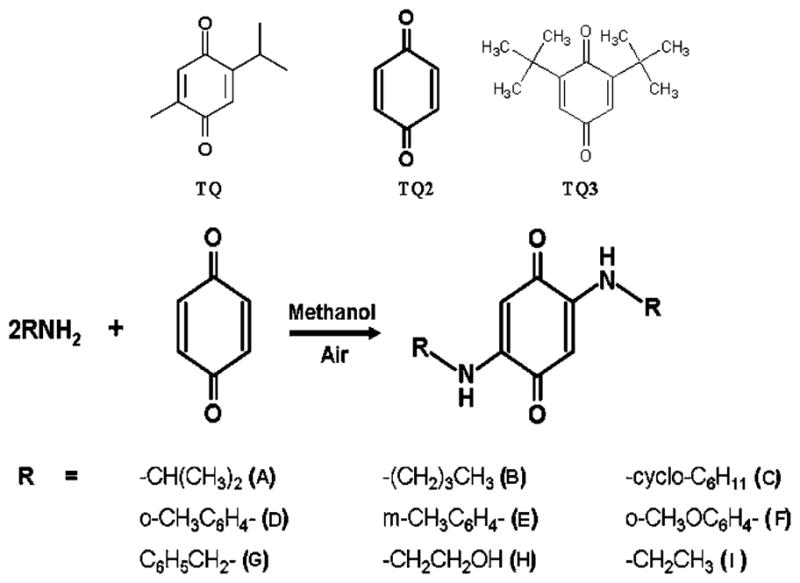
One-pot chemical synthesis of TQ analogs. TQ-2G, TQ-4A1 and TQ-5A1 are our lead compounds with IC50 values of 3 μM, 7 μM and 5 μM, respectively, in PC cells.
Table I.
TQ Analogs and Their Acronyms, Molecular Structures, MW and IC50 Values
| CODE | STRUCTURE | MOL WT | IC50 |
|---|---|---|---|
| (4ATPTHA) TQ-7A |
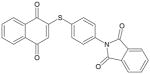
|
411 | > 10 μM |
| (4ATPSCl2) TQ-8A |
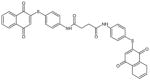
|
644 | > 10 μM |
| (NAQTGAEA) TQ-9A |
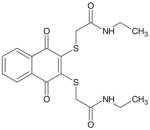
|
392 | > 10 μM |
| (MNAQ1,2BDT) TQ-10A |
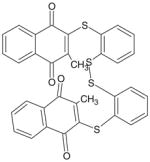
|
622 | > 10 μM |
| (MNAQ1,3PDT) TQ-11A |
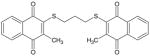
|
448 | > 10 μM |
| (BQBuNH2) TQ-1A |
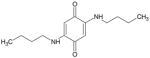
|
250 | > 10 μM |
| (BQ Ethanolamine) TQ-2A |
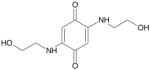
|
226 | > 10 μM |
| (BQ Isopropylamine) TQ-3A |
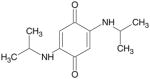
|
198 | > 10 μM |
| TQ-4A1 | Patent pending | 318 | < 10 μM |
| TQ-5A1 | Patent pending | 320 | < 10 μM |
| TQ-2G | Patent pending | 320 | < 10 μM |
Cell Viability Inhibition by TQ and TQ Analogs
Pancreatic cancer cells (3×103 cells/well) harboring different molecular signatures (MiaPaCa-2, BxPC-3, AsPC-1, Colo 357, L3.6pl and HPAC cells) were seeded in 96-well culture plates and replaced the next day with fresh medium containing either TQ (10 μM) or the investigational 11 analogs, diluted from stock solution. The effect of TQ and the analogs on cell viability was examined by MTT assay. The formazan formed by metabolically viable cells was dissolved in isopropanol, and the absorbance was measured at 595 nm using a plate reader (TECAN, Durham, NC), and the data was plotted as percent viable cell relative to control.
Quantification of Apoptosis
Two protocols were employed to confirm apoptosis following treatments with TQ or our analogs. The Cell Apoptosis ELISA Detection Kit (Roche, Palo Alto, CA), which quantifies the cytoplasmic histone-associated DNA fragmentation, was used according to manufacturer’s protocol. Additionally, Annexin V-FITC assay was used, and apoptotic cells were evaluated according to the manufacturer’s instructions (BD Biosciences, San Jose, CA).
Protein Extraction and Western Blot Analysis
MiaPaCa-2 cells were plated and allowed to attach for 36 h. TQ or the analogs exhibiting promising results (TQ-2G, TQ-4A1 and TQ-5A1) (10 μM) were directly added to cell cultures at the indicated concentrations and incubated for 48 h. Cell lysates were prepared by suspending the cells in RIPA lysis buffer and subjected to routine Western blot analysis as described earlier (6). Thirty to forty micrograms of total protein was separated on SDS-PAGE, electro-transferred and probed with specific antibodies.
Caspase Activity Assays
Caspase-3 activity was measured in MiaPaCa-2-treated cells by colorimetric assay according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN). The cells were treated as described under Western blot analysis.
Electrophoretic Mobility Shift Assay
Nuclear extracts were prepared from treated samples, and EMSA was performed by incubating 10 μg of nuclear extract with IRDye™–700-labeled NF-κB oligonucleotide as described earlier (6). The DNA-protein complex formed was visualized by Odyssey Infrared Imaging System using Odyssey Software Release 1.1. For loading control, 10 μg of nuclear protein from each sample was subjected to Western immunoblotting for retinoblastoma protein.
Determination of PGE2
BxPC-3 cells were seeded in 6-well plates and treated with TQ (0–10 μM) and the analogs (TQ-2G, TQ-4A1 and TQ-5A1; 10 μM) for 24 h in serum-free media. Conditioned medium was collected and analyzed to determine the levels of PGE2, using PGE2 high-sensitivity immunoassay kit (R&D Systems, Minneapolis, MN).).
COX Activity Assay
COX-1 and -2 activities were determined using the COX activity assay that utilizes the peroxidase component of cyclooxygenases. The peroxidase activity is typically assayed colorimetrically by monitoring the appearance of oxidized N, N, N′, N′-tetramethyl-p-phenylenediamine (TMPD) at 590 nm. Briefly, 1×106 cells were seeded in the dark clear-bottom 96-well plate, allowed to grow for 24 h, and further assayed for COX-1 and -2 activity following manufacturer’s recommendations (Cayman Chemicals, Ann Arbor, MI).
DNA Cell Cycle Analysis
MiaPaCa-2 cells were treated with TQ or the analogs (TQ-2G, TQ-4A1 and TQ-5A1) for 72 h using equimolar concentrations (10 μM). After treatments, the cells were collected by trypsinization, washed with cold PBS and subsequently fixed in 450 μl of ice-cold ethanol by incubating them for 1 h at 4°C. The cells were then centrifuged at 500 × g for 5 min, and the pellet was washed twice with cold PBS, suspended in 500 μl of PBS, and incubated with RNase (20 μg/ml, final concentration) for 30 min at 37°C. The cells were then stained with propidium iodide (50 μg/ml final concentration) for 1 h, and analyzed by flow cytometry.
Chemosensitization by TQ and TQ Analogs
MiaPaCa-2 cells were plated and incubated with medium containing either TQ or the analogs (TQ-2G, TQ-4A1 and TQ-5A1) at 10 μM concentrations for 48 h and exposed to 0.5 μM gemcitabine or 6.0 μg/ml of oxaliplatin for an additional 36 h. The effect of pretreatment with TQ or the analogs on cell viability and apoptosis was examined by MTT assay and ELISA cell apoptosis detection kit as described earlier, and the absorbance was measured using a plate reader (TECAN, Durham, NC).
Initial Toxicity Testing in SCID Mice
All in vivo studies were conducted in accordance with Wayne State University approved animal care and ethics committee guidelines and procedures. TQ analogs were administered both intravenously and as oral gavages to determine the maximum tolerated dose (MTD).
Statistical Analysis
Data are represented as mean ± SD for the absolute values or percent of controls as indicated in the vertical axis legend of figures. Statistical significance was determined by ANOVA test. Results of p<0.05 was considered statistically significant.
RESULTS
Effect of TQ and TQ Analogs on Cell Viability and Apoptosis Induction
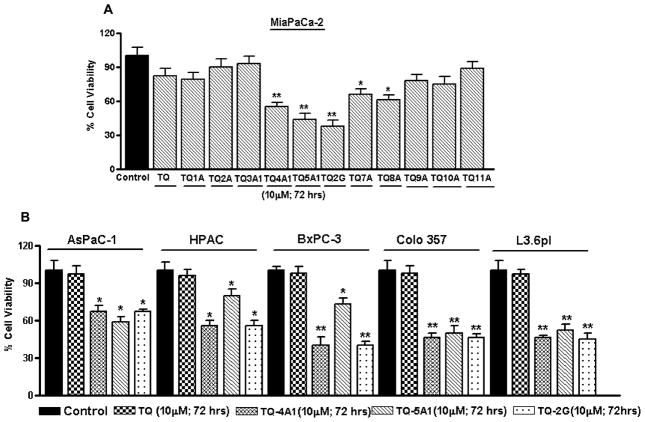
As shown in Fig. 2, using MTT assay, cell viability was initially screened in MiaPaCa-2 cell line (Fig. 2A) with fixed concentration of TQ and its analogs as described in Materials and Methods. Analogs TQ-4A1, TQ-5A1 and TQ-2G were found to be more effective than the parental TQ, and these analogs suppressed cell viability in almost all PC cell lines further evaluated (Fig. 2B) with ≤50% loss of cell viability at 10 μM concentration treatment for 72 h, respectively. In contrast, the rest of the analogs were found to be either ineffective or exhibited comparatively less reduction in cell viability (>60% relative to control) when exposed to equimolar concentrations as TQ (10 μM) for similar period of time (72 h; data not shown), suggesting that the majority of our analogs were relatively non-toxic or less toxic on PC cells. Overall, we found that the relative activity was in the following order: TQ-2G > TQ-5A1 > TQ-4A1. These findings led us to test the effects of these three analogs on cell cycle parameters and induction of apoptosis. Therefore, we next investigated the effects of these compounds on cell cycle and apoptosis using gemcitabine-resistant MiaPaCa-2 cells as our model system.
Fig. 2.
A Evaluation of cell viability induced by treatment with TQ and synthesized analogs (72 h) as tested in MiaPaCa-2 cells by MTT assay. *p<0.01; **p<0.001. B MTT assay depicting loss of cell viability following treatment with the potent TQ analogs in different PC cell lines for 72 h.
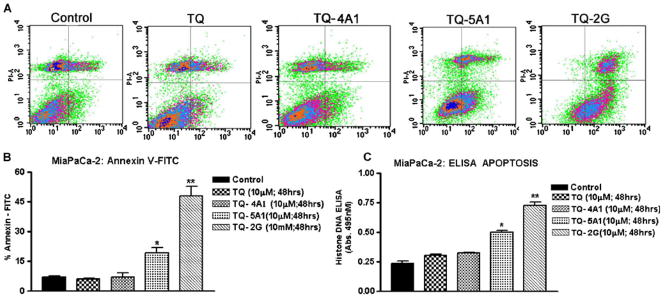
In order to determine apoptosis, we quantified cytoplasmic Histone-associated DNA fragmentation using ELISA-based kit and also by flow cytometric analysis of Annexin V-FITC/propidium iodide (PI), which stains samples of treated and untreated cells and is considered to be a highly specific indicator of apoptosis. As depicted in Fig. 3, after 48 h of treatment with 10 μM of TQ and each of the analogs TQ-4A1, TQ-5A1, and TQ-2G the percentage of apoptotic cells detected by Annexin V-FITC/PI staining and flow cytometry were 6%, 7% 19% and 48% (Fig. 3A: flow data; lower left panel Fig. 3B represents quantitation of flow data). Similar trend towards apoptosis was also noticed following quantitation by ELISA for detection of apoptosis (Fig. 3C). Our results clearly show that the analogs TQ-5A1 and TQ-2G are much more potent in inducing apoptosis relative to untreated control, as well as in comparison to the parental TQ (p <0.01; p <0.001).
Fig. 3.
A Flow scan results depicting Annexin-V/FITC-positive cells indicating apoptosis in MiaPaCa-2 cells exposed to TQ or synthesized analogs for 48 h. B Histograph representing % apoptotic cells in treatment groups as observed with flow cytometry. (*p<0.01; **p<0.001, relative to control). C Apoptosis as determined by Histone-DNA ELISA in MiaPaCa-2 cells following incubation with compounds as shown in embedded legend. Significantly increased apoptosis is evident in analog treated cells relative to untreated control (*p<0.01;**p<0.001, relative to control).
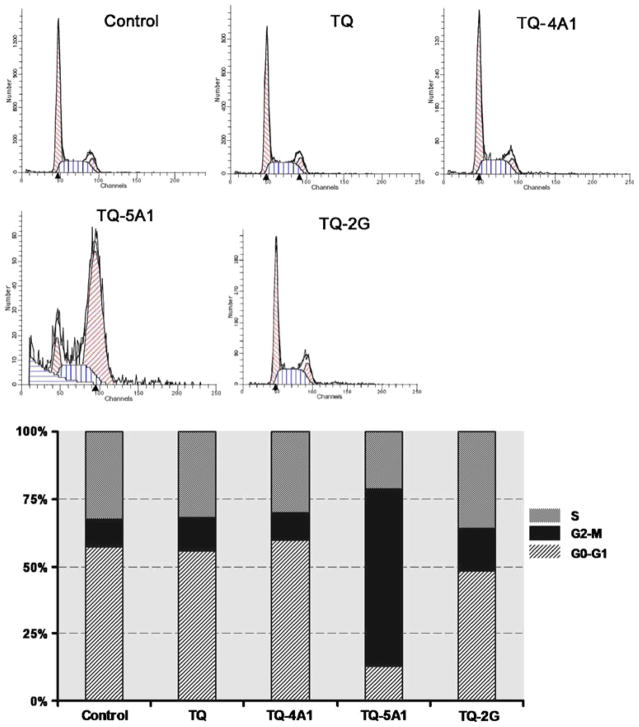
Effect of TQ and Analogs on Cell Cycle Progression
Untreated MiaPaCa-2 cells demonstrate a pattern with most cells in the G0-G1 phase (57%), a lower G2/M (10%) and S phase (32%). The alterations in the cell cycle distribution of MiaPaCa-2 cells treated for 72 h with 10 μM TQ and the selective analogs are shown in Fig. 4. Analog TQ-5A1 alone caused significant G2/M phase cell-cycle arrest (65.73% versus 10.28% in control), while the analog TQ-2G resulted in a minor increase in G2/M phase arrest (16% versus 10.28% in control), and the proportion of G0-G1 phase cells decreased significantly after 72 h of treatment. Interestingly, neither TQ nor the analog TQ-4A1 at the concentration tested (10 μM) exhibited any major effect on cell cycle progression. Overall, these results support the notion that the observed decline in cell viability by these analogs, especially the analogs TQ-5A1 and TQ-2G, execute differential effects on cell cycle progression. To gain better molecular insight, we next investigated the status on cell survival and apoptosis-related molecules in MiaPaCa-2 cells treated with these analogs.
Fig. 4.
Cell cycle analysis by flow cytometry and histogram showing % distribution of cells in G0/G1, G2/M or S phases of cell cycle (lower panel).
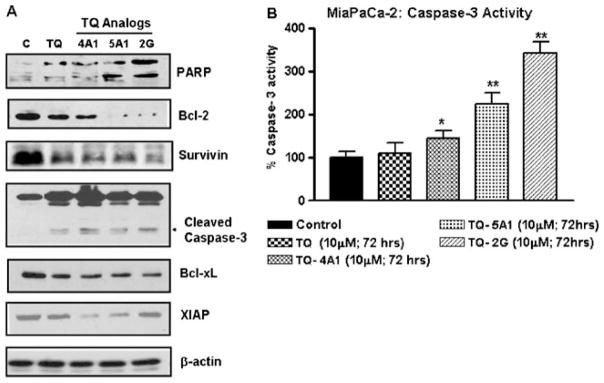
TQ Analogs Inhibit Anti-apoptotic Molecules in MiaPaCa-2 Cells
We performed Western immunoblotting using MiaPaCa-2 cells as a representative model to investigate the molecular effects of TQ and its novel analogs. As shown in Fig. 5A, the anti-apoptotic protein Bcl-2 was significantly inhibited by the analogs TQ-5A1 and TQ-2G, indicating that the apoptosis-inducing effects of these analogs could in part be due to up-regulation of the Bax/Bcl-2 protein ratio, which is a critical determinant of the induction of apoptosis. Additionally, other anti-apoptotic molecules, Bcl-xL, survivin and XIAP, were also evaluated. We found that relative to control, survivin and XIAP expression were down-regulated in cells exposed to the analogs (TQ-2G, TQ-4A1 and TQ-5A1). These results provide molecular evidence that complements apoptosis-inducing ability of TQ and its analogs. Western immunoblotting also revealed that only the analog treatment, but not TQ at an equivalent concentration, resulted in the appearance of cleaved active component of caspase-3 and PARP in MiaPaCa-2 cells (Fig. 5A right panel). In parallel, caspase-3 activity was also determined and found to be significantly elevated (Fig. 5B). An upstream event in the activation of the caspase cascade is the release of cytochrome c from mitochondria. As reported previously by us (6), TQ induced the release of cytochrome-c, which suggests that the induction of apoptosis by TQ and its analogs is in part mediated by the mitochondrial pathway.
Fig. 5.
A Western immunoblots depicting alterations in the expression of apoptosis-related proteins in whole cell lysates prepared from MiaPaCa-2 cells after treatment with different TQ analogs and the parental TQ (10 μM for 72 h). β-actin protein was used as loading control. B Caspase-3 activity in cell lysates derived from MiaPaCa-2 cells under the conditions of treatment with the analogs and the parental TQ as described above. A significant increase in caspase-3 activity was observed in cells treated with TQ analogs compared to untreated control (*p<0.05;**p<0.001, relative to control).
TQ Analogs Inhibit Activation of NF-κB in MiaPaCa-2 Cells
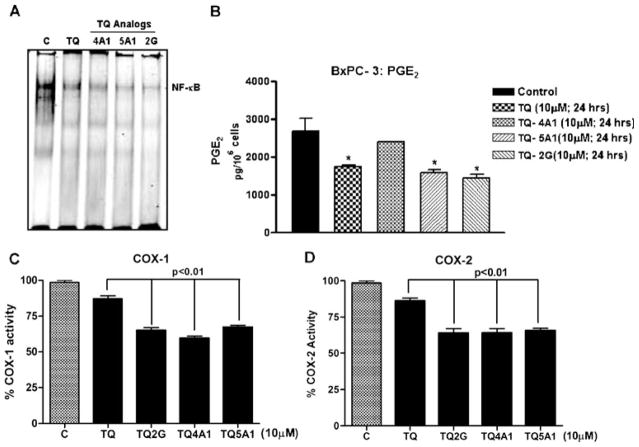
To investigate whether TQ and the analogs could abrogate constitutively expressed NF-κB in vitro in MiaPaCa-2, cells were treated with 10 μM dose of TQ and the analogs for 48 h, and subjected to gel shift assay (EMSA) for assessing the DNA binding activity of NF-κB. As shown in Fig. 4A, the analogs (TQ-2G, TQ-4A1 and TQ-5A1) resulted in down regulation of the DNA binding effect of NF-κB in MiaPaCa-2 cells, which again is consistent with down-regulation of the transcriptional target genes of NF-κB, such as Bcl-2 family of anti-apoptotic proteins, survivin and XIAP.
Suppression of COX Activity and Prostaglandin (PGE2) Synthesis in BxPC3 Cells
In our previous study, we showed using Western immunoblotting that TQ inhibits COX-2 protein expression in HPAC pancreatic cancer cells (6). We confirmed the molecular interaction between the parent TQ and cycloxygenase enzyme by molecular docking studies (personal observation, non-communicated). Based on preliminary computer modeling data acquired, it was concluded that TQ binds into the active site of COX-2 with binding energy of −7.68 Kcl/mole, which indicates that the compound might be a good candidate for COX-2 inhibition (personal observation, non-communicated). Thus, building from our preliminary molecular docking studies, we anticipated that the analogs will show more potent docking to COX-2 and subsequently inhibit COX-2; therefore, we carried further studies related to these molecules of interest in PC cells with high basal expression of these enzymes.
Inhibition of COX-2 and Prostaglandin E2 (PGE2) is considered a promising chemotherapeutic target for the treatment and reversal of chemo-resistance phenotype. We used BxPC-3 cells to determine the effects of TQ and its analog on the production of PGE2. The rationale for selecting BxPC-3 cells over that of MiaPaCa-2 cells was because of the high basal expression of COX-2 enzyme in these cells relative to MiaPaCa-2 cells. Thus, BxPC-3 cells were treated with either TQ or the analogs for 24 h, and the conditioned media was collected, centrifuged and assayed for quantifying PGE2 using commercial kit as described in Materials and Methods. Our results showed a significant decrease in PGE2 secretion by cells exposed to the analogs TQ-5A1 and TQ-2G. In order to further confirm our results on the effects of TQ and its analog on PGE2 production, we assessed the effects of our compounds on the enzymatic activity of both COX-1 and COX-2 enzymes using a COXovine colorimetric assay. We found that TQ and the investigated analogs TQ-2G TQ-4A1 and TQ-5A1 were all effective in inhibiting COX-1 and COX-2 enzyme activity at 10 μM concentrations (Fig. 6C, D).
Fig. 6.
A Gel Shift Assay showing down-regulation of NF-κB DNA binding activity in the nuclear extract of MiaPaCa-2 cells treated with 10 μM concentration of either TQ or analogs for 48 h. B PGE2 quantitation by ELISA in conditioned media collected from BxPC-3 cells. C Histogram depicting COX-1 and COX-2 enzyme activity after treatment of cells with TQ and its analogs as assessed by using Cayman’s colorimetric COX (ovine) Inhibitor Screening Assay Kit.
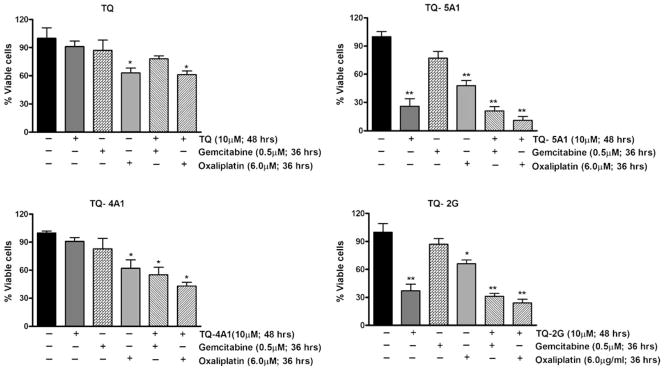
TQ Analogs Sensitize PC Cells to Chemotherapeutic Agents
Studies were undertaken from translational viewpoint to examine whether pretreatment of gemcitabine-resistant MiaPaCa-2 cells with our novel analogs could make the cells more sensitive to gemcitabine and oxaliplatin compared to parental TQ at equivalent concentration. Cells were treated with either TQ or the analogs (TQ-2G, TQ-4A1 and TQ-5A1) each at 10 μM concentration for 48 h for comparison and assessed whether the analogs are superior at concentrations lower than that of TQ as reported in our previous study (6). The pretreatment schedule was followed by incubation with suboptimal doses of either drug, gemcitabine (0.5 μM; 36 h) or oxaliplatin (6.0 μM for 36 h), and cell viability was determined by MTT assay (Fig. 7A–D). We found that treatment of cells initially with TQ followed by either gemcitabine or oxaliplatin treatment for 36 h caused only 22% (gemcitabine) and 39% (oxaliplatin) of cell killing, respectively. These results do not differ significantly with that of the cell killing by monotherapy regimens (gemcitabine −13% or oxaliplatin-37%). In contrast, our analogs caused significant loss of cell viability. In combination with gemcitabine, the loss of viable cells with the analogs TQ-2G, TQ-4A1 and TQ-5A was 45%, 78% and 69%, respectively, relative to the untreated control (p<0.001), and the corresponding data for gemcitabine alone ranged between 13–23% of cell killing. Similarly, the loss of viable cells upon treatment with analogs followed by oxaliplatin ranged between 57% (TQ-4A1+Oxaliplatin compared to 38% with oxaliplatin alone), 89% (TQ-5A1+ Oxaliplatin compared to 51% with oxaliplatin alone), and 76% (TQ-2G+ oxaliplatin compared to 34% with oxaliplatin alone). Intriguingly, as seen with the therapeutic regimens, oxaliplatin as well as gemcitabine, the analogs TQ-2G and TQ-5A1 showed better results relative to the analog TQ-4A1 and also showed superior effect compared to the parental TQ at 10 μM concentration.
Fig. 7.
Chemosensitization by TQ and its analogs were tested following pre-exposure (10 μM; 48 h) of MiaPaCa-2 cells followed by co-incubation with gemcitabine or oxaliplatin for additional 36 h. Viable cells were evaluated by MTT. *p<0.05; **p<0.001, relative to control.
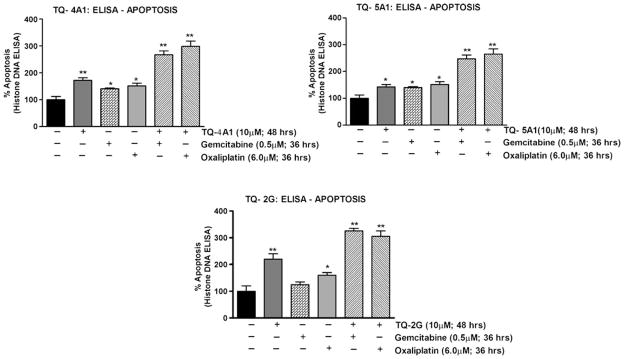
TQ Analogs Sensitize MiaPaCa-2 Cells to Apoptosis by Gemcitabine and Oxaliplatin
The aforementioned results showing superior chemo-sensitization effect by the analogs as concluded from the loss of viable cells are of paramount clinical interest in minimizing toxic side effects of chemotherapeutic agents on normal cells. Additionally, using the Histone DNA-ELISA assay, we confirmed that the analogs’ TQ-4A1, TQ-5A1 and TQ-2G pretreatment followed by cytotoxic chemo-therapeutic drugs induces more apoptosis (~50% more; p< 0.001) (Fig. 8), which provides strong support in favor of superior sensitizing effect of TQ analogs (TQ-2G, TQ-4A1 and TQ-5A1) with gemcitabine or oxaliplatin.
Fig. 8.
Sensitization of MiaPaCa-2 cells to apoptosis by the analogs TQ-4A1, TQ-5A1 and TQ-2G as determined by Histone-DNA ELISA. A significant increase in apoptosis was observed in the combination group relative to untreated control. (*p<0.05; **p<0.001, relative to control).
TQ and Its Analogs Are Non-toxic to Animals
Previously, we were able to administer TQ at 3 mg/mouse/day doses for 21 days without any signs of apparent toxicity to mice (6). Hence, we further evaluated the effect of one TQ analog (TQ-2G) on animal body weight and overall well being (data not shown). Based on empirical criterion, no evidence of any severe toxicity as inferred from body-weight loss criteria or signs of aversion to food intake or diarrhea were evident during the window of treatment. Furthermore, no macroscopic evidence of necrosis or hemorrhage in any visceral organs was seen under our experimental condition. It was inferred that TQ-2G, when tested in SCID mice at doses up to 50 mg/kg i.v. and up to 700 mg/kg by oral gavage, is non-toxic to mice, suggesting that this analog would be a non-toxic agent to normal cells. It also suggests the possibility of significant tumor-specific activity either alone or in combination with conventional therapeutic agents for the treatment of pancreatic tumors. However, further in-depth investigations are warranted for its safety.
DISCUSSION
Pancreatic cancer (PC) is one of the deadliest of the solid malignancies, with a five-year survival rate of only 4% (1). Growth inhibition and induction of apoptosis constitute the major mechanisms of action of most chemotherapeutics during cancer. Unfortunately, PC is inherently resistant to apoptosis in all conventional cancer therapeutic agents, which poses a great challenge to clinicians for the treatment of patients diagnosed with PC. Gemcitabine, oxaliplatin and 5-Flurouracil are the only approved drugs that have very modest activity against this disease. Studies from our laboratory and others have acknowledged that the underlying resistance of PC to these chemotherapeutic drugs could in part be due to the over-expression of chemo-resistant molecules such as NF-κB, Bcl-xL, survivin and COX-2. In recent years, increased attention has been directed towards naturally occurring compounds, particularly “dietary agents,” for their use as inhibitors of tumor progression and/or treatment due to their systemic, non-toxic nature and pleiotrophic molecular effects, which is believed to contribute to reversing drug resistance (30–33).
We recently reported the anti cancer properties of Thymoquinone (TQ), a natural non-toxic and bioactive compound derived from black seed (Nigella sativa) oil against PC (6,7). It has been proposed that TQ induces apoptosis in tumor cells by several mechanisms including NF-κB suppression, Akt activation and extracellular signal-regulated kinase signaling pathways, and also by inhibiting tumor angiogenesis (6,7,20,21). These studies clearly suggest that TQ could be useful as an adjunct to conventional chemotherapeutics, such as gemcitabine and oxaliplatin. Our data indicate that TQ alone at 25 μM was able to effectively induce cell growth inhibition and apoptosis in PC cells mediated through a mechanism involving inactivation of COX-2 and NF-κB (6). However, the concentration needed for such activity was viewed as practically high to be pharmacologically bioavailable for human clinical intervention studies, and thus we reasoned that novel synthetic analogs of TQ with proven bioactivity and lower IC50 than the parental TQ without systemic toxicity would be clinically relevant from translational viewpoint. This issue is of critical importance and highly relevant to cancer therapy, especially because any agent with low or no systemic normal tissue toxicity while retaining a robust anti-tumor activity is highly desirable.
To that end, we modified the original TQ to produce a series of 27 analogs, of which 11 are reported in this study. These modifications have been directed at the carbonyl sites or the benzenoid sites using single-pot synthesis that resulted in enhanced lipophilicity (for example the LogP for TQ-2G is 5.60 compared to 2.84 for TQ, and higher binding affinity to COX-2). Among these analogs investigated, three analogs, TQ-2G, TQ-4A1 and TQ-5A1 (patent pending), were found to be more potent than TQ in terms of their ability to inhibit cell growth and induce apoptosis in PC cells, and this superior biological activity of these novel analogs was found to be mechanistically linked to the down-regulation of transcription factor NF-κB and anti-apoptotic and cell survival-related molecules such as Bcl-2, Bcl-xL, survivin and XIAP. Additionally, our data provided new evidence in support of the activity of these analogs in inhibiting the production and secretion of PGE2 in high COX-2-expressing PC cells (BxPC-3). These selective analogs, TQ-2G, TQ-4A1 and TQ-5A1, have a much lower IC50 (<10 μM) compared to the parent TQ (IC50 ~25 μM), which is more favorable and acceptable for clinical therapy.
In our previous study, TQ was shown to exhibit chemosensitizing effects to gemcitabine and oxaliplatin (6). This effect was attributed to the inactivation of DNA binding activity of NF-κB, resulting in the inactivation of multiple NF-κB downstream survival factors. In this study, we found a similar chemosensitizing effect by TQ analogs. TQ-2G, TQ-4A1 and TQ-5A1 were more potent than the parental TQ against PC in the sensitization of PC cells to gemcitabine or oxaliplatin treatment. These analoges exhibited strong potential for the down-regulation of anti-apoptotic molecules, such as Bcl-2, Bcl-xL, survivin and XIAP, compared to parental TQ, which may represent the molecular mechanism by which these compounds elicit their biological activity; however, further in-depth molecular and animal experiments are needed for confirming the superiority of these novel analogs as anti-tumor agents compared to the parental TQ. It is exciting to see that these analogs showed increased caspase-3 activity, which was associated with increased apoptosis of PC cells. Furthermore, our observations with the cell cycle progression revealed that these analogs possess differential effects on cell cycle progression with the analog TQ-5A1 showing strong G2/M cell cycle arrest, which suggests that this analog could be useful for combination therapy using radiation, which of course requires further in-depth investigations. A broad spectrum of cell cycle genes trigger the disruption of the cell membrane integrity in response to stress condition elicited by cell cycle arrest. This can be viewed as one speculative reason for the differential trend between cell viability and cell cycle progression, as noted with the analogs in the present study.
Although complete understanding of the underlying apoptosis-inducing mechanism of these novel analogs is required, the initial findings reported here are encouraging, especially the chemosensitizing effects of these analogs to conventional agents that are not very effective in the treatment of PC. Our findings on chemoresistant MiaPaCa-2 cells clearly suggest that these compounds may hold promise for combination therapy toward better treatment outcome of patients diagnosed with PC. Interestingly, our preliminary data suggest that TQ-2G is non-toxic in SCID mice at doses up to 50 mg/kg when administered through i.v. route and a dose up to 700 mg/kg when administered orally by gavage, which suggests that this analog could indeed be useful in combination therapy without added toxicity when combined with conventional therapeutics such as gemcitabine or oxaliplatin. Of added interest, the synthesized analogs described closely mirror Lipinski’s Rule of Five.
In conclusion, we presented preliminary evidence for novel synthesis of TQ analogs with biological activity that is better than the parental TQ without systemic toxicity; thus, we believe that these and other novel analogs of TQ, could serve as promising agents for better treatment outcome of patients diagnosed with pancreatic cancer.
Acknowledgments
The authors express their sincere appreciation to Ms. Christine Wojewoda for her editorial assistance. Grant support from the National Institutes of Health RO1CA109389 (RM Mohammad) and NIH R01CA083695, R01CA131151, and R01CA132794 awarded to FHS is gratefully acknowledged. The authors also acknowledge the financial contribution of Guido Foundation.
Contributor Information
Sanjeev Banerjee, Department of Pathology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan 48201, USA.
Asfar S. Azmi, Department of Pathology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan 48201, USA
Subhash Padhye, D.Y. Patil University, Pune, Maharashtra 411018, India.
Marjit W. Singh, Department of Chemistry, Indian Institute of Technology, Guwahati, Assam 781 039, India
Jubaraj B. Baruah, Department of Chemistry, Indian Institute of Technology, Guwahati, Assam 781 039, India
Philip A. Philip, Division of Hematology and Oncology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Room-732 HWCRC Bldg, 4100 John R Street, Detroit, Michigan 48201, USA
Fazlul H. Sarkar, Department of Pathology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan 48201, USA
Ramzi M. Mohammad, Email: mohammar@karmanos.org, Division of Hematology and Oncology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Room-732 HWCRC Bldg, 4100 John R Street, Detroit, Michigan 48201, USA
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lage H, Dietel M. Multiple mechanisms confer different drug-resistant phenotypes in pancreatic carcinoma cells. J Cancer Res Clin Oncol. 2002;128:349–57. doi: 10.1007/s00432-002-0349-y. [DOI] [PubMed] [Google Scholar]
- 3.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, Xuan Y. Bypassing cancer drug resistance by activating multiple death pathways–a proposal from the study of circumventing cancer drug resistance by induction of necroptosis. Cancer Lett. 2008;259:127–37. doi: 10.1016/j.canlet.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Arlt A, Schafer H. NFkappaB-dependent chemoresistance in solid tumors. Int J Clin Pharmacol Ther. 2002;40:336–47. doi: 10.5414/cpp40336. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–83. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 7.Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity—the secret of Pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 8.Rooney S, Ryan MF. Effects of alpha-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005;25:2199–204. [PubMed] [Google Scholar]
- 9.Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–13. [PubMed] [Google Scholar]
- 10.Gali-Muhtasib H, ab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, et al. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–66. [PubMed] [Google Scholar]
- 11.Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, et al. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–9. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 12.Wilson-Simpson F, Vance S, Benghuzzi H. Physiological responses of ES-2 ovarian cell line following administration of epigallocatechin-3-gallate (EGCG), thymoquinone (TQ), and selenium (SE) Biomed Sci Instrum. 2007;43:378–83. [PubMed] [Google Scholar]
- 13.El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117:409–17. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 14.Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, et al. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782–8. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- 15.Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7:1789–96. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–70. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 17.Bauer L, Venz S, Junker H, Brandt R, Radons J. Nicotinamide phosphoribosyltransferase and prostaglandin H2 synthase 2 are up-regulated in human pancreatic adenocarcinoma cells after stimulation with interleukin-1. Int J Oncol. 2009;35:97–107. doi: 10.3892/ijo_00000317. [DOI] [PubMed] [Google Scholar]
- 18.Angst E, Reber HA, Hines OJ, Eibl G. Mononuclear cell-derived interleukin-1 beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery. 2008;144:57–65. doi: 10.1016/j.surg.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan MN, Lee YS. Cyclooxygenase inhibitors: Scope of their use and development in cancer chemotherapy. Med Res Rev. 2009 doi: 10.1002/med.20182. [DOI] [PubMed] [Google Scholar]
- 20.Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139–46. doi: 10.1093/carcin/21.2.139. [DOI] [PubMed] [Google Scholar]
- 21.Ali S, El-Rayes BF, Sarkar FH, Philip PA. Simultaneous targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways for pancreatic cancer therapy. Mol Cancer Ther. 2005;4:1943–51. doi: 10.1158/1535-7163.MCT-05-0065. [DOI] [PubMed] [Google Scholar]
- 22.Colby JK, Klein RD, McArthur MJ, Conti CJ, Kiguchi K, Kawamoto T, et al. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia. 2008;10:782–96. doi: 10.1593/neo.08330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zatelli MC, Mole D, Tagliati F, Minoia M, Ambrosio MR, Uberti ED. Cyclo-oxygenase 2 modulates chemoresistance in breast cancer cells involving NF-kappaB. Cell Oncol. 2009;31:457–65. doi: 10.3233/CLO-2009-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson FM, Mallery SR, Bergdall-Costell VK, Cheng M, Pei P, Prosperi JR, et al. Cyclooxygenase-2 directly induces MCF-7 breast tumor cells to develop into exponentially growing, highly angiogenic and regionally invasive human ductal carcinoma xenografts. Anticancer Res. 2007;27:719–27. [PubMed] [Google Scholar]
- 25.Stasinopoulos I, O’Brien DR, Wildes F, Glunde K, Bhujwalla ZM. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol Cancer Res. 2007;5:435–42. doi: 10.1158/1541-7786.MCR-07-0010. [DOI] [PubMed] [Google Scholar]
- 26.Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis G, Cohen C. COX-2 expression in invasive breast cancer: correlation with prognostic parameters and outcome. Appl Immunohistochem Mol Morphol. 2007;15:255–9. doi: 10.1097/01.pai.0000213130.63417.b3. [DOI] [PubMed] [Google Scholar]
- 27.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26:1393–9. [PubMed] [Google Scholar]
- 28.Valsecchi ME, Pomerantz SC, Jaslow R, Tester W. Reduced risk of bone metastasis for patients with breast cancer who use COX-2 inhibitors. Clin Breast Cancer. 2009;9:225–30. doi: 10.3816/CBC.2009.n.038. [DOI] [PubMed] [Google Scholar]
- 29.Simeone AM, Nieves-Alicea R, McMurtry VC, Colella S, Krahe R, Tari AM. Cyclooxygenase-2 uses the protein kinase C/interleukin-8/urokinase-type plasminogen activator pathway to increase the invasiveness of breast cancer cells. Int J Oncol. 2007;30:785–92. [PubMed] [Google Scholar]
- 30.Sarkar FH, Li Y. NF-kappaB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–9. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar FH, Li YW. Targeting multiple signal pathways by chemopreventive agents for cancer prevention and therapy. Acta Pharmacol Sin. 2007;28:1305–15. doi: 10.1111/j.1745-7254.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: pathogenesis, prevention and treatment. Toxicol Appl Pharmacol. 2007;224:326–36. doi: 10.1016/j.taap.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–50. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]