Abstract
The transcription factor HIF-1α activity is increased in adipose tissue to contribute to chronic inflammation in obesity. However, its upstream and downstream events remain to be characterized in adipose tissue in obesity. We addressed this issue by investigating adipocyte HIF-1α activity in response to obesity-associated factors, such as adipogenesis, insulin, and hypoxia. In adipose tissue, both HIF-1α mRNA and protein were increased by obesity. The underlying mechanism was investigated in 3T3-L1 adipocytes. HIF-1α mRNA and protein were augmented by adipocyte differentiation. In differentiated adipocytes, insulin further enhanced HIF-1α in both levels. Hypoxia enhanced only HIF-1α protein, not mRNA. PI3K and mTOR activities are required for the HIF-1α expression. Function of HIF-1α protein was investigated in the regulation of VEGF gene transcription. ChIP assay shows that HIF-1α binds to the proximal hypoxia response element in the VEGF gene promoter, and its function is inhibited by a corepressor composed of HDAC3 and SMRT. These observations suggest that of the three obesity-associated factors, all of them are able to augment HIF-1α protein levels, but only two (adipogenesis and insulin) are able to enhance HIF-1α mRNA activity. Adipose tissue HIF-1α activity is influenced by multiple signals, including adipogenesis, insulin, and hypoxia in obesity. The transcriptional activity of HIF-1α is inhibited by HDAC3-SMRT corepressor in the VEGF gene promoter.
Keywords: hypoxia-inducible factor-1α, adipocytes, histone deacetylase 3, hypoxia
a hypoxia response has been reported in adipose tissue during obesity by several laboratories (58). This finding provides a cellular mechanism underlying the chronic inflammation and adipose tissue dysfunction in obesity (50, 58). Adipose tissue hypoxia has led our attention to hypoxia marker genes such as hypoxia inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF). HIF-1α is a transcription factor whose activity is induced by hypoxia, and it has been used as an indicator of adipose tissue hypoxia (19, 23, 42, 60). Its qualification as a hypoxia-specific marker remains to be evaluated in obesity. HIF-1 is formed by two subunit proteins with basic helix-loop-helix structure. The α-subunit (HIF-1α) determines the transcriptional activity of HIF-1, and its protein abundance increases in response to oxygen deprivation (hypoxia). The β-subunit (HIF-1β) protein is constitutively expressed and known as aryl hydrocarbon receptor nuclear translocator (47). In the normoxic condition, HIF-1α is hydroxylated on proline and asparaginyl residues, which leads to a high-affinity binding to an ubiquitin ligase complex that contains von Hippel-Lindau. The ubiquitination induces proteasome-mediated HIF-1α degradation. Under hypoxia the degradation is inhibited, leading to an increase in HIF-1α protein abundance and transcriptional activity of HIF-1α. HIF-1α gene is expressed constantly in cells. The mRNA increase is not necessary for HIF-1α protein elevation. The transcriptional activity of HIF-1α is regulated by nuclear coactivator and corepressor. The coactivators include p300 and CBP, which catalyze acetylation of histone proteins and initiation of gene transcription (2, 11). The corepressor activity is determined by histone deacetylases (HDACs) (13), which have multiple isoforms. It is not clear which HDAC isoform specifically inhibits HIF-1 activity (25, 29, 41).
VEGF is a major target gene of HIF-1. VEGF promotes angiogenesis, a process that is required for adipocyte differentiation and adipose tissue growth as reviewed (6, 7, 9, 21). Angiogenic inhibitors suppress fat tissue growth in animal models (5, 15, 27, 45) and represent a potential class of antiobesity drugs. Interestingly, adipocytes express a high level of VEGF (8, 34), which provides a molecular mechanism for the high capacity of adipose tissue to induce angiogenesis (64). However, the molecular mechanism for VEGF expression in adipocytes remains poorly understood.
The present study explored the HIF-1α activity in adipocytes in response to obesity-associated factors such as preadipocyte differentiation, insulin, and hypoxia. The study suggests that the all three factors are able to induce HIF-1α protein. HIF-1 function is inhibited by the corepresor of HDAC3-silencing mediator for retinoic and thyroid hormone receptors (SMRT).
MATERIALS AND METHODS
Reagents.
The 5× HRE (quintuple repeats of a hypoxia-responsive element) luciferase reporter and pLZRS-HIF-1α were described previously (30, 62). The 1,340-bp (−1,286 to +50) VEGF-A promoter-driven luciferase reporter plasmid was a gift from Dr. Alan Knox at the University of Nottingham, City Hospital, Nottingham, UK (4). Antibody to HIF-1α (NB 100/105R) was purchased from Novus Biologicals. Antibodies against glucose transporter 4 (Glut4; ab654), p-Akt Thr308 (ab38449), tubulin (ab7291), β-actin (ab6276), and HDAC3 (ab2379) were obtained from Abcam (Cambridge, UK). Antibody against HDAC1 (H6287) was obtained from Sigma (St. Louis, MO). Antibodies to insulin receptor (IR)α (sc-710), IRβ (sc-711), insulin receptor substrate-1 (IRS-1; sc-7200), Akt (sc-8312), GSK-3β (sc-7291), p-IRS-1 Y632 (sc-17196-R), p-GSK-3β (sc-11757-R), and specific protein 3 (sc-644X) were obtained from the Santa Cruz Biotechnology (Santa Cruz, CA). RNAi expression vectors for SMRT, N-CoR, HDAC1, HDAC2, and HDAC3 were kindly provided by Drs. M. A. Lazar and D. Bohmann (24, 56). Isobutylmethylxanthine (I5879), dexamethasone (D8893), human insulin solution (I9278), collagenase (C6885), and CoCl2 (60818) were obtained from Sigma. Chemical inhibitors for MEK (098059, P-215), and p38 MAPK (SB203580, S-8307) were also purchased from Sigma. Phosphatidylinositol 3-kinase (PI3K) inhibitor LY-294002 (ST-420), JNK inhibitor SP-600125 (EI-305), mammalian target of rapamycin (mTOR) inhibitor rapamycin (A-275), and PKC inhibitor calphostin C (EI-198) were from Biomol (Plymouth Meeting, PA). Actinomycin D was purchased (114666; Calbiochem, EMD Biosciences, La Jolla, CA).
Mice.
Male C57BL/6 mice (5 wk of age) were purchased from the Jackson Laboratory (Bar Harbor, ME) and were housed in groups of 4 mice/cage in the animal facility at the Pennington Biomedical Research Center with a 12:12-h light-dark cycle and constant temperature (22–24°C). The mice had free access to water and diet. Dietary obesity was induced by feeding the mice with HFD (58% kcal in fat, D12331; Research Diets, New Brunswick, NJ) at 5 wk of age. This HFD contains 35% dietary fat in weight that includes soybean oil (7%) and hydrogenated coconut oil (93%). The lean control mice were fed the regular chow diet (11% kcal in fat). Body weight and fat composition were monitored for obesity. All procedures were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center.
Plasma VEGF.
Blood (30 μl) was sampled from each mouse without anesthesia through the tail vein using heparinized microhematocrit capillary tubes. The plasma was prepared from the blood and stored at −80°C. The VEGF protein was examined using an ELISA kit for VEGF-A (QIA52–1EA lot no. D32367; Calbiochem). The assay sensitivity was 7 pg/ml. The plasma sample was diluted 1:5 in calibrator diluent RD5T in the assay.
Cells.
Cell lines, including 3T3-L1 and human embryonic kidney-293, were purchased from American Type Culture Collection (Manassas, VA) and maintained in cell culture according to the guidelines of American Type Culture Collection. The mouse 3T3-L1 preadipocytes (CL-173) were maintained in DMEM culture medium supplemented with 10% fetal calf serum and 4 mM glutamine. For adipogenesis, 3T3-L1 preadipocytes were grown into confluence in a six-well or 100-mm plate and then differentiated into adipocytes in the adipogenic cocktail (10 μg/ml insulin, 0.5 mM isobutylmethylxanthine, and 4 μg/ml dexamethasone) for 3 days. This was followed by incubation in insulin-supplemented medium for additional 4 days. The normal medium was used on day 8 to maintain the adipocytes.
Primary adipocytes.
Primary adipocytes were prepared from epididymal fat pads of lean C57BL/6 mice at 15 wk of age in sterile conditions, using a protocol reported elsewhere (32). After cervical dislocation, the fat pads were collected immediately from the mice, minced, and digested with collagenase (1 mg/ml, Sigma C6885) at 37°C for 60 min. The cell fraction on top of the medium was collected as primary cells of adipose tissue and used in the study. The cells were incubated in 35-mm dishes in DMEM with 10% FBS after being washed two times in DMEM. Twenty-four hours later, the cells were treated with 1% oxygen in a sealed chamber for 24 h and then examined for VEGF gene expression.
Transfection and luciferase assay.
Human embryonic kidney-293 cells were transfected with Lipofectamine (18324-020; Invitrogen) according to the manufacturer's instructions. Transient transfection in mature 3T3-L1 adipocytes was done with electroporation using the Nucleofector Kit V (VCA-1003) and an electroperator (Nucleofector II AAD-1001N, device no. 400414; Amaxa Biosystems, Cologne, Germany). Mature adipocytes (5 × 106) were suspended in 100 μl of Nucleofector Solution and then mixed with 2.0 μg of DNA reporter plasmid. SV40 Renilla luciferase reporter plasmid was used at 1.0 μg as an internal control. The appropriate program (T 030) was selected for the electroporation. After transfection, the cells were divided into 12-well plates and cultured for 24 h. A mean value and a standard error of the triplicate samples were used to express the reporter activity.
Hypoxia treatment.
The hypoxia condition was generated in a sealed chamber, in which air was replaced with a gas of 1% O2, 5% CO2, and 94% N2. The cells were maintained in serum-free medium for 8 h before and during the hypoxia treatment to avoid insulin effect in the serum. The chamber was kept in a water bath of 37°C to maintain the temperature. The humidity was generated by water in the chamber. The control cells were under normoxia conditions (CO2 incubator) in a humidified 37°C incubator with 21% O2 and 5% CO2.
Quantitative real-time RT-PCR.
The mRNA levels of VEGF-A (Mm_00437304_m1), HIF-1α (Mm_00468869_m1), and HIF-1β (Mm_00507836_m1) were determined using TaqMan probes with the 7900 HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The total RNA was extracted using the TRIzol protocol (Sigma). Mouse ribosome 18S rRNA_s1 (without intron-exon junction) was used as a control for normalization of mRNA. The forward primer (5′-GGGAATCAGGGTTCGATTCC-3′), reverse primer (5′-CTGCCTTCCTTGGATGTGGTA-3′), and probe (5′-AGCCTGAGAAACGG-3′) were made for the ribosome 18S rRNA_s1 by Applied Biosystems. Each experiment was repeated at least three times.
Western blot.
The whole cell lysate, nuclear extracts, and cytoplasmic extracts were made as described elsewhere (17, 59). Western blotting was conducted according to protocols used previously (16). The detection system contains horseradish peroxidase-conjugated secondary antibodies (catalog no. NA934V or NA931; Amersham Biosciences) and chemiluminescence reagent (catalog no. NEL-105; PerkinElmer Life Sciences) for generation of the chemiluminescent signal.
Chromatin immunoprecipitation assay.
Differentiated 3T3-L1 adipocytes were cultured in a 100-mm cell culture plate and treated with hypoxia. The cells were treated with formaldehyde and collected for extraction of chromatin. The chromatin immunoprecipitation (ChIP) assay was performed as described elsewhere (16). The chromatin DNA was broken into fragments at 400–1,200 bp in length and immunoprecipitated with ChIP antibodies to HIF-1α (ab-1; Abcam). IgG was used as a control in immunoprecipitate for the nonspecific signal. The DNA in the immunoprecipitate product was quantified in qRT-PCR. The PCR primers were designed to cover the HIF-1 binding site (−975/−968) in the mouse VEGF gene promoter (37): forward, 5-CGAGGGTTGGCGGCAGGAC-3; reverse, 5-CAGTGGCGGGGAGTGAGACG-3. The relative signal strength was used to indicate interaction of HIF-1α and HDAC3 with the VEGF promoter DNA.
Statistical analysis.
Each experiment was conducted at least three times. Western blot was quantified with the Image J program, and the representative blots are presented. A mean value ± SE of three independent experiments is presented for the reporter assay, ChIP assay, and mRNA assay. The data were analyzed using Student's t-test, with significance at P < 0.05.
RESULTS
HIF-1α and VEGF increased in obese mice.
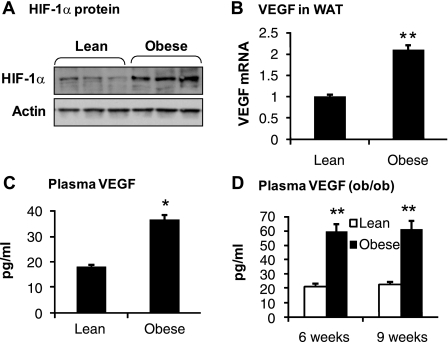
In an earlier study, we observed that HIF-1α protein is elevated in adipose tissue of obese mice (60). In an effort to characterize the HIF-1α activity, we repeated the experiment. HIF-1α protein was determined in the epididymal fat tissue homogenization in a Western blot. The obese mice exhibited a fourfold increase in HIF-1α protein (P < 0.001; Fig. 1A). The protein is associated with a 100% increase in VEGF mRNA in the adipose tissue (Fig. 1B), suggesting enhanced function of HIF-1 in the adipose tissue. The plasma VEGF protein was measured using an ELISA assay. The VEGF protein was elevated in both dietary obese mice (C57BL/6J) and genetically obese mice (ob/ob in C57BL/6J) (Fig. 1, C and D). The data suggest that the HIF-1α protein level may account for the VEGF expression in adipose tissue in the obese condition. Our data support that adipocyte VEGF expression is associated with the plasma VEGF elevation in obesity (33, 49).
Fig. 1.
Hypoxia-inducible factor-1 (HIF-1) function indicated by plasma vascular endothelial growth factor (VEGF) in obese mice. A: HIF-1α protein in the adipose tissue. Tissue homogenizer was made from epididymal fat of dietary obese mice and examined for HIF-1α protein in a Western blot. B: VEGF mRNA in white adipose tissue (WAT) of dietary obese mice. mRNA was quantified with quantitative real-time PCR (qRT-PCR) in epididymal fat of mice on high-fat diet (HFD) for 12 wk. C: plasma VEGF protein in dietary obese mice. The protein was determined using an ELISA assay in samples collected from mice on HFD at 12 wk (n = 10). D: Plasma VEGF in ob/ob mice. The test was conducted at 6 and 9 wk of age (n = 7). In the bar graph, each data point represents means ± SE. *P < 0.05, **P < 0.001 (compared with control).
Induction of HIF-1α activity by adipocyte differentiation.
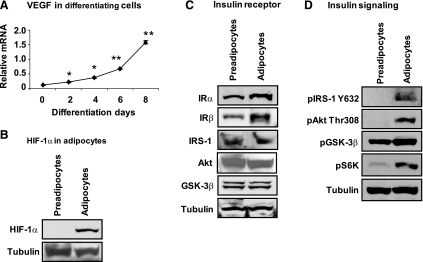
Although the upstream events of HIF-1 activation have been studied extensively in many types of cells, the work had not been done in adipocytes (26, 46). In some studies, HIF-1α was tested in adipocytes for hypoxia-induced gene expression (53, 54, 60). There is little information about nonhypoxia signal in the regulation of HIF-1α in adipocytes. To address this issue, we examined the impact of preadipocyte differentiation on HIF-1α activity. In 3T3-L1 cells, VEGF expression was used to monitor function of HIF-1α during differentiation. VEGF mRNA rose from the beginning of differentiation to reach its highest level at the end of the differentiation period (day 8; Fig. 2A). As a VEGF activator, HIF-1α protein was elevated 10-fold in the differentiated cells (P < 0.001; Fig. 2B).
Fig. 2.
HIF-1α regulation by cell differentiation. A: VEGF mRNA during adipogenesis of 3T3-L1. Total mRNA was prepared from cells collected at times as indicated and quantified for VEGF mRNA by qRT-PCR. B: HIF-1α protein in the whole cell lysate of differentiated 3T3-L1 cells. The protein was determined in a Western blot. C: proteins in the insulin-signaling pathway. The proteins were determined in the whole cell lysate of differentiated 3T3-L1 cells in a Western blot. D: phosphorylation status of the signaling proteins. Phosphospecific antibodies were used in the assay of whole cell lysate, as described in materials and methods. In the chart, each data point represents means ± SE (n = 3). *P < 0.05, **P < 0.001 (compared with control). IRα and -β, insulin receptor-α and -β, respectively; IRS-1, insulin receptor substrate-1.
To understand the mechanism underlying the HIF-1α induction by adipogenesis, we examined the insulin-signaling pathway. It was reported that HIF-1α is induced by insulin in several cell types (51, 63), but the adipocyte was not examined in those studies. HIF-1α protein reflects a balance between synthesis and degradation. HIF-1α protein synthesis is induced by PI3K in the insulin-signaling pathway. PI3K is activated by many other hormones/growth factors. Insulin is the most relevant hormone in the adipocyte differentiation model, in which insulin is required for adipogenesis. In the insulin-signaling pathway, several molecules were examined for their protein level and phosphorylation status, including insulin receptors (IRα and IRβ), IRS-1, Akt (PKB), and GSK-3β. In the differentiated adipocytes, IR is higher in both subunits (Fig. 2C). Proteins for IRS-1, Akt, and GSK-3β were not changed significantly (Fig. 2C). Phosphorylation status of the proteins was examined for the activity of the insulin-signaling pathway. An increase in phosphorylation was observed for IRS-1, Akt, GSK-3β, and S6K (Fig. 2D). The data suggest that insulin signal may be required for HIF-1α induction during adipocyte differentiation.
Elevation of HIF-1α by insulin.
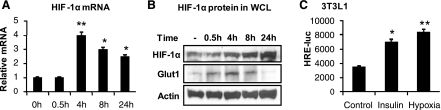
To test the role of insulin, we examined HIF-1α during insulin treatment of differentiated adipocytes. In the time course study, insulin induced HIF-1α mRNA and protein in a time-dependent manner, with peak expression at 4 h (Fig. 3, A and B). mRNA reached the peak at 4 h, and exhibited a reduction thereafter with time (Fig. 3A). The protein peaked at 4 h, and the level remained for 24 h (Fig. 3B). Glut1 is a HIF-1 target gene. Its protein expression was enhanced by hypoxia (Fig. 3B). The transcriptional activity of HIF-1 was further tested using a luciferase reporter construct whose activation is under the control of hypoxia response element (HRE). In the transient transfection assay, insulin induced the reporter activity in a way that is comparable with hypoxia (Fig. 3C), suggesting that HIF-1α mediates insulin signal to activate expression a hypoxia response gene.
Fig. 3.
HIF-1α regulation by insulin in differentiated 3T3-L1 cells. A: HIF-1α mRNA in cells treated with insulin (100 nM). B: HIF-1α and glucose transporter 1 (Glut1) proteins in whole cell lysate of differentiated 3T3-L1 adipocytes treated with insulin. C: HIF-1 function in hypoxia response element-luciferase (HRE-luc) reporter. Luciferase assay was performed in 3T3-L1 adipocytes that were transiently transfected with the reporter and treated with insulin for 16 h. Hypoxia treatment is a positive control. In the bar graph, each data point represents means ± SE (n = 3). *P < 0.05, **P < 0.001 (compared with control).
HIF-1α elevation by hypoxia.
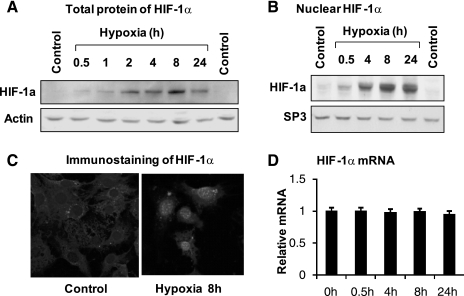
Insulin and hypoxia were compared in the stimulation of HIF-1α activity in adipocytes. To this point, HIF-1α was examined in response to hypoxia. HIF-1α protein and nuclear translocation were investigated in adipocytes. Total HIF-1α protein was increased in a time course study under hypoxia treatment. The protein increase exhibited at 0.5 h and reached a peak at 8 h (Fig. 4A). A similar pattern of increase was observed in the nuclear HIF-1α protein (Fig. 4B). The nuclear translocation of HIF-1α protein was investigated using immunofluorescent staining (Fig. 4C). Prior to hypoxia treatment, HIF-1α was located in the cytosol. Under the hypoxic condition, HIF-1α protein was detected in the nucleus. HIF-1α mRNA was examined at four time points between 0.5 and 24 h in adipocytes. We did not observe mRNA change for HIF-1α in adipocytes (Fig. 4D). These data suggest that hypoxia is able to induce total protein and nuclear translocation of HIF-1α in adipocytes in a time-dependent manner. The increase in HIF-1α protein is independent of mRNA level in response to hypoxia.
Fig. 4.
Regulation of HIF-1α by hypoxia. A: HIF-1α total protein. The protein was determined in the whole cell lysate in Western blot. Differentiated 3T3-L1 cells were treated with hypoxia (1% oxygen) for different times as indicated. B: nuclear HIF-1α protein. The nuclear extract was made from cells treated with hypoxia for different times and quantified for HIF-1α protein in a Western blot. Specific protein 3 (SP3) is a loading control. C: immunofluorescent staining of HIF-1α protein in 3T3-L1 adipocytes. D: mRNA of HIF-1α. The mRNA was determined in differentiated 3T3-L1 cells after hypoxia treatment. D: each data point represents means ± SE (n = 3).
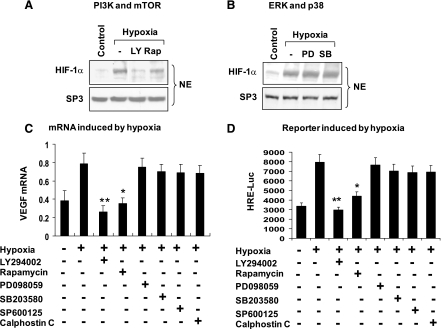
PI3K and mTOR in hypoxia-induced HIF-1 activity.
The HIF-1α protein is regulated by several serine kinases, including PI3K (3), mTOR (3), and ERK (43). To investigate these kinases in the HIF-1α regulation by hypoxia in adipocytes, we inhibited the kinases individually with chemical inhibitors. The hypoxia effect was significantly reduced by pretreatment of 3T3-L1 adipocytes with inhibitors to PI3K (LY-294002) or mTOR (rapamycin) (Fig. 5A). The inhibitors to MEK (PD-098059) and p38 (SB-203580) were also tested. They did not show any effect on hypoxia-induced HIF-1α (Fig. 5B). The HIF-1α inhibition was associated with suppression of the transcriptional activity of HIF-1 (Fig. 5, C and D). VEGF-Luc and HRE-Luc reporters were used in the functional assay for HIF-1α. In response to the PI3K and mTOR inhibitors, their activities were significantly reduced. In the reporter assay, inhibitors to JNK (SP-600125) and PKC (calphostin C) were tested together with those for MEK and p38. All of them failed to block the HIF-1 activities (Fig. 5, C and D). These data suggest that the PI3K-Akt-mTOR pathway is required for the hypoxia-induced HIF-1α activity in adipocytes.
Fig. 5.
Signaling for HIF-1α elevation. A: phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) in the control of HIF-1α activity. Differentiated 3T3-L1 cells were serum starved and pretreated with LY-294002 (LY) and rapamycin (Rap) for 0.5 h and then treated with hypoxia for 8 h. The HIF-1α protein was examined in the nuclear extract in a Western blot. B: ERK and p38. The nuclear HIF-1α was determined in differentiated 3T3-L1 cells treated with PD-098059 (PD) or SB-203580 (SB) for 0.5 h. C: inhibition of HIF-1 function by kinase inhibitors. Differentiated 3T3-L1 cells were pretreated with kinase inhibitors such as LY (PI3K), Rap (mTOR), PD (MEK/ERK), SB (p38), SP-600125 (JNK), and calphostin C (PKC) for 0.5 h, followed by hypoxia treatment. VEGF mRNA was examined after hypoxia treatment for 8 h. D: HRE-luc assay. The HRE-luc reporter was transfected into 293 cells and induced with hypoxia after the inhibitor treatment for 0.5 h. The luciferase activity was measured after hypoxia treatment for 8 h. In the bar graph, each data point represents means ± SE. *P < 0.05, **P < 0.001 (n = 3). NE, nuclear extract.
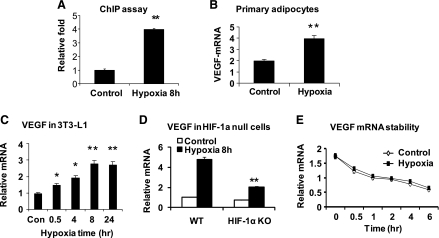
Enhanced transcriptional activity of HIF-1α.
To determine interaction of HIF-1 with target gene in adipocytes, we examined HIF-1 in the VEGF gene promoter. The protein-DNA interaction was examined at the proximal HRE using the ChIP assay. In response to hypoxia, the interaction was enhanced in adipocytes (Fig. 6A). The interaction was associated with VEGF mRNA expression in response to hypoxia (Fig. 6, B and C), which was observed in both primary adipocytes and 3T3-L1 adipocytes. The role of HIF-1α in VEGF transcription was tested in HIF-1α-null cells. In the wild-type mouse embryonic fibroblasts (MEFs), VEGF mRNA was elevated fourfold under hypoxia (Fig. 6D). In the HIF-1α-knockout MEFs, the increase in VEGF mRNA was reduced to onefold under the same condition, suggesting that the HIF-1α is required for a full response of VEGF gene to hypoxia. To test the role of gene transcription in the mRNA induction, we examined VEGF mRNA stability, in which the control and hypoxia-treated cells were compared using the actinomycin D protocol. The mRNA half-life was ∼4 h in both conditions (Fig. 6D), suggesting no change in mRNA stability under hypoxia. These data support a role of HIF-1α-mediated VEGF transcription in the VEGF protein expression in adipocytes.
Fig. 6.
HIF-1α in transcriptional expression of VEGF. A: chromatin immunoprecipitation (ChIP) assay for HIF-1α in the VEGF gene promoter. The assay was conducted in differentiated 3T3-L1 adipocytes after hypoxia treatment for 8 h. B: hypoxia induction of VEGF mRNA in primary adipocytes. C: VEGF induction in 3T3-L1 adipocytes. D: VEGF mRNA in HIF-1α-knockout (KO) cells. mouse embryonic fibroblasts from HIF-1α-KO mice were treated with hypoxia for 8 h in the experiment. E: VEGF mRNA stability in differentiated 3T3-L1 adipocytes. In the bar graph, each data point represents means ± SE. *P < 0.05, **P < 0.001 (n = 3–5). WT, wild type.
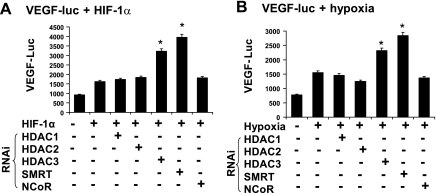
HIF-1α activity was inhibited by HDAC3 and SMRT.
In addition to the protein abundance, the transactivation function of HIF-1 is also regulated by HDAC (31). However, it was not known which isoform of HDAC acts in adipocytes (25, 29, 41). In this study, we explored the role of three HDACs (HDAC1, HDAC2, and HDAC3) in the regulation of HIF-1 function. The study was conducted by knocking down HDACs in cells through transient transfection of RNAi expression vectors. The HIF-1α transcriptional activity was monitored using the VEGF luciferase reporter. Knockdown of HDAC3 led to a significant induction in the transcriptional activity of HIF-1, as shown by the elevated reporter activity (Fig. 7, A and B). Knockdown of either HDAC1 or HDAC2 had no significant effect. The efficacy of HDAC knockdown was confirmed in Western blot. Our results suggest that HDAC3 is the major isoform in the inhibition of HIF-1 in adipocytes.
Fig. 7.
Inhibition of HIF-1α function by histone deacetylase (HDAC)3 and silencing mediator for retinoic and thyroid hormone receptors (SMRT). Functions of HDAC1, HDAC2, HDAC3, SMRT, and nuclear corepressor (NCoR) in the regulation of HIF-1 activity were examined using VEGF-luc reporter in the 293 cells. The corepressor proteins were knocked down with RNAi that was expressed from plasmid vectors cotransfected. The reporter activity was induced by either HIF-1α overexpression or hypoxia treatment. After transfection for 24 h, the cells were treated with hypoxia for 8 h in serum-free medium. A: VEGF-luc activity induced by HIF-1α expression. B: VEGF-luc activity induced by hypoxia. In the bar graph, each data point represents means ± SE. *P < 0.05 (n = 3).
SMRT and nuclear corepressor (NCoR) are components in the HDAC3-containing corepressor complex. Their activities were examined using the RNAi knockdown system. Whereas knockdown of SMRT induced the HIF-1 activity (Fig. 7, A and B), knockdown of NCoR had minimal impact on the HIF-1 activity. These data suggests that SMRT is a component of the corepressor complex for HIF-1. The efficacy of SMRT or NCoR knockdown was confirmed in Western blot (data not shown).
DISCUSSION
In the current study, the upstream signals that regulate HIF-1α in adipocytes were investigated, with a focus on obesity-associated factors such as cell differentiation, insulin, and hypoxia. HIF-1α activity is enhanced in mRNA and protein in adipose tissue in obesity. To understand the mechanism underlying the HIF-1α change, we tested the three obesity-associated factors. All of them were able to enhance HIF-1α protein expression, but only two of them (adipogenesis and insulin) were able to increase HIF-1α mRNA. Hypoxia did not induce HIF-1α mRNA in adipocytes. The increase in HIF-1α protein is likely a result of inhibition of protein degradation since the mRNA change occurs after the protein. Our data confirm that adipogenesis increases HIF-1α mRNA and protein in the mouse preadipocyte 3T3-L1, as reported by Floyd et al. (14), but is opposite of the observation in human preadipocytes by Wang et al. (53). This discrepancy might be related to differences in species (mouse vs. human) or experimental conditions. In the search for the mechanism of HIF-1α expression during adipogenesis, we found that expression of insulin receptor was enhanced by adipogenesis. The insulin receptor makes adipocytes to react actively to insulin with HIF-1α mRNA and protein expression. This effect of the insulin-signaling pathway is demonstrated in mature adipocytes. IGF (insulin-like growth factor) may stimulate the HIF-1α activity as well since it activates the insulin receptor. Given that both adipogenesis and insulin levels are elevated in obesity, the two factors should contribute to the HIF-1α upregulation in adipose tissue. Hypoxia exists in adipose tissue in obesity (60, 61) and contributes to the HIF-1α activity in adipocytes (53, 60), but hypoxia only stimulates the HIF-1α protein elevation in adipocytes. In addition to the three factors above, inflammation signal may induce HIF-1α expression in obesity. Activation of the IKK2-NF-κB pathway by TNFα leads to HIF-1α mRNA expression (44). These lines of evidence suggest that HIF-1α is not an exclusive biomarker for hypoxia in adipose tissue under obesity. To demonstrate hypoxia in adipose tissue in obesity, other parameters such as interstitial oxygen pressure, the chemical hypoxia probe (pimonidazole HCl), or lactate level are required (23, 60). Hypoxia induces lactate production through inhibition of mitochondria (61) and induction of monocarboxylate transporter 1 expression (39).
We tested several kinases that are reported to regulate HIF-1α. Our results suggest that the PI3K-Akt pathway is required by insulin and hypoxia in the stimulation of HIF-1α activities. The kinase inhibitors to PI3K and mTOR blocked HIF-1α protein activities in cytoplasm and nucleus in adipocytes. ERK is reported to enhance HIF-1 activity in nonadipocytes (43). However, this ERK activity is not supported by our data.
We examined HIF-1α downstream events by investigating VEGF gene promoter in adipocytes. VEGF secretion from adipocytes is associated with the plasma VEGF elevation in overweight or obese subjects (20, 33–35, 49). However, the VEGF production had not been comprehensively examined in adipocytes. The current study suggests that in obesity there are at least three factors to stimulate VEGF transcription in adipocytes. The first is adipogenesis. Adipocyte differentiation increases VEGF expression, which is consistent with the report by Claffey et al. (8). In obesity, adipogenesis is accelerated in response to quick turnover rate of adipocytes in adipose tissue (22). The adipogenesis will lead to more VEGF expression through HIF-1α. The second is insulin stimulation. Obesity leads to hyperinsulinemia from insulin resistance. We show that insulin induces HIF-1α expression in mRNA and protein in adipocytes, which is translated into the VEGF transcription. The third is hypoxia response. In adipocytes, HIF-1α activity is increased by hypoxia for VEGF expression, which is consistent with other reports (38, 42, 60). VEGF expression is also enhanced by inflammation (TNFα) (52) and sympathetic nerve signal (57). In obesity, all of these factors may contribute to VEGF production by adipose tissue. Our data show that stability of VEGF mRNA is not changed by hypoxia in adipocytes.
Our data suggest that HIF-1α is an activator of VEGF gene, but HIF-1α is not sufficient to induce VEGF expression in adipocytes in obesity. In an earlier study, we observed that VEGF mRNA was increased in epididymal fat of diet-induced obese mice but not ob/ob mice, although HIF-1α activity was enhanced in both models (60). This observation raised a question about role of HIF-1α in the control of VEGF expression in adipocytes. Here, we show that HIF-1α interacts with the VEGF gene promoter DNA in the ChIP assay, and VEGF expression is reduced significantly in HIF-1α-null cells. These data suggest that HIF-1α is a major transcriptional activator of VEGF gene in adipocytes. However, in ob/ob mice, this HIF-1α activity is not sufficient to induce VEGF expression. There may be an increase in VEGF expression in subcutaneous fat in ob/ob mice, which leads to VEGF elevation in plasma. VEGF transcription is controlled by several other transcription factors in addition to HIF-1α, such as STAT3 (36, 55), peroxisome proliferator-activated receptor (PPAR)γ coactivator-1α (PGC-1α) (1), and PPARγ (12). STAT3 is activated by leptin, which is absent in ob/ob mice. STAT3 activity may be decreased in ob/ob mice from leptin deficiency. This condition may prevent VEGF expression in response to HIF-1α. PGC-1α activity is enhanced by sympathetic nerve signal (cAMP) and inflammation (TNFα) (40, 57), both of which stimulate VEGF expression.
Our result suggests that HDAC3 and SMRT form a corepressor for HIF-1α in adipocytes, which was not found in the literature. In nonadipocytes, HIF-1α activity is regulated by HDACs, and HDAC isoforms in the regulation of HIF-1α are highly controversial (25, 29, 41). HDAC3, one of HDAC isoforms, belongs to the class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8) (10, 48). It was reported that HDAC1 suppresses the transcriptional activation of HIF-1α since HDAC1 expression was enhanced by hypoxia (28). HDAC1-3 was reported to inhibit the HIF-1α activity through an interaction with von Hippel-Lindau (31). Class II HDACs (HDAC4, HDAC6, and HDAC7) were reported to enhance the transcriptional activity of HIF-1α through a direct protein association (25, 41). The class II isoforms include HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10. In the current study, the activity of HDAC1-3 was examined using an RNAi-based gene knockdown strategy. The data suggest that in adipocytes HDAC3 is the primary isoform of HDACs in the inhibition of HIF-1 function, whereas HDAC1 and HDAC2 do not play a major role.
SMRT and NCoR are two different corepressor components that do not have any enzymatic activity. They act through triggering the catalytic activities of HDACs in the corepressor complex (18). Our data suggest that SMRT, but not NCoR, interacts with HDAC3 in the suppression of HIF-1 activity in adipocytes.
In summary, HIF-1α is increased in both mRNA and protein in adipose tissue in obesity. There are at least three factors to stimulate HIF-1α activity in obesity: adipogenesis, insulin, and hypoxia. In adipocytes, all of the three factors enhance HIF-1α protein. The PI3K-Akt pathway is required for HIF-1α activation by those factors. HIF-1α is a major transcriptional activator for VEGF gene, but it is not sufficient for activation of VEGF gene expression in adipose tissue. In adipocytes, the transcriptional activity of HIF-1α is inhibited by the corepressor composed of HDAC3 and SMRT. In adipose tissue, the increase in HIF-1α mRNA is likely a result of adipogenesis and hyperinsulinemia in obesity but not adipose tissue hypoxia.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-068036 and DK-085495 to J. Ye.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We appreciate critical comments and manuscript editing by Dr. Jeff Gimble. We are grateful to Dr. Alan Knox at the University of Nottingham, City Hospital, Nottingham, UK, for the gift of VEGF-luciferase vector.
REFERENCES
- 1. Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA 93: 12969–12973, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell 8: 443–454, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bradbury D, Clarke D, Seedhouse C, Corbett L, Stocks J, Knox A. Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J Biol Chem 280: 29993–30000, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res 94: 1579–1588, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov 9: 107–115, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol 318: 2–9, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Claffey KP, Wilkison WO, Spiegelman BM. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem 267: 16317–16322, 1992 [PubMed] [Google Scholar]
- 9. Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 4: 211–232, 1997 [DOI] [PubMed] [Google Scholar]
- 10. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J 18: 1905–1914, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emoto M, Anno T, Sato Y, Tanabe K, Okuya S, Tanizawa Y, Matsutani A, Oka Y. Troglitazone treatment increases plasma vascular endothelial growth factor in diabetic patients and its mRNA in 3T3-L1 adipocytes. Diabetes 50: 1166–1170, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Fath DM, Kong X, Liang D, Lin Z, Chou A, Jiang Y, Fang J, Caro J, Sang N. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-alpha. J Biol Chem 281: 13612–13619, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Floyd ZE, Kilroy G, Wu X, Gimble JM. Effects of prolyl hydroxylase inhibitors on adipogenesis and hypoxia inducible factor 1 alpha levels under normoxic conditions. J Cell Biochem 101: 1545–1557, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93: e88–e97, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao Z, Chiao P, Zhang X, Zhang X, Lazar MA, Seto E, Young HA, Ye J. Coactivators and corepressors of NF-kB in IkBalpha gene promoter. J Biol Chem 280: 21091–21098, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 (IRS-1) by inhibitor KappaB kinase (IKK) complex. J Biol Chem 277: 48115–48121, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Guenther MG, Barak O, Lazar MA. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21: 6091–6101, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hattori K, Sumi T, Yasui T, Morimura M, Nobeyama H, Okamoto E, Noriyuki M, Honda K, Kiyama H, Ishiko O. VEGF mRNA in adipocytes increase with rebound weight-gain after diet-restriction. Int J Mol Med 13: 395–399, 2004 [PubMed] [Google Scholar]
- 21. Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci 82: 925–934, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab 5: 299–311, 1976 [DOI] [PubMed] [Google Scholar]
- 23. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Ishizuka T, Lazar MA. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23: 5122–5131, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem 279: 41966–41974, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 129: 465–472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim DH, Woods SC, Seeley RJ. A peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes 59: 907–915, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med 7: 437–443, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell 38: 864–878, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem 281: 30678–30683, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malide D, Ramm G, Cushman SW, Slot JW. Immunoelectron microscopic evidence that GLUT4 translocation explains the stimulation of glucose transport in isolated rat white adipose cells. J Cell Sci 113: 4203–4210, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia 46: 1483–1488, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y. Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am J Physiol Endocrinol Metab 288: E1128–E1136, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Morimura M, Ishiko O, Sumi T, Yoshida H, Ogita S. Angiogenesis in adipose tissues and skeletal muscles with rebound weight-gain after diet-restriction in rabbits. Int J Mol Med 8: 499–503, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21: 2000–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Pages G, Pouyssegur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene—a concert of activating factors. Cardiovasc Res 65: 564–573, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58: 718–725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perez de Heredia F, Wood IS, Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch 459: 509–518, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 8: 971–982, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, Pili R. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res 66: 8814–8821, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem 274: 32631–32637, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453: 807–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 99: 10730–10735, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 64: 993–998, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev 14: 1983–1991, 2000 [PubMed] [Google Scholar]
- 48. Sengupta N, Seto E. Regulation of histone deacetylase activities. J Cell Biochem 93: 57–67, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 29: 1308–1314, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 277: 27975–27981, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Wang B, Trayhurn P. Acute and prolonged effects of TNF-alpha on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflugers Arch 452: 418–427, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455: 479–492, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol 198: 127–134, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22: 319–329, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Weiss C, Schneider S, Wagner EF, Zhang X, Seto E, Bohmann D. JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. EMBO J 22: 3686–3695, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, Cannon B, Cao Y. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 9: 99–109, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33: 54–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ye J, Cippitelli M, Dorman L, Ortaldo JR, Young HA. The nuclear factor YY1 suppresses the human gamma interferon promoter through two mechanisms: inhibition of AP1 binding and activation of a silencer element. Mol Cell Biol 16: 4744–4753, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Yin J, Gao Z, He Q, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab 296: E333–E342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol 25: 3040–3055, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17: 5085–5094, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res 67: 147–154, 1997 [DOI] [PubMed] [Google Scholar]