Abstract
The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are employed in the evaluation of patients with suspected septic arthritis, osteomyelitis, and acute rheumatic fever. The purpose of this study is to determine if one test has greater sensitivity (rises earlier) than the other. Laboratory data were retrieved for pediatric patients hospitalized with one of the above three conditions, who had both ESR and CRP tests done on or shortly prior to admission. Sensitivity calculations were performed for mild, moderate, and severe degrees of ESR and CRP elevation. Microcytic erythrocytes, as defined by mean corpuscular volume (MCV) <80 µL, were identified to see if this affects the ESR. ESR or CRP sensitivities depend on the cutoff value (threshold) chosen as a positive test. The sensitivities were similar for similar degrees of elevation. ESR and CRP discordance was not significantly related to MCV. We concluded that the CRP does not rise earlier than the ESR (their sensitivities are similar). Previously published conclusions are dependent on arbitrary thresholds. We could not find any evidence that MCV affects the ESR.
Key words: erythrocyte sedimentation rate, C-reactive protein, osteomyelitis, septic arthritis, acute rheumatic fever.
Introduction
The erythrocyte sedimentation rate (ESR)1–9 and C-reactive protein (CRP)3,6–9 are often employed in the diagnostic evaluation for acute inflammatory disease conditions such as septic arthritis (SA), osteomyelitis (Ost), and acute rheumatic fever (ARF). Some publications have indicated that the CRP is a superior test in the diagnostic evaluation for some of these inflammatory conditions,9 because it rises earlier than the ESR.3,10,11 If such were true, then the diagnostic sensitivity of the CRP should be superior to that of the ESR. However, there is no consensus on the superiority of one test over the other despite the fact that CRP is either ordered alone or in conjunction with ESR. Previous studies have concluded that the CRP is superior for monitoring the response of children to treatment, because its level declines earlier than the ESR with therapeutic improvement in the condition.3,6,12 In other words, as the patient improves, the CRP level declines earlier than the ESR. This finding is consistent among studies and is not disputed. However, one cannot infer that CRP levels would necessarily rise earlier with the progression of the inflammatory condition for this reason alone. It must be verified independently. This aspect is more difficult to confirm because patients typically do not present with symptoms that specifically precede SA, Ost, and ARF. As a result, the first ESR and CRP values that are obtained are typically when the patient presents with symptoms suggestive of SA, Ost, and ARF. The ESR and CRP are both non-specific inflammatory markers, but the magnitude of inflammation that is typical of SA, Ost, and ARF is very high (as opposed to moderately high), and they are two of the few simple tests that provide some indication that the patient may have one of these conditions, which will provoke clinicians to order more advanced studies such as magnetic resonance imaging (MRI), arthrocentesis, and/or bone aspirates. Thus, although the ESR and CRP are generally classified as non-specific tests, they provide modest predictive value among the simple tests currently available.
In addition, it is postulated that the mean corpuscular volume (MCV) can affect the ESR value but not the CRP, because the MCV affects the surface area to volume ratio of the red blood cells that could affect their motion (drag force) properties.13
The primary purpose of this study is to determine whether the ESR or CRP is more sensitive in the early diagnosis of SA, Ost, and ARF. ARF is included with SA and Ost because of its highly inflammatory nature, similar to that of SA and Ost. The secondary purpose of this study is to examine the relationship of MCV to the ESR in this cohort.
Materials and Methods
At a tertiary children's hospital, inpatient medical records were searched for ICD9 (International Classification of Diseases, 9th revision) diagnostic codes indicating a primary inpatient diagnosis of SA, Ost, and ARF during two study periods: (1) January 1997 to December 1999; (2) July 2000 to April 2006. These study periods were separate because the initial period did not yield a sufficient number of cases in which both tests were ordered simultaneously, and a second period was subsequently examined in a similar fashion. These medical record numbers and dates were linked to the laboratory information system to obtain laboratory values for these patients, without reviewing the patients' medical records. This method permits the calculation of sensitivity [the number of those with an abnormal test (ESR or CRP) divided by the total number of patients with the condition]. This assumes that all the patients with the condition were hospitalized and included in this inpatient study cohort. While it is possible that this is not the case (patients could have been transferred to other hospitals, or an unusual case could have been managed as an outpatient), the calculated sensitivity is still the sensitivity of the study cohort. Specificity, and positive and negative predictive values cannot be calculated using this study method because the group of patients who were tested (positive or negative) and did not have the condition were not included in this study cohort. It is possible that patients suspected of having SA, Ost, or ARF but who actually did not, could have been hospitalized, but these patients would not carry a final ICD9 diagnosis of these conditions as these conditions would have been ruled out prior to discharge.
Most patients with these three conditions had an ESR and/or a CRP obtained. However, only some patients had both test results performed on the initial diagnosis (i.e. at the onset of hospitalization). In comparing the sensitivities of the ESR and CRP, only the patients with both tests performed could be used for this study's purpose.
ESR and CRP elevation ranges were classified as mild, moderate, and severe as follows: mild (ESR >20 mm/hr, CRP >0.4 mg/dL), moderate (ESR >50 mm/hr, CRP >4 mg/dL), or severe (ESR >90 mm/hr, CRP >10 mg/dL), and compared. “Discordant” ESR/CRP elevations were defined as one value in the normal range or mild elevation range and the other value in the severe elevation range. Note that 1 mg/dL= 10 mg/L (CRP values are reported with either unit). Microcytosis was defined as a MCV <80 µL and leukocytosis was defined as a white blood count >12,000 per mm3.
Results
One hundred and fifty-seven cases of SA (n=34), Ost (n=84), ARF (n=30), or more than one of these disease categories (n=9) were identified with both ESR and CRP values obtained on or just prior to hospitalization (i.e. at the onset of the diagnosis and treatment). Five patients were diagnosed with SA and ARF, three patients were diagnosed with Ost and SA, and one patient was diagnosed with all three conditions. Only 141 patients had a complete blood count (CBC) study completed concurrently. While this was unexpected, it is likely that the 16 patients who did not have a CBC result in the laboratory system probably had a CBC performed prior to hospitalization at an outpatient laboratory or at the laboratory of a different hospital prior to hospitalization.
Age ranged from infants to 17 years (mean ± standard deviation: 8.5±4.5 yr). The mean ages, ESR and CRP values, and sensitivities in the mild, moderate, and severe elevation categories for ESR and CRP among the four disease categories above are summarized in Table 1.
Table 1. Mean age, C-reactive protein, and erythrocyte sedimentation rate values (± standard deviation) among the different disease groups.
| Septic arthritis | Osteomyelitis | Acute rheumatic fever | More than one | P | |
|---|---|---|---|---|---|
| N | 34 | 84 | 30 | 9 | |
| Age | 6.3±4.7 | 8.2±4.6 | 1.6±2.7 | 8.8±5.4 | 0.0001 |
| CRP (mg/dL) | 11.4±9.3 | 8.1±7.7 | 9.4±7.3 | 7.6±5.9 | NS |
| ESR (mm/hr) | 73.3±31.0 | 62.6±29.9 | 90.2±31.7 | 84.6±20.0 | 0.0002 |
| Sensitivity | |||||
| Mild CRP | 100% | 96% | 97% | 100% | |
| Mild ESR | 94% | 89% | 93% | 100% | |
| Mod CRP | 74% | 58% | 70% | 67% | |
| Mod ESR | 76% | 67% | 90% | 100% | |
| Severe CRP | 41% | 29% | 43% | 44% | |
| Severe ESR | 32% | 18% | 63% | 44% |
Patients with SA were significantly younger than those with Ost and ARF. Ost patients had the lowest mean ESR of the four disease groups, and cross-tabulation statistics demonstrated non-random distribution when cross-tabulating the disease groups with the ESR severity groups. The mean CRP values did not differ significantly between the four disease category groups.
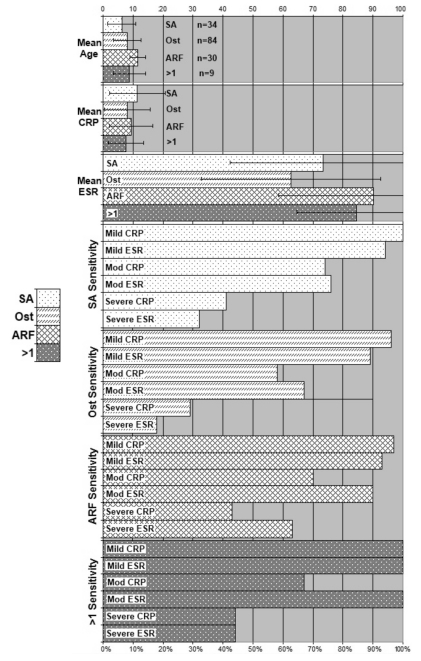
Table 1 lists the sensitivities for the ESR and CRP cutoff values for the four disease groups and although some differences are noted, a consistent pattern is difficult to identify. Figure 1 presents the same information in a bar graph. For example, a moderate ESR elevation has a better sensitivity than a moderate CRP elevation for Ost, but a severe CRP elevation has a better sensitivity than a severe ESR elevation for Ost.
Figure 1.
Mean age, CRP (mg/dL), and ESR (mm/hr) in 34 patients with septic arthritis, 84 patients with osteomyelitis, 30 patients with acute rheumatic fever, and 9 patients with more than one of these conditions. Sensitivity, mild elevation, moderate elevation, or severe elevations of CRP and ESR. SA, septic arthritis; Ost, osteomyelitis; ARF, acute rheumatic fever.
Table 2 summarizes the sensitivity of CRP, ESR, and CRP+ESR (defined as at least one of these meeting positive test criteria). CRP has superior sensitivity if mild elevation is considered a positive test. However, because a CRP >0.4 mg/dL and/or an ESR >20 mm/hr are not very impressive, it is not realistic to believe that this mild level of elevation would prompt a clinician to commit a patient to a more advanced test such as an MRI scan, arthrocentesis, or bone aspirate.
Table 2. Sensitivity of C-reactive protein and erythrocyte sedimentation rate.
| Positive test definition | CRP sensitivity | ESR sensitivity | CRP+ESR* sensitivity |
|---|---|---|---|
| Mild or greater elevation | 98% (153/157) | 92% (144/157) | 99% (155/157) |
| Moderate or greater elevation | 64% (101/157) | 75% (118/157) | 85% (133/157) |
| Severe elevation | 35% (55/157) | 31% (49/157) | 48% (75/157) |
CRP+ESR sensitivity means that either the CRP or the ESR were in or exceeded the required criterion value. In other words, the combined sensitivity should always be higher or equal to the better sensitivity of the two.
When moderate or greater elevation is considered a positive test, ESR has a superior sensitivity. A CRP >4 mg/dL and/or an ESR >50 mm/hr represent greater laboratory degrees of elevation and would be more likely to prompt a clinician to commit a patient to a more advanced test. When severe elevation is considered a positive test, CRP has superior sensitivity. These comparisons are highly dependent on the specific values that are assigned to “moderate” and “severe” values. Slight modifications in the chosen cutoff values will alter these comparisons.
There were 15 discordant pairs of CRP/ESR values. In nine cases, the ESR was severe, while the CRP was mild or normal (1 case of SA, 5 cases of Ost, 2 cases of ARF, and 1 case of SA and Ost). In six cases, the CRP was severe, while the ESR was mild or normal (2 cases of SA, 3 cases of Ost). This suggests that the ESR was more often sensitive when the CRP was insensitive, than vice versa.
Because the ESR can potentially be affected by the size of the red blood cells, the MCV of the normal, mild, moderate, and severe elevation groups were 78.4, 80.8, 82.4, and 82.3, respectively, which was not significantly different by analysis of variance. There were only four patients with an MCV <70 µL, and only 48 patients with an MCV <80 µL. Thus, if a true relationship existed between the MCV and the ESR, the MCV variation was probably insufficient to detect such a relationship.
Discussion
These data demonstrate that either test could have superior sensitivity depending on which value is used as the cutoff point (the definition of a positive test). Significantly disparate CRP/ESR results occurred both ways (mild CRP and severe ESR and vice versa). The CRP does not possess significantly superior sensitivity, which means that there is no evidence that the CRP rises earlier than the ESR.
In reality, clinicians do not utilize a single criterion value that defines a positive test. Clinicians appreciate greater risk with greater degrees of abnormality. This is a continuous decision-making process with degrees of risk, rather than a simple dichotomous process. Parents also contribute to the decision-making process and their decisions on whether to request or consent to a more advanced or invasive test is dependent on the recommendation of the clinician and their personal appreciation of risk level. The recommendation from the clinician is dependent on the degree of risk that is partially determined by the degree of CRP or ESR elevation. The clinician will strongly recommend a more advanced test if the degree of elevation is severe. The clinician will not be likely to recommend a more advanced test if both the CRP and ESR are normal. The clinician will render an intermediate recommendation for intermediate levels of elevation. The decision to commit to a more advanced test is a weighing of pros and cons. Parents can be advised on the degrees of risk of failing to diagnose the disease condition and degrees of risk sustained by a more advanced test.
Some previous studies comparing the CRP and ESR for the initial diagnosis of SA and Ost have concluded that one or the other is superior. However, the conclusion is highly dependent on which value is selected as the cutoff point. This is an artificial determination because such cutoff values do not reflect the true decision-making process of clinicians. Most studies have determined that the CRP and ESR are roughly the same.3,6–10,12
The report of Unkila-Kallio et al.,3 examining the CRP and ESR behavior in 44 children with Ost, concluded that the CRP rises earlier than the ESR. However, it should be noted that they used a CRP cutoff value of 1.9 mg/dL and a Westergren ESR cutoff value of 20 mm/hr. With this, the sensitivity of CRP was 98% and the sensitivity of ESR was 92%. It is not convincing that the difference between 98% and 92% is clinically important. This study did not present their original CRP and ESR values to permit an exact recalculation of sensitivities at different CRP and ESR cutoff values. Their mean initial CRP value was 7.1±4.5 mg/dL compared to their mean initial ESR value of 47±21 mm/hr.
The report by Dahl et al.,6 examining many clinical factors including the initial CRP and ESR values in 86 children with Ost, listed sensitivities of 89% and 96% for CRP and ESR, respectively. This study also did not present their original CRP and ESR values to permit an exact recalculation of sensitivities at different CRP and ESR cutoff values. Their mean initial CRP value was 6.3 mg/dL (95% CI of the mean 3.6–9.0), compared to their mean initial ESR value of 59 mm/hr (95% CI of the mean 52–66).
Gandini's report,7 examining many clinical factors including the initial CRP and ESR values in 11 children with SA of the hip, listed sensitivities of 100% for both CRP greater than 2 mg/dL and ESR greater than 20 mm/hr. Their mean initial CRP value was 14.4 mg/dL and their mean initial ESR was 65 mm/hr. Standard deviations and confidence intervals were not provided.
The report of Jung et al.,8 of 27 children with SA of the hip, listed sensitivities of 93% using an ESR cutoff of 20, 21% with an ESR cutoff of 40 mm/hr, and 89% with a CRP cutoff of 1 mg/dL. Their mean initial ESR was 79.2±42.7 mm/hr compared to their mean initial CRP value of 10.10±6.86 mg/dL.
Levine et al.9 compared the CRP and ESR in 39 children with SA and 94 children without SA. The sensitivity, specificity, and positive and negative predictive values were superior for the CRP or the ESR, depending on which cutoff values were used. A receiver operator curve methodology showed a greater area under the curve for the CRP compared to the ESR; however, the 95% confidence intervals overlapped. Their mean initial CRP value was 10.3±9.3 mg/dL and their mean initial ESR was 58.1±29.0 mm/hr.
Assuming a normal distribution, the means and standard deviations (or 95% CI) from four of the above studies3,6,8,9 permit CRP and ESR sensitivity recalculations utilizing the values used in our study, which are tabulated in Table 3. These values show that the comparisons between the CRP and ESR are similar to the comparisons found in our study. It is possible or perhaps likely that these values are not distributed in a normal fashion; however, this is the only way to recreate a data set from the previously published studies. Despite the possibility of non-normality, this does not necessarily favor one test over the other. The type of ESR is not specified (Westergren versus Wintrobe) in most of these studies.
Table 3. Published C-reactive protein and erythrocyte sedimentation rate sensitivities for septic arthritis and osteomyelitis from different studies and recalculated C-reactive protein and erythrocyte sedimentation rate sensitivities.
| Reference citation number | Cutoff values chosen by study | Mild elevation | Moderate elevation | Severe elevation | ||||
|---|---|---|---|---|---|---|---|---|
| CRP | ESR | CRP | ESR | CRP | ESR | CRP | ESR | |
| 3 (Ost only) | 1.9=98% | 20=92% | 93% | 90% | 75% | 44% | 26% | 2% |
| 6 (Ost only) | 5.0=89% | 25=96% | 68% | 88% | 57% | 61% | 39% | 17% |
| 8 (SA only) | 2.0=100% | 20=100% | 92% | 92% | 81% | 75% | 51% | 40% |
| 9 (SA only) | 1.0=90% | 25=92% | 86% | 91% | 75% | 61% | 51% | 14% |
Chosen cutoff values are those selected in the publication, mild elevation: CRP 0.4 mg/dL, ESR 20 mm/hr; moderate elevation: CRP 4 mg/dL, ESR 50 mm/hr; severe elevation: CRP 10 mg/dL, ESR 90 mm/hr;
SA, septic arthritis; Ost, osteomyelitis.
In all of the studies cited in Table 3, one could choose cutoff ESR and CRP values that demonstrate superior sensitivity for either test. However, most studies conclude that they are roughly the same. In our study and the other studies listed in Table 3, the sensitivities are similar.
Our study had the capability to examine the MCV relationship to the ESR, but no significant relationship was detected with this data set. The study by Barnes et al.,13 on patients with inflammatory bowel disease, showed that the MCV was somewhat larger in the children with discordant ESR and CRP than in the children with concordant ESR and CRP. Because our study had 15 pairs of discordant ESR/CRP values (nine one way and six the other way), we compared the MCV for these 15 patients and found the MCVs to be roughly the same in the high ESR group versus the low ESR group.
We concluded that the CRP does not rise earlier than the ESR and previously published conclusions are dependent on arbitrary thresholds. ESR and CRP should not be used in isolation as a decision-making tool for diagnostic testing for acute inflammatory diseases. Because their sensitivities are similar and the values are occasionally discordant, it is prudent to order both tests when evaluating for these inflammatory conditions. We could not find any evidence that the MCV affects the ESR.
References
- 1.Scott RJ, Christofersen MR, Robertson WW, et al. Acute Osteomyelitis in Children: A review of 116 cases. J Pediatr Orthop. 1990;10:649–52. doi: 10.1097/01241398-199009000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Del Baccaro MA, Champoux AN, Bockers T, et al. Septic arthritis versus tansient synovitis of the hip: the value of screening laboratory tests. Ann Emerg Med. 1992;21:1418–22. doi: 10.1016/s0196-0644(05)80052-6. [DOI] [PubMed] [Google Scholar]
- 3.Unkila-Kallio L, Kallio MJT, Eskola J, et al. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of Children. Pediatrics. 1994;93:59–62. [PubMed] [Google Scholar]
- 4.Taylor GR, Clarke NMP. Management of irritable hip: a review of hospital admission policy. Arch Dis Child. 1994;71:59–63. doi: 10.1136/adc.71.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein DM, Barbera C, Gray ST, et al. Sensitivity of objective parameters in the diagnosis of pediatric septic hips. Clin Orthop Relat Res. 1997;338:153–9. doi: 10.1097/00003086-199705000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Dahl LB, Hoyland AL, Dramsdahl H, et al. Acute osteomyelitis in children: a population-based retrospective study 1965 to 1994. Scand J Infect Dis. 1998;30:573–7. doi: 10.1080/00365549850161124. [DOI] [PubMed] [Google Scholar]
- 7.Gandini D. Acute septic arthritis of the hip in children in northern australia. ANZ J Surg. 2003;73:136–9. doi: 10.1046/j.1445-2197.2003.02574.x. [DOI] [PubMed] [Google Scholar]
- 8.Jung ST, Rowe SM, Moon E, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368–72. [PubMed] [Google Scholar]
- 9.Levine MJ, McGuire KJ, McGowan KL, et al. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatr Orthop. 2003;23:373–7. [PubMed] [Google Scholar]
- 10.Anderson MS, Burns J, Treadwell TA, et al. Erythrocyte sedimentation rate and C-reactive protein discrepancy and high prevalence of coronary artery abnormalities in Kawasaki disease. Pediatr Infect Dis J. 2001;20:698–702. doi: 10.1097/00006454-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Batlivala SP. Focus on Diagnosis: the erythrocyte sedimentation rate and the C-reactive protein test. Pediatr Rev. 2009;30:72–4. doi: 10.1542/pir.30-2-72. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Singh R, Soni M. C-reactive protein (CRP) as an indicator of sepsis in orthopaedic trauma. Indian J Med Sci. 2002;56:501–7. [PubMed] [Google Scholar]
- 13.Barnes BH, Borowitz SM, Saulsbury FT, et al. Discordant erythrocyte sedimentation rate and C-reactive protein in children with inflammatory bowel disease taking azathioprine or 6-mercaptopurine. J Pediatr Gastroenterol Nutr. 2004;38:509–12. doi: 10.1097/00005176-200405000-00009. [DOI] [PubMed] [Google Scholar]