Abstract
Oocyte cryopreservation is a promising technology that could benefit women undergoing assisted reproduction. Most studies examining the effects of cryopreservation on fertilization and developmental competence have been done using metaphase II-stage oocytes, while fewer studies have focused on freezing oocytes at the germinal vesicle (GV) stage, followed by in vitro maturation. Herein, we examined the effects of vitrifying GV-stage mouse oocytes on cytoplasmic structure and on the ability to undergo cytoplasmic changes necessary for proper fertilization and early embryonic development. We examined the endoplasmic reticulum (ER) as one indicator of cytoplasmic structure, as well as the ability of oocytes to develop Ca2+ release mechanisms following vitrification and in vitro maturation. Vitrified GV-stage oocytes matured in culture to metaphase II at a rate comparable to that of controls. These oocytes had the capacity to release Ca2+ following injection of inositol 1,4,5-trisphosphate, demonstrating that Ca2+ release mechanisms developed during meiotic maturation. The ER remained intact during the vitrification procedure as assessed using the lipophilic fluorescent dye DiI. However, the reorganization of the ER that occurs during in vivo maturation was impaired in oocytes that were vitrified before oocyte maturation. These results show that vitrification of GV-stage oocytes does not affect nuclear maturation or the continuity of the ER, but normal cytoplasmic maturation as assessed by the reorganization of the ER is disrupted. Deficiencies in factors that are responsible for proper ER reorganization during oocyte maturation could contribute to the low developmental potential previously reported in vitrified in vitro-matured oocytes.
Keywords: assisted reproductive technology, gamete biology, in vitro fertilization, meiosis
GV-stage oocytes mature to the MII stage following vitrification, develop the ability to release Ca2+, and can be fertilized; but cytoplasmic maturation is impaired by this process.
INTRODUCTION
Infertility is a widespread problem, affecting approximately 5%–15% of the population [1]. The most common form of assisted reproductive technology is in vitro fertilization (IVF), in which metaphase II (MII)-stage oocytes are obtained from women following hormonally induced ovarian stimulation and are fertilized with sperm. A few of the resulting embryos are usually implanted right away, but in many cases leftover embryos are frozen for later use. Cryopreservation of embryos is a well-established technique that has been used successfully for many years [2]. However, this method can introduce some practical and ethical issues, and the production of embryos requires that a woman have a partner's or donor's sperm. Therefore, it is not a viable option for all infertile women. For these reasons, it would be preferable to cryopreserve unfertilized eggs or immature oocytes.
To date, attempts to produce high-quality embryos that develop to term following freezing and thawing of MII-stage oocytes have had limited success [3–5]. Reasons for this may include low permeability of the oocyte membrane to cryoprotectants, susceptibility of the meiotic spindle to cooling [5–9], and toxic effects of cryoprotectants that affect various aspects of the oocyte's physiology [3, 7, 10, 11]. The two main methods that have been used for cryopreservation are slow freezing/thawing and ultrarapid freezing (vitrification). While both methods can yield high oocyte survival and fertilization rates following cryopreservation [4, 6, 10, 12–14], direct comparisons between slow and rapid freezing procedures have shown that vitrification is likely to be the more promising method [6, 7, 10, 15, 16]. Although survival and fertilization rates of vitrified eggs have improved over the past several years, this procedure has resulted in only ∼300–500 live births to date [3, 17, 18], suggesting that cryopreserved oocytes are compromised in other ways that do not affect survival and fertilizability.
Alternatively, the ability to cryopreserve immature oocytes that are at the germinal vesicle (GV) stage and then to mature them in vitro would represent a significant advance that could assist more groups of women. The process of in vitro oocyte maturation (IVM) has many advantages over current standard IVF protocols, which necessitate injecting women with large doses of hormones that can have unwanted adverse effects. With IVM, immature oocytes could be retrieved from the ovaries of women without prior hormone injection or with lower doses of hormones. In recent years, this procedure has improved and shows promise as a treatment for infertility [17, 19–22]. Because GV-stage oocytes have not yet formed meiotic spindles and their chromatin is decondensed within the nuclear envelope, they may be less susceptible to freezing damage that would otherwise disrupt the spindle in an MII-stage oocyte. Therefore, the cryopreservation of GV-stage oocytes is an attractive alternative for freezing female gametes. Although studies [23–29] have shown that bovine oocytes frozen at the GV stage have lower survival rates and developmental competence than oocytes frozen at the MII stage, live births have been obtained using this procedure [23, 24, 28–30]. Because the developmental competence of both cryopreserved GV-stage and MII-stage bovine oocytes is significantly lower than that of unfrozen controls, a more accurate comparison between freezing GV-stage vs. MII-stage bovine oocytes might be made after the overall cryopreservation procedure is improved.
There have been fewer studies examining the developmental competence in human and mouse oocytes following cryopreservation and IVM. In the limited studies that have been done in the mouse, it has been shown that GV-stage oocytes can be successfully frozen and thawed [31–33], that they can subsequently mature in culture and form morphologically normal spindles [33], and that they can develop after fertilization and produce live births [31, 32]. However, as in bovine oocytes, the developmental competence in terms of blastocyst formation and live births is approximately 50% lower than that of unfrozen controls [31, 32]. Therefore, more studies need to be done to examine the cause of this lower developmental competence. In addition, it is important to investigate other aspects of oocyte cryopreservation on oocyte physiology, particularly on components that are necessary for IVM. Defects in cytoplasmic structures and meiotic competence that arise due to cryopreservation could contribute to the low success rates following oocyte freezing.
During maturation, the oocyte undergoes many cytoplasmic changes that prepare it for successful fertilization and early embryonic development. One of these changes involves the development of the ability of the mature oocyte to release Ca2+ in response to sperm penetration [34–37]. Ca2+ release at fertilization is responsible for preventing polyspermy and for stimulating the oocyte to complete meiosis and to begin early development [38]. An important component of the Ca2+ release system is the endoplasmic reticulum (ER), which is a continuous membranous network throughout the egg that is the major site of Ca2+ storage [39–43]. The ER undergoes a dramatic change during oocyte maturation, such that clusters of ER form in the mature oocyte's cortex, and this reorganization is thought to be associated with the ability of the oocyte to release Ca2+ at fertilization [36, 44]. The objectives of this study were to examine the meiotic competence of vitrified oocytes, the ability of oocytes to develop to the MII stage following IVM, and the ability of immature oocytes to develop Ca2+ release mechanisms during oocyte maturation. We also used the structure of the ER as a cytoplasmic indicator to show the effects of vitrification on the ability of the ER to reorganize following IVM.
MATERIALS AND METHODS
Preparation and Culture of Gametes
All experiments were performed in accord with the Center for Laboratory Animal Care at the University of Connecticut Health Center. Except where noted, all chemicals were purchased from Sigma (St. Louis, MO).
CF1 mice (Harlan Sprague-Dawley, Indianapolis, IN) were used for all experiments, and oocytes and sperm were collected as previously described [45]. In brief, fully grown GV-stage oocytes were obtained from the ovaries of 6- to 10-wk-old female mice that had been primed 40–46 h earlier with 10 IU equine chorionic gonadotropin (eCG). Cumulus cells were removed by repeated pipetting through a small-bore pipette. Oocytes were cultured in 200-μl drops of medium under light mineral oil (Fisher Scientific, Pittsburgh, PA) at 37°C. The collection medium was modified Eagle medium (MEM) α (No. 12000–022; Invitrogen, Carlsbad, CA) supplemented with 20 mM Hepes, 75 μg/ml penicillin G, 50 μg/ml streptomycin sulfate, 0.1% polyvinyl alcohol, and 250 μM dibutyryl (db) cAMP to prevent spontaneous oocyte maturation. To initiate meiotic maturation, oocytes were washed into medium without dbcAMP. For overnight cultures, oocytes were incubated in bicarbonate-buffered MEMα in which 25 mM sodium bicarbonate was substituted for the Hepes and 5% fetal bovine serum (FBS) (Invitrogen) was substituted for the polyvinyl alcohol. Oocytes were assessed for the meiotic stage (MII, GV breakdown [GVBD], or GV intact) after 18–20 h.
Metaphase II-stage oocytes were collected from the ampullae of the oviduct from mice that were superovulated with 10 IU eCG, followed by 10 IU human chorionic gonadotropin 44–48 h later. Oocytes were collected in Hepes-buffered MEMα, and cumulus cells were removed using 0.3 mg/ml hyaluronidase (type IV-S). Oocytes were used on the same day they were collected.
Sperm were collected from the caudal epididymides and vas deferens of ≥12-wk-old CF1 mice in IVF medium [46] containing 3% bovine serum albumin (BSA) (fraction V; Calbiochem, La Jolla, CA). Sperm were capacitated for 1–2 h in a humidified atmosphere of 5% CO2/95% air before insemination. For insemination, sperm were diluted to a final concentration of ∼2–5 × 105 sperm/ml. Sperm and oocytes were incubated together for 1.5–2 h, and then the oocytes were washed out and incubated further in bicarbonate-buffered MEMα at 37°C in a humidified 5% CO2 incubator. Fertilized oocytes were observed periodically for the presence of second polar bodies and pronuclei.
Oocyte Vitrification and Warming
For vitrification, isolated GV-stage oocytes were placed into IVF medium supplemented with 20% FBS (holding medium) for ∼1 min. The oocytes were washed into holding medium containing 1.0 M ethylene glycol and 1.0 M 1,2-propanediol for 5 min and were then transferred to vitrification medium composed of holding medium containing 2.0 M ethylene glycol, 2.0 M 1,2-propanediol, and 0.5 M sucrose for 5 min. Oocytes were loaded onto the center of a vapor-chilled polyethylene terephthalate plastic strip (thickness, ∼100–130 μm) cut from the wall of a 0.5-L Poland Spring Water (Wilkes Barre, PA) bottle. The initial volume of the drops containing the oocytes was ∼1–2 μl; then the oocytes were spread out on the slide, and the remaining medium was removed using a small-bore pipette, such that the final volume on the strip was <1 μl. The strips were then plunged directly into liquid nitrogen and placed in cryovials for storage in liquid nitrogen. All steps before vitrification were carried out at 37°C.
For warming, the strips containing the vitrified oocytes were removed from liquid nitrogen and immediately immersed in holding medium containing 0.5 M sucrose for 1 min. Oocytes were transferred to holding medium containing 0.25 M sucrose for 3 min and then were transferred to holding medium containing 0.125 M sucrose for 3 min. All steps during warming were carried out at 37°C. Oocytes were washed twice in holding medium and twice in culture medium. The oocytes were then cultured in a 37°C incubator with 5% CO2/95% air.
Microinjection, Confocal Microscopy, and Ca2+ Measurements
Quantitative microinjection was performed as previously described using mercury-filled micropipettes [45]. A saturated solution of DiI (1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Invitrogen) was prepared in soybean oil (Wesson Oil; ConAgra Foods, Inc., Memphis, TN) and stored at 4°C. The DiI solution was front-loaded into a beveled mercury-filled pipette connected to a micrometer syringe system filled with Fluorinert FC-70 (Sigma). The same pipette was used to inject several oocytes with ∼2.5–5 pl of solution, which formed an oil droplet inside the oocytes. This 2-fold range in the amount of DiI did not affect the qualitative results to be described. The volume injected was calculated based on the diameter of the sphere that forms in the oocyte cytoplasm during microinjection. DiI-labeled GV-stage and MII-stage oocytes were observed using a confocal microscope (Pascal; Carl Zeiss Microimaging, Inc., Thornwood, NY) ∼30 min to 2 h after microinjection. Fluorescence was excited with the 543-nm line of a HeNe laser and was detected using a 560-nm emission filter. Images were collected using a 40× NA 1.2 water immersion objective (C-Apochromat; Carl Zeiss MicroImaging, Inc.). Cortical ER clusters were evaluated by examining the pattern of DiI labeling at the bottom edge of the cortex, just beneath the plasma membrane. The optical section thickness was ∼2 μm. The presence of cortical clusters, as defined by distinct aggregations of ER that were ≥0.5 μm in diameter, was evaluated blindly by two different people.
Injected concentrations of calcium green 10-kDa dextran (Invitrogen) and inositol 1,4,5-trisphosphate (IP3; Calbiochem) were based on an oocyte volume of 200 pl. Ca2+ measurements were performed as previously described [46] using a photodiode with a built-in amplifier (Oriel Instruments, Stratford, CT) mounted on an inverted microscope and connected to a chart recorder. Figures were made using Adobe Photoshop (Adobe Systems Inc., San Jose, CA) after scanning the chart records into a computer.
Immunofluorescence
In vitro-matured oocytes were fixed for 1 h in 2% formaldehyde in 100 mM Hepes, 50 mM ethyleneglycoltetracetic acid, 10 mM MgSO4, and 0.2% Triton X-100 at 37°C. After fixation, oocytes were washed into PBS containing 1% Triton-X, incubated for 5 h at 4°C, and then washed into blocking buffer (PBS containing 0.01% Triton X-100, 0.1% polyvinyl alcohol, and 3% BSA) for 15 min. Oocytes were incubated in primary antibody (anti-tubulin, YL1/2; Serotec Inc., Raleigh, NC) diluted 1:100 in blocking buffer overnight at room temperature. Following primary antibody incubation, oocytes were washed with blocking buffer and incubated for 2 h with secondary antibody (Alexa Fluor 488-conjugated anti-rat IgG; Invitrogen) diluted 1:200 in blocking buffer. Oocytes were then washed in PBS. In the first wash, 10 μg/ml Hoechst 33528 (Invitrogen) was included and incubated for 15 min. Labeled oocytes were observed with the 40× NA 1.2 lens on a Zeiss 510 confocal microscope (Carl Zeiss Microimaging, Inc.). Fluorescence was excited at 488 nm for tubulin and at 364 nm for Hoechst and was detected at 505 nm and 435–485 nm for tubulin and Hoechst, respectively.
Statistical Analysis
Tests of statistical significance were performed using InStat software (GraphPad Software, Inc., San Diego, CA). Differences between groups were determined by Student t-test or Fisher exact test. P < 0.05 was considered statistically significant.
RESULTS
Vitrified GV-Stage Oocytes Are Morphologically Normal and Are Meiotically Competent
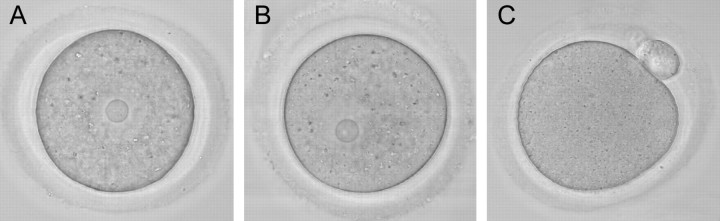
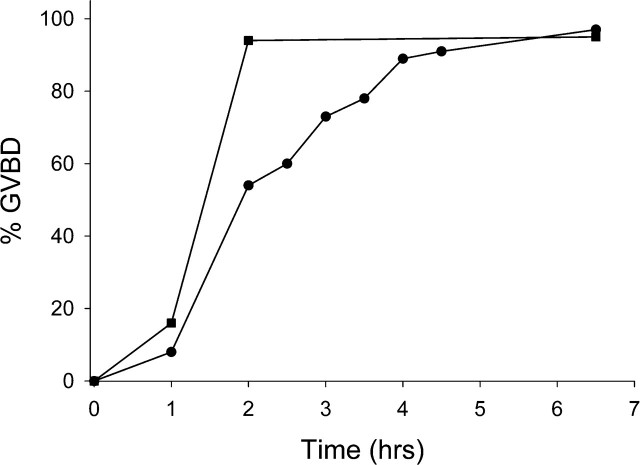
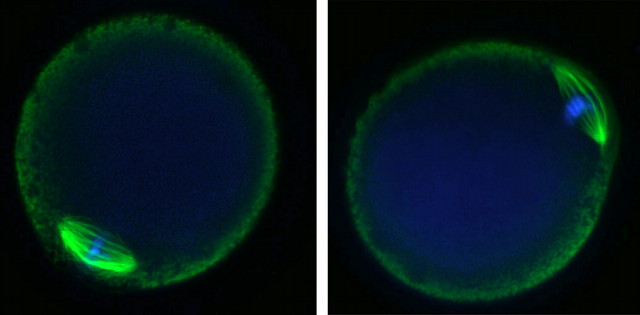
Vitrified, warmed (hereafter referred to as vitrified) GV-stage oocytes (n = 265) were morphologically indistinguishable from freshly isolated oocytes when examined live by transmitted light microscopy (Fig. 1). After washing dbcAMP from the culture medium, the vitrified oocytes resumed meiotic maturation, underwent GVBD, and extruded a first polar body (Fig. 1). The time to GVBD in vitrified oocytes was slightly slower than that in control fresh oocytes, with only 54% of vitrified oocytes undergoing GVBD within 2 h compared with 94% of controls (Fig. 2). However, by 6.5 h after removal of dbcAMP, similar numbers of oocytes in the vitrified (n = 89) and control (n = 400) groups had undergone GVBD (97% and 95%, respectively) (Fig. 2), demonstrating that almost all of the vitrified oocytes were meiotically competent. Seventy-eight percent of those GV-stage oocytes (n = 253) that underwent GVBD went on to extrude first polar bodies compared with 80% of fresh control oocytes (n = 221). Vitrified oocytes (n = 12) that were matured in vitro to the MII stage formed MII spindles that were morphologically identical to those of freshly isolated in vivo-matured MII-stage oocytes (n = 21) (92% vs. 95%) (Fig. 3).
FIG. 1.
Vitrified GV-stage oocytes are morphologically indistinguishable from fresh oocytes and mature to the MII stage in culture. A) Fresh oocyte. B) Vitrified oocyte. C) Vitrified MII-stage oocyte following IVM. Note GVBD and first polar body formation. Representative photographs from 265 vitrified oocytes are shown in B and C. Original magnification ×120.
FIG. 2.
Time course of GVBD following vitrification and in vitro maturation. Fresh (n = 400) or frozen (n = 89) oocytes were incubated in medium containing dbcAMP, and the time to GVBD was noted after washing the dbcAMP out of the culture medium. Black squares, control oocytes; black circles, vitrified oocytes.
FIG. 3.
In vitro-matured oocytes form morphologically normal MII spindles. Left: in vivo-matured MII-stage oocyte. Right: vitrified in vitro-matured MII-stage oocyte. Green, tubulin; blue, chromosomes. Original magnification ×120.
Vitrified Oocytes Develop the Ability to Release Ca2+ in Response to IP3 Following In Vitro Maturation and Are Fertilizable
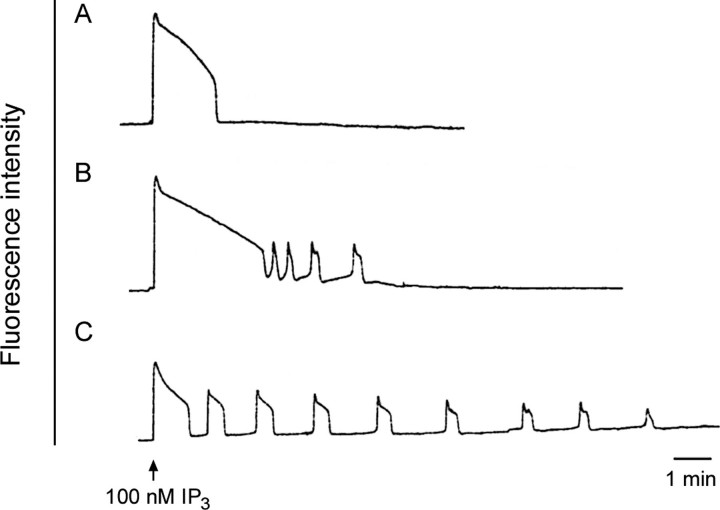
Release of intracellular Ca2+ at fertilization is critical for polyspermy prevention, meiotic resumption, and initiation of early embryonic development. The ability to release Ca2+ develops during oocyte maturation; immature GV-stage oocytes are unable to release a comparable amount of Ca2+ as mature oocytes at the MII stage [35, 36]. To examine if vitrified oocytes matured in vitro can initiate a normal pattern of Ca2+ release in response to a physiological stimulus, we injected oocytes with the Ca2+-sensitive indicator dye calcium green dextran and monitored intracellular Ca2+ during injection of 100 nM IP3. This concentration of IP3 has previously been shown to induce a transient Ca2+ release in MII-stage oocytes that is occasionally followed by a series of repetitive Ca2+ oscillations lasting ∼10–15 min [36]. Injection of IP3 into freshly ovulated MII-stage oocytes stimulated the following two types of Ca2+ release: (1) a single transient (Fig. 4A) or (2) an initial Ca2+ transient that is followed by one or more oscillations (Fig. 4B). IP3 injection into vitrified oocytes that were matured in vitro also stimulated Ca2+ release, with an initial Ca2+ transient that was followed by repetitive Ca2+ oscillations that were more prolonged than in fresh in vivo-matured MII-stage oocytes (Fig. 4C). The mean ± SD duration of the first transients was not significantly different between the freshly ovulated and vitrified in vitro-matured GV-stage oocytes (2.3 ± 0.5 and 1.6 ± 0.2 min, respectively; P > 0.05). However, the average number of transients was significantly higher in the vitrified group, with an average of eight oscillations vs. two oscillations in freshly ovulated MII-stage oocytes. The amplitude of the first transient above baseline was variable but did not differ significantly between the two groups. Although the number of oscillations differed between the fresh and vitrified groups, these data show that vitrified in vitro-matured oocytes can produce a series of Ca2+ transients in response to IP3.
FIG. 4.
Vitrified oocytes matured in vitro develop the ability to release Ca2+ in response to IP3. Oocytes were injected with the Ca2+ indicator dye calcium green 10-kDa dextran. The fluorescence intensity showing the relative Ca2+ level in the oocyte cytoplasm was measured during a subsequent injection of IP3 (100 nM total in the oocyte). A and B) Representative tracings from control freshly ovulated MII-stage oocytes (n = 8). C) Representative tracing from vitrified oocytes matured in vitro (n = 5).
We also fertilized vitrified in vitro-matured MII-stage oocytes and examined the presence of second polar bodies and pronuclei (Table 1). For these experiments, we used zona-free oocytes because of previous evidence that the zona can harden in response to vitrification [3, 10, 11, 15, 47]. Consistent with these observations, we found that it was necessary to remove the zonae before vitrification, as we were unable to remove them with acid Tyrode or chymotrypsin following vitrification. Eight-six percent of control freshly ovulated MII-stage oocytes (n = 35) formed second polar bodies within 2 h after insemination, and 87% of those oocytes with second polar bodies went on to form pronuclei (Table 1). Similarly, 72% of control (unfrozen) in vitro-matured MII-stage oocytes (n = 29) formed second polar bodies, and 67% of those went on to form pronuclei. Vitrified oocytes (n = 43) that were matured in vitro had 67% polar body formation, and 76% of those formed pronuclei (Table 1). No statistical differences were found among any of these groups. These results show that vitrified in vitro-matured oocytes are able to initiate the early events of egg activation following fertilization and, by extension, suggest that the Ca2+ releasing ability of vitrified oocytes is functional because of this.
TABLE 1.
Comparison of second polar body and pronuclear formation in fresh or vitrified oocytes at the MII stage.*
The Continuity of the ER Is Preserved Following Oocyte Vitrification
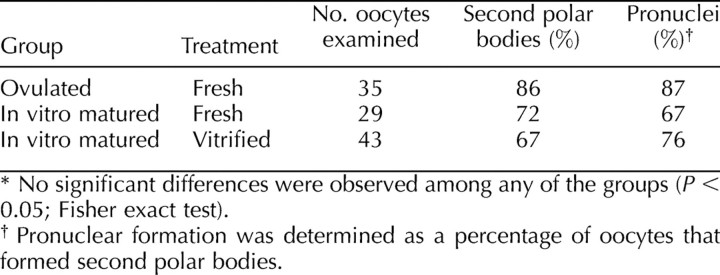
To examine the effects of cryopreservation on ER structure, we observed the structure of the ER following vitrification. The ER was visualized using the lipophilic fluorescent dye DiI and confocal microscopy [40–44, 47–49]. To label the ER, DiI was prepared as a saturated solution in soybean oil and microinjected into the oocyte, where it contacts intracellular membranes. Because the ER is a continuous network, the dye will spread throughout the entire ER if it is intact. As reported previously [40, 48], control, unfrozen GV-stage oocytes contained a fine reticular network throughout the oocyte that had no distinct clusters of ER in the cortex (Fig. 5, A and B). Clusters of ER were present throughout the oocyte interior (Fig. 5B). The ER in vitrified GV-stage oocytes remained intact, as indicated by dye spreading, and the structure was similar to controls, with no distinct ER clusters in the oocyte cortex but with clusters throughout the cytoplasm (Fig. 5, C and D).
FIG. 5.
The ER structure in GV-stage oocytes. The ER was labeled using the lipophilic fluorescent dye DiI. A and B) Representative photograph from 25 fresh oocytes showing a continuous ER but the absence of cortical ER clusters that are characteristic of MII-stage oocytes [40, 48] in the cortex (A) and equator (B). C and D) Representative photograph from 19 vitrified oocytes showing the absence of cortical clusters in sections of the cortex (C) and equator (D). Bar = 5 μm (A and C) and 10 μm for (B and D).
Optimization of Culture Conditions to Obtain ER Reorganization During In Vitro Maturation
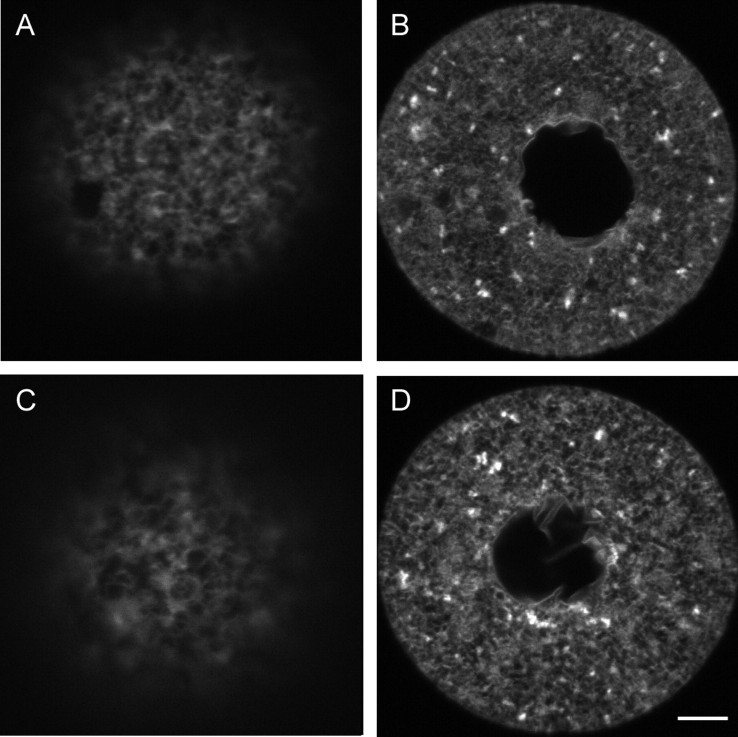
The ER undergoes dramatic reorganization during meiotic maturation in vivo, such that large clusters of ER form in the cortex opposite the meiotic spindle [39] (Fig. 6A). Therefore, we next examined the ability of the ER to reorganize during spontaneous maturation of fresh (unfrozen) oocytes in vitro. Unexpectedly, we found that only 42% of the oocytes cultured in MEMα (n = 48) formed distinct cortical ER clusters following IVM, while the rest contained no apparent ER clusters (Table 2). Because of this, we next examined the ER structure in oocytes that were matured in a different culture medium, CZB [50]. In contrast to oocytes matured in MEMα, 78% of oocytes matured in CZB (n = 46) formed cortical ER clusters (Fig. 6B and Table 2) compared with 94% of freshly ovulated MII-stage oocytes (n = 18) (Table 2). This is not significantly different from the number of cortical clusters present in freshly ovulated MII-stage oocytes.
FIG. 6.
The structure of the ER following in vitro maturation in fresh oocytes. A) Distinct ER clusters in an in vivo-matured oocyte are shown for reference. B and C) Distinct ER clusters were found in the majority of oocytes matured in CZB medium (B) but were sometimes absent (C). Bar = 5 μm.
TABLE 2.
Comparison of cortical endoplasmic reticulum clusters in oocytes at the MII stage.*
Because CZB contained 20% FBS and our standard MEMα medium contained only 5% serum, we examined whether the amount of serum in the culture medium affected the formation of cortical clusters during in vitro maturation. Sixty-eight percent of oocytes (n = 47) matured in MEMα containing 20% FBS exhibited cortical ER clusters (Table 2). This percentage was not significantly different from that of CZB-matured oocytes but is somewhat lower than the percentage of clusters present in freshly ovulated MII-stage oocytes. These data show that the amount of serum and the particular culture medium used are important factors for successful reorganization of the ER during in vitro maturation of mouse oocytes.
Cryopreservation Adversely Affects the Reorganization of Cortical ER Clusters During In Vitro Maturation
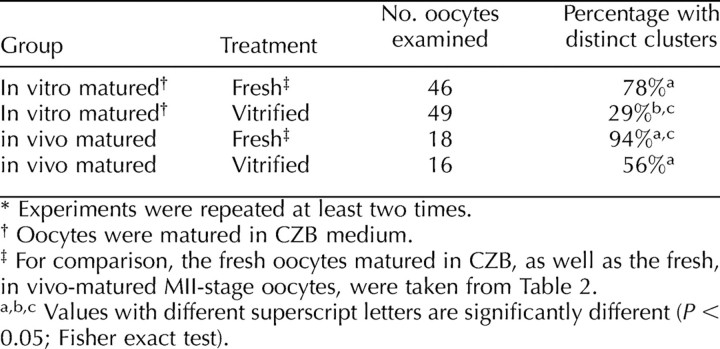
We next examined the formation of ER clusters in oocytes that were vitrified before IVM. For these experiments, we matured vitrified oocytes in CZB medium, as oocytes matured in CZB most closely resembled those of in vivo-matured oocytes. We found that a significantly lower percentage, only 29%, of vitrified oocytes had cortical ER clusters following in vitro maturation compared with 78% of controls (n = 46) (Table 3). This result was unexpected because the continuity of the ER was not disrupted in immature oocytes following vitrification (Fig. 5B) and suggests that some components necessary for the reorganization and stability of the ER during oocyte maturation are disrupted by the vitrification process. These factors could contribute to the lower developmental competence of vitrified in vitro-matured oocytes that has been reported previously [30, 31]. We also examined if vitrification affects the structure of cortical ER clusters in vitrified in vivo-matured MII-stage oocytes. Significantly fewer of these oocytes had cortical ER clusters compared with fresh oocytes (56% vs. 94%) (Table 3), showing that this process can also interfere with the ER organization in mature oocytes that have previously formed ER clusters.
TABLE 3.
Comparison of cortical endoplasmic reticulum clusters in in vivo- and in vitro-matured oocytes following vitrification.*
DISCUSSION
In this study, we confirmed previous reports showing that mouse oocytes cryopreserved at the GV stage can be vitrified to yield morphologically normal GV-stage oocytes, that they mature at a high rate in vitro after vitrification, and that they subsequently form morphologically normal bipolar meiotic spindles. We then extended previous studies by examining if cytoplasmic structure is preserved during the vitrification procedure. We found that the structure of the ER remains intact during vitrification of GV-stage oocytes, indicating that this process does not damage these intracellular membranes. In addition, oocytes vitrified at the GV stage and matured in vitro are capable of releasing Ca2+ in response to IP3 and can be fertilized. Because the ability to release Ca2+ develops during oocyte maturation, vitrification does not appear to adversely affect this important component of cytoplasmic maturation. However, the reorganization of the ER that normally occurs during oocyte maturation was impaired by this process. Although vitrification of immature oocytes followed by in vitro maturation yields morphologically normal oocytes that exhibit some of the properties of early development following fertilization, cytoplasmic maturation is disrupted, and this could account for the reduced developmental potential observed previously following vitrification and in vitro maturation [23, 25, 28–31].
During meiotic maturation in vivo, the ER undergoes reorganization, such that clusters of ER form in the oocyte cortex opposite the meiotic spindle [41, 44]. However, we found that the majority of oocytes that were not vitrified but were matured in our standard MEMα medium did not exhibit ER clusters in the mature oocyte cortex (Fig. 6 and Table 2). In contrast, the ER in oocytes matured in CZB and in MEMα containing 20% FBS reorganized into distinct cortical clusters, similar to those seen in freshly ovulated MII-stage oocytes (Fig. 6B). These latter results are in agreement with a recent study [44] that showed normal pattern and size of cortical ER accumulations in the mouse oocyte cortex following in vitro maturation, although this study did not indicate the percentage of oocytes that formed cortical ER. It is not clear in our study what effect an increased amount of FBS in the culture medium has on the ability of oocytes to exhibit ER reorganization during meiotic maturation. However, the difference in ER structure following IVM in different culture media herein underscores the importance of establishing culture conditions that yield consistent results during IVM before cryopreservation. The ability to form normal ER clusters that are retained after cryopreservation and in vitro maturation could be used as one criterion to assay how closely various culture conditions simulate in vivo meiotic maturation.
The structure of the ER in GV-stage oocytes was similar between control and vitrified oocytes, with continuous ER throughout the oocyte and around the GV in both groups, as well as aggregations of ER throughout the cytoplasm (Fig. 5). The ability of the ER to remain a continuous network throughout the vitrification process indicates that at least one important component of the cytoplasmic structure can remain intact following cryopreservation. However, the ability of the ER to reorganize into cortical ER clusters following vitrification and in vitro maturation was impaired, even in oocytes matured in CZB. This suggests that other components of cytoplasmic maturation could be affected by the vitrification process. The spindle morphology observed following vitrification and IVM appeared normal, suggesting that microtubules are possibly unaffected by this procedure. In oocytes maturing in vitro, microtubules are important for ER reorganization during GVBD, but microfilaments have been shown to be necessary for ER remodeling into cortical clusters [44]. It is possible that microfilaments or other proteins needed for actin polymerization could be disrupted by vitrification, such that they are no longer able to regulate ER reorganization during maturation. Moreover, a few studies [51–53] have shown that vitrification is detrimental to microfilament structure in bovine and porcine oocytes. To date, it is unknown what the role of microfilaments might be for ER reorganization. Other unknown proteins needed for the process of ER reorganization could also be impaired.
It is also possible that microtubules are damaged during vitrification, at least initially. This could explain why fewer oocytes vitrified at the MII stage displayed cortical ER clusters compared with fresh MII-stage oocytes, as the ER and microtubules are interdependent structures [54]. Some studies [4, 55, 56] have shown that, although microtubules are disrupted immediately after cryopreservation of MII-stage oocytes, they are able to reform after culturing at 37°, such that morphologically normal meiotic spindles become apparent. In our study, we examined the ER in vitrified MII-stage oocytes 1–2 h after oocyte warming. Even if microtubules are disrupted and a spindle is able to reform within a few hours after oocyte warming, it is possible that the association between the ER and microtubules does not reestablish within this time. However, other findings show no disruption of microtubules following cryopreservation [7]. Nevertheless, disruption of microtubules could contribute to the lack of cortical clusters seen in vitrified MII-stage oocytes and could help explain why cortical clusters do not form in vitrified oocytes following in vitro maturation of GV-stage oocytes.
Despite the inability of the majority of oocytes cultured in MEMα containing 5% FBS to form cortical ER clusters during IVM, vitrified in vitro-matured oocytes released Ca2+ on injection of IP3, which is the physiological stimulus for Ca2+ release at fertilization [57]. Indeed, these oocytes exhibited more transients than in vivo-matured freshly ovulated MII-stage oocytes (Fig. 4). Because Ca2+ release is essential for several aspects of successful fertilization, including polyspermy prevention, completion of meiosis, and early development [38], it is critical for vitrified/in vitro-matured oocytes to develop the ability to release Ca2+ during oocyte maturation. While the ER is a major site of Ca2+ storage [39, 43] and IP3 receptors have been localized to the cortical ER clusters [37, 42], IP3 receptors are also located on the ER that does not form clusters [40]. Because the amount of IP3 receptor protein doubles during oocyte maturation [37], it is possible that the increased number of IP3 receptors provides the oocyte with enough Ca2+ releasing ability to allow for a normal pattern of Ca2+ release in response to IP3 following IVM, even in the absence of cortical ER clusters. Moreover, vitrified in vitro-matured oocytes formed second polar bodies and pronuclei, events that depend on release of intracellular Ca2+ [38].
Deficiencies in the ability of the ER to reorganize following IVM and/or other components of cytoplasmic maturation could help explain the low developmental rates reported by others following oocyte cryopreservation and IVM [23, 25, 28–31]. Additional causes of low developmental competence in some cryopreserved oocytes could be that the zona pellucida is modified, such that it hardens and prevents fertilization in some cases [3, 7, 11, 15, 47]. In this study, we were unable to remove zonae from oocytes following cryopreservation using acid Tyrode, suggesting that the zonae were modified by some aspect of the vitrification procedure. The cause of zona hardening could be the use of the cryoprotectants ethylene glycol and/or 1,2-propanediol, both of which have been shown to cause Ca2+ release when added to unfrozen oocytes [7, 11]. This, in turn, could cause premature release of cortical granules and modification of the zona [58] and the plasma membrane. The ability of zona-free oocytes to be fertilized in this study demonstrates that, even if release of cortical granules modified the zona pellucida, sperm interaction with the plasma membrane was not impaired by the vitrification procedure in most cases. Nevertheless, release of Ca2+ by cryoprotectants could potentially affect other aspects of egg activation [3].
Additional components of cytoplasmic maturation that are independent of cryopreservation could also contribute to the low developmental potential following in vitro maturation. For example, mouse oocytes matured in vitro have been shown to have lower mitogen-activated protein kinase activity than those in controls, and this could contribute to a higher rate of parthenogenetic activation and reduced developmental competence seen in in vitro-matured oocytes [59]. More studies will be needed to clarify the normal events of cytoplasmic maturation (e.g., the effects on other organelles and protein synthesis) to determine how well IVM mimics these events. Development of culture media that more accurately simulate these normal cytoplasmic events will be essential for obtaining in vitro-matured oocytes, whether cryopreserved or not, that closely resemble those of freshly ovulated oocytes.
In summary, our results show that cryopreservation of GV-stage mammalian oocytes can preserve intracellular membranes and that nuclear maturation of immature oocytes matured following vitrification appears to be normal. However, this technique disrupts the ability of the ER to reorganize during oocyte maturation, and this could contribute to the low developmental competence that has been observed following cryopreservation. Before this technique can become a reliable method for treating infertility, further studies will need to be performed to improve the process of IVM in fresh and frozen oocytes, as well as to assess other aspects of cytoplasmic maturation that might contribute to the developmental competence of in vitro-matured oocytes.
Acknowledgments
We thank Susan Krueger and Rachael Norris for help with the confocal microscope, Melina Schuh for providing the immunofluorescence protocol, and Laurinda Jaffe for helpful comments on the manuscript.
Footnotes
1Supported by grant HD056366 to L.M.M.
REFERENCES
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007; 22: 1506 1512 [DOI] [PubMed] [Google Scholar]
- Borini A, Cattoli M, Bulletti C, Coticchio G. Clinical efficiency of oocyte and embryo cryopreservation. Ann N Y Acad Sci 2008; 1127: 49 58 [DOI] [PubMed] [Google Scholar]
- Gardner DK, Sheehan CB, Rienzi L, Katz-Jaffe M, Larman MG. Analysis of oocyte physiology to improve cryopreservation procedures. Theriogenology 2007; 67: 64 72 [DOI] [PubMed] [Google Scholar]
- Gomes CM, Silva CA, Acevedo N, Baracat E, Serafini P, Smith GD. Influence of vitrification on mouse metaphase II oocyte spindle dynamics and chromatin alignment. Fertil Steril 2008; 90: 1396 1404 [DOI] [PubMed] [Google Scholar]
- Huang JY, Chen HY, Park JY, Tan SL, Chian RC. Comparison of spindle and chromosome configuration in in vitro- and in vivo-matured mouse oocytes after vitrification. Fertil Steril 2008; 90: 1424 1432 [DOI] [PubMed] [Google Scholar]
- Huang JY, Chen HY, Tan SL, Chian RC. Effect of choline-supplemented sodium-depleted slow freezing versus vitrification on mouse oocyte meiotic spindles and chromosome abnormalities. Fertil Steril 2007; 88: 1093 1100 [DOI] [PubMed] [Google Scholar]
- Larman MG, Katz-Jaffe MG, Sheehan CB, Gardner DK. 1,2-Propanediol and the type of cryopreservation procedure adversely affect mouse oocyte physiology. Hum Reprod 2007; 22: 250 259 [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Ng SC, Trounson AO, Bongso A, Ratnam SS, Ho J, Mok H, Lee MN. The effects of ultrarapid freezing on meiotic and mitotic spindles of mouse oocytes and embryos. Gamete Res 1988; 21: 385 401 [DOI] [PubMed] [Google Scholar]
- Zenzes MT, Bielecki R, Casper RF, Leibo SP. Effects of chilling to 0 degrees C on the morphology of meiotic spindles in human metaphase II oocytes. Fertil Steril 2001; 75: 769 777 [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev 2001; 58: 342 347 [DOI] [PubMed] [Google Scholar]
- Larman MG, Sheehan CB, Gardner DK. Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction 2006; 131: 53 61 [DOI] [PubMed] [Google Scholar]
- Carroll J, Wood MJ, Whittingham DG. Normal fertilization and development of frozen-thawed mouse oocytes: protective action of certain macromolecules. Biol Reprod 1993; 48: 606 612 [DOI] [PubMed] [Google Scholar]
- Coticchio G, Bonu MA, Sciajno R, Sereni E, Bianchi V, Borini A. Truths and myths of oocyte sensitivity to controlled rate freezing. Reprod Biomed Online 2007; 15: 24 30 [DOI] [PubMed] [Google Scholar]
- Endoh K, Mochida K, Ogonuki N, Ohkawa M, Shinmen A, Ito M, Kashiwazaki N, Ogura A. The developmental ability of vitrified oocytes from different mouse strains assessed by parthenogenetic activation and intracytoplasmic sperm injection. J Reprod Dev 2007; 53: 1199 1206 [DOI] [PubMed] [Google Scholar]
- Ko CS, Ding DC, Chu TW, Chu YN, Chen IC, Chen WH, Wu GJ. Changes to the meiotic spindle and zona pellucida of mature mouse oocytes following different cryopreservation methods. Anim Reprod Sci 2008; 105: 272 282 [DOI] [PubMed] [Google Scholar]
- Larman MG, Minasi MG, Rienzi L, Gardner DK. Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod Biomed Online 2007; 15: 692 700 [DOI] [PubMed] [Google Scholar]
- Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril 2006; 86: 1277 1291 [DOI] [PubMed] [Google Scholar]
- Suikkari AM, Söderström-Anttila V. In-vitro maturation of eggs: is it really useful? Best Pract Res Clin Obstet Gynaecol 2007; 21: 145 155 [DOI] [PubMed] [Google Scholar]
- Holzer H, Scharf E, Chian RC, Demirtas E, Buckett W, Tan SL. In vitro maturation of oocytes collected from unstimulated ovaries for oocyte donation. Fertil Steril 2007; 88: 62 67 [DOI] [PubMed] [Google Scholar]
- Le Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier MC, Chevalier N, Fanchin R, Chian RC, Tachdjian G, Frydman R, Frydman N. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod 2005; 20: 420 424 [DOI] [PubMed] [Google Scholar]
- Mikkelsen AL. Strategies in human in-vitro maturation and their clinical outcome. Reprod Biomed Online 2005; 10: 593 599 [DOI] [PubMed] [Google Scholar]
- Söderstrom-Anttila V, Makinen S, Tuuri T, Suikkari AM. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod 2005; 20: 1534 1540 [DOI] [PubMed] [Google Scholar]
- Abe Y, Hara K, Matsumoto H, Kobayashi J, Sasada H, Ekwall H, Rodriguez-Martinez H, Sato E. Feasibility of a nylon-mesh holder for vitrification of bovine germinal vesicle oocytes in subsequent production of viable blastocysts. Biol Reprod 2005; 72: 1416 1420 [DOI] [PubMed] [Google Scholar]
- Diez C, Duque P, Gomez E, Hidalgo CO, Tamargo C, Rodriguez A, Fernandez L, de la Varga S, Fernandez A, Facal N, Carbajo M. Bovine oocyte vitrification before or after meiotic arrest: effects on ultrastructure and developmental ability. Theriogenology 2005; 64: 317 333 [DOI] [PubMed] [Google Scholar]
- Kubota C, Yang X, Dinnyes A, Todoroki J, Yamakuchi H, Mizoshita K, Inohae S, Tabara N. In vitro and in vivo survival of frozen-thawed bovine oocytes after IVF, nuclear transfer, and parthenogenetic activation. Mol Reprod Dev 1998; 51: 281 286 [DOI] [PubMed] [Google Scholar]
- Men H, Monson RL, Rutledge JJ. Effect of meiotic stages and maturation protocols on bovine oocyte's resistance to cryopreservation. Theriogenology 2002; 57: 1095 1103 [DOI] [PubMed] [Google Scholar]
- Otoi T, Yamamoto K, Koyama N, Suzuki T. In vitro fertilization and development of immature and mature bovine oocytes cryopreserved by ethylene glycol with sucrose. Cryobiology 1995; 32: 455 460 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Boediono A, Takagi M, Saha S, Sumantri C. Fertilization and development of frozen-thawed germinal vesicle bovine oocytes by a one-step dilution method in vitro. Cryobiology 1996; 33: 515 524 [DOI] [PubMed] [Google Scholar]
- Vieira AD, Forell F, Feltrin C, Rodrigues JL. Calves born after direct transfer of vitrified bovine in vitro-produced blastocysts derived from vitrified immature oocytes. Reprod Domest Anim 2008; 43: 314 318 [DOI] [PubMed] [Google Scholar]
- Vieira AD, Mezzalira A, Barbieri DP, Lehmkuhl RC, Rubin MI, Vajta G. Calves born after open pulled straw vitrification of immature bovine oocytes. Cryobiology 2002; 45: 91 94 [DOI] [PubMed] [Google Scholar]
- Aono N, Abe Y, Hara K, Sasada H, Sato E, Yoshida H. Production of live offspring from mouse germinal vesicle-stage oocytes vitrified by a modified stepwise method, SWEID. Fertil Steril 2005; 84 (suppl 2): 1078 1082 [DOI] [PubMed] [Google Scholar]
- Aono N, Naganuma T, Abe Y, Hara K, Sasada H, Sato E, Yoshida H. Successful production of blastocysts following ultrarapid vitrification with step-wise equilibriation of germinal vesicle-stage mouse oocytes. J Reprod Dev 2003; 49: 501 506 [DOI] [PubMed] [Google Scholar]
- Eroglu A, Toner M, Leykin L, Toth TL. Cytoskeleton and polyploidy after maturation and fertilization of cryopreserved germinal vesicle-stage mouse oocytes. J Assist Reprod Genet 1998; 15: 447 454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Nakada K, Shirakawa H, Miyazaki S. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev Biol 1993; 156: 69 79 [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem 1995; 270: 6671 6677 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod 1994; 51: 1088 1098 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol 1996; 180: 489 498 [DOI] [PubMed] [Google Scholar]
- Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol 2006; 17: 324 332 [DOI] [PubMed] [Google Scholar]
- Han JK, Nuccitelli R. Inositol 1,4,5-trisphosphate-induced calcium release in the organelle layers of the stratified, intact egg of Xenopus laevis. J Cell Biol 1990; 110: 1103 1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Mehlmann L, Fox C, Terasaki M. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol 1999; 215: 431 442 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol 1995; 170: 607 615 [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol 1995; 170: 594 606 [DOI] [PubMed] [Google Scholar]
- Terasaki M, Sardet C. Demonstration of calcium uptake and release by sea urchin egg cortical endoplasmic reticulum. J Cell Biol 1991; 115: 1031 1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol 2007; 305: 133 144 [DOI] [PubMed] [Google Scholar]
- Kline D. Quantitative microinjection of mouse eggs. Methods Mol Biol 2009; 518: 135 156 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cgamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol 1998; 203: 221 232 [DOI] [PubMed] [Google Scholar]
- Carroll J, Depypere H, Matthews CD. Freeze-thaw-induced changes of the zona pellucida explains decreased rates of fertilization in frozen-thawed mouse oocytes. J Reprod Fertil 1990; 90: 547 553 [DOI] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA. Imaging endoplasmic reticulum in living sea urchin eggs. Methods Cell Biol 1993; 38: 211 220 [DOI] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA. Labeling of cell membranes and compartments for live cell fluorescence microscopy. Methods Cell Biol 2004; 74: 469 489 [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679 688 [DOI] [PubMed] [Google Scholar]
- Albarracin JL, Morato R, Rojas C, Mogas T. Effects of vitrification in open pulled straws on the cytology of in vitro matured prepubertal and adult bovine oocytes. Theriogenology 2005; 63: 890 901 [DOI] [PubMed] [Google Scholar]
- Rojas C, Palomo MJ, Albarracin JL, Mogas T. Vitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology 2004; 49: 211 220 [DOI] [PubMed] [Google Scholar]
- Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, Lu X, Zheng X. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev 2006; 73: 1454 1462 [DOI] [PubMed] [Google Scholar]
- Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol 1986; 103: 1557 1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu A, Toth TL, Toner M. Alterations of the cytoskeleton and polyploidy induced by cryopreservation of metaphase II mouse oocytes. Fertil Steril 1998; 69: 944 957 [DOI] [PubMed] [Google Scholar]
- Rienzi L, Martinez F, Ubaldi F, Minasi MG, Iacobelli M, Tesarik J, Greco E. Polscope analysis of meiotic spindle changes in living metaphase II human oocytes during the freezing and thawing procedures. Hum Reprod 2004; 19: 655 659 [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol 2002; 245: 237 254 [DOI] [PubMed] [Google Scholar]
- Ghetler Y, Skutelsky E, Ben Nun I, Ben Dor L, Amihai D, Shalgi R. Human oocyte cryopreservation and the fate of cortical granules. Fertil Steril 2006; 86: 210 216 [DOI] [PubMed] [Google Scholar]
- Combelles CM, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod 2005; 20: 1349 1358 [DOI] [PubMed] [Google Scholar]