Abstract
We evaluated heat shock protein 70 (HSP70) changes in diabetes mellitus (DM) in a nonhuman primate model. To this end, two studies were conducted in DM vervet monkeys. 1) Normal control and streptozotocin-induced DM monkeys (Stz-DM) that were differentiated into moderately or poorly controlled DM by judicious insulin administration were evaluated. Liver was collected at 4, 8, 12, 16, and 20 wk after streptozotocin, exposed to ex vivo heat shock at 42°C, and immunoblotted for heat shock factor 1 (HSF1), HSP70, and phosphorylated HSF1. 2) Spontaneous DM monkeys that were not pharmacologically induced were included in a crossover study of the HSP70-inducing drug geranylgeranylacetone (GGA). GGA at 20 mg/kg was given for 14 days with a 6-wk washout period. Glucose tolerance testing and plasma and muscle HSP70 were the primary outcome measurements. In Stz-DM, hyperglycemia reduced hepatic HSP70 in a dose-dependent fashion. HSF1 was increased in livers of monkeys with Stz-DM, but responses to ex vivo heat shock were impaired vs. normal monkeys. Activation of HSF1 appears to be important, because the phosphorylation change with heat stress was nearly perfectly correlated with HSP70 increases. Impaired HSF1 activation was also seen in Stz-DM after chronic hyperglycemia (>12 wk). In naturally occurring DM, increased circulating HSP70 resulted in significantly improved glucose tolerance and significant, positive trends in other measurements of insulin resistance. No change in muscle HSP70 content was observed. We conclude that increasing HSP70, potentially through targeting hyperglycemia-related deficits in HSF1 induction and activation in the liver, is a potent and viable strategy to improve glucose tolerance.
Keywords: heat shock protein 70, chaperone proteins, geranylgeranylacetone, nonhuman primates, diabetes mellitus
heat shock proteins (HSPs) are the largest family of transcriptionally regulated chaperone proteins that respond to cellular stress. HSPs aid in repair of protein damage and survival of normal cellular functions. Heat shock factor 1 (HSF1) mediates most transcriptional activation by binding to heat shock elements in the promoter region of the HSP genes, with resultant transcription of HSP70 mRNA being most abundant. HSF1 undergoes modifications such as trimerization, phosphorylation, and acetylation as well as product feedback inhibition to modulate the activation-attenuation cycle (1). Induction of HSPs not only protects de novo proteins being translated through the endoplasmic reticulum but also protects oxidized proteins and upregulates intracellular antioxidant mechanisms, which together minimize the chronic inflammatory state associated with insulin resistance (21, 37).
Insulin-resistant and hyperglycemic people have reduced HSP70 protein and gene expression (7, 9, 29). Notably, new studies utilizing chemical chaperones show that insulin sensitivity may be restored in obese rodent models by limiting cellular stress in some tissues (39), and insulin signaling can be improved in obese people (24). Even more compelling is recent work (9) reporting that elevation of HSPs by various methods such as heat, pharmacological induction, and muscle overexpression prevented obesity-associated insulin resistance in rodents. In vitro systems and in vivo rodent models have provided important insights into the role of chaperone proteins in insulin resistance (9, 39), but this work has only just begun to be translated to human or nonhuman primates. An interesting pilot study in type 2 diabetic mellitus (DM) patients reported that clinically significant reductions in glycosylation of hemoglobin A1c (A1c%) were achieved with heat therapy (20). Although this study was uncontrolled and exploratory in nature, it documented a mean increase in body temperature of 0.8°C, sufficient for HSP induction (9, 23, 37). Furthermore, dosing for 4 wk with a HSP co-inducer in people improved glucose uptake by tissues; however, HSPs were not measured (31).
Our laboratory has reported significantly reduced expression of HSF1/HSPs in liver and reduced plasma HSP70 levels from nonhuman primates with type 2 DM (26). Induction of HSF1/HSPs is currently considered an exciting therapeutic approach to diabetes and metabolic syndrome disorders, since increasing the cellular stress response may reduce the low-grade inflammation associated with excess weight and insulin resistance (33).
In these studies, we extend our earlier findings that tissue and plasma HSP70 are lower in spontaneously diabetic monkeys by evaluating how the degree and duration of hyperglycemia affect basal and heat shock-induced expression of HSF1 and HSP70. Furthermore, we aim to demonstrate the importance of HSP70 by showing that improvement of HSP70 levels in spontaneously diabetic monkeys positively affects glucose metabolism and insulin resistance.
MATERIALS AND METHODS
Animal experiments.
All experimental procedures involving animals in this study were approved by and complied with the guidelines of the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences. All blood collections were done under sedation using ketamine hydrochloride 10–15 mg/kg im after an 18-h fast.
Study 1: experimentally induced hyperglycemia and effects on hepatic HSP70.
Fourteen mature male vervet monkeys (Chlorocebus aethiops), ranging from 6 to 12 yr of age (mean 7.1 ± 0.3 yr) were included in this study (Table 1) that has been described previously (44). Monkeys were stratified at baseline into three treatment groups on the basis of body weight, glycemic control (Hb A1c), and rate of glucose disposal derived from baseline glucose tolerance testing (data not shown). Study groups were control (n = 5), where monkeys maintained normal glycemic control, streptozotocin-induced DM (Stz-DM) moderate glycemic control (n = 4), and Stz-DM poor glycemic control (n = 5), wherein blood glucose after induction of DM was maintained under moderately good (postprandial plasma glucose ∼150 mg/dl or less) or poor control (postprandial glucose 250–300 mg/dl). All animals were fed a standard monkey chow diet (PMI Nutrition International, Brentwood, MO).
Table 1.
Study 1. Streptozotocin-induced hyperglycemia in vervet monkeys
| Control (n = 5) | Moderate Control (n = 4) | Poor Control (n = 5) | ANOVA (P Value) | |
|---|---|---|---|---|
| Age, yr | 8.6 ± 0.4 | 10.2 ± 1.1 | 7.4 ± 0.4 | 0.04 |
| Body weight, kg | 7.4 ± 0.4 | 6.6 ± 0.4 | 6.7 ± 0.4 | 0.17 |
| Fasting glucose, mg/dl | 49 ± 4a | 601 ± 105b | 473 ± 74b | <0.0001 |
| Fasting insulin, μIU/ml | 7.3 ± 2.1 | 2.5 ± 1 | 3.4 ± 0.9 | 0.06 |
| HOMA index, AU | 0.94 ± 0.31 | 3.6 ± 1.8 | 3.5 ± 0.83 | 0.11 |
| Fasting C-peptide, ng/ml | 36 ± 15 | 11.6 ± 7.9 | 12.5 ± 6.5 | 0.16 |
| Fructosamine, mEq/l | 142 ± 9.01a | 388 ± 33.5b | 507 ± 14.4c | <0.0001 |
| Total plasma cholesterol, mg/dl | 146 ± 13.5a | 330 ± 15.1b | 225 ± 13.5c | 0.0005 |
| Plasma triglycerides, mg/dl | 48 ± 13a | 206 ± 5b | 174 ± 52ab | 0.03 |
| Hepatic triglyceride, mg/mg protein | 18.31 ± 5.77a | 230 ± 91b | 42.18 ± 14.8c | 0.006 |
| Hepatic free cholesterol, mg/mg protein | 10.46 ± 0.59 | 10.31 ± 0.66 | 9.19 ± 0.59 | 0.29 |
Values are means ± SE. HOMA, homeostasis model assessment; AU, arbitrary units. Overall group differences at study's end are indicated by ANOVA P values, with post hoc results of specific intergroup differences indicated by different superscripted letters. P < 0.05 was considered significant.
Diabetes was induced by intravenous infusion of 55 mg/kg streptozotocin (Zanosar; SICOR Pharmaceuticals, Irvine, CA) as 100 mg/ml in normal saline. Control animals received an equivalent dose volume of saline. Hyperglycemia was confirmed in all Stz-DM monkeys within 48 h by measuring glucose concentrations from blood samples obtained by tail sticks in conscious monkeys (MediSense Precision Xtra glucometer; Abbott Laboratories, Abbott Park, IL). Insulin therapy was initiated as twice daily insulin injections [70% intermediate acting (neutral protamine Hagedorn), 30% short acting (regular insulin), Novolin 70/30; Novo Nordisk USA, Princeton, NJ]. Insulin doses were adjusted by small increments either up or down on the basis of whole blood glucose measurements determined via glucometer ∼3–4 h following insulin dosing and feeding (postprandial) at least twice weekly to achieve desired moderate (postprandial plasma glucose ∼150 mg/dl or less) or poor glycemic control (postprandial glucose 250–300 mg/dl) for the duration of the experiment.
Sample collections.
Body weight was recorded each week of study. Every 4 wk, all monkeys were sedated and blood samples collected. Stz-DM monkeys had insulin therapy withheld for 18 h before any blood sampling. Whole blood samples in EDTA-treated tubes were placed on ice immediately after collection, and plasma and whole blood were stored at −80°C until Hb A1c percentage was measured by HPLC (Primus PDQ; Primus Diagnostics, Kansas City, MO) and glucose by glucose oxidase colorimetric assay (Roche, Basel, Switzerland). Insulin concentrations were determined by ELISA (Mercodia, Uppsala, Sweden). Fructosamine concentrations were used as an additional indicator of average glycemic control (Roche), and C-peptide of endogenously produced insulin was used to additionally confirm pancreatic insufficiency (Mercodia).
Animals were euthanized after defined study periods by overdose of intravenous pentobarbital sodium. Two to four animals were chosen at 4, 8, 12, 16, and 20 wk post-DM induction for study termination with collection of liver tissue for analysis.
Liver from each monkey was immediately minced and placed in two cell culture flasks that contained modified Krebs-Henseleit buffer (Sigma-Aldrich, St. Louis, MO) that had been warmed using a temperature-controlled 37°C water bath. A 95:5% O2-CO2 gaseous mix was bubbled through each flask continuously until the experiment ended. Using conditions similar to those reported previously (17, 32), one sample was held at 37°C for 4 h, and the other was exposed to a 42°C water bath for 1 h before being transferred to 37°C for an additional 3 h (1999 no. 35; Gutsmann-Conrad). After 4 h, the liver tissue was blotted dry and frozen in liquid nitrogen prior to storage at −80°C until analysis.
Hepatic total protein was extracted for immunoblotting, as described previously (26). Briefly, tissue was homogenized in protein extraction and lysis buffer (GBiosciences, St. Louis, MO) supplemented with EDTA, DTT, and protease inhibitor cocktail (Sigma-Aldrich) with a PT 2100 Polytron electric blender (Kinematica, Littau-Lucerne, Switzerland). The homogenate was centrifuged for 30 min at 14,000 g and the supernatant retained for protein analysis (BCA; Pierce Biotechnology, Rockford, IL) with electrophoresis of 45 μg on a polyacrylamide gel (Invitrogen, Carlsbad, CA). A heat-shocked HeLa cell lysate (StressGen; Assay Designs, Ann Arbor, MI) was included as a positive control. Proteins were then transferred to a nitrocellulose membrane (Whatman, Sanford, ME) and blocked in 5% dry milk overnight. The membranes were probed with antibodies to HSF1, HSP70 (StressGen), phosphorylated HSF1 [HSF1p; at serine 326 (Abcam, Cambridge, MA)], and β-actin (Oncogene, Cambridge, MA) and appropriate horseradish peroxidase-conjugated secondary antibodies before detection by chemiluminescence (ECL Plus; GE Healthcare, Giles, UK). Membranes were scanned with a STORM 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA) and analyzed using ImageQuant 5.2 software (Molecular Dynamics).
Plasma lipids were enzymatically measured on an Alfa Wassermann ACE Alera Chemistry Analyzer (Alfa Wassermann, West Caldwell, NJ) with reagents supplied by the company. Hepatic lipids were extracted (14), with total and free cholesterol concentrations determined enzymatically (8). Triglyceride content was measured as described previously (30).
Study 2: pharmacological induction of HSP70 in spontaneously hyperglycemic monkeys.
Eight aged vervet monkeys [7 females, 1 male; average age 17 yr (SE = 2)] were identified at routine health screenings of the Vervet Research Colony (25) with fasting hyperglycemia [plasma glucose >110 mg/dl; spontaneous DM (Spont-DM)] on more than one occasion. Half of these monkeys required insulin to maintain body weight and avoid ketoacidosis, and insulin was administered to the monkeys as described above for moderate control. Their insulin doses were stabilized for ≥6 mo before study initiation and did not fluctuate during dosing periods by more than 10%.
Monkeys were randomized to treatment sequence for a placebo-controlled crossover study design (2 treatments and 2 dose periods, with a 6-wk washout period in between) utilizing geranylgeranylacetone (GGA; Selbex, Esai, Japan) to induce HSP70. The GGA was dosed as a liquid (98% pure) placed on an absorbent treat (Primatreat; Bio-Serv, Frenchtown, NJ) 20 min before administration. Placebo monkeys received a plain treat.
Monkeys were orally dosed with the drug at 20 mg/kg as a single daily dose for 14 days. Preliminary studies of this dose level in monkeys for 7 days had demonstrated increased HSP70 in muscle tissue (data not shown). In humans, the drug is dosed orally at 150 mg/day (or ∼2–3 mg/kg) with significant increases in tissue HSP70 concentrations (average elevation of 48%) detectable after 2 wk of dosing without adverse side effects (50). Higher doses have been used in animal studies (≤600 mg/kg), leading to greater induction (200–400%) of HSP70 (15). The mechanism of drug action is still unknown, but the drug target is thought to be HSP70 itself (41).
Blood sampling, muscle biopsy, blood pressure measurement, and a modified intravenous glucose tolerance test were performed after 2 wk, followed by a 6-wk washout period between treatment phases. For the intravenous glucose tolerance test, monkeys were sedated with ketamine as described above, intubated, and maintained on isoflurane anesthesia. A temporary peripheral catheter was placed for infusion of 500 mg/kg of 50% dextrose solution, and timed blood samples were collected through a vascular access port connected to an indwelling jugular vein catheter (placed more than 6 mo before study initiation). Samples were collected at 0, 2, 3, 4, 5, 8, 10, 12, 18, 20, 22, 24, 28, 30, 40, 50, and 60 min into EDTA-treated tubes and kept on ice until they were processed for plasma. Plasma was stored at −80°C until analysis of glucose and insulin concentrations at all time points and HSP70 concentrations at time 0 (StressGen). Results of the glucose tolerance testing [K values for glucose disappearance rate between 2 and 18 minutes were analyzed using MinMod Millenium software (University of Pittsburgh, Pittsburgh, PA) (6)]. The acute insulin response was calculated as the average insulin concentrations measured between 1 and 10 min minus the baseline value. Homeostasis model assessment (HOMA) index was calculated from the product of glucose (mmol/l) and insulin [(μUI/l)/22.5] and used as an indicator of insulin resistance (5). Muscle biopsies were collected from the biceps femoris, frozen in liquid nitrogen, and stored at −80°C until immublotting for HSP70 as described above.
Statistical analysis.
All results are reported as means ± SE. Statistical analyses were performed using Statistica 9 (StatSoft, Tulsa, OK). Log transformation of variables (baseline liver HSP70, plasma triglycerides in the pharmacological study) was performed when normality assumptions were not met. Intergroup comparisons were performed on all variables by one-way ANOVA in the streptozotocin-induced hyperglycemia study. When an overall significant group effect or group-by-time interaction was indicated by P < 0.05, Tukey's honestly significant differences test was used in post hoc testing to determine specific differences. The outcomes of the pharmacological study were assessed by paired t-tests after a two-way ANOVA (factors being treatment sequence and treatment group) indicated lack of sequence effect, so an assumption of no carryover effects was made. A P value of <0.05 was considered significant for all analysis, and a P value of <0.10 was determined to constitute a trend in value differences in study 2. Trends in study 1 were considered significant when individual end points had an association with the mean fructosamine value for each group. Correlational analyses were reported as r values for Pearson's correlation coefficients, with P < 0.05 indicating a significant association.
RESULTS
Study 1: experimentally induced hyperglycemia effects on HSP70.
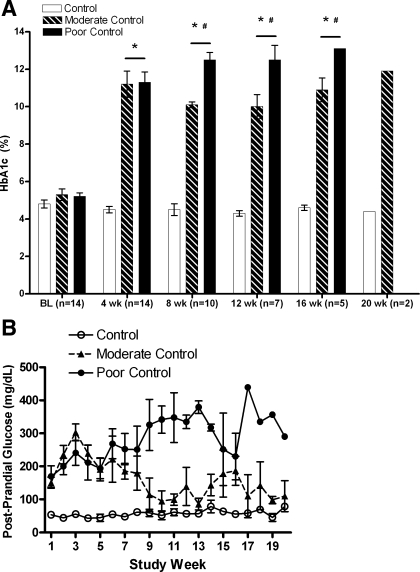
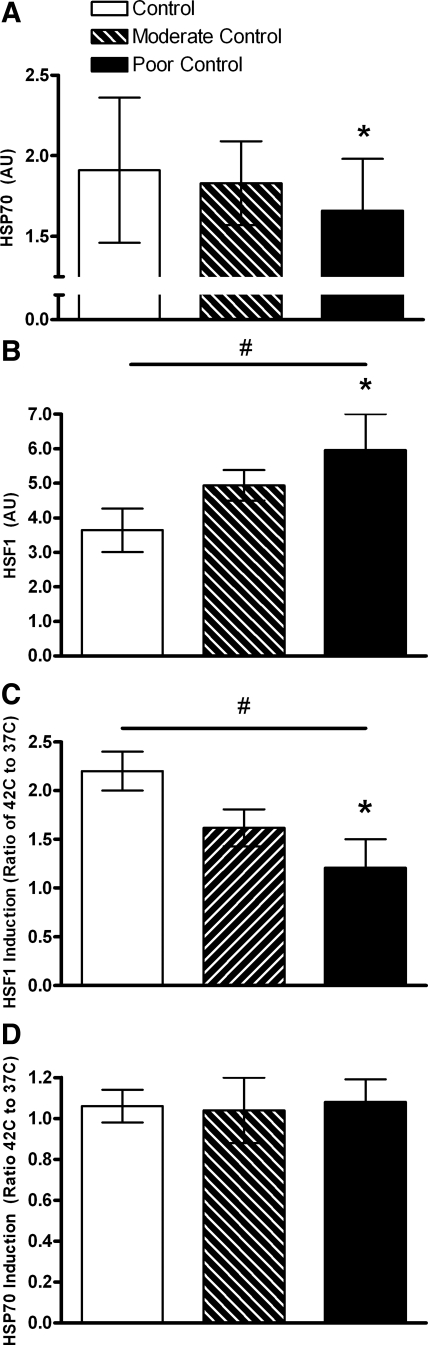
Hb A1c percent glycosylation and postprandial glucose levels verified successful differentiation of glycemic control by judicious insulin administration (Fig. 1). Consistent with the half-life of hemoglobin, separation of Stz-DM groups was not evident until 8 wk after streptozotocin administration, but groups remained distinct though week 16. At week 20, only two monkeys were evaluated. Fructosamine concentrations (Table 1) reflect mainly glycated albumin and also confirm the significant intergroup differences. In terms of clinical standards both hyperglycemic groups were poorly controlled, since Hb A1c levels were >8% in all monkeys. Fasting glucose and insulin values were variable (Table 1), with significant hyperglycemia seen in the two Stz-DM groups, along with lower insulin and C-peptide concentrations. Hepatic tissue levels of HSF1 and HSP70 were discordant, with the highest level of the transcription factor being observed in the most hyperglycemic animals, whereas the gene product HSP70 was significantly lower (Fig. 2, A and B). Both end points demonstrated intermediate values for the moderately controlled group, indicating that total glucose exposure to hepatocytes may be important in mediating this relationship. Activated HSF1 (HSF1p) followed the same pattern, with the percentage of total HSF1p being 7.6 and 16.7% lower in the moderate and poor control groups, respectively, compared with control monkeys (P = 0.20). HSF1p levels were closely associated with HSP70 levels (r = 0.87, P < 0.001).
Fig. 1.
A: average glycemic control measured in streptozotocin-induced diabetes mellitus (Stz-DM) and control monkeys as %glycosylation of Hb A1c. Open bars, control monkeys (n = 5 at baseline); hatched bars, moderately controlled hyperglycemic monkeys (n = 4); black bars, poorly controlled hyperglycemic monkeys (n = 5). *After baseline, all diabetic animals were significantly higher than control; #from weeks 8 through 16, the poor control group had significantly higher Hb A1c values than moderate control. At 20 wk, only 2 monkeys were evaluated. Duration of diabetes correlated with Hb A1c (r = 0.82, P < 0.001). B: postprandial glucose concentrations measured in whole blood from Stz-DM and control monkeys. Postprandial glucose was measured twice weekly to adjust insulin doses to achieve a postprandial glucose value of <150 mg/dl in the moderate control group (▴ with dotted line) or between 250 and 350 mg/dl in the poor control group (● with solid line). ○, Control animals.
Fig. 2.
A: baseline hepatic tissue heat shock protein 70 (HSP70) levels measured from samples maintained at 37°C by Western blotting in control (n = 5; open bar), moderate glycemic control (n = 4; hatched bar), and poor glycemic control (n = 5; black bar, *P = 0.03). B: baseline heat shock factor 1 (HSF1) levels measured from samples maintained at 37°C by Western blotting in control (n = 5; open bar), moderate glycemic control (n = 4; hatched bar), and poor glycemic control (n = 5; black bar, *P = 0.02). A significant trend (#P < 0.05) was seen with glycemic control and hepatic HSF1 levels. C: induction of HSF1 in liver tissue following 1 h of exposure at 42°C and 3 h at 37°C compared with 4 h at 37°C. Increases in HSF1 were significantly less (45% lower, *P = 0.03) in tissues from monkeys experiencing poor glycemic control (n = 5; black bar) compared with control animals (n = 5; open bar). Moderate glycemic control resulted in 26% lower HSF1 induction (n = 4; hatched bar) following ex vivo heat shock compared with control. A significant trend (#P = 0.08) was seen with glycemic control and hepatic HSF1 levels. D: induction of HSP70 in liver tissue following 1 h of exposure at 42°C and 3 h at 37°C compared with 4 h at 37°C. No differences in induction between control animals (n = 5; open bar), moderate glycemic control (n = 4; hatched bar), and poor glycemic control (n = 5; black bar).
Responses to ex vivo heat stress indicated that liver tissue from Stz-DM monkeys has significantly impaired ability to increase HSF1, with the poor glycemic control group having approximately one-half the increase as that seen in control monkeys (Fig. 2C). Basal levels of the gene product HSP70 were lower in Stz-DM monkeys (Fig. 2A); despite similar induction of HSP70 from this experimental standardized stressor (Fig. 2D), tissues from Stz-DM animals remained deficient in this chaperone protein.
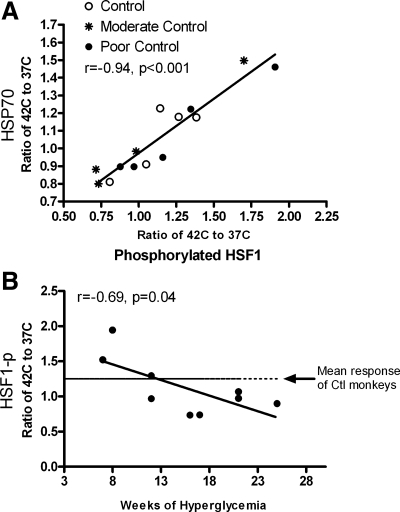
Stz-DM monkeys also induced less HSF1 in response to stress to perpetuate a lower transcription rate of this protein product. Activation of HSF1 by phosphorylation in response to heat stress across both Stz-DM groups was also ∼10% lower. Activation of HSF1 following heat shock correlated nearly perfectly with the resultant change in HSP70 (r = 0.94, P < 0.001; Fig. 3A). Induction of HSF1p with heat shock appeared to be related to the duration of diabetes. After 12 wk of hyperglycemia, the average increase in HSF1p in Stz-DM monkeys following heat stress was lower than in control animals (r = −0.69, P = 0.04; Fig. 3B).
Fig. 3.
A: scatterplot of the relationship between HSF1 activation by phosphorylation with ex vivo heat shock and resultant change in HSP70 (r = 0.94, P < 0.001). Stz-DM (n = 9) monkeys had 10% lower induction of phosphorylated HSF1 (HSF1p; ratio of %HSF1p at 42°C vs. 37°C), which was not significant compared with control (n = 5; P = 0.12). B: scatterplot of the relationship between HSF1 activation by phosphorylation with ex vivo heat shock and duration of hyperglycemia in Stz-DM monkeys (n = 9; r = −0.69, P = 0.04). During more subacute periods (<12 wk), the response may be upregulated compared with control (Ctl; mean response was 1.25 ± 0.06, indicated by the broken line); however, as the exposure to high glucose continues, the response reduces to below normal values such that effectively no overall increase in HSF1 phosphorylation is seen with ex vivo heat shock.
Hepatic and plasma lipids demonstrated changes typical of DM (Table 1). Hepatic free cholesterol was measured. Free cholesterol may represent the membrane-associated cellular cholesterol that may impart structural rigidity and affect membrane fluidity, which has been proposed as a signal for modulating the heat shock protein response (10, 18, 46). Although plasma cholesterol concentrations increased in animals with Stz-DM, this was not reflected in changes in the free cholesterol content of liver tissue (Table 1).
Study 2: pharmacological induction of HSP70 in spontaneously hyperglycemic monkeys.
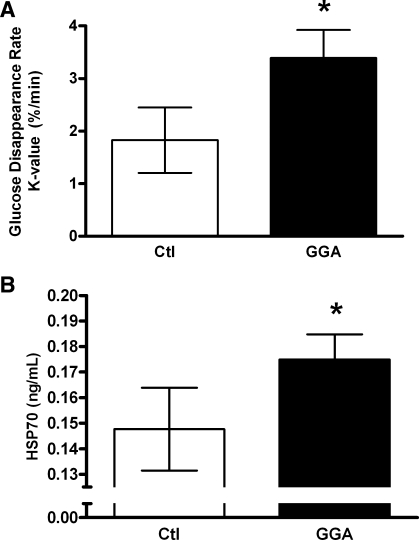
To further generate proof of concept regarding HSF/HSP deficiency in DM, monkeys that were known to have spontaneously developed hyperglycemia without dietary or pharmacological manipulation were chosen for evaluation. Comparable with the condition seen in humans, these were older, insulin-resistant animals (Table 2). GGA was chosen as the HSP70-inducing agent since the target appears to be reduced HSP70-HSF1 negative feedback (38) rather than HSF1 induction. Our hypothesis was that, by bypassing the impaired HSF1 induction process observed in the monkeys with induced DM, HSP70 levels could be restored to normal. After just 2 wk of dosing, we observed an 85% improvement in glucose tolerance (P = 0.008; Fig. 4A). This was corroborated by significant trends toward improvement in fasting glucose (16% reduction) and fasting insulin (14% reduction) and thus a 42% lower and improved HOMA index for insulin resistance. Glucose tolerance, which was nearly doubled in this experiment, indicates the ability of tissues to dispose of circulatory glucose; this improvement occurred in the context of a significant trend toward lower acute insulin response (Table 2). This further substantiates calculated HOMA values that indicate improved insulin sensitivity.
Table 2.
Study 2. Spontaneously diabetic and insulin-resistant vervet monkeys vs. controls after GGA therapy
| Control | GGA Treated | P Value | |
|---|---|---|---|
| Age, yr | 17 ± 2 | 17 ± 2 | NA |
| Body weight, kg | 6.6 ± 1.1 | 6.6 ± 1.1 | 0.30 |
| Fasting glucose, mg/dl | 154 ± 31 | 130 ± 18 | 0.09 |
| Fasting insulin, μIU/ml | 17.1 ± 7.9 | 14.7 ± 6.3 | 0.08 |
| HOMA index, AU | 9.2 ± 5.9 | 5.3 ± 2.9 | 0.08 |
| Acute insulin response, μIU·ml exp−1·min−1 | 5.22 ± 4.22 | 1.19 ± 3.41 | 0.07 |
| Systolic blood pressure, mmHg | 131 ± 9 | 132 ± 12 | 0.93 |
| Diastolic blood pressure, mmHg | 68 ± 4 | 69 ± 5 | 0.77 |
| Total plasma cholesterol, mg/dl | 192 ± 15 | 209 ± 22 | 0.22 |
| Plasma high-density lipoprotein cholesterol, mg/dl | 58 ± 2 | 66 ± 5 | 0.12 |
| Plasma triglycerides, mg/dl | 119 ± 13 | 99 ± 12 | 0.01 |
Values are means ± SE; n = 8. GGA, geranylgeranylacetone; NA, not applicable. P < 0.05 was considered significant, and P < 0.10 was considered a trend.
Fig. 4.
A: disappearance rates for glucose (K value) following intravenous glucose challenge in 8 spontaneously hyperglycemic and insulin-resistant vervet monkeys after treatment with geranylgeranylacetone (GGA; 20 mg·kg−1·day−1 for 14 days; black bar) or placebo (Ctl; open bar). Glucose tolerance increased by 85% (*P = 0.008) after treatment. B: plasma HSP70 concentrations measured in 8 spontaneously hyperglycemic and insulin-resistant vervet monkeys after treatment with GGA (20 mg·kg−1·day−1 for 14 days; black bar) or placebo (Ctl; open bar). HSP70 concentrations increased by 24% increase (*P = 0.02).
In this study, changes in glucose tolerance and insulin resistance occurred without changes in body weight, blood pressure, or plasma lipids. Plasma HSP70 levels increased by 24% as a result of GGA treatment (P = 0.02; Fig. 4B). There were no changes in muscle HSP70 protein content with treatment (control 2.29 ± 0.27 vs. GGA 2.74 ± 0.36, P = 0.47). It has been shown that, with exercise-induced plasma HSP70 elevations, the skeletal musculature was not the source of the increase, but the hepatosplanchic tissues were (13). This was in part our rationale for evaluating hepatic responses in study 1.
DISCUSSION
These two studies demonstrate that hyperglycemia leads to downregulation of the protective chaperone protein response through reduced induction and activation of the transcription factor HSF1 and that restoration of HSP70 improves indices of glucose metabolism and insulin sensitivity. These findings are inherently translational because we used highly relevant nonhuman primate models of hyperglycemia and a human therapeutic drug. Both the degree and the duration of hyperglycemia were related to the level of HSP70 impairment. This is the only study known to date where tissues from diabetic animals (both induced and naturally occurring), rather than blood components, were assessed more dynamically by ex vivo heat shock for HSP responses. The rapid and profound change in glucose tolerance seen with HSP70 induction in a naturally occurring primate model of insulin-resistant type 2 DM verifies the significance and relevance of the chaperone system in metabolic health.
Insulin resistance and overt DM are associated with cellular stress. Increased free fatty acids, glucose, oxidation products, and inflammatory cytokines initiate signaling pathways that activate endoplasmic reticular stress, which results in c-Jun NH2-terminal kinase (JNK) activation and in turn further inhibits insulin signaling through inhibitory serine phosphorylation of the insulin receptor substrate (21). This sets up a feed-forward cycle that perpetuates insulin resistance and suboptimal glycemic control. Chaperone proteins such as HSP70 may reduce some of the stimulus for JNK activation and thus restore insulin sensitivity in tissues such as liver and muscle (9, 39).
Hyperglycemia leads to decreased HSP70 in tissues such as skeletal muscle in people (7, 9, 29), liver in monkeys (26), and both liver and muscle tissues from rodents (3, 49). However, not all reports are consistent. Rodent studies after 30 days of hyperglycemia (the point where our investigation began) have demonstrated both exaggerated and deficient hepatic HSP70 responses to in vivo heat stress (36, 49). Muscle HSP70 protein in insulin-resistant people is estimated to be 30–40% lower than in age-matched controls (7, 9), with gene expression differences being three times greater (29). Previously, we showed ∼50% lower levels of HSP70 in liver tissue of insulin-resistant primates (26). The more moderate attenuation (<20%) seen in these monkeys with induced DM could be the result of the experimental system, since tissues were evaluated only after 4 h of incubation, with anticipated loss of protein. The lesser reduction in liver HSP70 may also result from the relatively short duration of hyperglycemia (<20 wk) compared with years of metabolic dysfunction in people or in our insulin-resistant monkeys or other differences that may exist with natural vs. experimentally induced DM.
The duration of insulin resistance or hyperglycemia is relevant to HSP reductions seen with normal aging processes. Diabetes has been termed “accelerated aging” because of the increased accumulation of oxidized proteins and premature incidence of age-associated morbidities such as cardiovascular disease. Stress protection through maintenance of HSP expression and healthy aging processes is supported by observations in invertebrate models, where overexpression of HSF1 in Caenorhabiditis elegans leads to lifespan extension (22, 47).
In rodent models of aging, basal HSF1 is normal or increased, but DNA binding and effective HSP70 expression is reduced. This was presumed to be related to HSF1 phosphorylation deficits (17, 19) in a manner similar to that seen here in Stz-DM primate tissue. Lower HSP70 concentrations are also found in the circulation of aged people (42), just as they are in primates with DM (26); however, healthy centenarians demonstrate the ability to induce HSP70 with ex vivo heat shock in a manner comparable with young healthy controls (32). Our study of naturally occurring DM supports this concept, since normal aging is associated with the development of insulin resistance (45) and both conditions exhibit reduced HSP70 (26, 42), whereas improved insulin sensitivity results from HSP70 induction. The 24% increase in circulating HSP70 seen with GGA therapy may reflect restoration to a more healthy state, since a reduction of similar magnitude (32%) was observed in Spont-DM monkeys compared with healthy controls (26).
Although here we demonstrate the effect of subacute and chronic moderate to severe hyperglycemia on the HSP response, acute and moderate hyperglycemic states also impair the chaperone system. In exercise-induced stress, HSP70 is released from the hepatosplanchnic circulation rather than the musculature of humans, and this response was reduced if only 1 calorie/kg or less of simple carbohydrate was ingested before stress. Furthermore, the specificity of the tissue response was substantiated by lack of change in HSP70 protein levels of muscle biopsies taken pre- or postexercise (12). Consistent with this finding, a single dose of 10 calories/kg of glucose resulted in a blunted HSP70 response (<20% of saline-administered controls) in healthy rats when they were exposed to transient but severe hepatic ischemia (4). In this experimental model, a single administration of glucose did not alter basal HSP70 expression in the absence of an experimental stressor. Our results indicate that prolonged exposure to high glucose may be necessary to impair HSP responses and may represent a maladaptive hepatic response. Other organs such as kidney, brain, peripheral blood mononuclear cells, and pancreas show increased HSP levels or responses in a hyperglycemic environment (4, 26, 48) This may be attributed to their relative insulin independence and subsequent accumulation of O-linked β-N-acetylglucosamine modification of glycogen synthase kinase-3, which reduces inhibitory serine phosphorylation of HSF1 and should result in greater HSP70 expression (27). The lack of increase in Spont-DM muscle HSP70 levels with GGA treatment but greater plasma concentrations suggests the importance of the liver, which is known to increase hepatic HSP70 levels in response to GGA, in the maintenance of total body insulin sensitivity.
Ex vivo heat shock allows tissues to be removed from the individual's physiological microenvironments and evaluated under comparable conditions. Typically, such experiments have used circulating white cell populations, where HSP70 has a distinct role in immunological processes by binding to an extracellular receptor and stimulating a proinflammatory response (2). Our small study of GGA resulted in increased circulating HSP70 from baseline; thus, the small potential of proinflammatory effects of this change needs to be weighed against the benefits of improved glucose tolerance. However, experiments with exogenously administered human recombinant HSP70 demonstrate that the protein is taken up into muscle tissue, and this results in improved muscle performance and density of innervated neuromuscular junctions (16). Additionally, increasing skeletal muscle HSPs through heat or overexpression has increased muscle mass in rat models (11, 28). Our monkeys were sedentary, and it is possible that GGA-induced HSP70 in the circulation would have acted independently on peripheral muscle tissues. Improved muscle mass and function also would be expected to increase the capacity to dispose of glucose during intravenous glucose tolerance testing. No changes in body weight were noted during our studies; however, more sensitive body composition testing would be required to detect small changes in muscle weight that may have occurred.
Limitations to our study include a small sample size for both the experimentally and naturally induced DM monkey models. Also, the streptozotocin model used for experimental DM represents type 1 DM and is without the inflammatory and hyperinsulinemic features of the spontaneous type 2 DM model. However, we detected biologically and statistically significant differences in HSPs. GGA is a human drug widely prescribed in Japan for the treatment of gastric ulceration, and thus demonstration of beneficial effects in nonhuman primates is justification for a clinical trial evaluating this drug's ability to improve glucose metabolism. We also evaluated the liver only in experimental DM despite HSP70 deficiencies that are important for glucoregulation being noted in other tissues, such as skeletal muscle. Both GGA oral administration and exercise-related stress increase HSP70 in liver (13, 15); thus, we chose to study hepatic tissue. Moreover, skeletal muscle is notorious for failing to show metabolic stress (24) and consistent upregulation of HSPs with stressors such as exercise (34, 35). The lack of change in muscle HSP70 protein with GGA shown here supports an important role for the liver in glucose tolerance. We also measured only HSP70 despite HSF1 controlling a range of small and large HSPs. In our prior investigation, HSP90 and HSP32 mirrored HSP70 in control and DM primate liver tissue, substantiating coordinated control of the chaperone proteins by HSF1 (26, 43). A number of kinases and phosphatases act on HSF1 to modulate its activation (1, 40), but the larger effect seen in our study of hyperglycemia was that of reduced HSF1 levels available for trimerization, phosphorylation, and translocation to the nuclear stress bodies. GGA therapy may also increase the abundance of free HSF1 (38) with the ability to improve HSP70 levels that are deficient in Spont-DM (7, 9, 26, 29).
In summary, we have shown in nonhuman primates that hyperglycemia impairs the heat shock response through significantly reduced HSF1 levels and lower HSF1 activation by phosphorylation as hyperglycemia becomes chronic. These studies highlight the importance of glycemic control in maintenance of normal cellular stress responses, which aid in preserving health and, by extension, longevity. Pharmacological induction of HSP70 using clinically relevant dosages of GGA also rapidly increases insulin sensitivity and glucose tolerance and provides important proof of concept regarding the viability of HSP70-inducing strategies to treat DM.
GRANTS
Funding for these studies came from National Institute on Aging Grant K01-AG-033641 (K. Kavanagh), Wake Forest University's Claude Pepper Older Americans Independence Center (P30-AG-21332), and the Juvenile Diabetes Research Foundation (Butler 7-2005-1152).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Esai of Japan for the generous gift of the geranylgeranylacetone. Part of this work was presented in abstract form at the American Diabetes Association Scientific Sessions, 2008.
REFERENCES
- 1. Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11: 545–555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6: 435–442, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol 97: 605–611, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Behrends M, Martinez-Palli G, Niemann CU, Cohen S, Ramachandran R, Hirose R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg 14: 528–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23: 57–63, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5: 1003–1015, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52: 2338–2345, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem 26: 39–42, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 105: 1739–1744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Csont T, Balogh G, Csonka C, Boros I, Horvath I, Vigh L, Ferdinandy P. Hyperlipidemia induced by high cholesterol diet inhibits heat shock response in rat hearts. Biochem Biophys Res Commun 290: 1535–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Dodd S, Hain B, Judge A. Hsp70 prevents disuse muscle atrophy in senescent rats. Biogerontology 10: 605–611, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Febbraio MA, Mesa JL, Chung J, Steensberg A, Keller C, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones 9: 390–396, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544: 957–962, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 15. Fudaba Y, Ohdan H, Tashiro H, Ito H, Fukuda Y, Dohi K, Asahara T. Geranylgeranylacetone, a heat shock protein inducer, prevents primary graft nonfunction in rat liver transplantation. Transplantation 72: 184–189, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Gifondorwa DJ, Robinson MB, Hayes CD, Taylor AR, Prevette DM, Oppenheim RW, Caress J, Milligan CE. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J Neurosci 27: 13173–13180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutsmann-Conrad A, Pahlavani MA, Heydari AR, Richardson A. Expression of heat shock protein 70 decreases with age in hepatocytes and splenocytes from female rats. Mech Ageing Dev 107: 255–270, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto M, Hossain S, Masumura S. Effect of aging on plasma membrane fluidity of rat aortic endothelial cells. Exp Gerontol 34: 687–698, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Heydari AR, You S, Takahashi R, Gutsmann-Conrad A, Sarge KD, Richardson A. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res 256: 83–93, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 341: 924–925, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300: 1142–1145, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Hutter MM, Sievers RE, Barbosa V, Wolfe CL. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation 89: 355–360, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59: 1899–1905, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 15: 1666–1674, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones 14: 291–299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE. O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem 285: 39096–39107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobayashi T, Goto K, Kojima A, Akema T, Uehara K, Aoki H, Sugiura T, Ohira Y, Yoshioka T. Possible role of calcineurin in heating-related increase of rat muscle mass. Biochem Biophys Res Commun 331: 1301–1309, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51: 1102–1109, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Lee RG, Kelley KL, Sawyer JK, Farese RV, Jr, Parks JS, Rudel LL. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ Res 95: 998–1004, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Literati-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, Tory K, Kolonics A, Fleming A, Mandl J, Koranyi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res 41: 374–380, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Marini M, Lapalombella R, Canaider S, Farina A, Monti D, De Vescovi V, Morellini M, Bellizzi D, Dato S, De Benedictis G, Passarino G, Moresi R, Tesei S, Franceschi C. Heat shock response by EBV-immortalized B-lymphocytes from centenarians and control subjects: a model to study the relevance of stress response in longevity. Exp Gerontol 39: 83–90, 2004 [DOI] [PubMed] [Google Scholar]
- 33. McCarty MF. Induction of heat shock proteins may combat insulin resistance. Med Hypotheses 66: 527–534, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Morton JP, MacLaren DP, Cable NT, Bongers T, Griffiths RD, Campbell IT, Evans L, Kayani A, McArdle A, Drust B. Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J Appl Physiol 101: 176–182, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Morton JP, Maclaren DP, Cable NT, Campbell IT, Evans L, Bongers T, Griffiths RD, Kayani AC, McArdle A, Drust B. Elevated core and muscle temperature to levels comparable to exercise do not increase heat shock protein content of skeletal muscle of physically active men. Acta Physiol (Oxf) 190: 319–327, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Najemnikova E, Rodgers CD, Locke M. Altered heat stress response following streptozotocin-induced diabetes. Cell Stress Chaperones 12: 342–352, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okada M, Hasebe N, Aizawa Y, Izawa K, Kawabe J, Kikuchi K. Thermal treatment attenuates neointimal thickening with enhanced expression of heat-shock protein 72 and suppression of oxidative stress. Circulation 109: 1763–1768, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Otaka M, Yamamoto S, Ogasawara K, Takaoka Y, Noguchi S, Miyazaki T, Nakai A, Odashima M, Matsuhashi T, Watanabe S, Itoh H. The induction mechanism of the molecular chaperone HSP70 in the gastric mucosa by Geranylgeranylacetone (HSP-inducer). Biochem Biophys Res Commun 353: 399–404, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park J, Liu AY. JNK phosphorylates the HSF1 transcriptional activation domain: role of JNK in the regulation of the heat shock response. J Cell Biochem 82: 326–338, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Patury S, Miyata Y, Gestwicki JE. Pharmacological targeting of the Hsp70 chaperone. Curr Top Med Chem 9: 1337–1351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol 36: 341–352, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Rowe PA, Kavanagh K, Zhang L, Wagner JD. Elevated plasma ferritin and impaired heme oxygenase-1 response in arteries from diabetic monkeys (Abstract). Diabetes 55: A174, 2006. [Google Scholar]
- 44. Saisho Y, Manesso E, Butler AE, Galasso R, Kavanagh K, Flynn M, Zhang L, Clark P, Gurlo T, Toffolo GM, Cobelli C, Wagner JD, Butler PC. Ongoing {beta}-Cell Turnover in Adult Nonhuman Primates Is Not Adaptively Increased in Streptozotocin-Induced Diabetes. Diabetes 60: 848–856, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Skilton MR, Lai NT, Griffiths KA, Molyneaux LM, Yue DK, Sullivan DR, Celermajer DS. Meal-related increases in vascular reactivity are impaired in older and diabetic adults: insights into roles of aging and insulin in vascular flow. Am J Physiol Heart Circ Physiol 288: H1404–H1410, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Vigh L, Horvath I, Maresca B, Harwood JL. Can the stress protein response be controlled by “membrane-lipid therapy”? Trends Biochem Sci 32: 357–363, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2: 131–139, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Yabunaka N, Ohtsuka Y, Watanabe I, Noro H, Fujisawa H, Agishi Y. Elevated levels of heat-shock protein 70 (HSP70) in the mononuclear cells of patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 30: 143–147, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Yamagishi N, Nakayama K, Wakatsuki T, Hatayama T. Characteristic changes of stress protein expression in streptozotocin-induced diabetic rats. Life Sci 69: 2603–2609, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Yanaka A, Zhang S, Sato D, Tauchi M, Suzuki H, Shibahara T, Matsui H, Nakahara A, Hyodo I. Geranylgeranylacetone protects the human gastric mucosa from diclofenac-induced injury via induction of heat shock protein 70. Digestion 75: 148–155, 2007 [DOI] [PubMed] [Google Scholar]