INTRODUCTION
In 1955, I began graduate studies at the University of Chicago in the Committee on Biophysics. The program allowed considerable freedom in choosing a research project. When I had made up my mind, I went to see Dr. William Bloom, the distinguished histologist, and told him that I wanted to work on the mechanism of mitosis. He handed me his copy of Wilson's (1925) book, The Cell in Development and Heredity, and said, “Read it, and when you finish, if you still want to work on such a difficult problem, come back and see me, and we'll talk.”
On his retirement, Dr. Bloom gave me this copy of Wilson (1925) and I have been rereading parts of it while preparing this lecture. My title was intended to make the topic appear fashionable but the idea of the cell as a machine is not new. Loeb in 1906 referred to the cell as a “chemical machine” and Wilson tells us that “the specificity of each kind of cell depends essentially on what we call its organization, i.e., upon the construction of the cell machine” but “ the differences between the cell and even the most intricate artificial machine still remain too vast by far to be bridged by our present knowledge.”
At that time, the research group of William Bloom, Raymond Zirkle, and Robert Uretz was investigating the mechanism of mitosis by using microbeam irradiation by protons and UV light. The newt was used as the source of material because the cells are big and beautiful. They do not round up during mitosis and one can see the stages of the process in the living cell with a clarity that is without parallel. Mitosis is the prime example of the operation of the cell as a molecular machine.
The explanation of the formation of the mitotic apparatus and the movements of the chromosomes had made little progress since 1925, the year of the third edition of Wilson's (1925) book. Franz Schrader's (1953) critical discussion of the current state of the field was a particularly valuable introduction to the problem. The contrast between the mode of thought at that time and the molecular biology of today is very striking. A description in terms of the controlled assembly and disassembly of fibers consisting of globular protein subunits and the action of local force generators, which move organelles along tracks, would not have been possible in 1955. In this lecture I wish to focus more on the development of these ideas than on an account of the work in my laboratory.
My Ph.D. dissertation was a very modest contribution to solving the mechanism of mitosis. There was a shortage of actual numbers to test models of mitosis and my work was primarily concerned with measuring the rates of the processes by using polarized light microscopy to visualize the spindle. The rate of growth of the spindle in prophase and the elongation in late anaphase and the rate of chromosome-to-pole motion for various conditions provide data for thinking quantitatively about the mechanism. A constant growth rate of the spindle of 1 to 2 μm/min could be explained by assembly of filaments from a pool of subunits. Later studies on microtubule polymerization did yield velocities in this range. The amount of energy expended against viscous drag on the chromosomes was calculated from the velocity of chromosome movement and an estimate of cell viscosity obtained from the Brownian movement of vesicles. The amount of energy is very small, equal to the hydrolysis of 25 to 50 molecules of ATP for a movement of 20 μm. The velocity did not depend on the size of the chromosome, as had been noted previously, and it also did not depend on the viscosity of the cell. I concluded that mechanical forces did not determine the velocity and that the important energy term was “the change in internal energy of the spindle itself.”
Perhaps I was on the right track. Today we would say that velocity is determined by the ATP turnover rate of the motor and that the viscous drag on the organelle is usually small compared with the free energy of ATP hydrolysis. In this system the rate of disassembly of the microtubules could also be significant in limiting the velocity.
I spent 2 yr in the laboratory of Francis Schmitt at Massachusetts Institute of Technology as a postdoctoral fellow, learning protein chemistry and tracking down the giant squid in Chile that was our source of axoplasm. Peter Davison and I worked on the properties of the neurofilament protein that is now known to be a member of the family of intermediate filaments. Its function in neurons was not clear then and it may still be in doubt.
On my return to the University of Chicago I set up my own laboratory and initially my students and I studied the birefringence of filamentous proteins but my main interest was still mitosis. I decided that the two most important problems to investigate were first, What is the protein that is assembled into the fibers of the spindle and second, What is the force generator?
When I began to work in the field in the mid-1950s, models of mitosis were mainly field theories (electric, magnetic, hydrodynamic, or diffusion fields) or some variation on the idea of fibers that pull or push chromosomes. There was no quantitative experimental evidence for the former and cytologists objected to the latter because the fibers did not thicken during shortening. Models of muscle contraction might have been expected to provide a basis for understanding movement, but at that time contraction was still thought of as a folding of protein chains.
The mitotic apparatus had been isolated from sea urchin eggs by Mazia and Dan in 1952. However, it was only a small fraction of the cytoplasmic proteins and in our hands it did not appear to be enriched in any particular protein compared with the cytoplasm. I thought, perhaps wrongly, that by following the dynamics of spindle formation we might find a protein that was made specifically for the spindle. For this purpose the egg is not suitable and we tried to synchronize the KB cell line that grows in suspension. Although we had some success in obtaining ∼20% of the cells in mitosis, we did not obtain a reasonable yield of the mitotic apparatus.
It was known that colchicine blocks cells in mitosis. Treatment of our suspension cultures with colchicine led to an accumulation of 80% of the cells in mitosis, whereas there was little or no effect on the metabolism of the cells that were moving through interphase. The spindles of the blocked cells were disordered but because the effect was so specific I suspected the drug had some effect on the assembly of the spindle fiber protein.
I prepared a tritium-labeled colchicine to determine its interaction with cells. It was taken up and bound to some constituent of the cell because it was only slowly released from cells resuspended in fresh medium. I devised a binding assay based on fast separation on a sizing column. The target proved to be a protein and it probably represented 5% of the cytoplasmic protein, which suggested that it was a structural protein.
At this point an outstanding group of graduate students, Gary Borisy, Michael Shelanski, and Richard Weisenberg, joined my laboratory. They carried out experiments on cell cultures, sea urchin eggs, and cilia that showed that the 6S colchicine-binding protein is the subunit of microtubules. At first, we considered the possibility that the protein might be restricted to mitotic cells but a survey of various tissues yielded the highest colchicine-binding activity in brain, which was intended to be the control for zero mitotic rate. Brain then became the source for the preparation of large quantities of tubulin (Weisenberg et al., 1968). After he left my laboratory, Weisenberg succeeded in doing the clinching experiment of polymerizing crude tubulin into microtubules.
An interesting property of tubulin, also found by Ian Gibbons, was that each subunit of the tubulin dimer has a strongly bound GTP but only one GTP is hydrolized during polymerization. This aspect of the system was investigated by Harriet Smith and Mike Jacobs during my stay at the Medical Research Council Muscle Unit at Kings College, London, England. We introduced the E and N site terminology to refer to the exchangeable and nonexchangeable sites.
The second problem was what moves the chromosomes. In the 1960s there were only two candidates, myosin and dynein, but only dynein interacts with microtubules. Richard Weisenberg showed that the isolated spindle from sea urchin eggs contains a 13S ATPase whose nucleotide specificity resembled dynein (Weisenberg and Taylor, 1968). At that time a cytoplasmic dynein had not been described and 90% of the 13S ATPase was in the cytoplasm of the egg. It was possible that this dynein was a cilia precursor that was a contamination of the mitotic apparatus. We did not think that our evidence that the ATPase was functioning in mitosis was convincing and we put the problem aside.
In the 1960s various laboratories had begun the search for nonmuscle actin and myosin. As early as 1952, Ariel Loewy had found evidence for actomyosin-like activity in Physarum but the proteins had not been properly purified. Hatano and Oosawa were the first to obtain convincing evidence for a nonmuscle actin. Only part of the actin was polymerizable, probably because of impurities that included actin-binding proteins that blocked polymerization. In 1968, Mark Adelman in my laboratory purified an actin from slime mold that polymerized quantitatively and hydrolyzed one ATP per subunit incorporated into polymer. This finding was strong evidence for the close similarity of nonmuscle and muscle actin. Mark went on to purify a myosin from slime mold, which was activated by actin. The field advanced rapidly with the finding of the first single- headed myosin in Acanthameba by Tom Pollard and Ed Korn. This was a surprise. Although we were not sure why muscle myosin had two heads, an ameba with a one-headed myosin must somehow be a defective creature. We did not realize that this was the beginning of the end of the isolation of the muscle community from the rest of cell biology.
In 1965 we did not really understand how any motile system worked. I felt that it was necessary to first concentrate on muscle actomyosin to understand the principles of mechano-chemical coupling. The history of the study of muscle contraction makes an interesting story that has been recounted by Huxley (1980). Although the constant length of the A band was known in 1880, this knowledge was lost and had to be rediscovered by A. Huxley and R. Neidergerke and by H. Huxley and J. Hanson in the 1950s.
If you were not there at the time it is difficult to appreciate why certain problems were being studied and why the right questions were not being asked. Reading the articles and particularly reading the discussions at symposia are very helpful in trying to understand what was happening in the field. The symposium Fibrous Proteins and their Biological Significance (Symposia of the Society for Experimental Biology IX (1955) contains the beginnings of the new ideas. H. Huxley and J. Hanson presented the evidence for the sliding filament model and proposed that “the myosin–actin linkage can pull the actin filament … by the contraction of a branch of the myosin molecule.” This model was formulated quantitatively by Huxley (1957) and some form of this model has dominated the field ever since.
The structural models forced a change in the way of thinking about contractility but it was not yet clear what ATP was doing. The muscle biochemists met in Japan in 1957 at a conference on the chemistry of muscle contraction. They were blissfully ignorant of the changes brought about by the progress in structure. A large-scale change in length of a polymer was still considered to be a reasonable model of contraction. It was not clear whether ATP acted in contraction or relaxation because it dissociates actomyosin and this effect might be related to relaxation. ATP is hydrolyzed in actin polymerization but it had not yet been shown that ATP was hydrolyzed in a twitch contraction. Contraction might then be a cyclic change in length of actin filaments driven by ATP hydrolysis. It is not surprising that in the mid-1950s an outsider like myself was totally confused about the biochemistry of muscle contraction.
It took about 10 yr to straighten out the biochemical concepts and to begin to ask the right questions. In the meantime the sliding filament–rotating cross bridge model was well-established (Huxley, 1969), and there was some experimental evidence that the myosin cross bridges might rotate (Reedy et al., 1965).
A consequence of the cross bridge model is that an ATPase cycle must be coupled to the cross bridge cycle and that each cycle contributes a small movement or a local contribution to the force. At the Aspects of Cell Motility symposium (Symposia of the Society for Experimental Biology, 1968), the biochemists were talking a different language. Andrew Szent-Gyorgyi suggested that there are four steps in the cycle, that “ATP hydrolysis … is not necessarily the point of mechano-chemical coupling” and that “one may imagine, for instance, that the structure associated with AM.ATP is different from AM.ADP.” Thus, the people in the field were beginning to think in terms of a molecular motor that couples ATP hydrolysis to changes in conformation.
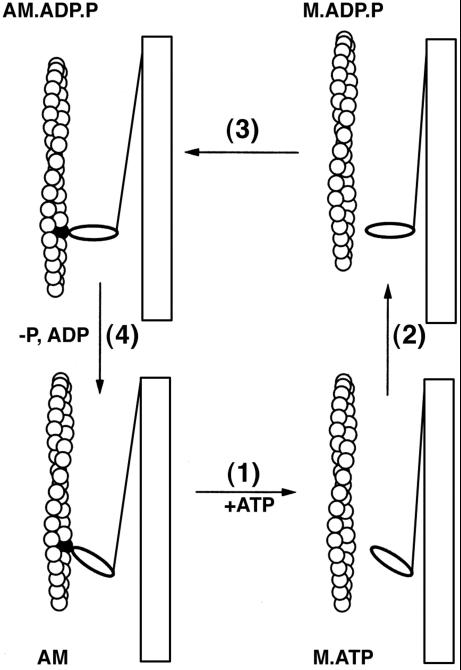
In my laboratory we concluded that to determine the steps in the actomyosin cycle one should apply the methods of transient kinetics. We had no experience in this field and the necessary equipment was not commercially available but what we did have was an excellent machine shop. In 1965 we decided to build a stop flow and then a quench flow apparatus. After the usual period of making mistakes and modifying the apparatus we were able to begin the study of the mechanism. The first work on myosin was done by the late Birdwell Finlayson and continued by Richard Lymn. Although kinetic studies were being done in other laboratories, notably by Tonomura in Japan, the models that were being proposed were too complex. Our goal was to arrive at a simple model, one that could be correlated with the steps in the contraction cycle. The important lesson was learned that the rate constants could establish a dominant pathway, and in the actomyosin case, a simple model (Lymn and Taylor, 1971) accounted for the features of the system that had been confusing in previous studies. ATP plays two roles in the mechanism. The binding of ATP dissociates actomyosin to complete the cycle, whereas the actual bond cleavage step occurs primarily in the dissociated state and resets the mechanical cycle. The Lymn–Taylor model is shown in Figure 1. It is surprisingly similar to current models of the contraction cycle.
Figure 1.
The Lymn—Taylor model.
In the early 1970s I moved to the Medical Research Council Muscle Biophysics Unit at Kings College to collaborate with Jean Hanson. Our simple model was extended by work in my group by Jane Koretz, Howard White, and John Sleep, and by the laboratories of David Trentham and Evan Eisenberg, who emphasized the importance of weak versus strong binding states. I thought that the biochemical scheme together with the mechanical model of Huxley and Simmons (1971) and the structural evidence provided a basis for understanding muscle contraction. There were important details to add, particularly on the mechanism of relaxation and we continued to refine the mechanism through the work of excellent students and postdoctoral fellows in my laboratory, particularly Kathy Trybus, Ken Johnson, Steve Marston, Steve Rosenfeld, and Y.Z. Ma. However, the field of striated muscle contraction continued to struggle with the basic problem of proving that a large-scale conformation change was coupled to some step in the mechanism. Kinetic studies could contribute little to this problem and it was time to explore other systems.
Nonmuscle myosins and cytoplasmic dynein were clearly involved in cell and organelle movements but these motors could not account for observations such as bidirectional particle movement along microtubules. A new motor protein, kinesin, was identified as the anteriograde transporter of vesicles in axons (Vale et al., 1985). A microtubule-based motor of reasonable size and complexity that might by involved in mitosis was just what we wanted. Kuznetzov and Gelfant (1986) showed that the ATPase activity of kinesin increased with microtubule concentration to a saturating value, which is a familiar property of actomyosin. This suggested that the kinetic mechanism might be similar and several laboratories that had worked on actomyosin switched to kinesin (David Hackney, Ken Johnson, Rob Cross, and my own).
Studies of single motors soon showed that there are significant differences in the properties of kinesin compared with a typical fast muscle myosin, although both systems have similar ATP turnover rates. Three important properties distinguish kinesin from myosin II. The motility depends on a two-headed kinesin; a single kinesin dimer produces as large a velocity of movement of microtubules as many kinesins; individual kinesin molecules can take 50 to 100 steps before dissociating from the microtubule. A kinetic mechanism was worked out, based on contributions from several laboratories. A key result was the finding by Hackney (1994) that the binding of kinesin to the microtubule released only one ADP from the kinesin dimer. The binding of ATP to the other head of the dimer was necessary to release the second ADP. The electron microscopy evidence on the microtubule–kinesin complex and the crystal structure of the kinesin dimer suggested that only one head of the dimer could bind to the microtubule. In terms of kinetics, a complex with both heads bound is a transient intermediate that is only present for a small fraction of the cycle time.
The kinetic schemes from various laboratories differ in some of the details but the kinetic pathway can account for the processive motion of kinesin. The main problem is the one that held up the myosin field for so long, that of identifying a change in structure with a step in the cycle. Myosin undergoes a large structural change in the absence of actin. Thus, the crystallographic evidence on myosin alone allows us to visualize the structure of two different nucleotide states that are intermediates of the cycle that correspond to two positions of a long lever arm. In the kinesin case only one structure, kinesin.ADP, is known (although it may exist in two conformations), and there are no large conformational changes. Possibly the change in structure that gives rise to a movement step may only occur in association with the microtubule. An interesting model based on structural and kinetic data is that the neck linker region of a kinesin–microtubule complex undergoes a disorder to order transition induced by ATP, which allows the second head to bind to the next tubulin site on the microtubule (Rice et al., 1999). However, much work remains to be done to test this model and other models of the movement step.
I have had the pleasure of observing and participating in the development of ideas in the field of cell movement. When I began my graduate work, our concepts were primitive and mostly incorrect, based as they were on analogies to nonbiological systems and the folding of protein chains. By concentrating on simple in vitro systems, actomyosin and microtubule-kinesin and also on the polymerization of actin and tubulin, the field has arrived at an understanding of the basic mechanisms for generating force and movement by means of conformational machines driven by the hydrolysis of ATP or GTP.
Wilson's statement still holds true that the differences between the cell and our artificial machines, actomyosin and microtubule-kinesin, “remain too vast … to be bridged by our present knowledge.” I never expected that the cell would need 15 different myosins and a still uncounted number of kinesins. Although we do have a reasonable understanding of the function of the moving parts, the challenge of the future is to understand how the parts work together in the cell. Some notable progress has already been made in understanding how actin filaments and microtubules form and break down in the cell and how these processes can lead to organelle and cell movement.
It is 45 years since I first watched mitosis in the newt cell and I continue to be amazed by what Wilson called “this spectacular feat”, which we are still a long way from understanding.
Footnotes
Corresponding author. E-mail address: ewt1@midway.uchicago.edu.
REFERENCES
- Committee of Muscle Chemistry of Japan. Conference on the Chemistry of Muscle Contraction. Tokyo: Igaku Shoin; 1957. [Google Scholar]
- Hackney DD. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc Nat Acad Sci USA. 1994;91:6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscle contraction. Science. 1969;164:1356–1366. [PubMed] [Google Scholar]
- Huxley Sir Andrew. Reflections on Muscle. Princeton, NJ: Princeton University Press; 1980. [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Gelfand VI. Bovine brain kinesin is a microtubule-activated ATPase. Proc Nat Acad Sci USA. 1986;83:8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10:4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Holmes KC, Tregear RT. Induced changes in orientation of the cross bridges of glycerinated insect flight muscle. Nature. 1965;207:1276–1282. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Rice S, et al. A structural change in the kinesin motor that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- Schrader F. Mitosis. 2nd ed. New York: Columbia University Press; 1953. [Google Scholar]
- Symposia of the Society for Experimental Biology IX. Fibrous Proteins and their Biological Significance. New York: Academic Press; 1955. [Google Scholar]
- Symposia of the Society for Experimental Biology XXII. Aspects of Cell Motility. New York: Academic Press; 1968. [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force generating protein, kinesin, involved in microtubule based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg RC, Borisy GG, Taylor EW. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968;7:4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg RC, Taylor EW. Studies on the ATPase activity of sea urchin eggs and the isolated mitotic apparatus. Exp Cell Res. 1968;53:372–384. [Google Scholar]
- Wilson EB. The Cell in Development and Heredity. 3rd ed. New York: The Macmillan Company; 1925. [Google Scholar]