Abstract
The differentiation of uterine stromal fibroblasts into decidual cells is critical for establishing pregnancy. This process, called decidualization, requires the reorganization of the actin cytoskeleton, which mainly depends on actin dynamics and the phosphorylation status of the myosin light chain. We manipulated actin dynamics with jasplakinolide (100 nM) and latrunculin B (1 μM), both of which significantly inhibited the synthesis of decidualization markers induced by 6 days of treatment with embryo-mimicking stimulus interleukin 1beta (IL1B) and steroid hormones (SHs; 17beta-estradiol and medroxyprogesterone acetate) in the human uterine fibroblast (HuF) in vitro model. However, only jasplakinolide had long-lasting effects on the G-actin:F-actin ratio and prevented decidualization induced by the artificial stimulus cAMP (and SHs). Actin-binding protein cofilin mainly colocalized with G-actin in the nucleus as well as the cytoplasm. Only some spots of colocalization between cofilin and F-actin were detected in the cytoplasm. Brief extraction of cytosolic proteins from living cells revealed that in cells treated with IL1B or cAMP (and SHs) for 6 days, cofilin was mainly detected in the nucleus. The translocation of cofilin from cytosol to nucleus was also detected in HuFs treated for 12 days with SHs, IL1B and SHs, and cAMP and SHs. The same significant translocation was confirmed in primary baboon stromal uterine fibroblasts. We conclude that changes in actin dynamics, particularly the stabilization of F-actin, have a significant negative impact on decidualization, and the translocation of cofilin to the nucleus is a key feature of this process in the primate.
Keywords: actin dynamics, baboon, cofilin, cytoskeleton, decidua, decidualization, human, uterus
The manipulation of actin dynamics, and stabilization of F-actin, has significant negative impact on decidualization; the translocation of the actin-binding protein cofilin to the nucleus is an important feature of this process.
INTRODUCTION
The cytoskeleton plays a crucial role in basic cellular processes, such as mitosis, growth, motility, aging, smooth muscle contraction, and apoptosis (see review in Ayscough and Winder [1]). Decidualization (the differentiation of uterine fibroblasts into decidual cells) is critical to the successful establishment and maintenance of pregnancy in humans. It represents a complex cell transformation that requires morphological and functional changes in cell structure and physiology and involves the reorganization of the cytoskeleton. Proper cytoskeletal organization and function depend on cytoskeletal dynamics—that is, the interactions between actin and myosin II. Cytoskeletal dynamics have been implicated previously in decidualization [2–4]. Downregulation of alpha-smooth muscle actin was demonstrated in stromal cells undergoing decidualization at the early implantation site in the baboon (Day 15 of pregnancy), which is in close contact with the conceptus [2]. It also has been shown that the disruption of actin filaments by cytochalasin D leads to a significant enhancement of decidualization in vitro [3]. We have demonstrated recently that changes occur in cytoskeletal proteins during decidualization and that upregulation of myosin light chain (MLC) phosphorylation prevents in vitro decidualization of human stromal fibroblasts [4]. Furthermore, a decrease in MLC phosphorylation precedes decidualization induced by an exogenous stimulus (cAMP), whereas decidualization induced by a stimulus of embryonic origin, interleukin 1beta (IL1B), appears to depend on changes in actin dynamics [4].
Actin dynamics—the interactions between actin polymerization and depolymerization—are also regulated by actin-binding proteins, such as cofilin. Cofilin binds to both monomeric (G) and filamentous (F) actin, and its major known function is the depolymerization of F-actin (see review in Paavilainen et al. [5]). The ability of cofilin to bind to actin is regulated by its phosphorylation, which depends on at least two enzymes: serine-threonine-specific LIM kinases 1 and 2 (LIMK1 and LIMK2) [6]. The mRNAs for LIMK1 and LIMK2 have been detected during rat development in the extraembryonic structure containing the maternal decidual membrane [7], suggesting the involvement of the LIMK-cofilin pathway during decidualization. Phosphorylation and dephosphorylation of cofilin could enable the cell to respond rapidly to signals and to remodel the actin cytoskeleton (see reviews in Paavilainen et al. [5] and dos Remedios et al. [8]). Therefore, we hypothesized that cofilin might play an important role in the changes in actin dynamics during decidualization. The aim of this study was to investigate the importance of actin dynamics, particularly the involvement of cofilin, in decidualization induced by a widely used exogenous stimulus (cAMP) and by a stimulus of embryonic origin (IL1B) in the in vitro primate decidualization model.
MATERIALS AND METHODS
Materials
Recombinant human IL1B was obtained from R & D Systems Inc. (Minneapolis, MN). Jasplakinolide and latrunculin B were purchased from Biomol (Plymouth Meeting, PA). Monoclonal clone AC 15 beta-actin antibody, the rabbit polyclonal cofilin antibody used for immunofluorescence, and N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (cAMP) were purchased from Sigma Chemical Co. (St. Louis, MO). Rabbit polyclonal antibodies to cofilin and phosphocofilin used in Western blot detections were from Cell Signaling Technology Inc. (Beverly, MA). Deoxyribonuclease I Texas Red conjugate (DNAse I), rhodamine-conjugated phalloidin, Prolong Antifade mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI), and all cell culture supplies were purchased from Invitrogen (Grand Island, NY). Other reagents of cell culture grade were purchased from Fisher Scientific (Itasca, IL) or Sigma (St. Louis, MO).
Cell Culture
Human placenta tissue was obtained from the Human Female Reproductive Tissue bank in the Center for Women's Health and Reproduction at the University of Illinois at Chicago. All studies were approved by the Institutional Review Board of the University of Illinois. Baboon endometrial tissue (midsecretory phase, 9–12 days after ovulation) was obtained from adult female baboons (Papio anubis) by endometriectomy or after hysterectomy (n = 3). The animal study was approved by the Animal Care Committee at the University of Illinois at Chicago.
Human uterine fibroblasts (HuFs) were isolated from the decidua parietalis dissected from placental membranes after normal vaginal delivery at term, as described previously [9]. These cells represent a proliferating population of nondifferentiated fibroblastic cells, which are maintained in the decidualized uterine endometrium and closely resemble endometrial stromal cells [10, 11]. Although HuFs are not identical to stromal cells isolated from human endometrium, an advantage lies in their easy availability from term placenta, their robust proliferative abilities, and their confirmed use as a model for in vitro decidualization in several studies [4, 9–13].
Baboon uterine fibroblasts were isolated from baboon endometrial tissues according to a similar protocol described in detail previously [14].
Cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated and charcoal-stripped fetal bovine serum (SFBS), 0.1 mM sodium pyruvate, and 1% penicillin-streptomycin. At confluence, cells were trypsinized, propagated, and used for experiments in the first passage (baboon uterine fibroblasts) or in passage numbers three to five (HuF cells). Cell purity was assessed by immunocytochemistry using antibodies against cytokeratin (Dako, Glastrup, Denmark) and vimentin (Zymed Laboratories Inc., San Francisco, CA). The purity of fibroblast cells used in the studies was greater than 97%.
Treatment of Cells
Treatment with decidualization stimuli.
Decidualization studies were carried out in RPMI-1640 with 2% SFBS. Confluent cells were subjected to vehicle treatment (control) or were treated for 6 days or 12 days with the decidualization stimuli: 10 ng/ml IL1B or 0.1 mM cAMP, both in the presence of hormones. The word “hormones” in this article indicates treatment with a mix of (final concentration) 36 nM 17beta-estradiol and 1 μM medroxyprogesterone acetate (steroid hormones [SHs]). Cell culture medium was changed every 2 days.
Treatment with inhibitors.
All drug experiments were carried out at 80%–90% cell confluence in six-well plates in serum-free RPMI-1640 supplemented with sodium pyruvate and penicillin-streptomycin. The HuF cells were pretreated for 1 h with 100 nM jasplakinolide or 1 μM latrunculin B. After the pretreatment with inhibitors, medium was exchanged for RPMI-1640 for 24 h or for RPMI-1640 with 2% SFBS, and the cells were treated with decidualization stimuli as described above. After 24 h of incubation, cells were subjected to G-actin and F-actin fractionation. In the decidualization experiments, the medium was collected on Day 6 of treatment and frozen at −70°C until further analysis. All experiments were repeated a minimum of three times, and each experiment was done in triplicate.
Detection of IGFBP1 and Prolactin in Cell Supernatants
IGFBP1 and prolactin concentrations were measured in cell media collected as described above using ELISA kits (R & D Systems).
Actin Dynamics as Evaluated by G-actin:F-actin Ratio
Human uterine fibroblasts were plated into six-well plates at the density 1 × 105 cells/well and cultured until 80%–90% confluency. After decidualization treatments as described above, fractionation of cells into G-actin and F-actin fractions was performed using the G-actin:F-actin in vivo assay kit (catalogue no. BK037; Cytoskeleton, Denver, CO). Western blots for beta-actin were used to estimate the amounts of actin in each fraction. The immunoreactive bands were detected by enhanced chemiluminescence, digital images were captured by Quantity One 1-D analysis software on a ChemiDoc XRS System (Bio-Rad, Hercules, CA), and the G-actin:F-actin ratio was calculated.
Isolation of Nuclear and Cytosolic Fractions
Human uterine fibroblasts grown in 100-mm plates were subjected to decidualization stimuli for 6 days. The cells were scraped into ice-cold PBS and washed three times. The pellet was resuspended in buffer A (10 mM HEPES, pH 7.9; 1.5 mM MgCl2; 10 mM KCl; 1 mM dithiothreitol [DTT]; and protease inhibitor cocktail set III [EMD Chemicals Inc., San Diego, CA]). After 10 min of incubation in buffer A, the homogenate was centrifuged at 10 000 × g for 10 min at 4°C. The supernatant was collected and considered the cytosolic fraction. The pellet was resuspended in buffer C (20 mM HEPES, pH 7.9; 330 mM NaCl; 1.5 mM MgCl2; 25% v/v glycerol; 1 mM DTT; and protease inhibitor cocktail), incubated for 15 min at 4°C, and centrifuged at 10 000 × g for 10 min at 4°C. The supernatant was collected and considered the nuclear extract fraction. Equal volumes of the nuclear and the cytosolic fractions were loaded and separated by 4%–20% SDS-PAGE, and the amounts of p-cofilin and cofilin were detected by Western blot analysis. After the transfer onto polyvinylidene difluoride membranes, the blots were probed with specific antibodies against cofilin, p-cofilin (Cell Signaling Technology), and beta-actin (Sigma) according to the protocols provided by the manufacturers. The immunoreactive bands were detected by enhanced chemiluminescence, and digital images were captured by Quantity One 1-D analysis software on a ChemiDoc XRS System (Bio-Rad).
Immunofluorescence Staining
Cells grown on glass coverslips and exposed to decidualization treatments for 6 and 12 days were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and then blocked with 5% bovine serum albumin at room temperature. Incubations with the primary antibody against cofilin (1:1000) were conducted at 4°C overnight, followed by incubation for 1 h with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (1:100). For double staining of cofilin and F-actin or G-actin, rhodamine-conjugated phalloidin or DNAse I, respectively, were included with a secondary FITC-conjugated antibody. Coverslips were mounted with Prolong Antifade containing DAPI and were examined using a Zeiss LSM 510 laser confocal microscope.
Extraction of Cytosolic Proteins from Living Cells
Human uterine fibroblast cells grown on glass coverslips and treated with decidualization stimuli for 6 days were subjected to an extraction of soluble cytosolic proteins according to a modification of the previously described protocol [15]. Briefly, cytosolic proteins were extracted from living cells growing on coverslips with an exactly 1-min immersion in extraction solution (0.5% Triton X-100, 4% polyethylene glycol 40 000) in PEM buffer (100 mM PIPES, pH 6.9; 1 mM ethylene glycol tetraacetic acid; and 1 mM MgCl2) with protease inhibitor cocktail (EMD Chemicals Inc.) at room temperature, followed by fixation in 4% paraformaldehyde and staining as described above.
Evaluation of Cofilin Translocation
The intensity of nuclear and cytosolic staining of cofilin after decidualization treatments was quantified in at least 50 cells from each treatment group in three independent experiments by ImageJ software (National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij). Translocation of cofilin into the nucleus was expressed by the cytosol:nucleus ratio of densitometric units of staining.
Statistical Analysis
Statistical analyses were performed using SPSS 15.0 (SPSS Inc., Chicago, IL). Results are expressed as mean ± SD. One-way ANOVA was used to test the null hypothesis of group differences, followed by a two-tailed t-test for pairwise comparison or by post hoc tests using Tukey and Bonferroni correction for multiple comparisons.
RESULTS
The Effect of Changes in Actin Dynamics on Decidualization In Vitro
We have demonstrated previously that stabilizing the cytoskeleton by increasing MLC phosphorylation leads to a significant decline in decidualization in vitro [4]. Because actin and myosin work together to provide and maintain proper cytoskeletal organization and function, we investigated the role of actin dynamics in the process of the transformation of HuFs into decidual cells in our current study.
First, we analyzed whether manipulation of actin polymerization might affect the process of in vitro decidualization in the HuF model system. Two drugs regulating actin dynamics were used: jasplakinolide, an actin-stabilizing drug causing de novo polymerization of F-actin, and latrunculin B, an actin-depolymerizing drug. After 1 h of pretreating HuF cells with either jasplakinolide or latrunculin B, the drugs were removed, and decidualization treatments in 2% SFBS continued for 6 days. Measurement of the synthesis of biochemical markers for decidualization, insulin-like growth factor-binding protein 1 (IGFBP1; Fig. 1) and prolactin (data not shown), revealed that both drugs inhibited decidualization induced by IL1B and SHs (Fig. 1A). On the other hand, cAMP (and SH)-induced decidualization was inhibited only by jasplakinolide pretreatment, with latrunculin B failing to have any effect (Fig. 1B). These data demonstrate that increasing/stabilizing actin polymerization significantly decreases the process of decidualization in vitro.
FIG. 1.
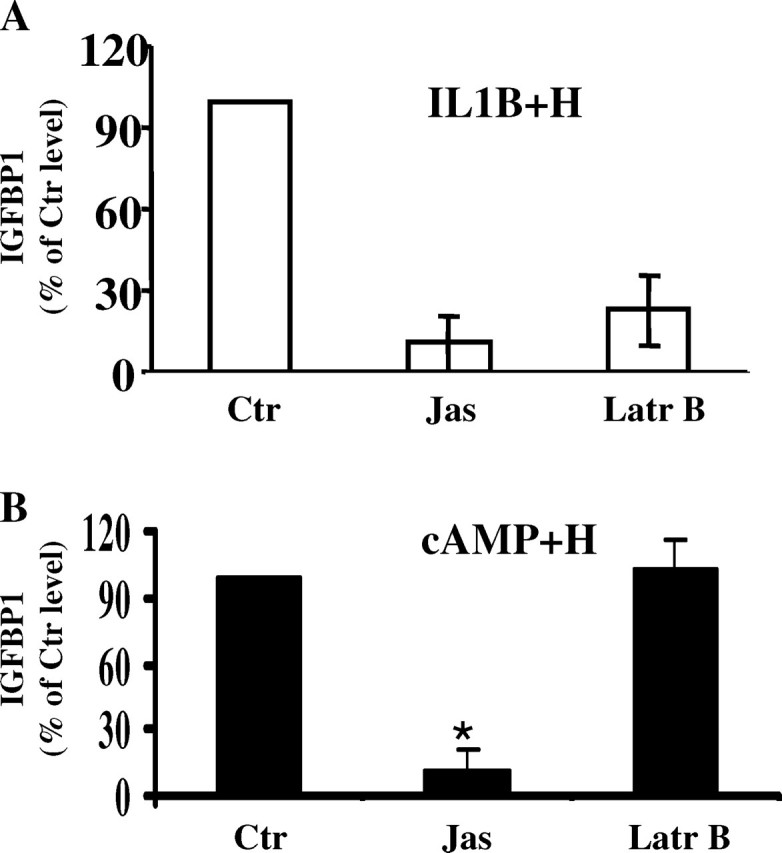
Effect of actin dynamics drug manipulation on decidualization. Human uterine fibroblast cells were pretreated for 1 h with latrunculin B (Latr B; 1 μM), jasplakinolide (Jas; 100 nM), or vehicle control (Ctr), followed by treatment for 6 days with (A) IL1B and hormones (IL1B + H) or (B) cAMP and hormones (cAMP + H). The IGFBP1 protein released into the medium was measured using ELISA. Note that although pretreatment with both inhibitors caused a significant decrease in the synthesis of IGFBP1 in HuFs undergoing decidualization induced by IL1B and hormones (A), only jasplakinolide decreased the level of IGFBP1 in HuFs treated with cAMP and hormones (B) compared with HuFs with vehicle control pretreatment (Ctr). *P < 0.05.
Because both latrunculin B and jasplakinolide affect actin dynamics, the ratio of monomeric actin (G-actin) to filamentous actin (F-actin) was measured: 1) immediately after 1 h of drug treatment in serum-free medium (Fig. 2A, upper blot); 2) after 1 h of drug treatment followed by 24 h of recovery of the cells in 2% SFBS (Fig. 2A, lower blot); and 3) after 1 h of drug treatment followed by 6 days of decidualization treatment (as seen for jasplakinolide treatment in Fig. 2B). Treatment with latrunculin B caused an increase in the G-actin level after 1 h of treatment (Fig. 2A, upper blot); however, the levels of G- and F-actin returned to levels comparable to the control after 24 h of cell recovery (Fig. 2A, lower blot). The effect of latrunculin B was completely diminished after 6 days of decidualization treatment (data not shown). On the other hand, treatment with jasplakinolide for 1 h resulted in a significant decline in G-actin, suggesting that more actin is kept in the F-actin form (Fig. 2A, upper blot). This trend of G-actin decline was still evident even after 24 h of cell recovery (Fig. 2A, lower blot) and, truly remarkably, it was still noticeable after 6 days of treatment with decidualization stimuli (Fig. 2B, lower blot, Jas). Decidualization treatment itself did not affect the G-actin:F-actin ratio (Fig. 2B, upper blot). These results demonstrate that although both drugs change the G-actin:F-actin ratio, only jasplakinolide, an actin-stabilizing drug, has a long-lasting effect.
FIG. 2.

Effect of latrunculin B and jasplakinolide on actin dynamics in HuF cells. A) The densitometric evaluation of the G-actin:F-actin ratio (labeled as G-/F-actin ratio) in HuF cells pretreated with latrunculin B (Latr B; 1 μM) or jasplakinolide (Jas; 100 nM) and either harvested immediately after 1 h of treatment with the drugs (1 h; white bars) or collected after 1 h of treatment with the drugs followed by incubation with 2% SFBS-RPMI for 24 h (1 h/24 h; black bars). The G-actin:F-actin ratio in untreated cells (Ctr) was set as 1, and other treatments were compared with the control. The blot at the top shows representative immunoblots of the distribution of beta-actin (ACTB) in the G-actin and F-actin fractions of HuF cells detected after 1 h of treatment with the drugs (1 h; upper blot) and after 1 h of treatment with the drugs followed by incubation with 2% SFBS-RPMI for 24 h (1 h/24 h; lower blot). B) The densitometric evaluation of the G-actin:F-actin ratio (labeled as G-/F-actin ratio) in HuF cells pretreated for 1 h with jasplakinolide (Jas; black bars) or with vehicle control (Ctr; white bars), followed by the treatment for 6 days with hormones (H), IL1B and hormones (IL1B + H), cAMP and hormones (cAMP + H), or untreated controls (Ctr). The blot at the top shows representative immunoblots of the distribution of ACTB in the G-actin and F-actin fractions of HuF cells treated with jasplakinolide (Jas; lower blot) or without pretreatment (upper blot) as detected after 6 days of decidualization treatment of the cells. Note the overall decrease in the G-actin:F-actin ratio detected after 6 days of decidualization treatment in HuF cells pretreated with jasplakinolide compared with HuFs without pretreatment.
Association of Cofilin with G- and F-Actin During In Vitro Decidualization
The dynamics between actin polymerization and depolymerization are regulated by actin-binding proteins. Because the actin-binding protein cofilin is known to bind to both monomeric and filamentous actin, we next investigated its association with G- and F-actin in HuF cells during decidualization. Double staining with cofilin and DNAse I (which binds to G-actin with a Kd between 10−8 and 10−10 M and to F-actin with a Kd between 10−3 and 10−6 M [16]) demonstrated a strong colocalization of cofilin and G-actin both in the nucleus and the cytoplasm in all groups of cells (Fig. 3). In contrast, the spots of colocalization between cofilin and F-actin were barely detectable in control cells or in HuFs undergoing decidualization for 6 days (Fig. 4A). The analysis of cofilin and phalloidin colocalization was significantly complicated because of the high level of monomeric actin and cytoskeletal-binding proteins present in the cytoplasm. To increase the resolution of polymeric actin and to improve the detection of cofilin associated with it, we used a fast extraction (1 min) of live cells' cytosolic proteins with a solution of Triton X-100 and polyethylene glycol. Immunofluorescent staining of cofilin and phalloidin in HuFs after extraction revealed that some fraction of endogenous cofilin was not extracted from the living cells and remained colocalized with phalloidin. Several spots of cofilin-phalloidin colocalization were detected in control cells and in HuFs treated with SHs for 6 days (Fig. 4B, two upper rows, please see arrows in detail images). In contrast to these two groups of cells, no cofilin-phalloidin colocalization was detected in the cytoplasm of HuFs treated with IL1B and SHs and with cAMP and SHs (Fig. 4B, two lower rows). Also, there was noticeably less cofilin left in the cytoplasm after the extraction. In HuF cells undergoing decidualization by IL1B and cAMP, cofilin was detected mainly in the nucleus (Fig. 4B, two lower rows, green staining for cofilin).
FIG. 3.

Association of cofilin with monomeric actin. Confocal microscopy of double-immunofluorescent staining for G-actin (visualized with DNAse I Texas Red dye; DNAse I; middle column) and cofilin (green; cofilin; left column) of HuF cells treated with hormones (H), IL1B and hormones (IL1B + H), cAMP and hormones (cAMP + H), or vehicle control (Ctr) for 6 days. Merged image is shown in the right column. All images were taken at the same magnification. Bar = 20 μm.
FIG. 4.

Association of cofilin with filamentous actin during cell decidualization and after extraction of cytosolic proteins. A) Confocal microscopy of double-immunofluorescent staining for F-actin (visualized with rhodamine-conjugated phalloidin [red; Phalloidin column]) and cofilin (green; Cofilin column) in vehicle-treated HuF cells (Ctr) and in HuFs treated for 6 days with hormones (H), IL1B and hormones (IL1B + H), or cAMP and hormones (cAMP + H). Images in rightmost column provide higher-magnification detail of areas close to arrowheads on merge images (Detail). Bar = 20 μm. B) Confocal microscopy (after a 1-min extraction of the soluble cytosolic proteins in live cells) of double-immunofluorescent staining for F-actin (visualized with rhodamine-conjugated phalloidin (red; Phalloidin column]) and cofilin (green; Cofilin column) in vehicle-treated HuF cells (Ctr) and in HuFs treated for 6 days with hormones (H), IL1B and hormones (IL1B + H), or cAMP and hormones (cAMP + H). Images in rightmost column (Detail) provide higher-magnification detail. Arrows in details indicate cofilin-phalloidin colocalization. Bar = 20 μm.
Subcellular Redistribution of Cofilin in HuFs During Decidualization
Cofilin localization and activity (its ability to bind to monomeric and filamentous actin) depend on its phosphorylation. Phosphorylated cofilin becomes inactive and is translocated from the nucleus into the cytosol [17]. To study changes in cofilin distribution and phosphorylation during decidualization, we detected cofilin and phosphocofilin (p-cofilin) in the cytosolic and the nuclear extract fractions of the cells. Immunoblotting data revealed increased amounts of p-cofilin in cytosolic fractions of HuFs treated for 6 days with IL1B plus SHs as well as with SHs alone compared with untreated controls (Fig. 5A, upper blot). As a result of the translocation of p-cofilin from the nucleus into the cytosol, the cytosol:nuclear extract ratio for p-cofilin notably increased after these treatments (Fig. 5B, upper blot), although it did not reach statistical significance. In contrast, cAMP plus SH-treated HuFs at this time point (6 days) did not display a change in the subcellular distribution of p-cofilin, and thus this ratio was not affected (Fig. 5, A and B, upper graph).
FIG. 5.

Changes in phosphorylation and localization of cofilin during decidualization. A) Distribution of p-cofilin and cofilin in the cytosolic (c) and nuclear extract (n) fractions of HuFs treated for 6 days with hormones (H), IL1B and hormones (IL1B + H), cAMP and hormones (cAMP + H), and vehicle controls (Ctr) as detected by Western blotting with specific antibodies. The cytosolic:nuclear fraction ratio in untreated cells was set as 1, and other treatments were compared with the control. B) The densitometric evaluation of the cytosol:nuclear extract ratio (labeled as Cyt./Nucl. extr) for p-cofilin (pCofilin; upper graph; white bars) and cofilin (lower graph; black bars) in HuFs treated for 6 days with hormones (H), IL1B and hormones (IL1B + H), cAMP and hormones (cAMP + H), and vehicle-treated controls (Ctr). The ratio in untreated cells was set as 1, and other treatments were compared with the control. Note the significant decrease (*P < 0.05) in the cytosol:nucleus ratio for cofilin in HuFs treated for 6 days with cAMP and hormones in comparison with Ctr.
Interestingly, the cytosol:nuclear extract ratio for total cofilin significantly decreased after cAMP treatment in comparison with untreated controls (Fig. 5B, lower graph). The change in the cytosol:nuclear ratio for cofilin suggested that there was more active (localized in the nucleus) cofilin after cAMP treatment for 6 days. Redistribution (visible translocation to the nucleus) of cofilin after this treatment was further confirmed by immunofluorescence (Fig. 6A). The treatment with cAMP resulted in the faster appearance of decidualization markers than did IL1B and hormonal treatment. Although there was no visible translocation of cofilin after 6 days of SH (Fig. 6A) or IL1B and SH (Fig. 6A) treatment, clear translocation was noticed after 12 days in all treatment groups except the untreated controls (Fig. 6B). On Day 6 of treatment, the translocation of cofilin into the nucleus as expressed by the cytosol:nucleus ratio of densitometric units was significantly decreased with cAMP treatment. However, on Day 12 of treatment, a significant decrease in this ratio was observed in all treatment groups (Fig. 6C).
FIG. 6.
Localization of cofilin in HuF cells treated with decidualization stimuli. A and B) Confocal microscopy of immunofluorescent staining of cofilin (green; left columns) in vehicle-treated HuF cells (Ctr) and in HuFs undergoing decidualization for 6 days (A) and 12 days (B) induced by hormones (H), IL1B and hormones (IL1B + H), and cAMP and hormones (cAMP + H). Nuclear staining with DAPI (blue; middle columns) and merged images (right columns) are shown. Bar = 20 μm. C) The densitometric evaluation of the ratio of immunofluorescent staining of cofilin in the cytosol to immunofluorescent staining of cofilin in the nucleus in vehicle-treated HuF cells (Ctr) and in HuFs undergoing decidualization for 6 days (white bars) and 12 days (black bars) induced by hormones (H), IL1B and hormones (IL1B + H), and cAMP and hormones (cAMP + H). Note the significant decrease (*P < 0.05) in the cytosol:nucleus ratio (labeled as Cyt./Nucl.) for cofilin staining in HuFs treated for 6 days with cAMP and hormones in comparison with control, and for cofilin staining in all 12-day decidualization-inducing treatments in comparison with control.
To confirm that decidualization accompanied by the translocation of cofilin from the cytosol into the nucleus is typical not only for the HuF model system, we used primary baboon (P. anubis) stromal fibroblasts isolated from the endometrium of the midsecretory phase of the menstrual cycle as an additional primate model. As in HuFs, after 12 days of treatment cofilin was mainly localized in the nucleus in all treatment groups except the controls in baboon stromal fibroblasts (Fig. 7).
FIG. 7.

Localization of cofilin in primary baboon uterine stromal cells treated with decidualization stimuli for 12 days. A) Confocal microscopy of immunofluorescent staining of cofilin (green; left column) in vehicle-treated cells (Ctr) and in cells undergoing decidualization induced by hormones (H), IL1B and hormones (IL1B + H), and cAMP and hormones (cAMP + H) for 12 days. Nuclear staining with DAPI (blue; middle column) is shown. Merged images are shown in the right column. Bar = 20 μm. B) The densitometric evaluation of the ratio of immunofluorescent staining of cofilin in the cytosol to immunofluorescent staining of cofilin in the nucleus in control-treated HuF cells (Ctr) and in HuFs undergoing decidualization for 12 days induced by hormones (H), IL1B and hormones (IL1B + H), and cAMP and hormones (cAMP + H). Note the significant decrease (*P < 0.05) in the cytosol:nucleus ratio (labeled as Cyt./Nucl.) for cofilin staining in all decidualization-inducing treatments in comparison with control.
DISCUSSION
The current study analyzes the role of actin dynamics and the involvement of cofilin in particular during in vitro decidualization in the human model of HuF cells as well as primary baboon endometrial stromal fibroblasts. Our data demonstrate for the first time that the stabilization of F-actin prevents decidualization and that the translocation of cofilin from the cytosol to the nucleus is a key common feature of in vitro primate decidualization.
Initiation of the decidualization process in stromal fibroblasts is detected by their ability to synthesize biochemical markers. Two primary markers of primate decidualization are IGFBP1 and prolactin. The signaling pathways leading to decidualization induced by embryonic origin stimulus IL1B and exogenous stimulus cAMP (both in the presence of SHs) are different [4, 9]. The synthesis of decidualization marker IGFBP1 protein by the second messenger cAMP is much faster (detectable after 2–3 days of treatment) than the synthesis induced by IL1B (detectable after 6 days and in approximately 100-fold lower amounts than with cAMP, with maximal levels at 12 days). The presence of IL1B increases the effect of SHs, treatment with which appears to result in detectable levels of biochemical decidualization markers after 12 days. The time course of IL1B action in the in vitro model is reminiscent of the time frame seen during the decidualization response in vivo in humans. We have described previously the ability of IL1B to contribute to in vivo decidualization in the baboon model of simulated pregnancy [2]. However, the IL1B and cAMP pathways do not work in synergy, because we reported previously that the addition of IL1B antagonizes the ability of cAMP to induce decidualization in vitro [9]. In our present study, we investigated how changes in actin dynamics would affect decidualization. We again noticed the differences and similarities between IL1B- and cAMP-induced decidualization. Although IL1B-induced decidualization (initially dependent on intact cytoskeleton organization to transduce signals and initiate differentiation) was significantly inhibited by both actin polymerization-destabilizing and actin polymerization-stabilizing drugs (latrunculin B and jasplakinolide, respectively), only jasplakinolide negatively affected second messenger cAMP-induced decidualization.
It is generally recognized that the organization of the actin cytoskeleton in most mammalian cells is regulated by the RHO guanosine triphosphatases (GTPases; RHO, RAC, CDC42) and their downstream effectors [18]. It was suggested that RHOA is important in decidual cells during implantation [19]. Furthermore, IL1B has been described as activating small GTPases [20, 21]. RHOA/RHO kinase signaling also results in focal adhesion kinase (FAK, official symbol PTK2) activation [22, 23]. We noticed previously the activation of PTK2 during decidualization of HuF cells with IL1B (and hormones) [4]. Also, cAMP was described through protein kinase A activation to regulate RHOA and LIMK/cofilin activity [24]. LIMK expression was detected in rat embryo Embryonic Day 12 extraembryonic tissue containing the decidual membrane [7], and the LIMK-cofilin pathway has been shown to be involved in differentiation processes, such as spermatogenesis [25] and differentiating layers of epidermis [26].
The activity of cofilin depends on its phosphorylation at a conserved N-terminal serine, Ser3 [27]. The phosphorylation of cofilin is regulated by the balance between the activities of its known kinases (LIMKs and testicular kinase) and its recently described phosphatases: slingshot and chronophin (see review by Huang et al. [28]). Early studies revealed that cofilin in its phosphorylated form is unable to bind actin and that dephosphorylation of this site reactivated the actin-depolymerizing potential of cofilin [29], thus providing a simple phosphoregulatory mechanism for actin reorganization.
The difference in the signaling pathways between IL1B- and cAMP-induced decidualization was noticed in the subcellular localization of cofilin and p-cofilin. Whereas decidualization induced by 6-day treatment with IL1B (and hormones), as well as by hormones alone, was accompanied by the translocation of p-cofilin to the cytosol, decidualization induced by 6-day treatment with the much faster exogenous stimulus (cAMP and hormones) was characterized by the translocation of cofilin to the nucleus. Importantly, 12-day treatment with all decidualization stimuli led to a notable translocation of cofilin to the nucleus, suggesting similar endpoints of the signaling pathways leading to decidualization and emphasizing the role of cofilin in this process. The HuF cells provide an excellent in vitro model for decidualization [9–13]. However, their origin in the decidual tissue of term placenta and the recently described presence of pluripotency markers and mesenchymal stem properties [30] might raise questions about the accuracy of this model for early pregnancy decidualization. Therefore, we used stromal cells isolated from the midsecretory-phase endometrium of a close nonhuman primate model, baboon (P. anubis), to confirm the translocation of cofilin into the nucleus during decidualization. The similarity of this model with decidualization in primary human uterine endometrium fibroblasts was confirmed in earlier studies [14], and we have used them previously to study the decidualization process [9, 31].
The translocation of cofilin during decidualization is similar to the previously noticed translocation from cytosol to nucleus in T lymphocytes after accessory receptor stimulation [32]. For that model system, it was suggested that this translocation may play an important role in the “decision” between T-cell activation and T-cell unresponsiveness [32]. Modulation of the equilibrium between monomeric globular and filamentous actin has been known to occur during cell transformation [33, 34]. Also, increasing cellular G-actin in rat peritoneal mast cells caused the translocation of both actin and cofilin into the nuclei [35]. The translocation of cofilin was also noticed to be promoted by a variety of adverse cellular conditions, including heat shock [36, 37], or by ATP depletion [35].
We hypothesize that one of the reasons for cofilin translocation during decidualization is to stop cofilin activity in the cytosol once the reorganization of actin, which is necessary for the change to decidual cells, is accomplished. Another reason cofilin translocates during decidualization may be to transport G-actin to the nucleus, and thus contribute to actin action in the nucleus. Although evidence of actin action in the nucleus remains controversial, actin appears to be involved in the regulation of gene expression [38, 39], chromatin remodeling [40], and the nuclear export of protein [41] and mRNA [42]. Our data demonstrate that cofilin also colocalizes with G-actin in the nucleus of HuF cells. Because the actin sequence lacks a nuclear translocation signal and cofilin has a nuclear import sequence, it is likely that actin enters the nucleus as a complex with cofilin. A recent study analyzing the interactions of cofilin and G-actin within the nucleus and cytoplasm determined that in Vero African green monkey fibroblasts, almost all G-actin in the nucleus is bound to cofilin, whereas about one half is bound in the cytoplasm [43]. These data suggest that there is significantly more cofilin in a complex with G-actin and less free cofilin in the nucleus than in the cytoplasm [43].
In conclusion, the presented results further expand and confirm our previous finding that stabilization of the actin cytoskeleton, either by increasing MLC phosphorylation [4] or by the stabilization of F-actin (this study), prevents the differentiation of HuFs into decidual cells. The translocation of cofilin to the nucleus seems to be a universal feature of in vitro decidualization in the primate.
Acknowledgments
We thank Professor A.T. Fazleabas (director of the Center for Women's Health, University of Illinois at Chicago) for his generous help with baboon primary cells; S. Ferguson-Gottschall (Human Female Reproductive Tissue Bank at the Center for Women's Health, University of Illinois at Chicago) for obtaining placenta and cell preparation, and Dr. M.L. Chen for helpful technical advice with confocal microscopy.
Footnotes
1Supported by National Institutes of Health grant HD-44713 to Z.S.
REFERENCES
- Ayscough KR, Winder SJ. Two billion years of actin. Meeting on cytoskeletal dynamics: from cell biology to developmental disease. EMBO Rep 2004; 5: 947 952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. In vivo infusion of interleukin-1β and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology 2005; 146: 4097 4104 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jaffe RC, Fazleabas AT. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology 1999; 140: 997 1004 [DOI] [PubMed] [Google Scholar]
- Ihnatovych I, Hu W, Martin JL, Fazleabas AT, de Lanerolle P, Strakova Z. Increased phosphorylation of myosin light chain prevents in vitro decidualization. Endocrinology 2007; 148: 3176 3184 [DOI] [PubMed] [Google Scholar]
- Paavilainen VO, Bertling E, Falck S, Lappalainen P. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol 2004; 14: 386 394 [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998; 393: 805 809 [DOI] [PubMed] [Google Scholar]
- Mori T, Okano I, Mizuno K, Tohyama M, Wanaka A. Comparison of tissue distribution of two novel serine/threonine kinase genes containing the LIM motif (LIMK-1 and LIMK-2) in the developing rat. Brain Res Mol Brain Res 1997; 45: 247 254 [DOI] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 2003; 83: 433 473 [DOI] [PubMed] [Google Scholar]
- Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1β induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology 2000; 141: 4664 4670 [DOI] [PubMed] [Google Scholar]
- Markoff E, Zeitler P, Peleg S, Hardwerger S. Characterization of the synthesis and release of prolactin by an enriched fraction of human decidual cells. J Clin Endocrinol Metab 1983; 56: 962 968 [DOI] [PubMed] [Google Scholar]
- Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod 1995; 52: 609 615 [DOI] [PubMed] [Google Scholar]
- Brar AK, Handwerger S, Kessler CA, Aronow BJ. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 2001; 7: 135 148 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG. Regulation of insulin-like growth factor binding protein-1 (IGFBP1) promoter activity by FKRH and HOXA-10 in primate endometrial cells. Biol Reprod 2003; 68: 24 30 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod 1998; 59: 160 168 [DOI] [PubMed] [Google Scholar]
- Gorovoy M, Niu J, Bernard O, Profirovic J, Minshall R, Neamu R, Voyno-Yasenetskaya T. LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J Biol Chem 2005; 280: 26533 26542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland RP, You W, Paragas VB, Wells KS, DuBose DA. Simultaneous visualization of G- and F-actin in endothelial cells. J Histochem Cytochem 1994; 42: 345 350 [DOI] [PubMed] [Google Scholar]
- Nebl G, Meuer SC, Samstag Y. Dephosphorylation of serine 3 regulates nuclear translocation of cofilin. J Biol Chem 1996; 271: 26276 26280 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420: 629 635 [DOI] [PubMed] [Google Scholar]
- Shiokawa S, Sakai K, Akimoto Y, Suzuki N, Hanashi H, Nagamatsu S, Iwashita M, Nakamura Y, Hirano H, Yoshimura Y. Function of the small guanosine triphosphate-binding protein RhoA in the process of implantation. J Clin Endocrinol Metab 2000; 85: 4742 4749 [DOI] [PubMed] [Google Scholar]
- Puls A, Eliopoulos AG, Nobes CD, Bridges T, Young LS, Hall A. Activation of the small GTPase Cdc42 by the inflammatory cytokines TNF(alpha) and IL1B, and by the Epstein-Barr virus transforming protein LMP1. J Cell Sci 1999; 112: 2983 2992 [DOI] [PubMed] [Google Scholar]
- Singh R, Wang B, Shirvaikar A, Khan S, Kamat S, Schelling JR, Konieczkowski M, Sedor JR. The IL1B receptor and Rho directly associate to drive cell activation in inflammation. J Clin Invest 1999; 103: 1561 1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Morii N, Narumiya S, Rozengurt E. Botulinum C3 exoenzyme blocks the tyrosine phosphorylation of p125FAK and paxillin induced by bombesin and endothelin. FEBS Lett 1994; 354: 315 319 [DOI] [PubMed] [Google Scholar]
- Torsoni AS, Marin TM, Velloso LA, Franchini KG. RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. Am J Physiol Heart Circ Physiol 2005; 289: H1488 H496 [DOI] [PubMed] [Google Scholar]
- Goeckeler ZM, Wysolmerski RB. Myosin phosphatase and cofilin mediate cAMP/cAMP-dependent protein kinase-induced decline in endothelial cell isometric tension and myosin II regulatory light chain phosphorylation. J Biol Chem 2005; 280: 33083 33095 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Funakoshi H, Nakamura T. LIM-kinase as a regulator of actin dynamics in spermatogenesis. Cytogenet Genome Res 2003; 103: 290 298 [DOI] [PubMed] [Google Scholar]
- Honma M, Benitah SA, Watt FM. Role of LIM kinases in normal and psoriatic human epidermis. Mol Biol Cell 2006; 17: 1888 1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 1999; 15: 185 230 [DOI] [PubMed] [Google Scholar]
- Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol 2006; 18: 26 31 [DOI] [PubMed] [Google Scholar]
- Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem 1995; 270: 17582 17587 [DOI] [PubMed] [Google Scholar]
- Strakova Z, Livak M, Krezalek M, Ihnatovych I. Multipotent properties of myofibroblast cells derived from human placenta. Cell Tissue Res 2008; 332: 479 488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakova Z, Szmidt M, Srisuparp S, Fazleabas AT. Inhibition of matrix metalloproteinases prevents the synthesis of insulin-like growth factor binding protein-1 during decidualization in primate. Endocrinology 2003; 144: 5339 5346 [DOI] [PubMed] [Google Scholar]
- Samstag Y, Eckerskorn C, Wesselborg S, Henning S, Wallich R, Meuer SC. Costimulatory signals for human T-cell activation induce nuclear translocation of pp19/cofilin. Proc Natl Acad Sci U S A 1994; 91: 4494 4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol 1998; 10: 123 130 [DOI] [PubMed] [Google Scholar]
- Stournaras C, Stiakaki E, Koukouritaki SB, Theodoropoulos PA, Kalmanti M, Fostinis Y, Gravanis A. Altered actin polymerization dynamics in various malignant cell types: evidence for differential sensitivity to cytochalasin B. Biochem Pharmacol 1996; 52: 1339 1346 [DOI] [PubMed] [Google Scholar]
- Pendleton A, Pope B, Weeds A, Koffer A. Latrunculin B or ATP depletion induces cofilin-dependent translocation of actin into nuclei of mast cells. J Biol Chem 2003; 278: 14394 14400 [DOI] [PubMed] [Google Scholar]
- Iida K, Matsumoto S, Yahara I. The KKRKK sequence is involved in heat shock-induced nuclear translocation of the 18-kDa actin-binding protein, cofilin. Cell Struct Funct 1992; 17: 39 46 [DOI] [PubMed] [Google Scholar]
- Nishida E, Iida K, Yonezawa N, Koyasu S, Yahara I, Sakai H. Cofilin is a component of intranuclear and cytoplasmic actin rods induced in cultured cells. Proc Natl Acad Sci U S A 1987; 84: 5262 5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egly JM, Miyamoto NG, Moncollin V, Chambon P. Is actin a transcription initiation factor for RNA polymerase B? EMBO J 1984; 3: 2363 2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U, Hinssen H, Franke WW, Jockusch BM. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell 1984; 39: 111 122 [DOI] [PubMed] [Google Scholar]
- Olave IA, Reck-Peterson SL, Crabtree GR. Nuclear actin and actin-related proteins in chromatin remodelling. Annu Rev Biochem 2002; 71: 755 781 [DOI] [PubMed] [Google Scholar]
- Hofmann W, Reichart B, Ewald A, Muller E, Schmitt I, Stauber RH, Lottspeich F, Jockusch BM, Scheer U, Hauber J, Dabauvalle MC. Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin. J Cell Biol 2001; 152: 895 910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HH, Robert F, Young RA, Struhl K. Genome-wide location and regulated recruitment of the RSC nucleosome-remodeling complex. Genes Dev 2002; 16: 806 819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra D, Dos Remedios CG. Cofilin, actin and their complex observed in vivo using fluorescence resonance energy transfer. Biophys J 2005; 89: 1902 1908 [DOI] [PMC free article] [PubMed] [Google Scholar]



