Abstract
Drugs that improve chronic hyperglycemia independently of insulin signaling or reduction of adiposity or dietary fat intake may be highly desirable. Ad36, a human adenovirus, promotes glucose uptake in vitro independently of adiposity or proximal insulin signaling. We tested the ability of Ad36 to improve glycemic control in vivo and determined if the natural Ad36 infection in humans is associated with better glycemic control. C57BL/6J mice fed a chow diet or made diabetic with a high-fat (HF) diet were mock infected or infected with Ad36 or adenovirus Ad2 as a control for infection. Postinfection (pi), systemic glycemic control, hepatic lipid content, and cell signaling in tissues pertinent to glucose metabolism were determined. Next, sera of 1,507 adults and children were screened for Ad36 antibodies as an indicator of past natural infection. In chow-fed mice, Ad36 significantly improved glycemic control for 12 wk pi. In HF-fed mice, Ad36 improved glycemic control and hepatic steatosis up to 20 wk pi. In adipose tissue (AT), skeletal muscle (SM), and liver, Ad36 upregulated distal insulin signaling without recruiting the proximal insulin signaling. Cell signaling suggested that Ad36 increases AT and SM glucose uptake and reduces hepatic glucose release. In humans, Ad36 infection predicted better glycemic control and lower hepatic lipid content independently of age, sex, or adiposity. We conclude that Ad36 offers a novel tool to understand the pathways to improve hyperglycemia and hepatic steatosis independently of proximal insulin signaling, and despite a HF diet. This metabolic engineering by Ad36 appears relevant to humans for developing more practical and effective antidiabetic approaches.
Keywords: infectobesity, insulin resistance, diabetes, adenovirus-36, adipose tissue, metabolic remodeling, glucose uptake, glucose disposal, hepatic steatosis, nonalcoholic fatty liver disease
impaired glycemic control is associated with serious health conditions, including diabetes and cardiovascular risks (23). Lifestyle changes to reduce dietary fat intake and obesity can substantially ameliorate the impairment or further deterioration of glycemic control (61). Unfortunately, despite their obvious health benefits, compliance with lifestyle changes to achieve sustained improvements in diet or obesity has proved challenging for the general population. Hence, drugs often play an important role in controlling hyperglycemia. Particularly, antidiabetic agents that improve chronic hyperglycemia, independent of reduction of adiposity or dietary fat intake would be extremely attractive and of practical benefit.
Most of the currently available antihyperglycemic agents target the insulin signaling pathway, which could be broadly divided into proximal signaling [binding of insulin to its receptor (IR)], followed by the activation of insulin receptor substrate (IRS)-1 and IRS-2, and distal signaling, which includes the activation of phosphatidylinositol 3-kinase (PI3K) pathway by IRS-1 and IRS-2 and which via the activation of Akt2 leads to glucose transporter-mediated glucose disposal. However, in diabetes, proximal insulin signaling is often impaired (45, 53), which could render a proximal insulin signaling-based agent less effective. Therefore, drugs that improve hyperglycemia through a mechanism that is independent of insulin signaling, or at least independent of proximal insulin signaling, may be more effective. Interestingly, studies of human adenovirus Ad36 offer a new model to develop therapeutic approaches to enhance glycemic control despite adiposity and high-fat intake and independently of proximal insulin signaling.
Ad36 was first isolated from a human fecal sample (63). It is among 51 human adenovirus serotypes (63), which are associated with infections of the respiratory and gastrointestinal tracts. Experimental Ad36 infection of chickens, mice, rats, and nonhuman primates increases adiposity in chow-fed animals (14, 15, 43, 47) yet improves systemic glycemic control (43). In vitro, Ad36 downregulates IR and IRS-1 activation yet robustly upregulates the distal insulin signaling via a Ras- and PI3K-dependent mechanism, leading to up regulation of glucose transporters and, consequentially, enhanced glucose uptake (49, 62). These findings have potentially valuable applications. Ad36 may offer a novel tool to reveal how to manipulate host metabolism to improve glycemic control in the presence of excess adiposity and without recruiting proximal insulin signaling.
To accomplish this, we first tested whether Ad36 would improve glycemic control in vivo in a mouse model. Furthermore, to evaluate the human relevance of the metabolic effects of Ad36, we determined whether in humans natural Ad36 infection is associated with better glycemic control, adjusted for age, sex, and adiposity. Considering the close association of glycemic control with hepatic steatosis (HS), we also determined the effect of Ad36 on hepatic lipid accumulation in mice and humans, as described below.
METHODS
Animal Experiments
The Institutional Animal Care and Use Committee (IACUC) of the Pennington Biomedical Research Center approved the protocols for animal studies. Mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and placed on a 12:12-h light-dark cycle at 25°C and housed in microisolator cages under Biosafety level 2 containment with ad libitum access to food and water. Protective clothing, gloves, shoes, hairnets, and masks were worn by research personnel when entering the rooms, and utmost care was taken to prevent cross contamination.
Experiment 1: effect of experimental Ad36 infection on glycemic control in chow-fed mice.
Four-week-old female C57B6/6J mice were received. They were offered rodent chow (Purina Lab Diet 5001). After 1 wk of acclimatization, total body fat was determined by Bruker Minispec mq10-NMR (nuclear magnetic resonance) analyzer. Mice were divided into three groups matched for body weight and body fat and were inoculated intranasally, orally, and intraperitoneally with 107 PFU of Ad36 (n = 3) or Ad2 (a common human adenovirus used as a control for infection; n = 4) or mock infected with tissue culture medium (n = 6). The body weights were measured weekly, and blood samples were obtained from the intraorbital retrobular sinus from anesthetized mice fasted for 4 h. The mice were killed 12 wk postinfection (PI). Trunk blood was collected, and serum was separated and used for glucose and insulin determinations. Liver and retroperitoneal fat depots were carefully separated, weighed, and flash-frozen in liquid nitrogen and stored at −80°C until used for qRT-PCR assay or for Western blot or histology studies as described below in Techniques and Assays. Additional details of the assays are included in supplementary information (supplementary information is found linked to the online version of this article).
Experiment 2: effect of experimental Ad36 infection on glycemic control in high-fat-fed mice.
Fourteen-week-old male C57B6/6J mice who were fed a high-fat (HF, 60% kcal) diet (Research Diets D12492i) starting at 6 wk of age were received. Upon 1 wk acclimatization, baseline body fat was determined by NMR, and mice were divided into three groups (n = 10/group) matched for body fat and body weight. The groups were infected with Ad36 (0.6 × 106 PFU), Ad2 (3 × 106 PFU), or mock infected intranasally, intraperitoneally, and orally and continued on HF diet (60% kcal) for an additional 20 wk. Food disappearance and body weights were measured weekly for 16 wk, and blood samples were obtained from the intraorbital retrobular sinus in anesthetized mice. Fasting samples were collected after removing food for 4 h. The mice were killed 20 wk PI in the free-fed state. Trunk blood was collected. Liver and epididymal and retroperitoneal fat depots were carefully separated, weighed, flash-frozen in liquid nitrogen, and stored at −80°C until use for qRT-PCR assay, or for Western blot or histology studies as described in Techniques and Assays.
Statistical analyses.
Differences in food intake, body weights, liver, fat pad weights, and glucose and insulin levels were analyzed by Student's t-test for both animal experiments. Probability levels were set at P ≤ 0.05.
Human Studies: Experiment 3. Association of Natural Infection of Ad36 with Glycemic Control in Humans
Human serum samples from four cohorts were screened post hoc for the presence of Ad36-neutralizing antibodies as described (3) and were analyzed for differences in available measures of glycemic control between the seropositive and seronegative groups. For each of the cohorts, approvals were obtained from Institutional Review Boards. Detailed information about the individual cohorts is presented in supplementary information.
The cohorts included subjects from the following: 1) HERITAGE (HEalth, RIsk factors, exercise Training And GEnetics) Family Study (8) (n = 671 white and black men and women), 2) PBRC (Pennington Biomedical Research Center) Study (n = 206 white and black men and women), 3) MET (Mechanisms of the Metabolic Syndrome in Prepubertal Youth) study (31, 59) (n = 45 prepubertal healthy white and black boys and girls), and 4) VIVA LA FAMILIA Study (10) (n = 585 Hispanic boys and girls). All results were adjusted for age, sex, race, and available measures of adiposity as applicable.
Techniques And Assays
Additional details about the techniques and assays are described in supplementary information.
Viruses.
Ad36 and Ad2 were obtained from American Type Culture collection (ATCC cat. nos. VR913 and VR846, respectively). Ad36 was plaque purified and propagated in A549 cells (human lung cancer cell line) as described (13, 14).
Biochemical assays.
Glucose was measured using the Raichem glucose Oxidase method (R80038), and insulin was measured with an ultrasensitive mouse insulin ELISA kit (Crystal Chem no. 90090). Triglycerides were determined using Cardiochek Lipid panel test strips.
qRT-PCR.
mRNA was extracted from the livers of chow- and HF-fed mice, and quantitative RT-PCR was conducted as described (48).
Neutralizing antibody titer.
The presence of neutralizing antibodies in serum was determined by the “constant virus-decreasing serum” method as described (3).
Glucose tolerance test.
Subsequent to a 16-h fast, conscious mice were injected intraperitoneally with d-glucose (2.5 mg/g body wt). Blood was collected from the tail vein prior to glucose injection (time 0) and at 10, 20, 30, 60, 120, and 150 min PI. Blood glucose was determined using a glucometer (Contour, Bayer).
Western blot analyses.
Proteins from adipose tissue, liver, and skeletal muscle were used for Western blot analyses by standard procedure (49). For immunoprecipitation of IR, IRS-1, and IRS-2, tissue samples were homogenized in a buffer containing 50 mM HEPES (pH 7.4), 2 mM sodium orthovanadate, 10 mM sodium fluoride, 2 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate, and protease inhibitors. Homogenates (250 μg) were then immunoprecipitated with 3 μg of primary antibody. Samples were subjected to SDS-PAGE using 4–20% gradient gel and transferred to PVDF membranes. The membranes were immunoblotted with anti-phospho antibodies. Antibodies for phoshphorylated Tyr1322-IRβ (Millipore, no. 04-300) and total IRβ receptor (Millipore, no. 05-1104), total IRS-1 (Santa Cruz, no. Sc-559) and p-IRS-1 (Tyr989; Santa Cruz, no. Sc-17200), p-IRS-1 (Ser307; Cell Signaling, no. 2381), IRS-2 (Millipore, no. 06-506), and p-IRS-2 (Tyr612) (Santa Cruz, no. Sc-17195-R), respectively.
Protein concentrations were quantitated by bicinchoninic acid assay and loaded onto the 4–20% or polyacrylamide gel in equal amounts. Proteins were then transferred to PVDF membranes. Membranes were blocked in PBS-Tween-20 containing 3% BSA and incubated with polyclonal or monoclonal antibodies that recognize total PKB (protein kinase B; Cell Signaling, no. 4691), p-PKB (Ser473; Cell Signaling, no. 9271), Ras (Cell Signaling, no. 3965), GLUT1 (Abcam, no. 35826), GLUT4 (Abcam, no. 14683), GLUT2 (Santa Cruz, no. 9117), glucose-6-phosphatase (G-6-Pase; Santa Cruz, no. 7291), total glycogen synthase kinase (Santa Cruz, no. 27198), p-glycogen synthase kinase (Ser21; Santa Cruz, no. 16308), total AMPKα (Cell Signaling, no. 2603), p-AMPKα (Thr172; Cell Signaling, no. 2535), and leptin (Abcam, no. 2095) antibodies, respectively. Followed by secondary antibody conjugated with horseradish peroxidase, signals were detected by enhanced chemiluminiscence. The specific bands were quantitated with scanning densitometry using AlphaEaseFC analyzer software, and equal loading was assessed by normalization to GAPDH (Ambion, no. 4300) abundance.
Histochemistry.
Glycogen was stained using periodic acid-Schiff (PAS) stain on flash-frozen liver samples of three mice each from Ad36, Ad2, and mock infected HF-fed mice, and one mock-infected chow-fed mouse as a control as described (4). Glycogen staining gives a magenta color to sections; a darker stain indicates more glycogen. Lipid leaves the sample during fixation; thus, a white blank area on the slide indicates lipid droplets (2).
RESULTS
Experiment 1: Ad36 Improves Glycemic Control in Chow-Fed Mice
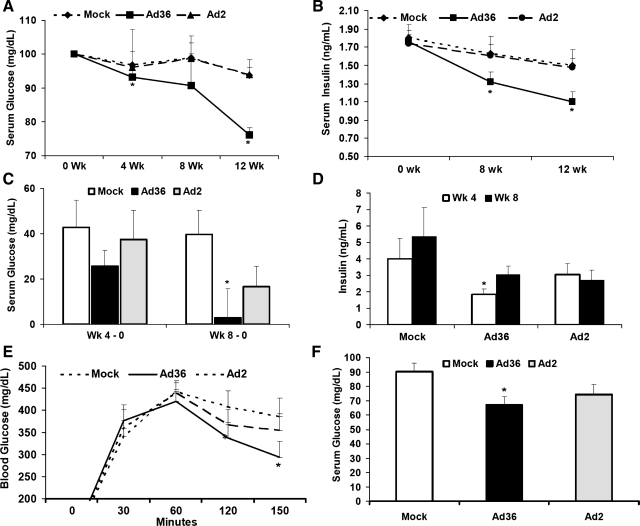
In both animal experiments, neutralizing antibodies to the adenoviruses and/or PCR analyses for viral DNA and/or mRNA in various mouse tissues confirmed mock infection or infection by the expected viruses (Suppl. Table S1). In experiment 1, body weights did not differ among the groups during the 12-wk study. Despite similar baseline levels, fasting serum glucose and insulin progressively decreased only in Ad36-infected mice over the duration of the experiment (Fig. 1, A and B). Furthermore, at 12 wk PI, mean retroperitoneal fat pad weight was twofold greater (P < 0.03) and the mean liver weight was 10% lower (P < 0.04) in Ad36-infected mice than in the mock-infected mice (Table 1). Thus, in chow-fed mice, Ad36 infection, but not Ad2 infection, increased adiposity yet improved systemic glycemic control.
Fig. 1.
Adenovirus (Ad)36 reduces fasting serum glucose and insulin in chow-fed and high-fat (HF)-fed mice. Glycemic response of mice infected with Ad36 or Ad2 or mock infected. A: fasting serum glucose adjusted to baseline. B: fasting serum insulin at baseline (week 0) and up to 12 wk postinfection in chow-fed mice. C–F: glycemic response of HF-fed mice. C: weeks 4 and 8 postinfection, change in fasting serum glucose compared with baseline (week 0); D: weeks 4 and 8, change in fasting insulin from week 0; E: week 12 ip glucose tolerance test after 2.5 mg/g body wt glucose. F: week 20, free-fed serum glucose adjusted to baseline. *P < 0.05 or less vs. mock of respective studies. Values are means ± SE.
Table 1.
Response of chow-fed and HF-fed mice to Ad36
| Mock Infected | Ad36 | Ad2 | P | |
|---|---|---|---|---|
| Baseline and final characteristics of chow-fed mice, means ± SE | ||||
| n | 6 | 3 | 4 | |
| Body weight, g, week 0 | 16.3 ± 0.44 | 16.9 ± 0.12 | 16.2 ± 0.22 | NS |
| Body fat, g, week 0 | 2.1 ± 0.08 | 2.3 ± 0.11 | 2.0 ± 0.05 | NS |
| Body weight, g, week 12 | 22.3 ± 0.34 | 23.8 ± 1.02 | 22.5 ± 0.72 | NS |
| Retroperitoneal fat, g, week 12 | 0.18 ± 0.02 | 0.36 ± 0.08* | 0.25 ± 0.06 | *P < 0.05 vs. Mock |
| Liver, g | 1.00 ± 0.04 | 0.9 ± 0.05* | 1.0 ± 0.1 | *P < 0.05 vs. Mock |
| Baseline and final characteristics of HF-fed mice, means ± SE | ||||
| n | 10 | 10 | 10 | |
| Body weight, g, week 0 | 36.2 ± 0.9 | 36.1 ± 1.1 | 37.3 ± 1.0 | NS |
| Body weight, g, week 20 | 51.5 ± 0.8 | 48.3 ± 2.1 | 50.7 ± 1.2 | NS |
| Cumulative food Intake, g, measured 16 wk postinfection | 186 ± 9.2 | 186 ± 13.3 | 184 ± 13.7 | NS |
| Total body fat, g, week 0 | 10.5 ± 0.8 | 10.7 ± 0.7 | 11.5 ± 1.0 | NS |
| Body fat, g, week 20 | ||||
| Epididymal | 1.41 ± 0.05 | 1.36 ± 0.13 | 1.46 ± 0.18 | NS |
| Retroperitoneal | 0.49 ± 0.02 | 0.52 ± 0.05 | 0.47 ± 0.02 | NS |
| Liver, g | 2.73 ± 0.1 | 2.34 ± 0.17* | 2.36 ± 0.24 | *P < .05 vs. Mock |
| Serum triglycerides, mg/dl | ||||
| Week 0 | 71.6 ± 1.7 | 71.2 ± 0.9 | 71.5 ± 1.2 | |
| Week 8 | 70.4 ± 2.1 | 68.3 ± 1.2* | 69.3 ± 1.0 | *P < .05 vs. week 0 of Ad36 |
| qRT-PCR data of expression of genes in livers of mice fed chow or HF diet | ||||
| Lipogenesis |
Lipid Oxidation |
Lipid Export |
Inflammation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | FAS | SREBP1c | FoxO1 | AdipoR1 | AdipoR2 | CPT I | LXR | PPARα | apoB | MTP | IL-6 | IL-10 | INFγ | TNFα |
| Chow | NS | NS | ↑* | ↑* | ↑* | ↑* | NS | ↑* | ↑* | ↑* | ↓(0.06) | NS | NS | NS |
| HF | NS | ↓(0.08) | ↑(0.07) | NS | NS | NS | NS | NS | ↑* | NS | ↓* | NS | ↓* | ↓* |
Arrows indicate direction of changes induced by Ad36 compared with mock-infected group. HF, high fat; NS, no significant difference.
P < 0.05 or better. To indicate trend, P value is also denoted, if between 0.05 and 0.1.
Experiment 2: Ad36 Improves HF Diet-Induced Hyperglycemia Despite Continued HF Diet and Without Requiring Weight Loss
Experiment 2 investigated whether Ad36 would improve HF diet-induced hyperglycemia. Fourteen-week-old mice fed a HF diet for the prior 8 wk developed a diabetic state as evidenced by high fasting serum glucose levels (>200 mg/dl). At that point, mice were either mock infected or infected with Ad36 or Ad2. By 20 wk PI, all three groups had similar cumulative food intake as well as total body weight and fat pad masses (Table 1; Suppl. Fig. S1). The adipogenic effect of Ad36 was probably overwhelmed by adipogenic effects of HF diet (Table 1). Over the 20-wk period PI, glycemic control was assessed in various ways: by determining fasting glucose and insulin, by glucose tolerance test, and by determining glucose levels in free-fed state. Overall, Ad36, but not Ad2, significantly improved glycemic control compared with the mock-infected group, and the effect lasted for the 20-wk duration of the experiment (Fig. 1).
Specifically, due to the HF diet-induced insulin resistance, mock-infected mice had an expected increase in fasting serum glucose and insulin. Whereas Ad36 significantly attenuated these increases at 4 or 8 wk PI (Fig. 1, C and D, and Suppl. Fig. S2). Blood glucose clearance in response to an intraperitoneal glucose tolerance test (ipGTT) at 12 wk PI, from its peak at 60 min to the end of the assay 150 min post-glucose infusion, was significantly quicker in Ad36-infected mice (Fig. 1E). At 20 wk PI, Ad36-infected mice had lower free-fed serum glucose levels (Fig. 1F). These free-fed serum glucose levels of all Ad36-infected mice were in the lower 50th percentile compared with those of mock-infected mice (Suppl. Fig. S2; χ-test, P = 0.01).
The phenotypic effect of Ad36 on glucose metabolism correlated to the load of infection. Serum glucose levels at the termination of the experiment (20 wk) significantly and negatively correlated with the amount of Ad36 DNA in livers of respective mice from the Ad36 group (r = −0.94, 2-tailed P = 0.001) by the standard curve method and r = −0.91, 2-tailed P = 0.001 by ΔΔCT method of real-time PCR (Suppl. Fig. S3; methods described in supplementary information). The correlation was not significant for the Ad2 group (P = 0.5 or 0.7 for the two methods, respectively). Thus, Ad36 improves glycemic responses in diabetic mice under both fasted and fed conditions in a viral load-dependent manner. Probably due to the semiquantitative nature of antibody titer, no significant correlation was observed between antibody titers and phenotypic outcomes for Ad36 or Ad2 (Suppl. Fig. S4). Only four of ten mice from the Ad2 group were seropositive at 20 wk PI. Despite a fivefold higher dose of inoculation used for Ad2 vs. Ad36, Ad2 failed to significantly improve hyperglycemia compared with the mock group. Modulation of glycemic control by Ad2 did not reach statistical significance yet showed some trend. A virus would exert a set of characteristic influences on its host. It would be important to identify which difference in the action of the two adenoviruses determines their metabolic effects. This would help in screening other members of human adenovirus group for actions similar to Ad36.
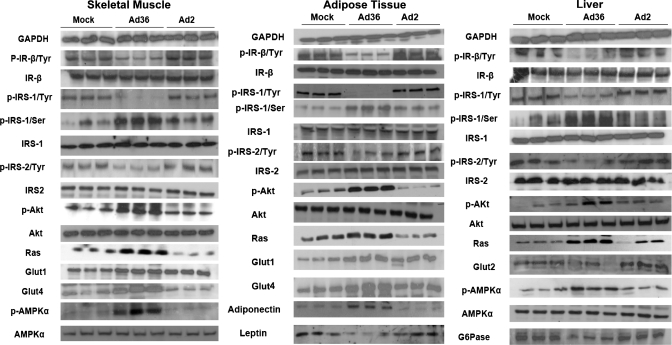
Western blot analyses conducted for mice tissues from experiment 2 showed that, in agreement with our in vitro data (49, 62), Ad36 upregulated the Ras-PI3K pathway as indicated by significantly greater abundance of Ras and phospho-Akt in skeletal muscle, adipose tissue, and liver compared with mock-infected mice (Fig. 2). Probably via this upregulation of distal insulin signaling, Ad36 increased abundance of Glut4 and Glut1 proteins in skeletal muscle and adipose tissue (Fig. 2), which may contribute to enhanced glucose uptake, whereas Ad36 lowered Glut2 abundance and Glucose-6-phosphatase (G-6-Pase) in the liver (Fig. 2), indicators of a reduction in hepatic glucose release. Thus, increased Ad36 appears to improve systemic glycemic control by upregulating skeletal muscle and adipose tissue glucose uptake and by reducing hepatic glucose release. Future experiments, including hyeperinsulinemic-euglycemic clamp studies and tissue-specific glucose disposal, should further clarify this model.
Fig. 2.
Protein abundance and lipid accumulation in tissues of HF-fed mice. Mice were killed in free fed state (to elicit insulin response) 20 wk postinfection. Harvested tissues were flash-frozen and used for Western blots; 3 mice per group were compared. GAPDH was the loading control. Densitometry analysis was used to quantitate protein abundance and compare changes. Changes in Ad36 compared with mock (P < 0.05 or better): skeletal muscle, liver, and adipose tissue. Decreased, phospho-IR, Tyr-phospho-IRS-1 and -2; increased, Ser-phospho-IRS-1, Ras, phospho-Akt. Skeletal muscle and adipose tissue: increased, Glut1 and Glut4. Adipose tissue: decreased, leptin; increased, adiponectin. Liver: increased, phosphor-AMPK; decreased, Glut2, G-6-Pase. Change in Ad2 vs. mock: no significant changes.
Insulin induces IRS signaling (proximal insulin signaling) to activate the PI3K pathway (distal insulin signaling), which upregulates glucose uptake. Consistent with previous in vitro findings (49, 62), Ad36 downregulated levels of activated, tyrosine-phosphorylated IR, IRS-1, and IRS-2 and upregulated levels of inactivated, serine-phosphorylated IRS-1 (Fig. 2) yet upregulated glucose disposal. Although the precise reason for the downregulation of IRS-1 action is unknown, it may be due to the effect of Ad36 on tumor necrosis factor-α (TNFα), which functionally inhibits IRS-1 by inducing its serine phosphorylation (20). Adipose tissue from Ad36-infected mice expressed 2.5-fold higher TNFα mRNA levels than did mock-infected mice (Suppl. Fig. S5). Likewise, Ad36 also significantly increased mRNA levels of other inflammatory cytokines such as MCP1 and CD68 (Suppl. Fig. S5), which have also been implicated in downregulation of insulin receptor signaling and insulin resistance (46). Ad36 enhances glucose disposal despite the impaired proximal insulin signaling pathway, probably because the robust upregulation of the distal insulin signaling by Ad36, including the PI3K and Glut4 pathways, compensate for the effect.
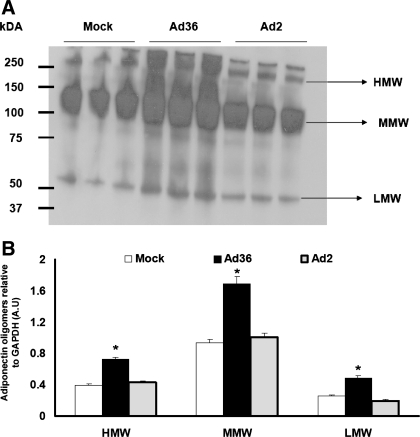
Although Ad36 DNA/mRNA was detected in various tissues of the mice, it is unclear whether Ad36 alters metabolism directly in these tissues. For instance, changes in glucose metabolism in the liver and skeletal muscle could be secondary to the effects of adiponectin, a key adipose tissue-expressed adipokine that improves glycemic control (41). Adiponectin exists as higher-, medium-, and lower-molecular-weight (MW) forms, although the higher MW form is most strongly linked to insulin sensitivity (51). PI3K is a strong promoter of adiponectin secretion (7, 44). Via the activation of the PI3K pathway, Ad36 appears to significantly increase levels of total adiponectin and all its molecular weight forms in adipose tissue of HF diet-fed mice (Figs. 2 and 3). Adiponectin acts via adiponectin receptors AdipoR1 and AdipoR2 to activate AMPK (AMP-activated protein kinase) in skeletal muscle and liver to promote glucose uptake in skeletal muscle (64) and to protect liver against steatosis (66). Furthermore, adiponectin upregulates PPARα pathways (25), decreases systemic and hepatic insulin resistance, and attenuates liver inflammation and fibrosis (25). Ad36-infected HF-fed mice showed higher levels of the activated, phosphorylated-AMPK in skeletal muscle and liver compared with mock-infected or Ad2-infected mice (Fig. 2). AMPK activation promotes insulin-independent glucose disposal (68). Therefore, we postulate that Ad36 directly increases adiponectin expression in adipose tissue, which contributes to the systemic improvement in metabolic profile.
Fig. 3.
Ad36 increases adiponectin abundance in adipose tissue of HF-fed mice. Proteins were isolated from adipose tissue of HF-fed mice killed 20 wk postinoculation with Ad36 or Ad2 or mock infected (n = 3 mice/group). HMW, high molecular weight; MMW, medium molecular weight; LMW, low molecular weight isoforms. A: Western blot showing adiponectin oligomers assayed under nonreducing, nondenaturing conditions. B: densitometry analyses of adiponectin oligomers from samples (A). Values are means ± SE. P < 0.03 or better.
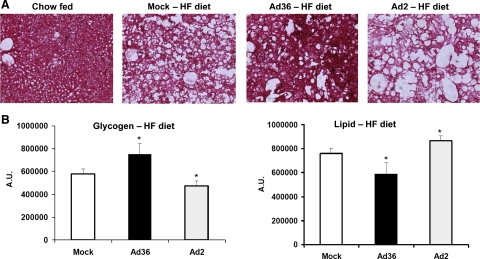
Ad36 may alter determinants of hepatic lipid accumulation. As expected, liver sections of HF diet-fed mice displayed less glycogen and more lipid than the liver section of a chow-fed mouse (Fig. 4). Within the HF diet-fed groups, however, Ad36 appeared to protect from the hepatic effects of the HF diet, as evidenced by significantly increased glycogen and lower lipid content in livers compared with mock-infected mice (P < 0.02; Fig. 4).
Fig. 4.
Ad36 increases glycogen and reduces lipid content in the liver. Mice were killed in free-fed condition, and liver samples obtained were flash-frozen at −80°C. Liver samples from 3 mice each from Ad36, Ad2, or mock infected HF-fed mice were stained with periodic acid-Schiff (PAS) stain for glycogen. Liver samples from one chow-fed mouse were used as a positive control for staining. Pink stain indicates glycogen, and blank area indicates stored lipid. Three specimens per sample and three images per specimen were analyzed using Image J software for quantification of glycogen and lipid. Images were converted to 8 bit, and threshold was determined where only glycogen-specific staining was visible and the amount of glycogen was calculated as pixels2 at this threshold. This number was subtracted from the total area to obtain the area of blank space to quantitate lipid. Values are means ± SD. *P < 0.03 or better.
The metabolic regulation of hepatic lipid storage is complex. Potential determinants of hepatic lipid levels include synthesis (lipogenesis), utilization (fat oxidation), and export of hepatic lipids (26). Due to the reduction in hepatic lipid accumulations by Ad36, we tested the differences in expressions of selected hepatic genes of chow-fed and HF-fed mice. Although these molecules have overlapping roles in multiple pathways, we considered FAS (fatty acid synthase), SREBP-1c (sterol response element-binding protein-1c), and FOXO1 (forkhead box O1) as modulators of lipogenesis (67); AdipoR1 and AdipoR2, CPT I (carnitine palmityl acyltransferase), LXR (liver X receptor), and PPARα to indicate lipid oxidation (29, 34, 65) and MTP (microsomal triglyceride transfer protein) and apoB (apolipoprotein B) as indicative of lipid export (32, 57). Since hepatic steatosis coupled with inflammation may signal the progression to nonalcoholic steatohepatitis (NASH), markers of inflammation were also determined.
The overall gene expression results are summarized in Table 1. The hepatic gene expressions were determined 12–20 wk PI. Although such a long-period PI and a HF diet may mask some changes, gene expressions from the chow-fed and HF-fed mice collectively suggest that Ad36 increases adiponectin receptor expression and reduces lipogenesis, upregulates lipid oxidation and export, and reduces inflammation in the liver (Table 1). Interestingly, a recent study showed that AMPK activation in the liver downregulates IL-6-mediated inflammatory response (38). This appears to be the case in livers of HF fed mice of the Ad36 group.
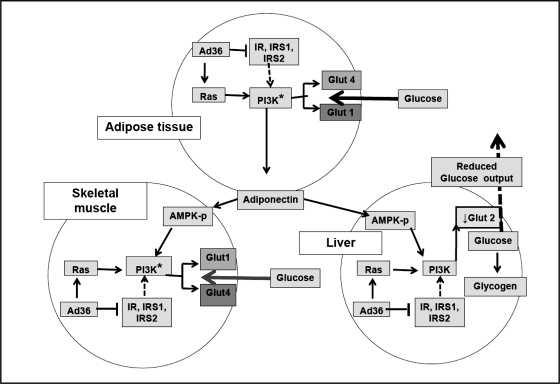
These findings present a new animal model, wherein Ad36 may increase glucose uptake by skeletal muscle and adipose tissue and reduce glucose release and lipid accumulation by the liver, thereby significantly improving systemic glycemic control and HS (Fig. 5).
Fig. 5.
Working model to explain the antidiabetic effect of Ad36. Overall, data from Figs. 1–3 suggest that, in adipose tissue, skeletal muscle, and liver of mice, Ad36 downregulates insulin signaling yet upregulates the Ras-PI3K pathway, which upregulates Glut1 and Glut4 in skeletal muscle and adipose tissue and downregulates Glut2 in liver. Furthermore, Ad36 increases adiponectin, which may activate AMPK. Collectively, this leads to greater glucose uptake by adipose tissue and skeletal muscle and reduces hepatic glucose release, which may contribute to Ad36-induced improvement in systemic glycemic control.
Experiment 3: Ad36 Is Associated with Better Glycemic Control and Lower HS in Humans
To methodically test the association of Ad36 with adult and pediatric serum samples from four cohorts that covered a wide range of age, race, and BMI were screened retrospectively for Ad36 antibodies. Presence of Ad36 antibodies indicated past natural infection with the virus. Serum neutralization assay, the highly specific and sensitive but labor-intensive gold standard for determining neutralizing antibodies, was used. Until the results of antibody testing were available, the investigators were blinded to the available phenotypic data.
The stability of Ad36 antibodies after natural infection was assumed in subjects. About 13% of individuals from the HERITAGE Family Study (8) scored positive for Ad36 antibodies (Table 2), and such individuals had better insulin sensitivity (3.65 ± 0.23 vs. 3.06 ± 0.09, 10−4 min/mU/ml, P = 0.007) than did Ad36 seronegative individuals. Ad36 infection was associated with better glycemic control as indicated by greater insulin sensitivity in whites (P = 0.026) and greater disposition index (P = 0.004) in blacks. In the PBRC cohort, 18% subjects were seropositive for Ad36 and had significantly lower fasting glucose and fasting insulin levels and lower insulin resistance as indicated by HOMA-IR (homeostatic model of insulin resistance) index (Table 2). In the MET cohort, 22% of these healthy, exclusively prepubertal children were Ad36 seropositive and showed significantly lower fasting glucose, fasting insulin, and lower HOMA-IR (Table 2) than did Ad36 seronegative children. Only 7.1% of samples from the Viva La Familia study (9) scored positive for Ad36 antibodies and had better glycemic control as indicated by significantly lower fasting glucose (P = 0.04) and greater odds ratio (OR) to have fasting glucose (P = 0.05), fasting insulin (P = 0.01), and HOMA-IR (P = 0.01), below the median of the overall group, compared with that for the Ad36 seronegative group (Table 2). Some indexes of glycemic control varied based on their availability for a cohort. However, remarkably, the association of Ad36 infection with better glycemic control was consistently valid across cohorts of diverse age groups and races of over 1,500 individuals. It is interesting that Ad36 seropositivity-based differences in insulin emerged even in children, whose mean insulin levels were well within the normal range. It is also intriguing that, despite the numerous potential factors that might influence glycemic control in adults, past Ad36 infection was a significant protective factor, independent of age, sex, and adiposity. It is unknown whether the relatively lower prevalence of Ad36 infection observed in Hispanic children contributes to the greater risk of diabetes reported in Hispanic population (11).
Table 2.
Association of Ad36 seropositivity with measures of glycemic control
| White Subjects |
Black Subjects |
|||||
|---|---|---|---|---|---|---|
| Ad36− | Ad36+ | P | Ad36− | Ad36+ | P | |
| HERITAGE family study: adult men and women (n = 671; black/white, 221/450) mean (95% CI) adjusted for age, sex, and BMI. NS: P > 0.05 | ||||||
| n = 671 | 406 | 44 | 174 | 47 | ||
| Fasting insulin, pmol/l | 56.8 (54.3–59.4) | 56.8 (49.5–65.1) | NS | 63.8 (59.2–68.7) | 70.6 (61.0–81.6) | NS |
| Fasting glucose, mg/dl | 90.7 (89.8–91.5) | 91.4 (89.0–94.0) | NS | 91.9 (90.4–93.4) | 92.7 (89.8–95.6) | NS |
| Insulin sensitivity, 10−4 min/mU/ml | 3.85 (3.63–4.08) | 4.62 (3.88–5.42) | 0.026 | 2.26 (2.02–2.52) | 2.69 (2.20–3.26) | NS |
| Disposition index, si × AIRg | 2214 (2,075–2,358) | 2037 (1,637–2,481) | NS | 2794 (2,451–3,159) | 3909 (3,153–3,469) | 0.004 |
| AIRg, 10 min/mU/ml | 627 (589–668) | 521 (418–636) | 0.042 | 1379 (1,212–1,532) | 1627 (1,278–1,926) | NS |
| Glucose effectiveness, min−1 | 0.016 (0.015–0.017) | 0.017 (0.014–0.019) | NS | 0.019 (0.017–0.021) | 0.022 (0.019–0.026) | 0.043 |
| PBRC study: adult men and women (n = 206; black/white/other, 74/118/14); mean (95% CI) adjusted for age, sex, race, and body fat mass | ||||||
| n = 206 | n = 169 | n = 37 | ||||
| Fasting glucose, mg/dl | 96.9 (92.6, 101.3) | 92.3 (87.1, 97.6) | 0.004 | |||
| Fasting insulin, μU/ml | 11.6 (9.6, 14.0) | 9.4 (7.1, 12.4) | 0.04 | |||
| HOMA IR | 2.7 (2.2, 3.3) | 2.1 (1.6, 2.8) | 0.01 | |||
| Liver density* (HU) | 10.7 (8.5, 13.4) | 13.0 (9.9, 17.2) | 0.02 | |||
| *Normalized to spleen density. Higher HU value equates to lower lipid content | ||||||
| MET study: prepubertal boys and girls (n = 45; black/white/other, 10/32/3); meana (95% CI) adjusted for sex, and body fat mass | ||||||
| n = 42 | n = 35 | n = 10 | ||||
| Fasting glucose, mg/dl | 74.5 (71.6–77.4) | 68.7 (62.9–74.6) | 0.04 | |||
| Fasting insulin, μU/ml | 3.1 (2.4–4.1) | 1.8 (1.1–3.0) | 0.04 | |||
| HOMA IR | 0.51 (0.39–0.69) | 0.28 (0.16–0.48) | 0.03 | |||
| Intrahepatic lipid, %water peak | 0.005 (0.002–.006) | 0.003 (0.004–0.008) | 0.04 | |||
| aArithmetic mean for glucose; geometric mean for insulin, HOMA-IR, and intrahepatic lipid | ||||||
| VIVA LA FAMILIA study. Hispanic boys and girls (n = 585). Odds ratio (OR) adjusted for sex, body fat mass, and family; meana (95% CI) adjusted for adiposity and familyb. NS, P > 0.05 | ||||||
| n = 585 | n = 543 | n = 42 | ||||
| Fasting glucose, mg/dl | 92.2 (91.3–93.0) | 89.1 (85.8–92.4) | 0.04 | |||
| Fasting insulin, μU/ml | 15.9 (15.2–16.6) | 16.6 (13.9–19.8) | NS | |||
| HOMA-IR | 3.58 (3.42–3.76) | 3.65 (3.03–4.40) | NS | |||
| Fasting glucose, mg/dl | 92.2 (91.3–93.0) | 89.1 (85.8–92.4) | 0.04 | |||
| OR for Ad36+ to be below the median of the overall group | ||||||
| OR | 95% CI | P | ||||
| Fasting glucose | 2.5 | 1.1–5.7 | 0.02 | |||
| Fasting insulin | 1.8 | 0.9–3.4 | 0.04 | |||
| HOMA IR | 2.4 | 1.1–5.3 | 0.02 | |||
Arithmetic mean for glucose; geometric means for insulin and HOMA IR.
Initial models included age and sex, but neither was a significant predictor, so both were excluded from the final analytical model; however, the resulting P values were unchanged.
Measures assessing intrahepatic lipid (IHL) content were available in the PBRC and MET cohorts. In both cohorts, Ad36 infection was associated with significantly lower IHL (Table 1). These findings agree well with a recent report of protection from nonalcoholic fatty liver disease (NAFLD) in Ad36 seropositive people (60). These findings suggest that Ad36 infection may protect individuals from impaired glucose tolerance (IGT) and HS, two key risk factors associated with type 2 diabetes and serious liver diseases.
Thus, experimental Ad36 infection of mice improved glycemic control and HS, and those humans who were naturally infected with Ad36 mirrored these findings.
DISCUSSION
Some salient features of this study are that 1) despite increased adiposity (experiment 1) or continued HF diet (experiment 2), Ad36 robustly and lastingly improved systemic glycemic control and reduced HS; 2) the improvement in systemic glycemic control was a nontransient and specific response to Ad36 infection; 3) robust upregulation of adiponectin and distal insulin signaling by Ad36 appears to contribute to these metabolic improvements; 4) improvement in glycemic control by Ad36 was despite the downregulation of proximal insulin signaling; 5) natural Ad36 infection in humans mirrors the effects induced by the virus in experimentally infected animals. These aspects are discussed below.
1) Excess adiposity, HF-diet, impaired insulin signaling, and adipose tissue inflammation are important risk factors for several serious conditions including type 2 diabetes, NAFLD and
NASH and cardiovascular diseases.
Reducing dietary fat intake and obesity is the mainstay of the behavioral component and an adjunct to effective drug treatment of these health conditions. Despite their obvious health benefits, compliance with lifestyle changes to achieve sustained improvements in diet or obesity has proved challenging for the general population. Therefore, drugs that improve diabetes independently of adiposity or dietary fat intake would be extremely attractive and of practical benefit. Also, most of the currently available antidiabetic agents are mimetics, sensitizers, or secretagogues of insulin, that employ the insulin signaling pathway for their action. However, insulin-resistant states such as obesity or diabetes are often associated with impaired proximal insulin signaling (21, 40, 45, 52–55), which may limit the efficacy of such drugs. Thus, there is an urgent need to develop new drugs that improve glycemic control independent of insulin signaling, and independent of dietary fat intake or obesity. The unique capability of Ad36 offers a model to creatively negate the ill effects of excess adiposity or excess dietary fat intake without the need to reduce it.
2) In both animal experiments, the improvement in glycemic control persisted for the entire duration of the study. Ad2 infection did not improve glycemic control indicates, which suggests that the phenotypic changes were not a nonspecific response to an infection but were specific to the proteins of Ad36. The specific role of Ad36 is further highlighted by a strong correlation between the viral DNA load and the improved glycemic control observed.
3) By upregulating adiponectin, its receptors in the liver (AdipoR1 and AdipoR2), and its target molecule (AMPK), Ad36 appears to improve glucose and hepatic metabolism independently of adiposity. In fact, adiponectin accounts for a subtype of “insulin-sensitive obesity”, independent of total fat mass (28). Overexpression of adiponectin increases body fat yet improves glycemic control (27). Likewise, the thiazolidinedione (TZD) class of drugs upregulate adiponectin and improve hyperglycemia and HS (35). But better drugs are needed, as some serious side effects of TZDs have been recently reported (33, 39). To that effect, Ad36 does not cause morbidity or unintended mortality in animals. In addition, Ad36 appears to have distinct advantages over the action of the TZDs, particularly in the presence of a HF diet. Unlike the TZDs, Ad36 does not increase adiposity in HF-fed mice (19). Moreover, in presence of a HF diet, TZDs improve glycemic control, but they concurrently promote lipid storage in various organs, including the liver (30, 58). This may limit the scope of TZDs, if fat intake is not reduced, which is not a limitation for Ad36.
4) Ad36 enhances glucose disposal despite the impaired proximal insulin signaling, probably because the robust upregulation of the distal insulin signaling by Ad36 compensates for the effect. The downregulation of proximal insulin signaling by Ad36 does not induce insulin resistance, which would have been characterized by elevated fasting insulin with normal or elevated fasting glucose. Instead, Ad36 reduces fasting glucose and insulin. This is probably because less insulin is required to maintain glucose homeostasis due to enhanced glucose disposal by the virus. Thus, the improvement in glucose disposal by Ad36 should be regarded as its “insulin sparing action”. Hence, as observed in vitro (49, 62), the in vivo effect of Ad36 on glucose disposal also appears independent of proximal insulin signaling. The distal insulin signaling pathway recruited by Ad36 may not require insulin action. Such a potential agent may especially benefit advanced-stage type 2 diabetics, who usually require insulin therapy but often respond weakly due to impaired insulin signaling. Similarly, insulin-independent glucose disposal may also be useful for the treatment of type 1 diabetes as an adjunct to insulin treatment.
5) Importantly, we revealed a link between natural Ad36 infections of humans and better glycemic control and lower hepatic lipid, which may have valuable health implications. It is suggested that hepatic lipid, and not visceral fat, is a determinant of metabolic syndrome (18, 36). Moreover, hepatic fat accumulation could lead to NAFLD or further serious consequences. The prevalence of NAFLD is about 70–80% in adults with type 2 diabetes or obesity (5, 42, 56), 3–10%, in all children, and up to 40–70% in obese children (5). NAFLD is associated with greater overall and liver-related mortality (1, 17). In addition to steatosis, inflammation and fibrosis can develop, and NAFLD may progress to NASH, cirrhosis, liver failure, and hepatocellular carcinoma. Although steatosis is potentially reversible, once it progresses to NASH, there is no established treatment, and the few available medications show limited success (22, 50); therefore, the timely prevention and/or treatment of hepatic steatosis is critical. However, even for NAFLD, drug treatment has marginal success (16), and reducing dietary fat intake and obesity are recommended (37), which is challenging. Therefore, agents to lower hepatic steatosis independently of adiposity or dietary fat intake would be extremely attractive and of practical benefit. Ad36 may provide a template to develop such an approach.
The congruence of results from the human and animal studies particularly strengthens the findings. Also, these findings underscore the need to consider Ad36 infection status as a potential confounder in epidemiological studies that investigate parameters related to glucose metabolism. Importantly, the association of Ad36 with better glyemic control in humans strongly supports the human relevance of the antidiabetic attributes of Ad36 proteins.
Although viruses are generally considered causative agents for disease, we report surprising evidence suggesting that an infection with Ad36 favorably alters host glucose metabolism. The long-term goal of this study is not to recommend Ad36 infection for improving metabolic profile but to use it as a tool and exploit its properties to develop more effective strategies for managing hyperglycemia. Such an approach of harnessing certain properties of viruses for beneficial purposes has been creatively used for several years, including the use of bactericidal properties of a bacteriophage virus (24), the oncolytic ability of a mutant adenovirus (6), or the use of Herpes simplex virus and several other viruses for the treatment of cancers (12). Similarly, Ad36 offers a new model. This model may be valuable due to its unique attributes that are desirable for the effective and clinically more practical management of IGT or HS. Future experiments are required to carefully determine the precise molecular signaling modulated by Ad36 and to identify the candidate viral protein (s) responsible for the effect.
In summary, excess adiposity and HF diet are associated with impaired insulin signaling and are risk factors for conditions such as type 2 diabetes or NAFLD. Despite the risk factors, Ad36 improves IGT or HS in mice and, possibly, in humans as well. This offers an opportunity to use Ad36 as a new tool to reveal how the host metabolism could be manipulated without reducing dietary fat or adiposity, a highly desirable attribute for the treatment/prevention of IGT or HS. This information may provide a template to develop novel antidiabetic approaches that are clinically more attractive, hence, potentially more effective.
GRANTS
The research was funded in part by 1) the American Diabetes Association (1-09-IN-13) and The Mathile Institute for the Advancement of Human Nutrition grants awarded to N. V. Dhurandhar; 2) The HERITAGE Family Study has been funded by multiple grants from National Institutes of Health (HL-45670, HL-47323, HL-47317, HL-47327, HL47321) to C. Bouchard, T. Rankinen, and others. C. Bouchard is partially funded by the George A. Bray Chair in Nutrition; 3) National Institute of Child Health and Human Development 1R01 HD-41071-01A2, 1R01 HD-49046-05, National Institute of Diabetes and Digestive and Kidney Diseases (CNRU) 1P30 DK-072476, and the LSU Health Sciences Center and Tulane University CTRC; 4) the Viva La Familia Study and related work were funded by National Institute of Diabetes and Digestive and Kidney Diseases R01 DK-59264 and USDA/ARS 58-6250-51000-037 to N. B; 5) This work used Genomics core facilities at the Pennington Biomedical Research Center that are supported in part by COBRE (P20-RR 021945) and NORC (1P30 DK-072476) grants from the National Institutes of Health.
DISCLOSURES
No conflicts of interest are reported by the authors.
Supplementary Material
REFERENCES
- 1. Adams LA, St Lymp JF, Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129: 113–121, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Aoki F, Honda S, Kishida H, Kitano M, Arai N, Tanaka H, Yokota S, Nakagawa K, Asakura T, Nakai Y, Mae T. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci Biotechnol Biochem 71: 206–214, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, Augustus AS. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond) 29: 281–286, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bajpeyi S, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Hickner RC, Kraus WE, Houmard JA. Effect of exercise intensity and volume on persistence of insulin sensitivity during training cessation. J Appl Physiol 106: 1079–1085, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 28: 155–161, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274: 373–376, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Blumer RM, van Roomen CP, Meijer AJ, Houben-Weerts JH, Sauerwein HP, Dubbelhuis PF. Regulation of adiponectin secretion by insulin and amino acids in 3T3–L1 adipocytes. Metabolism 57: 1655–1662, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc 27: 721–729, 1995 [PubMed] [Google Scholar]
- 9. Butte NF, Cai G, Cole SA, Comuzzie AG. Viva la Familia Study: genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. Am J Clin Nutr 84: 646–654; quiz 673–644, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF, Ellis KJ, Comuzzie AG. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr 85: 1478–1485, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U. S. population in 1988–1994 and 2005–2006. Diabetes Care 32: 287–294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crompton AM, Kirn DH. From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets 7: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Dhurandhar NV, Israel BA, Kolesar JM, Mayhew G, Cook ME, Atkinson RL. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord 25: 990–996, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord 24: 989–996, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM, Kemnitz JW, Allison DB, Atkinson RL. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr 132: 3155–3160, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Duvnjak M, Tomasic V, Gomercic M, Smircic Duvnjak L, Barsic N, Lerotic I. Therapy of nonalcoholic fatty liver disease: current status. J Physiol Pharmacol 60, Suppl 7: 57–66, 2009 [PubMed] [Google Scholar]
- 17. Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 106: 15430–15435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandes-Santos C, Carneiro RE, de Souza Mendonca L, Aguila MB, Mandarim-de-Lacerda CA. Pan-PPAR agonist beneficial effects in overweight mice fed a high-fat high-sucrose diet. Nutrition 25: 818–827, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277: 48115–48121, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95: 2195–2204, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta AK, Bray GA, Greenway FL, Martin CK, Johnson WD, Smith SR. Pioglitazone, but not metformin, reduces liver fat in Type-2 diabetes mellitus independent of weight changes. J Diabetes Complications 24: 289–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 263: 2893–2898, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Hanlon GW. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int J Antimicrob Agents 30: 118–128, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Heiker JT, Kosel D, Beck-Sickinger AG. Molecular advances of adiponectin and adiponectin receptors. Biol Chem 391: 1005–1018, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Kantartzis K, Schick F, Haring HU, Stefan N. Environmental and genetic determinants of fatty liver in humans. Dig Dis 28: 169–178, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. Insulin sensitive obesity. Am J Physiol Endocrinol Metab 299: E506–E515, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Kotokorpi P, Ellis E, Parini P, Nilsson LM, Strom S, Steffensen KR, Gustafsson JA, Mode A. Physiological differences between human and rat primary hepatocytes in response to liver X receptor activation by 3-[3-[N-(2-chloro-3-trifluoromethylbenzyl)-(2,2-diphenylethyl)amino]propyloxy]phenylacetic acid hydrochloride (GW3965). Mol Pharmacol 72: 947–955, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kuda O, Stankova B, Tvrzicka E, Hensler M, Jelenik T, Rossmeisl M, Flachs P, Kopecky J. Prominent role of liver in elevated plasma palmitoleate levels in response to rosiglitazone in mice fed high-fat diet. J Physiol Pharmacol 60: 135–140, 2009 [PubMed] [Google Scholar]
- 31. Larson-Meyer DE, Newcomer BR, VanVrancken-Tompkins CL, Sothern M. Feasibility of assessing liver lipid by proton magnetic resonance spectroscopy in healthy normal and overweight prepubertal children. Diabetes Technol Ther 12: 207–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liao W, Kobayashi K, Chan L. Adenovirus-mediated overexpression of microsomal triglyceride transfer protein (MTP): mechanistic studies on the role of MTP in apolipoprotein B-100 biogenesis. Biochemistry 38: 7532–7544, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA 298: 2634–2643, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Louet JF, Le May C, Pegorier JP, Decaux JF, Girard J. Regulation of liver carnitine palmitoyltransferase I gene expression by hormones and fatty acids. Biochem Soc Trans 29: 310–316, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Lutchman G, Promrat K, Kleiner DE, Heller T, Ghany MG, Yanovski JA, Liang TJ, Hoofnagle JH. Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol 4: 1048–1052, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity (Silver Spring) 18: 1510–1515, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mishra P, Younossi ZM. Current treatment strategies for non-alcoholic fatty liver disease (NAFLD). Curr Drug Discov Technol 4: 133–140, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Nerstedt A, Johansson A, Andersson CX, Cansby E, Smith U, Mahlapuu M. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3). Diabetologia 53: 2406–2416, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Pagliassotti MJ, Kang J, Thresher JS, Sung CK, Bizeau ME. Elevated basal PI 3-kinase activity and reduced insulin signaling in sucrose-induced hepatic insulin resistance. Am J Physiol Endocrinol Metab 282: E170–E176, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep 3: 207–213, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology 132: 2191–2207, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL, MohanKumar S, MohanKumar PS, Markward N, Dhurandhar NV. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity (Silver Spring) 14: 1905–1913, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Pereira RI, Leitner JW, Erickson C, Draznin B. Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3′-kinase. Life Sci 83: 638–643, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest 106: 165–169, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev 21: 1443–1455, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Raben A, Haulrik N, Dhurandhar N, Atkinson R, Astrup A. Minor role of human adenovirus 36 in the obesity epidemic in Denmark. Int J Obes 25, Suppl 2: S46, 2001 [Google Scholar]
- 48. Rathod M, Rogers PM, Vangipuram SD, McAllister EJ, NVD Adipogenic cascade can be induced without adipogenic media by a human adenovirus. Obesity 17: 657–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rogers PMMN, Rathod MA, Dubuisson O, Wang ZQ, Dasuri K, Babin S, Gupta A, Markward N, Cefalu WT, Dhurandhar NV. Metabolically favorable remodeling of human adipose tissue by human adenovirus Ad-36. Diabetes 57: 2321–2331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149: 2270–2282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Semple RK, Cochran EK, Soos MA, Burling KA, Savage DB, Gorden P, O'Rahilly S. Plasma adiponectin as a marker of insulin receptor dysfunction: clinical utility in severe insulin resistance. Diabetes Care 31: 977–979, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, Soos MA, Carpenter TA, Lomas DJ, Cochran EK, Gorden P, O'Rahilly S, Savage DB. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 119: 315–322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sesti G, Federici M, Hribal ML, Lauro D, Sbraccia P, Lauro R. Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J 15: 2099–2111, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Sesti G, Federici M, Lauro D, Sbraccia P, Lauro R. Molecular mechanism of insulin resistance in type 2 diabetes mellitus: role of the insulin receptor variant forms. Diabetes Metab Res Rev 17: 363–373, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30: 1212–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Tietge UJ, Bakillah A, Maugeais C, Tsukamoto K, Hussain M, Rader DJ. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J Lipid Res 40: 2134–2139, 1999 [PubMed] [Google Scholar]
- 58. Todd MK, Watt MJ, Le J, Hevener AL, Turcotte LP. Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab 292: E485–E493, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Tompkins CL, Cefalu W, Ravussin E, Goran M, Soros A, Volaufova J, Vargas A, Sothern MS. Feasibility of intravenous glucose tolerance testing prior to puberty. Int J Pediatr Obes 5: 51–55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trovato GM, Martines GF, Garozzo A, Tonzuso A, Timpanaro R, Pirri C, Trovato FM, Catalano D. Ad36 adipogenic adenovirus in human non-alcoholic fatty liver disease. Liver Int 30: 184–190, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Wang ZQCW, Zhang XH, Yongmei Y, Qin J, Son L, Rogers PM, Mashtalir N, Bordelon JR, Ye J, Dhurandhar NV. Human adenovirus type 36 enhances glucose uptake in diabetic and non-diabetic human skeletal muscle cells independent of insulin signaling. Diabetes 57 1805–1813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wigand R, Gelderblom H, Wadell G. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol 64: 225–233, 1980 [DOI] [PubMed] [Google Scholar]
- 64. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002 [DOI] [PubMed] [Google Scholar]
- 65. Yoon M. The role of PPARalpha in lipid metabolism and obesity: focusing on the effects of estrogen on PPARalpha actions. Pharmacol Res 60: 151–159, 2009 [DOI] [PubMed] [Google Scholar]
- 66. You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281: 10105–10117, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.