Abstract
Background
Particulate air pollution has been linked to heart disease and stroke, possibly resulting from enhanced coagulation and arterial thrombosis. Whether particulate air pollution exposure is related to venous thrombosis is unknown.
Methods
We examined the association of exposure to particulate matter of less than 10 µm in aerodynamic diameter (PM10) with DVT risk in 870 patients and 1210 controls from Lombardia Region, Italy examined between 1995–2005. We estimated exposure to particulate matter of less than 10 µm in aerodynamic diameter (PM10) in the year before DVT diagnosis (cases) or examination (controls) through area-specific average levels obtained from ambient monitors.
Results
Higher average PM10 level in the year before the examination was associated with shortened Prothrombin Time (PT) in DVT cases (beta=−0.12; 95% CI −0.23, 0.00; p=0.04) and controls (beta=-0.06; 95% CI −0.11, 0.00, p=0.04). Each PM10 increase of 10 µg/m3 was associated with a 70% increase in DVT risk (OR=1.70; 95% CI, 1.30–2.23; p=0.0001) in models adjusting for clinical and environmental covariates. The exposure-response relationship was approximately linear over the observed PM10 range. The association between PM10 and DVT was weaker in women (OR=1.40; 95% CI, 1.02–1.92; p=0.02 for the interaction between PM10 and sex), particularly in those using oral contraceptives or hormone replacement therapy (OR=0.97; 95% CI 0.58–1.61; p=0.048 for the interaction between PM10 and hormone use).
Conclusions
Long-term exposure to particulate air pollution is associated with altered coagulation function and DVT risk. Other risk factors for DVT may modulate the effect of particulate air pollution.
INTRODUCTION
Exposure to particulate air pollution has been associated with increased short- and long-term morbidity and mortality from heart disease and stroke.1–4 Hypercoagulability and enhanced thrombosis have been indicated as one mechanistic pathway that mediates such effects,4, 5 as higher plasma levels of coagulation proteins such as factor VIII, von Willebrand factor, and fibrinogen have been associated with the exposure. 5–7 Although arterial atherosclerosis and venous thrombosis share common underlying mechanisms, as well as the connection between pollution-induced systemic inflammation and coagulation,
Recently, changes in coagulation function resulting in shortened Prothrombin Time (PT) have been observed in association with higher average particulate air pollution of <10 µm in aerodynamic diameter (PM10) in the 30 days before the examination, suggesting that extended PM10 exposure may cause effects on blood clotting.8
Procoagulant abnormalities are stronger determinants of venous than arterial thrombosis,9 and increased risk of deep vein thrombosis (DVT) has been associated with a series of heritable or acquired risk factors that cause hypercoagulability, including factor V Leiden and G20210A prothrombin mutations, deficiencies of the natural anticoagulant proteins antithrombin, protein C and protein S, and use of oral contraceptive or hormone replacement therapy.10, 11 In rat and hamster models developed to investigate mechanisms involved in arterial thrombosis, inhalation or intravenous administration of air pollution constituents — such as diesel exhausts and ultrafine particles — induces thrombosis of the femoral6 and ear12 veins. In human subjects, no evidence is currently available relating air pollution exposure to DVT. In the present study, we investigated whether long-term ambient PM10 exposure was associated with increased thrombotic susceptibility and higher DVT risk in a large epidemiology investigation conducted in Lombardia Region, Northern Italy.
STUDY SUBJECTS AND METHODS
Study population
Eight hundred-seventy cases (420 men, 490 women), who were Lombardia Region residents and had been diagnosed between January 1995 through September 2005 with lower-limb DVT with or without symptomatic pulmonary embolism, were included in the study. Cases were referred to the Thrombosis Center, University of Milan and IRCCS Maggiore Hospital, Milan, Italy, for a thrombophilia screening and were asked to bring all available clinical documentation. DVT was diagnosed on this documentation based on evidence from objective methods such as compression ultrasonography or venography. Cases were asked about the presence of transient DVT risk factors in the month preceding the event, including surgery, trauma, immobilization (>=10 days), oral contraceptives or hormone replacement therapy, pregnancy/puerperium (6 week period after delivery). DVT was complicated by symptomatic pulmonary embolism in 170 cases (19.2%). Seven hundred and sixty-one cases (87.4%) had a first DVT during the study period, whereas 110 (12.6%) had recurrent DVT. Cases with recurrent DVT had not been included in the study after their first diagnosis, as they were recruited after their first visit at the Thrombosis Center.
Controls were 1210 healthy Lombardia Region residents (451 males, 720 females), who volunteered to be investigated at the Thrombosis Center in the same study period of the cases. Controls were recruited by asking each of the cases a list of friends or nonblood relatives and were randomly selected from this lists using an algorythm that balanced the age distribution of the controls to that of the cases. To increase power to evaluate a potential effect modification by oral contraceptives and hormone replacement therapy, female controls were recruited in excess of female cases. Thrombosis was excluded in controls with a structured questionnaire validated for the retrospective diagnosis of venous thromboembolism. Information on clinical, lifestyle, and reproductive factors was collected from all participants during an in-person interview. For practical reasons, because most of the interviews took place while the DVT patients were in the hospital, interviewers could not be blinded to the case status. However, the subjects address’ was confirmed on administrative or other official records, and exposure was assigned, based on the address, by a statistician blinded to the case status. Participants’ written informed consent and local Institutional Review Board approvals were obtained before the study. Prothrombin Time (PT) and activated Partial Thromboplastin Time (aPTT) were measured using laboratory methods previously described.16 DVT cases, if on anticoagulant drugs, were not asked to stop the treatment.
Air pollution and weather data
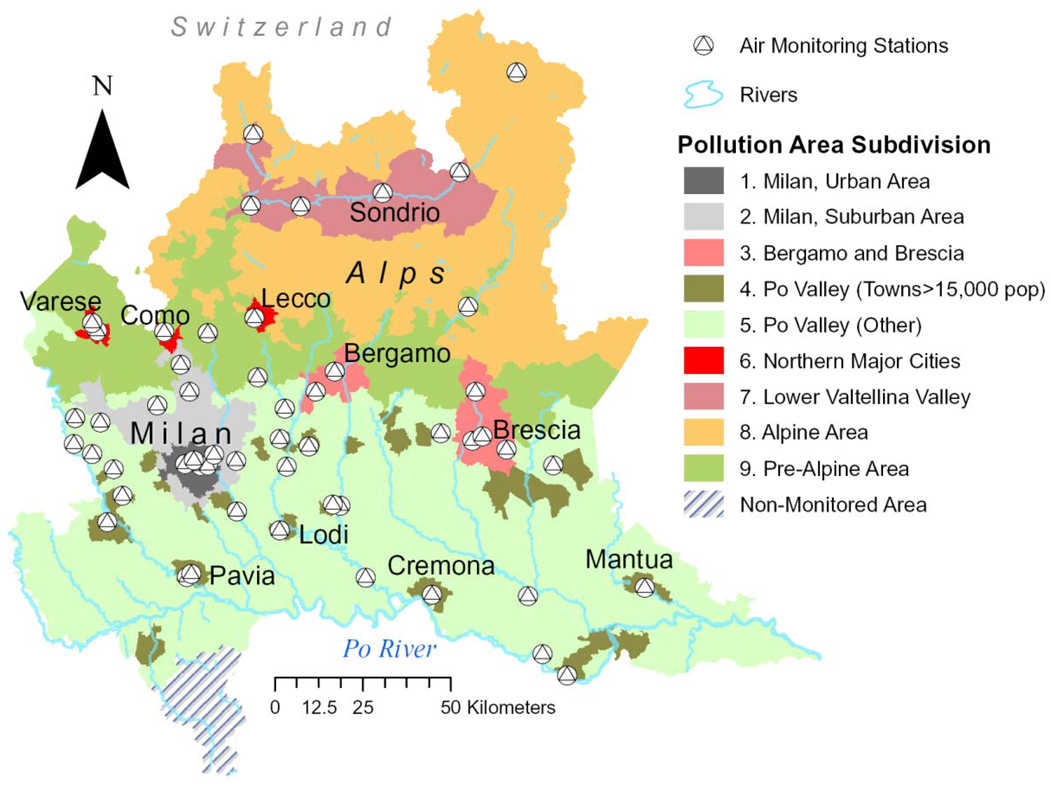
Methods for exposure assignment were previously described in detail.8, 13 Briefly, for each of the nine geographic areas shown in Figure 1, average mean concentrations of PM10 were computed using data from monitors located at 53 different sites throughout the Region. All participants were assigned to one of nine geographic areas based on their residence at the time of the study. PM10 level was evaluated in our statistical analyses using ambient PM10 concentrations averaged over the 365 days preceding the index date, taking also into account changes of residence during the same time-window. Index dates were the date of diagnosis for DVT cases and the date of the examination for controls.
Figure 1.
Lombardia Region Map showing the location of the 53 air pollution monitors in the nine areas identified for the study.
Statistical analysis
In previous work, we tested the association of shorter-term PM10 exposure with PT and aPTT in the same healthy subjects who served as controls in the present analyis.16 Here, to evaluate the association between PM10 and PT/aPTT among DVT cases or controls, we used the same statistical methods we previously described.16
Differences between cases and controls after stratification by geographic area or study period were tested using the Mantel-Haenszel method. The association of PM10 level with DVT risk was tested in a case-control analysis using unconditional logistic regression models including as independent variables sex, area of residence, education (elementary/middle school, high school, college), factor V Leiden or G20210A prothrombin mutation (yes/no), and current use of oral contraceptives or hormone replacement therapy (yes/no). Variables with potentially non linear associations with risk, including age, body mass index, day of the year (for seasonality), index date (for long-term time trends), and ambient temperature, were controlled using penalized regression splines with 4 degrees of freedom each.14 Unconditional regression was performed to allow for the use of penalized splines, which are not available for use in conditional logistic models in available software packages, as well as to avoid the loss from the analyses of subjects in incomplete sets due to participation refusal or missing exposure or covariate information. As approximation of the relative risk of DVT, we reported Odds Ratios (ORs) and 95% confidence intervals (CI) for each increase of 10 µg/m3 in the average level of PM10. We used Stata, version 9.0 (Stata Corp., College Station, TX) for descriptive analyses and R, version 2.2.0 (R Project for Statistical Computing, Vienna, Austria) to fit regression models. All statistical tests were two-sided.
RESULTS
Characteristics of the study population
Table 1 shows the characteristics of the 870 DVT cases (420 males, 451 females) and 1,210 controls (490 males, 720 females). Cases and controls had similar distributions by age (p=0.12) and area of residence (p=0.59). Cases had higher body mass index (p<0.001), lower education (p=0.01), more frequent use of oral contraceptives or hormone replacement therapy (p<0.001), higher prevalence of factor V Leiden (p<0.001), G20210 prothrombin mutation (p<0.001), inherited deficiencies of natural anticoagulant proteins (p<0.001), hyperhomocysteinemia (p<0.001), and of any of the causes of thrombophilia (p<0.001). Controls were less likely to be examined for the study during the summer, and their examinations were more frequent in the fall, while no major seasonal pattern was found for DVT diagnoses (p<0.001). Mean ambient temperatures were higher on the days of diagnosis of DVT cases than on the days of examination of controls (p<0.001). A larger proportion of controls were entered earlier in the study than cases (p<0.001). This difference in recruitment was accounted for by performing analyses stratified by year and through the use of non-linear terms for long-time trends in multivariable models.
Table 1.
Characteristics of deep vein thrombosis in cases and controls.
| Cases (n=870) |
Controls (n=1210) |
|||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Sex | ||||
| Male | 420 | (48.2) | 490 | (40.5) |
| Female | 451 | (51.8) | 720 | (59.5) |
| Age | ||||
| 18–35 years | 303 | (34.8) | 373 | (30.8) |
| 35–50 years | 275 | (31.6) | 424 | (35.0) |
| 50–84 years | 293 | (33.6) | 413 | (34.1) |
| Area of residence | ||||
| Milan, Urban Area | 381 | (43.7) | 525 | (43.4) |
| Milan, Suburban Area | 201 | (23.1) | 272 | (22.5) |
| Bergamo/Brescia | 8 | (0.9) | 18 | (1.5) |
| Po River Valley (towns>15,000 pop) | 47 | (5.4) | 72 | (6.0) |
| Po River Valley (other territory) | 119 | (13.7) | 152 | (12.6) |
| Varese, Como, Lecco | 26 | (3.0) | 24 | (2.0) |
| Lower Valtellina Valley | 14 | (1.6) | 29 | (2.4) |
| Alpine territory | 10 | (1.2) | 15 | (1.2) |
| Pre-Alpine territory | 65 | (7.5) | 103 | (8.5) |
| Body Mass Index | ||||
| 13.3–22.0 kg/m2 | 195 | (23.6) | 396 | (32.9) |
| 22.0–24.9 kg/m2 | 202 | (24.5) | 399 | (33.1) |
| 24.9–53.3 kg/m2 | 428 | (51.9) | 409 | (34.0) |
| Education | ||||
| Elementary/Middle School | 288 | (34.4) | 343 | (28.6) |
| High School | 384 | (45.9) | 579 | (48.3) |
| College | 165 | (19.7) | 278 | (23.2) |
| Pre-Menopausal women with current use of oral contraceptives† | ||||
| No | 117 | 39.3 | 267 | 71.4 |
| Yes | 181 | 60.7 | 107 | 28.6 |
| Post-Menopausal women with current use of hormone-replacement therapy† | ||||
| No | 112 | 73.7 | 255 | 81.0 |
| Yes | 40 | 26.3 | 60 | 19.0 |
| Current use of oral contraceptives or hormone replacement therapy† | ||||
| No | 229 | (50.9) | 522 | (75.8) |
| Yes | 221 | (49.1) | 167 | (24.2) |
| Factor V Leiden | ||||
| −/− | 721 | (82.8) | 1180 | (97.5) |
| −/+ | 140 | (16.1) | 30 | (2.5) |
| +/+ | 10 | (1.2) | 0 | (0.0) |
| G20210A prothrombin mutation | ||||
| −/− | 767 | (88.1) | 1166 | (96.4) |
| −/+ | 99 | (11.4) | 44 | (3.6) |
| +/+ | 5 | (0.6) | 0 | (0.0) |
| Factor V Leiden or G20210A prothrombin mutation | ||||
| No mutation | 638 | (73.3) | 1137 | (94.0) |
| Any mutation | 233 | (26.8) | 73 | (6.0) |
| Deficiencies of natural anticoagulant proteins | ||||
| No | 809 | (92.9) | 1185 | (97.9) |
| Yes | 62 | (7.1) | 25 | (2.1) |
| Hyperhomocysteinemia | ||||
| No | 738 | (84.7) | 1104 | (91.2) |
| Yes | 133 | (15.3) | 106 | (8.8) |
| Any cause of thrombophilia* | ||||
| No | 364 | (41.8) | 877 | (73.5) |
| Yes | 507 | (58.2) | 333 | (27.5) |
| Year of study‡ | ||||
| 1995–1997 | 253 | (29.0) | 471 | (38.9) |
| 1998–2000 | 308 | (35.4) | 384 | (31.7) |
| 2001–2005 | 310 | (35.6) | 355 | (29.3) |
| Season at diagnosis (cases) or examination (controls) | ||||
| Nov-Feb | 227 | (26.1) | 282 | (23.3) |
| Mar-May | 206 | (23.7) | 309 | (25.5) |
| Jun-Aug | 228 | (26.2) | 216 | (17.9) |
| Aug-Nov | 210 | (24.1) | 403 | (33.3) |
| Mean temperature on the day of diagnosis (cases) or examination (controls) | ||||
| −6.1 to 8.4 °C | 277 | (31.8) | 403 | (33.3) |
| 8.4 to 16.5 °C | 229 | (26.3) | 403 | (33.3) |
| 16.5 to 32.7 °C | 365 | (41.9) | 404 | (33.4) |
Subjects with deficiencies of the natural anticoagulant proteins, factor V Leiden, G20210A prothrombin mutation, or hyperhomocysteinemia
Only female subjects are shown
Year of DVT diagnosis for cases or date of examination for controls
Effects of long-term PM10 exposure on coagulation times
Average PM10 level over the one year before the examination was significantly associated with shortened PT in both cases (standardized regression coefficient [beta]=−0.12; 95% CI −0.23, 0.00; p=0.04) and controls (beta=−0.06; 95% CI −0.11, 0.00, p=0.04). While the negative effects of PM10 average levels on PT showed no major differences across averages taken over 30, 60, 90, 180, 270 days, or 1 year (data not shown), the one-year PM10 average was the only time-window significantly associated with shortened PT among cases. A non significant aPTT shortening was observed in association with the one-year PM10 average among controls (beta=−0.09; 95% CI −0.19, 0.01; p=0.07), but not in cases (beta=0.01; 95% CI −0.03, 0.004; p=0.78).
Particle Exposure and Relative Risk of Deep Vein Thrombosis
Table 3 presents the tertiles of the average PM10 level measured in the area of residence during the year before the date of DVT diagnosis (cases) or date of examination (controls), according to their area of residence and year of study. Both in cases and in controls the level of PM10 was highest in the Milan urban and suburban areas (p<0.001). Subjects from the Alpine territory and the Lower Valtellina area were all in the lowest tertile of PM10 level. Level of PM10 in the highest tertile was more frequent in the earlier years of the study in both cases and controls, and decreased throughout the study period to reach the lowest frequency in the most recent years (p<0.001).
Table 3.
Tertile of exposure to Particulate Matter (PM10) in deep vein thrombosis cases and controls, by area of residence and year of examination*
| Tertile of Level of PM10(µg/m3)† | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||||||
| 12.0–44.2 | 44.2–48.1 | 48.1–51.5 | 12.0–44.2 | 44.2–48.1 | 48.1–51.5 | |||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Area of residence | ||||||||||||
| Milan, Urban Area | 72 | (18.9) | 156 | (40.9) | 153 | (40.2) | 70 | (13.3) | 265 | (50.5) | 190 | (36.2) |
| Milan, Suburban Area | 47 | (23.4) | 68 | (33.8) | 86 | (42.8) | 70 | (25.7) | 80 | (29.4) | 122 | (44.9) |
| Bergamo/Brescia | 3 | (37.5) | 1 | (12.5) | 4 | (50.0) | 10 | (55.6) | 3 | (16.7) | 5 | (27.8) |
| Po River Valley (towns>15,000 pop) | 16 | (34.0) | 12 | (25.5) | 19 | (40.4) | 33 | (45.8) | 12 | (16.7) | 27 | (37.5) |
| Po River Valley (other territory) | 42 | (35.3) | 28 | (23.5) | 49 | (41.2) | 62 | (40.8) | 35 | (23.0) | 55 | (36.2) |
| Varese, Como, Lecco | 24 | (92.3) | 1 | (3.9) | 1 | (3.9) | 20 | (83.3) | 1 | (4.2) | 3 | (12.5) |
| Lower Valtellina Valley | 14 | (100.0) | 0 | (0.0) | 0 | (0.0) | 29 | (100.0) | 0 | (0.0) | 0 | (0.0) |
| Alpine territory | 10 | (100.0) | 0 | (0.0) | 0 | (0.0) | 15 | (100.0) | 0 | (0.0) | 0 | (0.0) |
| Pre-Alpine territory | 56 | (86.2) | 9 | (13.9) | 0 | (0.0) | 94 | (91.3) | 9 | (8.7) | 0 | (0.0) |
| Year of study* | ||||||||||||
| 1995–1997 | 27 | (10.7) | 66 | (26.1) | 160 | (63.2) | 59 | (12.5) | 185 | (39.3) | 227 | (48.2) |
| 1998–2000 | 81 | (26.3) | 124 | (40.3) | 103 | (33.4) | 121 | (31.5) | 150 | (39.1) | 113 | (29.4) |
| 2001–2005 | 176 | (56.8) | 85 | (27.4) | 49 | (15.8) | 223 | (62.8) | 70 | (19.7) | 62 | (17.5) |
Year of DVT diagnosis for cases or date of examination for controls
Tertile of level of Particulate Matter<10 µm in aerodynamic diameter in control subjects during the year before the examination
DVT cases tended to have higher level of PM10 than controls and this pattern was more evident after the data were stratified by year of the study (Table 3). The test for the difference between cases and controls by tertile of PM10 was significant in the analysis stratified by year of the study (Mantel-Haenszel p-value=0.002). Such difference was not statistically significant without stratification by year of the study (Fisher's exact p=0.44), indicating that inequalities in the distribution of cases and controls by study period, if not controlled, would have biased the results.
We estimated the relative risk of DVT associated with the level of PM10 in a multivariable model controlled for age, sex, year of diagnosis, area of residence, body mass index, education, current use of oral contraceptives or hormone-replacement therapy, Leiden V or prothrombin mutations, season, and ambient temperature. In this model, an increase of 10 µg/m3 in the level of PM10 was associated with an adjusted OR of 1.70 (95% CI 1.30–2.23; p=0.0001). The increase in DVT risk was nearly linear across the range of exposure concentrations that were measured (Figure 2).
Figure 2.
Level of Exposure to Fine Particulate Matter and the Risk of Deep Vein Thrombosis.
The graph demonstrates the observed relationship between the relative risk of deep vein thrombosis and the level of particulate matter of less than 10 µm in aerodynamic diameter (PM10) in the year preceding the diagnosis. These results suggest a linear relationship between exposure and risk, though the 95% confidence intervals (shaded areas) are wide at the extremes of exposure. Risk is depicted in comparison with a reference value of 12.0 µg per cubic meter (minimum observed PM10 level). The histogram in the bottom part illustrates the density of exposure distribution for air pollution. Risk estimates are adjusted for age, sex, year of diagnosis, area of residence, body mass index, education, current use of oral contraceptives or hormone-replacement therapy, Leiden V or prothrombin mutations, season, and ambient temperature
We also examined DVT risk in association with different exposure time-windows using PM10 level averaged over 90 days-2 years before diagnosis (see Appendix: Supplementary Figure 1 and Table 1; available at URL: http://www.cdldevoto.it/didattic/materiale/Appendix_Archives_online.pdf). DVT risk increased progressively with the duration of the time window evaluated, and was statistically significant in association with the 270-day, 1-year, and 2 year PM10 average levels.
Susceptibility to Air Pollution Effects
Differences in the relationship between DVT risk and the level of PM10 according to the characteristics of the study subjects are summarized in Table 4. The association between PM10 and DVT was significantly attenuated in female subjects (p=0.021 for the interaction between PM10 and sex). An increase of 10 µg/m3 in PM10 level was associated with an adjusted OR for DVT of 2.07 (95% CI, 1.50 to 2.84; p<0.00001) in males and 1.40 (95% CI, 1.02–1.92; p=0.04) in females. No PM10 effect was observed in women taking oral contraceptives or hormone replacement therapy (OR=0.97; 95% CI 0.58–1.61; p=0.89 for PM10 effect; p=0.048 for the interaction between PM10 and hormone use). The other characteristics evaluated, including also year of diagnosis, did not significantly modify the association between PM10 exposure and DVT risk (Table 4).
Table 4.
Relative risk* of deep vein thrombosis associated with a 10 µg/m3 increase in particulate matter (PM10) in the year preceding the diagnosis, by subjects characteristics.
| OR | (95% CI) | p-value† | p-interaction‡ | |
|---|---|---|---|---|
| All subjects | 1.70 | (1.30–2.23) | 0.0001 | - |
| Sex | ||||
| Male | 2.07 | (1.50–2.84) | <0.00001 | |
| Female | 1.40 | (1.02–1.92) | 0.04 | 0.021 |
| Age | ||||
| 18–35 years | 1.57 | (1.11–2.24) | 0.01 | |
| 35–50 years | 1.97 | (1.41–2.77) | 0.00008 | |
| 50–84 years | 1.54 | (0.90–2.63) | 0.12 | 0.99 |
| Pre-Menopausal women with current use of oral contraceptives | ||||
| No | 1.53 | (0.86–2.72) | 0.14 | |
| Yes | 0.87 | (0.46–1.67) | 0.68 | 0.11 |
| Post-Menopausal women with current use of hormone-replacement therapy | ||||
| No | 1.60 | (0.72–3.54) | 0.24 | |
| Yes | 0.85 | (0.29–2.45) | 0.76 | 0.27 |
| Current use of oral contraceptives or hormone replacement therapy | ||||
| No | 1.64 | (1.05–2.57) | 0.03 | |
| Yes | 0.97 | (0.58–1.61) | 0.89 | 0.048 |
| Body Mass Index§ | ||||
| 13.3–22.0 kg/m2 | 1.47 | (0.97–2.23) | 0.07 | |
| 22.0–24.9 kg/m2 | 1.72 | (1.17–2.54) | 0.006 | |
| 24.9–53.3 kg/m2 | 1.83 | (1.03–3.24) | 0.04 | 0.37 |
| Education | ||||
| Elementary/Middle School | 1.93 | (1.35–2.76) | 0.0003 | |
| High School | 1.72 | (1.24–2.39) | 0.001 | |
| College | 1.35 | (0.74–2.45) | 0.33 | 0.21 |
| Deficiencies of natural anticoagulant proteins | ||||
| None | 1.66 | (1.26–2.18) | 0.0003 | |
| Any | 2.56 | (0.91–7.18) | 0.07 | 0.41 |
| Factor V Leiden or G20210A prothrombin mutation | ||||
| None | 1.69 | (1.27–2.23) | 0.0003 | |
| Any | 1.79 | (1.05–3.05) | 0.03 | 0.83 |
| Hyperhomocysteinemia | ||||
| No | 1.66 | (1.26–2.19) | 0.0004 | |
| Yes | 2.19 | (1.33–3.61) | 0.002 | 0.25 |
| Any cause of thrombophilia¶ | ||||
| No | 1.59 | (1.19–2.13) | 0.0017 | |
| Yes | 1.96 | (1.34–2.87) | 0.0005 | 0.27 |
| Year of diagnosis | ||||
| 1995–1997 | 1.61 | (1.06–2.46) | 0.03 | |
| 1998–2000 | 1.34 | (0.90–1.99) | 0.15 | |
| 2001–2005 | 2.14 | (1.04–4.39) | 0.04 | 0.12 |
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) adjusted for age, sex, year of diagnosis, area of residence, body mass index, education, current use of oral contraceptives or hormone-replacement therapy, Leiden V or prothrombin mutations, season, and ambient temperature.
Test for the association of PM10 with DVT risk; Test for differences by subjects' characteristics of the association between PM10 and DVT.
Body Mass Index categorized according to controls' tertiles.
Subjects with deficiencies of the natural anticoagulant proteins, factor V Leiden, G20210A prothrombin mutation, or hyperhomocysteinemia
Sensitivity analyses
We repeated all the analyses after excluding cases with a recurrent (non-first) episode of DVT (n=110). Risk estimates were very similar to those obtained on the entire study population.
Each increase of 10 µg/m3 in the level of PM10 was associated with an OR of 1.67 (95% CI, 1.27–2.22; p=0.0003), adjusting for multiple variables. In the subsample of 760 cases with a single episode of DVT, the variations in the association between PM10 and the risk of DVT due to demographic characteristics, presence of thrombophilia or use of hormone therapies were similar to those observed in the entire study population.
To evaluate the influence of splines selection in fitting non-liner terms in the logistic models, we repeated all statistical analyses by using natural splines instead of penalized splines. The use of natural splines did not modify the statistical significance of the results, with only small changes in the risk estimates.
We also evaluated the influence of different methods for adjusting for long-term time trends during the study period (Supplementary Table 2, available at URL http://www.cdldevoto.it/didattic/materiale/Appendix_Archives_online.pdf). As also shown above, ignoring long-term time trends in the analyses would have almost completely obscure the association between PM10 level and DVT risk. However, all of the methods for adjustment for long-term time trends that we evaluated in our sensitivity analysis (fitting dummy variables for each year of the study period, as well as linear term or penalized splines for index date with degrees of freedom varying between 2–8) produced risk estimates all indicating a significant association between PM10 level and DVT risk, with only small changes due to different handling of the time-trends for most of the methods. However, it is worth noting that using only a linear variable for the long-term trend would have produced a lower OR, likely reflecting less-than-optimal fitting of the time relationships present in our data.
DISCUSSION
In this study of DVT cases and healthy controls, exposure to increased concentrations of particulate air pollution in the year before diagnosis was associated with increased DVT risk after controlling for clinical and environmental covariates. PM10 average level before the examination was also correlated with shorter PT in both cases and controls. The DVT risk increase associated with PM10 level was smaller in women and limited to those who were not using oral contraceptives or hormone replacement therapy at the time of diagnosis.
Long-term exposure to particulate air pollution has been associated with increased risk of coronary heart and cerebrovascular disease in multiple investigations conducted in several countries.4 A systemic increase in thrombotic tendency, secondary to the induction of inflammatory mediators produced in the lungs and released in the circulation, or to the translocation of particles of smaller diameter from the lungs into the circulation,6 has been frequently proposed to account for the cardiac and cerebrovascular effects of particulate air pollution. In contrast, venous thrombosis has received little attention in studies on the cardiovascular outcomes of air pollution. In a time-series analysis from the Netherlands, Hoek et al.15 reported an association of short-term exposure to ambient ozone, and to lesser extent to black smoke and PM10, with increased mortality from embolism and thrombosis, a broad category that included arterial and venous thromboses in various sites. To date, no study has specifically addressed the association between particulate air pollution and DVT. In our population, we estimated an overall 70% increase in DVT risk with each increase of 10 µg/m3 in PM10 level during the year before diagnosis. For comparison, in the Harvard Six Cities Study the risk of death from cardiopulmonary diseases was 37% higher in the most polluted compared with the least polluted cities.1 In the Women Health Initiative Study, an increase of 10 µg/m3 of annual average concentrations of PM2.5 — which is considered a stronger predictor than PM10 level of air pollution effects — was associated with a 24% increase in the risk of cardiovascular events and a 76% increase in the risk of death from cardiovascular disease.2 The estimated increase in risk of death from all cardiovascular causes associated with 10 µg/m3 in long-term PM2.5 level was 19% in the Harvard Six Cities study and 13% in the American Cancer Society's study.3
In the present study, PM10 exposure did not increase the risk of DVT in women as much as in men. By evaluating additional risk factors, we found that part — if not all — of such risk attenuation was due to the lack of association between PM10 and the risk of DVT among women using oral contraceptives or hormone replacement therapy. Such hormone therapies are independent risk factors for DVT,10 as also confirmed in this study by the higher prevalence of oral contraceptives and hormone replacement use in cases than controls. Use of oral contraceptives and hormone replacement therapy induces changes in coagulation factors, such as increased levels of the procoagulant factors VII, IX, X, XII, XII, von Willebrand factor and fibrinogen, and a reduced level of the anticoagulant proteins antithrombin and protein S, 16–18 that are similar in characteristics and degree to the coagulation changes observed after exposure to air particles. 7, 8, 19–22 We surmise that prothrombotic mechanisms are already activated in hormone users, so that they undergo less or no further induction after air particle exposure.
In our analyses, we evaluated DVT risk in association with the level of PM10 measured during the year before diagnosis. In this study, the use of short term (hourly or daily) air pollution levels would not have been appropriate, because DVT presentation is often subtle and its diagnosis has been shown to lag as long as four weeks after the initial DVT symptoms.23 In the present work, we demonstrated that average PM10 level in the year before the examination was associated with shortened PT, extending our previous observation of an association with shorter exposure time windows.16 Interestingly, while the negative effects of PM10 average levels on PT were rather independent of the time-window selected, the one-year PM10 average was the only exposure metric significantly associated with shortened PT among cases. In addition, while in our previous work we could not find any relation between 30-day average PM10 level and aPTT, a suggestive, though non significant aPTT shortening was observed in association with the one-year PM10 average among controls in the present analysis. Thus, the use of PM10 level in the year before diagnosis appeared to capture a fuller range of prothrombotic effects, while also contributing to reduce the risks of confounding by seasonal patterns and ambient temperature. This study has the advantage of being based on a large number of DVT cases and controls recruited using a standardized protocol over a long time span. Cases had objective diagnoses of DVT, and both cases and controls were well characterized for inherited and acquired risk factors for DVT. In the statistical analysis, we used models that included non-linear regression terms to adjust for long-term time trends and day of the year — thus controlling for confounding from year of the study and seasonal variations —, in addition to age, sex, area of residence, education, factor V Leiden, G20210A prothrombin mutation, use of oral contraceptive or hormone-replacement therapy, body mass index, and ambient temperature.
Healthy controls, also due to their selection among nonblood relatives and friends of the DVT cases, tended to be distributed in the nine study areas with proportions that were very similar to those of the DVT cases. This might have generated overmatching in our study, i.e., the exposure levels of controls may have been more similar to those of DVT cases than they actually are in the population at risk. Therefore, it is possible that risk estimates were underestimated in our study. A limitation of this study is that we used ambient air pollution estimated at the subjects’ address as a surrogate for personal exposure, which may have resulted in measurement error, as most subjects conduct a large part of their daily activities away from their residence. A detailed questionnaire was used to ascertain known risk factors for DVT, but no information was collected concerning daily activities, such as time spent outside or in traffic, that could have refined the assessment of PM10 exposure. In addition, our exposure assessment was done by dividing Lombardia region in nine areas for which average PM10 levels were assigned by averaging measurements from multiple monitors. Although these areas were selected to group together territories with similar population density and geographical characteristics, thus likely reducing within-area variations of the exposure, we were not able to obtain PM10 level estimates at a smaller scale. However, PM10 levels tend to be spatially homogenous and a recent study comparing personal exposures to site monitoring in Boston reported that monitor readings and personal exposure are highly correlated.24 In addition, it has been shown that using ambient measures to estimate individual exposure is likely to produce an underestimation of pollution effects25 rather than causing the increased risk of DVT found in our study population.
In conclusion, this study provides evidence in support of an association of exposure to particulate air pollution with enhanced prothrombotic mechanisms and risk of DVT. Given the magnitude of the observed effects and the widespread diffusion of particulate pollutants, our findings introduce a novel and common risk factor into the pathogenesis of DVT and, at the same time, give further substance to the call for tighter standards and continued efforts aimed at reducing the impact of urban air pollutants on human health.
Supplementary Material
Table 2.
Estimated changes of prothrombin time (PT) and activated partial thromboplastin time (APTT) associated with particulate matter (PM10) levels in the year before the examination in Deep Vein Thrombosis (DVT) cases and controls.
| Case Status | PM10 association with Prothrombin Time (PT)† |
PM10 association with activated Partial Tromboplastin Time (aPTT)† |
||||
|---|---|---|---|---|---|---|
| Beta* | (95% CI) | p-value | Beta* | (95% CI) | p-value | |
| Controls | −0.12 | (−0.23, 0.00) | 0.04 | −0.09 | (−0.19, 0.01) | 0.07 |
| DVT cases | −0.06 | (−0.11, 0.00) | 0.04 | 0.01 | (−0.03, 0.04) | 0.7807 |
Standardized regression coefficients, expressing the fraction of a standard deviation change in PT or aPTT associated with a standard deviation change in exposure, adjusted for age, gender, body mass index, cigarette smoking, alcohol consumption, oral contraceptives, and non linear terms for seasonality, long-term time trend, and temperature.
ACKNOWLEDGMENTS
Work supported by the following research grants: EPA PM Center R83241601 and R827353; NIEHS ES0002 and ES015172-01 and PO1 ES009825U.S; MIUR Internationalization Program 2004-2006/97-C; CARIPLO Foundation and Lombardy Region UniMi 8614/2006 and UniMi 9167/2007.
We thank Guido Lanzani, Nadia Carfagno, and Anna Cazzullo, ARPA Lombardia for support in air-monitoring data handling; and Steve Melly, Harvard School of Public Health for assistance in the creation of geographic maps.
Footnotes
The Authors did not declare any financial interest to disclose
REFERENCES
- 1.Dockery DW, Pope CA, 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 2.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA, 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 5.Utell MJ, Frampton MW, Zareba W, Devlin RB, Cascio WE. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol. 2002;14(12):1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- 6.Nemmar A, Hoylaerts MF, Nemery B. Effects of particulate air pollution on hemostasis. Clin Occup Environ Med. 2006;5(4):865–881. doi: 10.1016/j.coem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Ruckerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173(4):432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 8.Baccarelli A, Zanobetti A, Martinelli I, et al. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost. 2007;5(2):252–260. doi: 10.1111/j.1538-7836.2007.02300.x. [DOI] [PubMed] [Google Scholar]
- 9.Lowe GD. Arterial disease and venous thrombosis: are they related, and if so, what should we do about it? J Thromb Haemost. 2006;4(9):1882–1885. doi: 10.1111/j.1538-7836.2006.02130.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353(9159):1167–1173. doi: 10.1016/s0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344(20):1527–1535. doi: 10.1056/NEJM200105173442007. [DOI] [PubMed] [Google Scholar]
- 12.Silva VM, Corson N, Elder A, Oberdorster G. The rat ear vein model for investigating in vivo thrombogenicity of ultrafine particles (UFP) Toxicol Sci. 2005;85(2):983–989. doi: 10.1093/toxsci/kfi142. [DOI] [PubMed] [Google Scholar]
- 13.Baccarelli A, Zanobetti A, Martinelli I, et al. Air Pollution, Smoking, and Plasma Homocysteine. Environ Health Perspect. 2007;115(2):176–181. doi: 10.1289/ehp.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood SN. Modelling and smoothing parameter estimation with multiple quadratic splines. J R Stat Soc Ser B. 2000;62(1):95–114. [Google Scholar]
- 15.Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12(3):355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 16.van Rooijen M, Silveira A, Thomassen S, et al. Rapid activation of haemostasis after hormonal emergency contraception. Thromb Haemost. 2007;97(1):15–20. [PubMed] [Google Scholar]
- 17.Rosendaal FR, Van Hylckama Vlieg A, Tanis BC, Helmerhorst FM. Estrogens, progestogens and thrombosis. J Thromb Haemost. 2003;1(7):1371–1380. doi: 10.1046/j.1538-7836.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 18.Rabbani LE, Seminario NA, Sciacca RR, Chen HJ, Giardina EG. Oral conjugated equine estrogen increases plasma von Willebrand factor in postmenopausal women. J Am Coll Cardiol. 2002;40(11):1991–1999. doi: 10.1016/s0735-1097(02)02565-2. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz J. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect. 2001;109 Suppl 3:405–409. doi: 10.1289/ehp.01109s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhal Toxicol. 2003;15(14):1465–1478. doi: 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- 21.Riediker M, Cascio WE, Griggs TR, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169(8):934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- 22.Liao D, Heiss G, Chinchilli VM, et al. Association of criteria pollutants with plasma hemostatic/inflammatory markers: a population-based study. J Expo Anal Environ Epidemiol. 2004;15(4):319–328. doi: 10.1038/sj.jea.7500408. [DOI] [PubMed] [Google Scholar]
- 23.Elliott CG, Goldhaber SZ, Jensen RL. Delays in diagnosis of deep vein thrombosis and pulmonary embolism. Chest. 2005;128(5):3372–3376. doi: 10.1378/chest.128.5.3372. [DOI] [PubMed] [Google Scholar]
- 24.Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16(3):385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.