Abstract
Ossabaw swine fed excess kilocalorie diet develop metabolic syndrome (MS) characterized by obesity, hypertension, insulin resistance, and glucose intolerance with/without dyslipidemia. The purpose of this study was to test the hypothesis that MS would have a detrimental effect on skeletal muscle structure and cause changes in the expression of myosin heavy chains (MHCs). Adult male Ossabaw swine were fed for 24 wk high-fructose or high-fat/cholesterol/fructose diets to induce normolipidemic MS (MetS) or dyslipidemic MS (DMetS), respectively, and were compared with the lean swine on control diet. MetS swine showed mild MS, lacking increases in total and low density lipoprotein (LDL) cholesterol, both of which were highly upregulated in DMetS swine. There was an ∼1.2-fold increase in the cross-sectional areas of muscle fibers in MetS and DMetS groups compared with control for biceps femoris and plantaris muscles. In plantaris muscles, DMetS diet caused an ∼2-fold decrease in slow MHC mRNA and protein expression and an ∼1.2- to 1.8-fold increase in the number of intramyocellular lipid (IMCL) droplets without large changes in the size of the droplets. There was a trend to the decrease in slow MHC expression in muscles of swine on MetS diet. The number of IMCL droplets in muscle fibers of the MetS group was comparable to controls. These data correlate well with the data on total plasma cholesterol (control = 60, MetS = 70, and DMetS = 298 mg/dl) and LDL (control = 29, MetS = 30, and DMetS = 232 mg/dl). We conclude that structural changes observed in skeletal muscle of obese Ossabaw swine correlate with those previously reported for obese humans.
Keywords: obesity, fiber type composition, intramyocellular lipids, myosin heavy chains
skeletal muscle constitutes more than 40% of the total body weight and is one of the most important organs for glucose homeostasis. The mass and composition of skeletal muscle are critical for its functions. Obesity is a major risk factor for the development of both insulin resistance and non-insulin-dependent diabetes mellitus (NIDDM). Obesity-related abnormalities lead to excessive accumulation of triglycerides and fatty acids in skeletal muscle fibers in the form of intramyocellular lipids (IMCL). IMCL accumulation is strongly associated with the impairment of glucose metabolism, oxidative stress, deficiency in energy production, insulin resistance, and eventually the development of type 2 diabetes (reviewed in Refs. 3 and 26).
The fiber type composition of skeletal muscle has strong effects on metabolism and utilization of glucose and lipids. Lillioja et al. (22) showed that in vivo insulin action determined by the euglycemic clamp was positively correlated with the percentage of type 1 fibers and negatively correlated with the percentage of type 2b/x fibers in vastus lateralis muscles of humans. Several studies showed that obese individuals with or without type 2 diabetes had a significantly lower percentage of type 1 and a higher percentage of type 2b/x muscle fibers than lean individuals (2, 10, 17, 24, 25, 29, 35).
Not only fiber type composition but also the size of skeletal muscle fibers could have a major effect on muscle function. Increased fiber size could diminish oxygen and substrate supply for the metabolic processes in the central area of the fiber. Skeletal muscles from obese compared with lean individuals had greater muscle cross-sectional areas (CSAs) of both type 1 and 2 fibers (9, 24). Weight loss resulted in a 14–25% decrease of the fiber CSAs of both type 1 and type 2 muscle fibers (12), supporting the idea that obesity correlates with an increase in muscle fiber area.
High-fat and high-carbohydrate diets are known to induce accumulation of IMCL in skeletal muscle fibers (reviewed in Ref. 3). Type 1 muscle fibers accumulate higher amounts of IMCL than type 2 fibers (12, 36). Muscle lipid content followed the order of type 1 > type 2a > type 2b/x, but, within each fiber type, skeletal muscle from obese and NIDDM women and men had greater lipid content than muscle from lean subjects (16, 24). The size of lipid aggregates was not affected by obesity, but the number of lipid droplets within muscle fibers was two times as abundant and had a more central distribution in obese compared with lean individuals (24). Weight loss results in the decreased IMCL content (11, 12).
Miniature Ossabaw swine have a “thrifty genotype” predisposing them to the diet-induced obesity. For several years, Sturek's laboratory has used Ossabaw swine as a model to study the effect of diet-induced obesity on the development of insulin resistance, impaired glucose tolerance, dyslipidemia, metabolic syndrome (MS), nonalcoholic steatohepatitis, and cardiovascular complications (1, 7, 8, 21, 27). Swine fed hypercaloric diet high in fructose (MetS diet) develop MS defined by the presence of three or more of the risk factors, but not dyslipidemia, while high-fat/cholesterol/fructose diet (DMetS diet) renders MS with the dyslipidemia component (21).
The effect of the diet-induced obesity and MS on the structural composition of the skeletal muscles of the Ossabaw swine has not been studied previously. We hypothesized that obesity and MS will cause changes of the fiber CSAs and fiber type composition in muscles of Ossabaw swine similar to those found in muscles of obese humans. We also predicted increased accumulation of the IMCL droplets in the skeletal muscle fibers of obese Ossabaw swine on MetS and DMetS diets compared with lean swine on a control diet. These changes could have a negative effect on the biochemical and physiological functions of the muscles. Our goal was to assess whether changes in the skeletal muscles of obese vs. lean Ossabaw swine with MS are consistent with those found in obese vs. lean humans.
MATERIALS AND METHODS
Animal model.
Male Ossabaw swine (N = 7) on the control diet were fed standard pig chow (LabDiet Mini-Pig HF Grower 5L80) that contained 16% kcal from protein, 72% kcal from carbohydrates, and 12% kcal from fat (Purina Test Diet, Richmond, IN). The MetS group (N = 7) was fed a hypercaloric, high-fructose diet containing 16% kcal from protein, 72% kcal from carbohydrates (20% kcal from fructose), and 12% kcal from fat. The DMetS group (N = 7) was fed a hypercaloric, high-fat/cholesterol/fructose atherogenic diet containing 8% kcal from protein, 46% kcal from carbohydrates (20% kcal from fructose), 46% kcal from fat (hydrogenated soybean oil; 56% trans fatty acid), and supplemented with 2% cholesterol by weight (21). All three groups of male Ossabaw swine were gonadally intact. Swine were housed and fed in individual pens and provided a 12:12-h light-dark cycle. Water was provided ad libitum.
At the day of dissection, swine were sedated with atropine (2.2 mg/kg), telazol (3.5 mg/kg), and xylazine (1.5 mg/kg), and anesthesia was maintained with isoflurane (1–5%). Biceps femoris (Biceps), plantaris (PLN), and soleus (Sol) muscles were dissected, frozen in isopentane (for sectioning) or liquid nitrogen (RNA isolation), and stored at −800C. At the end of dissection, animals were killed (under full anesthesia) by excision of the heart.
All experiments with live animals, including skeletal muscle dissections, were performed by Sturek's laboratory at the Indiana University School of Medicine (Indianapolis, IN). Frozen skeletal muscle samples were shipped on dry ice to the Indiana University School of Medicine-Northwest (Gary, IN). All subsequent skeletal muscle analysis was performed in Kostrominova's laboratory at the Indiana University School of Medicine-Northwest.
The facilities and all of the animal use policies and practices were approved by the United States Department of Agriculture and the American Association for the Accreditation of Laboratory Animal Care. The Ossabaw swine were housed at the breeding colony at Purdue University for 80% of the experimental protocol and at Indiana University School of Medicine, Indianapolis for 20% of the experimental protocol. The animal facilities at Purdue University and at Indiana University School of Medicine were directed by licensed veterinarians and operated by a highly trained staff. All animal care and animal surgeries were performed in accordance with The Guide for Care and Use of Laboratory Animals (Washington, DC: National Academy Press, 2010); the experimental protocol was approved by the Indiana University Committee for the Use and Care of Animals.
Histochemical and immunohistochemical analysis.
For the histochemical analysis, unfixed samples were placed in a TBS medium (Triangle Biological Sciences, Durham, NC), frozen in cold isopentane, and stored at −800C until needed. Samples were sliced with a cryostat at a thickness of ∼12 μm, adhered to Superfrost Plus microscopy slides, and used for staining.
For lipid staining, tissue sections were air-dried, incubated in 3% paraformaldehyde for 20 min at room temperature, washed three times with PBS, and incubated with 1 μg/ml BODIPY 493/503 or LipidTOX Red neutral lipid stains (Molecular Probes, Eugene, OR) for 20 min at room temperature. The sections were examined and photographed using confocal microscopy (Olympus FV 300; Olympus). The quantitative analysis of the IMCL droplets area and size was done using Image J software (http://rsb.info.nih.gov/ij/; NIH, Bethesda, MD). Type 1 and type 2a muscle fibers accumulated a much higher amount of the IMCL droplet than type 2b/x fibers. For the quantitative analysis of the IMCL droplets area and size type 1 and type 2a muscle fibers were combined into one group and type 2b/x fibers into a separate group.
For immunostaining, frozen sections were fixed with ice-cold methanol for 10 min and rinsed three times with PBS. Sections were blocked for 30 min with PBS-0.05% Tween 20 (PBST) containing 20% calf serum (PBST-S) at room temperature. Sections were incubated overnight at 40C with the primary antibodies in PBST-S. The following primary antibodies were used: mouse anti-slow (type 1) myosin heavy chain (MHC; clone A4.84) and mouse anti-fast (type 2) MHC (clone A4.74) obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA) and rabbit anti-laminin (Chemicon International, Temecula, CA). A 1-h room temperature incubation with Cy3-conjugated anti-mouse and Cy2-conjugated anti-rabbit antibody (Jackson ImmunoResearch, West Grove, PA) was used for visualization. After immunostaining with mouse anti-fast MHC antibody, sections displayed bright red staining of type 2a fibers and dark red staining of type 2b/x fibers. Sections incubated only with Cy2- and Cy3-conjugated anti-mouse or anti-rabbit antibody were used as negative controls. Nuclei were stained by 5 min incubation with a DAPI solution (Sigma, St. Louis, MO) in PBST. The sections were examined and photographed with a Leica microscope.
Morphometric analysis.
Muscle fiber CSAs were measured in PLN, Sol, and Biceps muscles using Image J software. Samples for measurements of the fiber CSAs were obtained from the midportion of PLN, Sol, and Bicep muscles. The samples were stained with anti-MHC antibodies to identify type 1 and type 2a and 2b/x muscle fibers. Anti-laminin antibody was used to visualize basement membrane surrounding each of the muscle fibers. Each sample section stained for the type 1 fibers was compared with the adjacent section stained for type 2a and 2b/x muscle fibers. Muscle sections at magnification ×100 were used for analysis of the CSAs using Image J software.
Western blotting.
Muscles were dissected, frozen in liquid nitrogen, and homogenized in a solution containing 20 mM Tris·HCl (pH 6.8), 4% (wt/vol) SDS, 1 mM of phenylmethylsulfonyl fluoride, and 1 μm each of leupeptin and pepstatin A. Protein concentrations were determined using the Bio-Rad DC protein assay (Hercules, CA). Protein samples were mixed with loading buffer, subjected to 5% SDS-PAGE, and transferred electrophoretically to Immobilon-P membranes (Millipore, Bedford, MA). After transfer, Immobilon-P membranes were blocked in buffer containing 5% dry milk in PBST and then incubated overnight at 40C with primary antibodies. The following primary antibodies were used: mouse monoclonal IgG antibody recognizing all MHC isoforms (clone MF-20), mouse monoclonal IgM antibody recognizing type 1 MHC (clone 4.840), and mouse monoclonal IgG antibody recognizing type 2 MHC (clone 4.74). All primary antibodies were obtained from the Developmental Studies Hybridoma Bank, University of Iowa. Depending on the type of primary antibody, immunodetection was done using peroxidase-conjugated anti-mouse IgG or anti-mouse IgM antibodies (Jackson ImmunoResearch Laboratories) with subsequent chemiluminescence (Thermo Scientific, Rockford, IL). The same blots were sequentially probed with antibodies against type 2 MHC (clone 4.74), type 1 MHC (clone 4.840), and types 1 and 2 MHC (clone MF-20). The blots were washed in PBST after incubation with anti-type 2 MHC antibody and before incubation with anti-type 1 MHC antibody. The blots were washed in 0.2 M NaOH for 10 min and washed extensively with PBST after incubation with anti-type 1 MHC antibody and before incubation with anti-type 1 and 2 MHC antibody. Images were scanned, and band intensity was evaluated using Image J software. The relative expression levels of type 1 and type 2 MHC were calculated after normalization to the levels of total MHC in each sample.
RNA isolation, reverse transcription, and quantitative RT-PCR analysis.
Total RNA was isolated by homogenizing muscles in Trizol (GIBCO-BRL, Grand Island, NY) followed by the single-step purification method described by the manufacturer's protocol. DNA contamination was removed by digestion with RNase-free DNase I using the DNA-free kit (Ambion, Austin, TX). RNA concentrations were estimated with a spectrophotometer. Reverse transcription and quantitative real-time PCR (QRT-PCR) analysis was performed as follows: 1 μg of total RNA was reverse transcribed using a ThermoScript RT kit (Invitrogen, Carlsbad, CA). QRT-PCR was performed with a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Amplifications were performed in a 20-μl total volume. Nucleotides, Taq DNA polymerase, and buffer were included in the Master SYBR Green mix (Qiagen, Valencia, CA). An amplification protocol incorporated an initial incubation at 95°C for 10 min for the activation of FastStartTaq DNA polymerase followed by 40 cycles, with a 94°C denaturation for 15 s, 56–60°C annealing for 30 s, and 72°C extension for 30 s. Detection of the fluorescent product was performed at the end of the 72°C extension period in each cycle. To confirm the amplification specificity, the PCR products from each primer pair were subjected to a melting curve analysis and subsequent agarose gel electrophoresis. Relative quantification was performed based on the threshold cycle (CT value) for each of the PCR samples (23). Initially two housekeeping genes were evaluated for the normalization of QRT-PCR data: 18S and muscle-specific isoform of creatine kinase (MCK). Normalization to the level of expression of both of these genes showed similar results (data not shown). MCK was chosen for the normalization of all QRT-PCR experiments in this study. The sequences of the primers used for the QRT-PCR analysis were designed using Primer3 software and are summarized in Table 1.
Table 1.
Primers used for QRT-PCR study
| GeneBank Accession No. | Gene Description | Forward Primer | Reverse Primer |
|---|---|---|---|
| NM 001129949 | MCK | TTCGGCAACACCCACAACAA | GTCATGATGAAGGGGTGACCT |
| L10129 | MHC 1 | TCTTTCCTTGCTGCTCTCAG | GCTGCGCCTTGGTTTCTC |
| NM 214136.1 | MHC 2a | TCCTGCTTTAAAAAGCTCCAA | AAATATGGCCATTTCCTGGTC |
| AK347242 | MHC 2b | TTTAAGTAGTTGTCTGCCTTGAGC | ACTCATGGCTGCGGGTTAT |
| NM 001104951.1 | MHC 2x/d | ACTGGGCTGCCATCAATAAC | TGCGCTCCTTTTCAGACTTT |
| NR 002170.3 | 18S | TCAACTTTCGATGGTAGTCGC | CCTCCAATGGATCCTCGTTAA |
QRT-PCR, quantitative real-time PCR; MCK, muscle-specific isoform of creatine kinase; MHC, myosin heavy chain.
Assessment of statistical significance.
Statistical significance of the results was assessed using SigmaStat software (Systat Software, Point Richmond, CA). The statistical analysis of the data was done using two different approaches: Student's t-test and one-way repeated-measures ANOVA with pairwise multiple-comparison procedures (Holm-Sidak or Dunn's method). Differences in values with P < 0.05 were considered statistically significant.
RESULTS
Comparison of total body weight and levels of blood triglycerides and cholesterol at the end of dietary intervention.
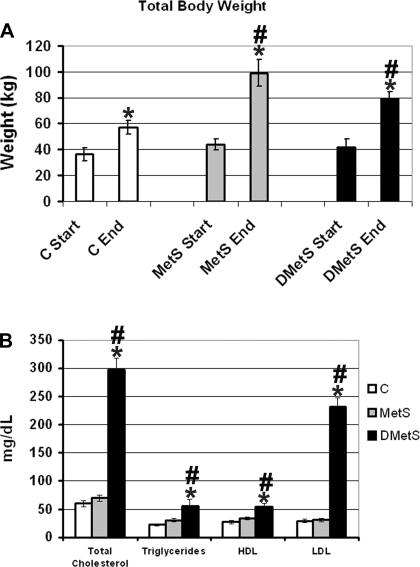
The age (70–80 wk) and total body weight at the beginning of the dietary intervention for the Ossabaw swine in all three diet groups were similar. Swine in all three groups continued to increase in weight through the 24-wk dietary intervention period. The swine had a significantly higher total body weight at the end compared with the start of the dietary intervention (Fig. 1A). At the end of the dietary intervention, swine on the MetS and DMetS diet accumulated significantly more total body weight than swine in the control group (Fig. 1A).
Fig. 1.
Total body weight at the beginning and the end of the experiment (A) and blood triglycerides and cholesterol levels (B) for each diet group. *Significant difference between weight at the beginning and the end of the experiment in each group according to the t-test (A) and significant difference between control and dyslipidemic metabolic syndrome (DMetS) diet groups at the end of the experiment according to the t-test (B). #Significant difference between control and normolipidemic metabolic syndrome (MetS) and DMetS diet groups at the end of the experiment according to the ANOVA. C, control; HDL, high density lipoprotein; LDL, low density lipoprotein.
At the end of the dietary intervention period, swine from the MetS and control groups had similar levels of blood triglycerides and cholesterol levels (Fig. 1B). Swine on the DMetS diet showed an approximately twofold increase in the level of blood triglycerides and an approximately sixfold increase in the total cholesterol level. The level of high-density lipoprotein (HDL) in the blood of DMetS swine increased approximately twofold, and the level of low density lipoprotein (LDL) increased approximately eightfold compared with the control group (Fig. 1B).
Fiber type composition and CSA comparison.
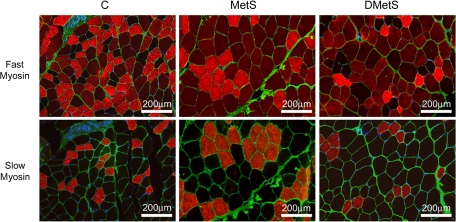
To evaluate fiber type composition of skeletal muscle of Ossabaw swine, antibodies against fast and slow MHCs were used to visualize type 2a and 2b/x and type 1 muscle fibers, respectively (Fig. 2). Antibody against fast MHC that were used in this study stained type 2a muscle fibers brighter than type 2b/x fibers in swine muscles, allowing these subtypes of fast muscle fibers to be distinguished (Fig. 2). We did not use antibodies to differentiate between 2b and 2x muscle fibers; therefore, they are represented by a combined 2b/x group in our evaluation.
Fig. 2.
Immunostaining of plantaris (PLN) muscles of control, MetS, and DMetS diets with antibodies against fast and slow myosins. Fast 2a muscle fibers were identified by bright red staining, 2b/x by dull red staining, and type 1 by no staining (black) with antibody against fast myosin. Slow type 1 muscle fibers were identified by bright red staining with antibody against slow myosin. Anti-laminin antibody (green) was used for visualization of the muscle fiber boundaries.
There was a statistically significant increase in the CSAs of fibers in PLN and Biceps muscles of swine on MetS and DMetS diets compared with swine on the control diet (Table 2). For PLN and Biceps, all three fiber types (types 1, 2a, and 2b/x) showed statistically significant increases of the CSAs in swine on MetS and DMetS diets (Table 2). Sol muscle showed similar results for swine on the MetS diet except for the type 1 muscle fibers in which CSAs did not change (Table 2). Unexpectedly, Sol muscle showed a statistically significant decrease of the CSAs in swine on the DMetS diet (Table 2).
Table 2.
Comparison of fiber cross-sectional areas in skeletal muscles of Ossabaw swine on different diets
| Plantaris Average CSA, μm2 |
Soleus Average CSA, μm2 |
Biceps Average CSA, μm2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | All Types | Type 1 | Type 2a | Type 2b/x | All Types | Type 1 | Type 2a | Type 2b/x | All Types | Type 1 | Type 2a | Type 2b/x |
| C | 4,420 ± 42 | 2,567 ± 44 | 3,296 ± 42 | 5,625 ± 59 | 5,032 ± 48 | 5,165 ± 73 | 4,903 ± 81 | 5,772 ± 116 | 4,845 ± 72 | 3,590 ± 74 | 4,291 ± 109 | 6,062 ± 131 |
| MetS | 5,283 ± 63* | 3,508 ± 135* | 3,768 ± 72* | 6,135 ± 80* | 6,604 ± 71* | 5,185 ± 85 | 6,171 ± 75* | 8,628 ± 162* | 5,653 ± 72* | 3,879 ± 108* | 5,285 ± 106* | 6,430 ± 112* |
| DMetS | 5,439 ± 62* | 3,247 ± 79* | 4,208 ± 83* | 6,370 ± 84* | 4,180 ± 42* | 4,961 ± 91* | 4,666 ± 92* | 4,924 ± 112* | 5,698 ± 73* | 4,379 ± 123* | 5,334 ± 144* | 6,447 ± 101* |
Values are means ± SE.
CSA, cross-sectional area; C, control; MetS, normolipidemic metabolic syndrome; DMet, dyslipidemic metabolic syndrome.
Statistically significant values according to the comparison with control using ANOVA.
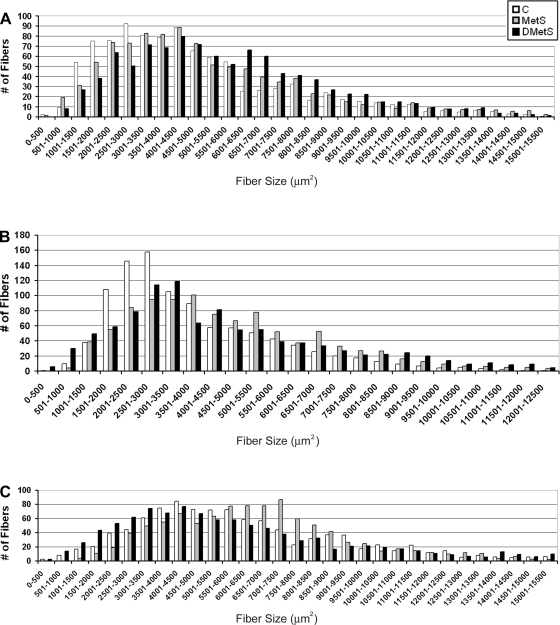
The frequency histogram of the fiber CSAs showed a similar increase in the proportion of larger fibers in the Biceps (Fig. 3A) and PLN (Fig. 3B) muscles of swine on MetS and DMetS diets. In PLN muscles of swine on the DMetS diet, the number of the very small fibers (<1,000 μm2) was also increased (Fig. 3B). For Sol muscle, there was an increase in the proportion of larger fibers in muscles of swine on MetS diet and a decrease in the proportion of the larger fibers in swine on DMetS diet (Fig. 3C).
Fig. 3.
Histogram of the muscle fiber cross-sectional areas (CSAs) in biceps femoris (Biceps, A), PLN (B), and soleus (Sol, C) muscles of Ossabaw swine on control, MetS, and DMetS diets.
The analysis of the fiber type composition showed a trend to the increase in the number of 2b/x fibers and decrease in type 1 fibers in Biceps, PLN, and Sol muscles of swine on MetS and DMetS diets compared with the swine on the control diet (Table 3). Nevertheless, this trend did not reach the statistically significant values. The only statistically significant change that was detected was an increase in the proportion of type 2a muscle fibers in the Biceps muscles of swine on MetS diet (Table 3).
Table 3.
Comparison of fiber type composition in skeletal muscles of Ossabaw swine on different diets
| Plantaris Fiber Type Composition, % |
Soleus Fiber Type Composition, % |
Biceps Fiber Type Composition, % |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Diet | Type 1 | Type 2a | Type 2b/x | Type 1 | Type 2a | Type 2b/x | Type 1 | Type 2a | Type 2b/x |
| C | 12.0 ± 7.6 | 34.2 ± 9.0 | 53.9 ± 12.6 | 32.9 ± 3.5 | 38.5 ± 5.3 | 28.7 ± 5.2 | 27.5 ± 5.8 | 26.7 ± 2.4 | 45.9 ± 6.6 |
| MetS | 7.0 ± 5.9 | 31.5 ± 11.3 | 61.5 ± 14.6 | 28.6 ± 3.8 | 40.4 ± 11.4 | 35.1 ± 19.4 | 14.8 ± 3.2 | 35.0 ± 4.2* | 50.2 ± 3.2 |
| DMetS | 10.6 ± 6.9 | 30.5 ± 9.8 | 58.9 ± 12.2 | 25.5 ± 7.3 | 40 ± 10.4 | 34.5 ± 15.8 | 23.0 ± 3.9 | 22.1 ± 2.7 | 54.9 ± 5.21 |
Values are means ± SE.
Statistically significant values according to the comparison with control using ANOVA.
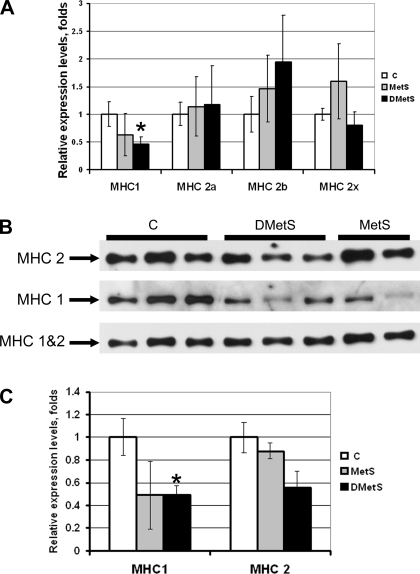
The evaluation of the MCH mRNA and protein expression in PLN muscles using QRT-PCR and Western blotting, respectively, showed a statistically significant decrease in type 1 MHC expression in muscles of swine DMetS diet compared with the swine on the control diet (Fig. 4). There was also a trend to the decrease in type 1 MHC expression in muscles of swine on MetS diet, but this trend did not reach the statistically significant values (Fig. 4). There was also a trend to the increase in type 2b MHC mRNA expression in muscles of swine on MetS and DMetS diets, but this trend also did not reach the statistically significant values (Fig. 4A).
Fig. 4.
Comparison of the mRNA and protein expression levels of myosin heavy chain (MHC) in PLN muscle of the Ossabaw swine on control, MetS, and DMetS diets. mRNA expression of different MHC isoforms was evaluated using quantitative real-time PCR (QRT-PCR, A). Representative image of Western blots evaluating protein expression of MHC1 and MHC2, as well as total MHC (MHC1&2) is shown in B. Relative protein expression level of MHC1 and MHC 2 after normalization for the total MHC expression in each sample is shown in C. *Significant difference between control and MetS and DMetS diet groups according to the t-test. There was no significant difference between control and MetS and DMetS diet groups according to the ANOVA.
IMCL accumulation.
Two different lipid stains LipidTOX Red and BODIPY 493/503 showed similar patterns of staining of muscle cross sections (Fig. 5, A and B, respectively). Staining for the IMCL droplets showed that type 1 and type 2a fibers accumulated a much higher amount of droplets than type 2b/x fibers (Fig. 5).
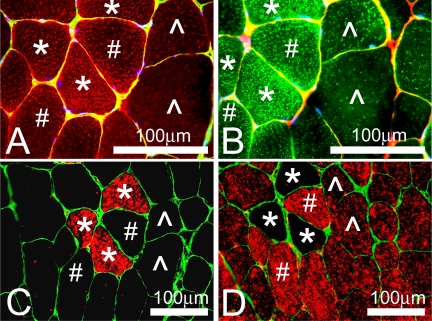
Fig. 5.
Staining of the intramyocellular lipid (IMCL) droplets and MHCs in PLN muscle of the Ossabaw swine on DMetS diet. Two different lipid stains were used for the visualization of IMCL droplets: LipidTOX Red (A) and BODIPY 493/503 (B). Costaining of the adjacent muscle sections with antibodies against slow (C) and fast (D) MCHs showed that type 1 (*) and type 2a (#) fibers accumulated a much higher amount of IMCL droplets than type 2b/x fibers (^).
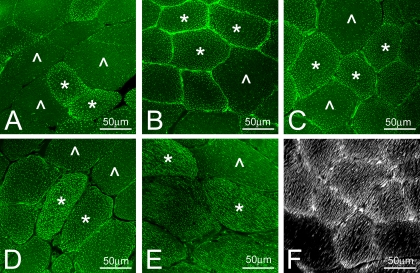
Comparison of the lipid staining in PLN muscles of Ossabaw swine on control, MetS, and DMetS diets showed a higher accumulation of IMCL droplets in fibers of swine on DMetS diet (Fig. 6). The higher amount of IMCL droplets was especially noticeable in type 2b/x fibers that usually have the lowest amount of IMCL (Fig. 6C). Several fibers in muscles of swine on DMetS diet showed an extremely high accumulation of lipid droplets that practically filled the entire fiber (Fig. 6D). Because only a few of such fibers were found, they were excluded from the quantitative analysis. As could be observed on the tangential sections in the fibers with a high amount of IMCL, the droplets were arranged in the continuous rows (Fig. 6E). Three-dimensional reconstruction of sections of PLN muscles from Ossabaw swine on DMetS diet using Image-Pro software also showed the arrangement of IMCL droplets in continuous rows (Fig. 6F).
Fig. 6.
Staining of the IMCL droplets with BODIPY 493/503 in PLN muscle of the Ossabaw swine on control (A), MetS (B), and DMetS (C-F) diets. Type 1 and type 2a muscle fibers (*) accumulated a much higher amount of IMCL droplets than type 2b/x fibers (^). Some of the type 1 and 2a muscle fibers had very high accumulation of the IMCL droplets (D). Tangential sections (E) and three-dimensional reconstruction (F) showed that IMCL droplets were arranged in continuous rows.
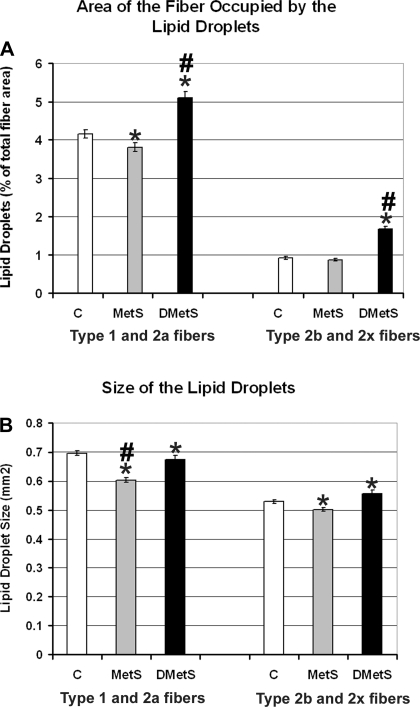
The quantitative analysis of the IMCL droplets showed that swine on DMetS diet accumulated ∼1.2- and ∼1.8-fold higher amounts of IMCL droplets in type 1/2a and type 2b/x fibers, respectively (Fig. 7A). These data correlate well with the data on total plasma cholesterol (control = 60, MetS = 70, and DMetS = 298 mg/dl) and LDL (control = 29, MetS = 30, and DMetS = 232 mg/dl). There were no large changes in size of the IMCL droplets in muscles of swine on DMetS diet compared with swine on the control diet (Fig. 7B). The size of the IMCL droplets in type 1/2a muscle fibers of swine on MetS diet was decreased ∼1.2-fold (Fig. 7B).
Fig. 7.
Comparison of the fiber areas occupied by the IMCL droplets (A) and IMCL droplet sizes (B) in PLN muscle of the Ossabaw swine on control, MetS, and DMetS diets. *Significant difference between control and MetS and DMetS diet groups according to the t-test. #Significant difference between control and MetS and DMetS diet groups according to the ANOVA.
DISCUSSION
Ossabaw swine model.
The Ossabaw swine model was selected for this study because of their thrifty genotype and their increased predisposition to the development of insulin resistance in response to high-fat/cholesterol and/or high-fructose diets (1, 7, 8, 21, 27). It is difficult to investigate the early events associated with the development of insulin resistance in obese and/or type 2 diabetic humans. When selected for the clinical studies, these individuals were already obese and/or had type 2 diabetes for a prolonged time and often had other associated comorbidities (13). Moreover, the small size and limited selection (only those muscles that could be accessed with a biopsy needle) of skeletal muscle biopsies obtained during clinical studies limits the experimental design.
For these reasons, it is important to have an animal model closely resembling changes observed in obese and/or type 2 diabetic humans. Rodent models are often used in studies of mechanisms involved in the development of obesity and insulin resistance in response to high-carbohydrate/high-fat diets (reviewed in Ref. 31). Although these models are relatively inexpensive and easy to use and a number of knockout and transgenic mice are available to the researches to test their hypotheses, these models are significantly different from humans in body size, skeletal muscle size, muscle fiber CSAs, and fiber type composition, all of which could result in the differences in muscle response to the diet-induced obesity between rodents and humans.
Nonhuman primates might be the model most closely resembling humans in studies of the mechanisms involved in the development of obesity and/or type 2 diabetes (reviewed in Refs. 5, 15, 32). Nonhuman primate studies, however, also have a number of limitations: they are expensive and only a limited number of Primate Research Centers are able to do them, and the small size of skeletal muscle biopsies obtained during these studies limits the experimental design.
Swine models, in contrast to nonhuman primates and rodents, are much closer to humans in body size, skeletal muscle fiber size, and fiber type composition. Skeletal muscle fiber CSAs of swine muscle (∼2,500–6,600 μm2; current study) are more comparable to fiber CSAs of human muscles (∼2,500–5,700 μm2; see Refs. 2 and 10) and are much larger than CSAs in muscle of rats and mice (∼1,000–3,000 μm2; see Refs. 6 and 19). Several studies evaluated the effect of diet-induced obesity on heart (20), liver (1), and muscle (14) of swine models. Swine models are less expensive than nonhuman primate models and are highly available, and the animals could be killed at the end of the study, providing a large amount of various muscle samples. The goal of our study was to assess whether the changes in skeletal muscles of Ossabaw swine on excess-calorie high-fat/cholesterol and/or high-fructose diets are consistent with those found in obese humans.
Changes in the fiber CSAs and fiber type composition in skeletal muscles in response to high-fat/cholesterol and/or high-fructose diet.
Our study showed a statistically significant increase in CSAs of both slow and fast fibers in PLN and Biceps muscles of Ossabaw swine on MetS and DMetS diets. Similar findings were previously reported for Biceps muscles of Yucatan swine on a “Western”-type diet (14). These data correlate well with previous studies in humans showing that skeletal muscles from obese compared with lean individuals have greater muscle CSAs (9, 24). The increased CSAs could have a negative effect on the oxygen and substrate supply to the center of the fiber, leading to the reduced muscle performance.
Interestingly, Sol muscles of Ossabaw swine on DMetS diet showed decreased CSAs for both slow and fast fibers. Sol muscles have the highest proportion of slow fibers than any other hindlimb muscle (28). Slow fibers are known to accumulate high amounts of IMCL (12, 36). The decreased CSAs in Sol muscles of Ossabaw swine on DMetS diet could be related to the increased IMCL accumulation.
Although there was a noticeable trend for the decrease in the percentage of type 1 fibers and an increase in the percentage of type 2b/x fibers using the immunostaining technique, our study found no statistically significant changes in fiber type composition of Biceps, PLN, and Sol muscles of Ossabaw swine on MetS and DMetS diets compared with swine on control diet. The only exception was the increase in the proportion of type 2a fibers in Biceps muscles of swine on MetS diet. Similarly, a study of Yucatan swine fed Western-type diet for 12 mo found no differences in the fiber-type composition of Biceps muscles compared with swine on the control diet, although there was a trend of decreased percentages of type 2b/x fibers (14). Using QRT-PCR and Western blot techniques, we were able to detect a decrease in type 1 MHC mRNA and protein expression levels in PLN muscles of swine on DMetS diet. Previous studies in humans showed that obese individuals with or without NIDDM had a significantly lower percentage of type 1 and higher percentage of type 2b/x muscle fibers than lean individuals (10, 17, 24, 25, 29, 35). Overall, the trends we noted in changes of fiber type composition in muscles of Ossabaw swine on MetS and DMetS diets are highly consistent with findings in obese and NIDDM humans. One of the plausible explanations for the differences in the magnitude of changes of fiber type composition between swine and human studies could be the differences in the duration of diet intervention (24 wk in our study vs. years in humans).
Effect of IMCL accumulation on the development of insulin resistance and type 2 diabetes.
Contrary to our initial hypothesis that diet-induced obesity will cause upregulation of IMCL droplets in both MetS and DMetS groups, our current study showed an increased accumulation of IMCL droplets in muscles of Ossabaw swine on the DMetS diet but not on the MetS diet. The size of the lipid droplets in muscles of Ossabaw swine on the DMetS diet was in the same range as lipid droplet size in control swine. Our data correlate well with previous publications showing that there were no changes in IMCL droplet size in highly trained endurance athletes, NIDDM patients, and overweight sedentary men (36). The accumulation of IMCL droplets in muscles of Ossabaw swine on DMetS diets correlated with high levels of total cholesterol, triglycerides, HDL, and LDL in the plasma. The muscle fiber area occupied by IMCL droplets and levels of total cholesterol, triglycerides, HDL, and LDL in the plasma did not differ between normolipidemic Ossabaw swine on the MetS diet and swine on the control diet. Insulin resistance and glucose intolerance were shown previously to be comparable in MetS and DMetS Ossabaw swine groups (21). Numerous adipokines not measured in our study could have contributed to the development of insulin resistance in the highly obese MetS group (33, 37). These data indicate that dyslipidemia and not obesity by itself is the most critical factor for the increased IMCL droplet accumulation in muscles of Ossabaw swine.
Increased IMCL accumulation could result from the excess of lipids in the diet and/or increased liver and adipose tissue lipolysis and, therefore, increased free fatty acid in the plasma, decreased lipid oxidation in the muscle mitochondria, or from a combination of all of these factors (reviewed in Ref. 38). In the current study, we did not measure the levels of liver and adipose tissue lipolysis and the levels of lipid oxidation in the muscle mitochondria, but we observed a clear correlation between the concentration of free fatty acid in the plasma and the amount of IMCL droplets in muscles of Ossabaw swine.
Type 2b/x fibers in muscles of Ossabaw swine on DMetS diet showed the highest increase in the amount of IMCL droplets (1.8-fold). Type 2b/x fibers in control swine had ∼1% of total fiber area occupied by the IMCL droplets, which is approximately fourfold lower than the area occupied by the IMCL droplets in type 1 and 2a fibers. This is a physiologically relevant difference: type 1 and 2a fibers are oxidative fibers with a high amount of mitochondria and have a high capacity for IMCL oxidation. IMCL are a major source of energy for ATP production in type 1 and 2a fibers. Type 2b/x fibers have a much smaller amount of mitochondria and mostly rely on glycolysis for ATP production. Increased accumulation of IMCL droplets in type 2b/x fibers without an increase in the amount of mitochondria and the capacity for IMCL oxidation could lead to a lower IMCL turnover rate and the accumulation of harmful intermediates of incomplete oxidation of fatty acid. In agreement with our findings, reduced oxidation of IMCL and reduced mitochondrial mass were shown to contribute independently to accumulation of IMCL under conditions of high extracellular free fatty acid availability in vitro in myotubes established from muscles of obese NIDDM individuals (4).
Although it is well documented that accumulation of IMCL in muscle of obese individuals correlates with the increased risk of development of NIDDM, the molecular mechanisms underlying this correlation are not completely understood. Some of the proposed mechanisms include: IMCL interference with insulin-sensitive signaling pathways because of the accumulation of lipid intermediates (diacylglycerols, ceramide, acyl-CoAs); increased oxidative stress and lipid peroxidation; induction of the inflammatory pathways (tumor necrosis factor-α, NF-kB, JNK); and activation of protein kinase C and IkB kinases (reviewed in Refs. 26 and 38). Endurance-trained athletes also have a high content of IMCL that does not interfere with insulin-sensitive signaling pathways. This is because of the high oxidation levels of IMCL by mitochondria and high IMCL turnover rate (18, 34). The preponderance of data supports the notion that it is not the increased levels of IMCL itself that are responsible for the development NIDDM in sedentary obese individuals but the accumulation of harmful intermediates of incomplete oxidation of glucose (lactate, methylglyoxal) and fatty acid (acyl-CoAs, hydroxyacyl-CoAs, ceramide) (reviewed in Refs. 26 and 30).
In conclusion, our experiments showed that swine on MetS diet had a greater increase in total body weight than swine on DMetS diet. Despite these findings, swine on MetS diet did not have upregulation of plasma cholesterol and triglycerides or increased accumulation of IMCL droplets. These data suggest that high total body weight is closely associated with the development of MS, whereas upregulation of plasma cholesterol and triglycerides is linked to increased accumulation of IMCL droplets in skeletal muscle without changes in droplet size.
Overall, the findings of the current study on muscles of Ossabaw swine on MetS and DMetS diets closely correlate with previously published data on obese and type 2 diabetic humans. We plan to use the Ossabaw swine model for future studies that examine mechanisms of the effect of diet-induced MS with or without dyslipidemia on the structural and physiological changes, as well as studies of changes in global gene expression profiles in skeletal muscles.
GRANTS
Design of the diet intervention study, skeletal muscle dissection, and all data on the intact animals presented here were performed/obtained by M. Sturek and his laboratory at the Indiana University School of Medicine (IUSM)-Indianapolis and was supported by National Institutes of Health Grants RR-013223 (M. Sturek) and HL-062552 (M. Sturek). Skeletal muscle study design and all of the muscle analysis data presented here were performed at the IUSM-Northwest and were supported by the funds from IUSM-Northwest (T. Y. Kostrominova). B. A. Clark, an undergraduate student working in T. Y. Kostrominova's laboratory, was supported by the IUSM-Northwest Undergraduate Student Summer Research Program.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Anastasia Kostrominova for proofreading the manuscript.
REFERENCES
- 1. Bell LN, Lee L, Saxena R, Bemis KG, Wang M, Theodorakis JL, Vuppalanchi R, Alloosh M, Sturek M, Chalasani N. Serum proteomic analysis of diet-induced steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Am J Physiol Gastrointest Liver Physiol 298: G746–G754, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coen PM, Dube JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, Goodpaster BH. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59: 80–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr 85: 662–677, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Corpeleijn E, Hessvik NP, Bakke SS, Levin K, Blaak EE, Thoresen GH, Gaster M, Rustan AC. Oxidation of intramyocellular lipids is dependent on mitochondrial function and the availability of extracellular fatty acids. Am J Physiol Endocrinol Metab 299: E14–E22, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Cruzen C, Colman RJ. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin Geriatr Med 25: 733–739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dow DE, Cederna PS, Hassett CA, Kostrominova TY, Faulkner JA, Dennis RG. Number of contractions to maintain mass and force of a denervated rat muscle. Muscle Nerve 30: 77–86, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56: 35–45, 2006 [PubMed] [Google Scholar]
- 8. Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. Exercise training decreases store-operated Ca2+entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res 85: 631–640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gavin TP, Stallings HW, III, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, Hickner RC. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol 98: 315–321, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Gerrits MF, Ghosh S, Kavaslar N, Hill B, Tour A, Seifert EL, Beauchamp B, Gorman S, Stuart J, Dent R, McPherson R, Harper ME. Distinct skeletal muscle fiber characteristics and gene expression in diet-sensitive versus diet-resistant obesity. J Lipid Res 51: 2394–2404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49: 467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA. Effect of weight loss on muscle lipid content in morbidly obese subjects. Am J Physiol Endocrinol Metab 284: E726–E732, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9: 88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guillerm-Regost C, Louveau I, Sebert SP, Damon M, Champ MM, Gondret F. Cellular and biochemical features of skeletal muscle in obese Yucatan minipigs. Obesity (Silver Spring) 14: 1700–1707, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hansen BC. Pathophysiology of obesity-associated type II diabetes (NIDDM): implications from longitudinal studies of non-human primates. Nutrition 5: 48–50, 1989 [PubMed] [Google Scholar]
- 16. He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50: 817–823, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Hickey MS, Weidner MD, Gavigan KE, Zheng D, Tyndall GL, Houmard JA. The insulin action-fiber type relationship in humans is muscle group specific. Am J Physiol Endocrinol Metab 269: E150–E154, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Jong-Yeon K, Hickner RC, Dohm GL, Houmard JA. Long- and medium-chain fatty acid oxidation is increased in exercise-trained human skeletal muscle. Metabolism 51: 460–464, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Kostrominova TY, Pasyk KA, Van RH, Richardson AG, Faulkner JA. Adaptive changes in structure of skeletal muscles from adult Sod1 homozygous knockout mice. Cell Tissue Res 327: 595–605, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lee J, Xu Y, Lu L, Bergman B, Leitner JW, Greyson C, Draznin B, Schwartz GG. Multiple abnormalities of myocardial insulin signaling in a porcine model of diet-induced obesity. Am J Physiol Heart Circ Physiol 298: H310–H319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee L, Alloosh M, Saxena R, Van AW, Watkins BA, Klaunig JE, Sturek M, Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 50: 56–67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 415–424, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Malenfant P, Joanisse DR, Theriault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord 25: 1316–1321, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Marin P, Andersson B, Krotkiewski M, Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17: 382–386, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 294: E203–E213, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Neeb ZP, Edwards JM, Alloosh MA, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in Ossabaw compared to Yucatan swine. Comp Med 60: 300–315, 2010 [PMC free article] [PubMed] [Google Scholar]
- 28. Novak P, Zacharova G, Soukup T. Individual, age and sex differences in fiber type composition of slow and fast muscles of adult Lewis strain rats. Comparison with other rat strains. Physiol Res 59: 783–801, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29: 895–900, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Ritov VB, Menshikova EV, Azuma K, Wood RJ, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298: E49–E58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Speakman J, Hambly C, Mitchell S, Krol E. The contribution of animal models to the study of obesity. Lab Anim 42: 413–432, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Standaert ML, Ortmeyer HK, Sajan MP, Kanoh Y, Bandyopadhyay G, Hansen BC, Farese RV. Skeletal muscle insulin resistance in obesity-associated type 2 diabetes in monkeys is linked to a defect in insulin activation of protein kinase C-zeta/lambda/iota. Diabetes 51: 2936–2943, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Stefanyk LE, Dyck DJ. The interaction between adipokines, diet and exercise on muscle insulin sensitivity. Curr Opin Clin Nutr Metab Care 13: 255–259, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Talanian JL, Holloway GP, Snook LA, Heigenhauser GJ, Bonen A, Spriet LL. Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am J Physiol Endocrinol Metab 299: E180–E188, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002 [DOI] [PubMed] [Google Scholar]
- 36. van Loon LJ, Koopman R, Manders R, van der WW, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab 287: E558–E565, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol 2: 31–56, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta 1801: 1–22, 2010 [DOI] [PubMed] [Google Scholar]