Abstract
Modern biomedical research requires access to high quality specimens of human tissue with or without extensive clinical annotation. Multiple types of organizations have developed to supply human tissues to support biomedical research. These organizations follow different models including the specific models of 1) prospective collection, 2) tissue banking, and 3) tissue collection associated with clinical trials as well as the model of 4) a tissue resource that incorporates features of the other models. These types of organizations devoted to supplying tissues for research have chosen different goals to meet the different tissue and informational needs of the investigators to whom they supply tissue. In order to provide high quality tissues to support research, all models should rely on a strong quality assurance program with extensive quality control of the tissues being provided to support research. In addition to facilities which collect, process, store and provide tissues, the need for a rigorous QA program applies to all resources and infrastructures used to support biomedical research. The UAB Tissue Collection and Banking Facility which provides human tissue to support biomedical research has been functioning and developing since 1979. To our knowledge, similar programs in providing tissues from animals are less developed, but could easily follow the models which UAB and other institutions providing human tissues have established, including the approaches of UAB and others to QA and QC. This manuscript reviews the current concepts of QA and QC in use in organizations supplying tissue to support biomedical research as well as new approaches in QA and QC that have been proposed.
INTRODUCTION
Modern biomedical research requires access to high quality specimens of human tissue with or without extensive clinical annotation. For some studies focusing on the extraction and characterization of a novel protein from breast cancer only a correct diagnosis of “breast cancer” may be needed; however, studies of a similar protein and the correlation of its phenotypic expression with clinical outcome of ductal carcinoma of the breast will require extensive clinical annotation and a more specific diagnosis of ductal carcinoma of the breast along with information regarding tumor grade and stage. Organizations devoted to supplying tissues for research have developed differing goals and standards to meet the varied tissue and informational needs of the researchers that they supply.
A strong program in quality assurance is important in any aspect of biomedical research and must be developed for facilities which collect, process, store and provide tissues to support biomedical research. Quality Assurance/Management (QA) is a general approach to management activities which focuses on operational standards for all aspects of all activities to ensure that a procedure or product is of the defined quality required. Quality Control (QC) is the system of technical activities that measures the attributes and performance of a process, or item, against defined standards, to verify that the stated requirements are fully met. Thus, QC is only one component of an overall QA program.
The organized program for providing human tissue to support biomedical research at UAB has been developing since 1977. Similar programs established to provide tissues from animals could easily follow the models which UAB and other institutions have established, including our approaches to QA and QC.
Models of Tissue Collection
Obtaining human tissues and bodily fluids to support biomedical research may be organized or disorganized. “Catch as catch can” is the best designation of the approach in which a surgeon, pathologist or other veterinary or medical personnel provides tissues to biomedical investigators. Such specimens characteristically have not been collected, processed, or stored using standard operating procedures and usually are not associated with quality control; thus these specimens may be of poor quality and their diagnosis may be incorrect. More worrisome is that such specimens of human tissue may be obtained without oversight of Privacy Boards or Institutional Review Boards and may violate the Common Rule and/or HIPAA regulations. Similarly, collection of such specimens may be under the purview of an animal use committee, especially if the animals are sacrificed specifically to obtain the tissue.
In general, programs which provide tissues to biomedical research tend to fall into the following four models:
An organized approach in which investigators specify exactly the tissue specimens they need as well as how the specimens are to be processed and stored is designated as the “prospective collection model”. The clear disadvantage of prospective collection is that large numbers of specimens are not available immediately when requested. Also outcome data are not available for several years as the specimens are collected at the time that they are requested. The advantage of the prospective collection model is that the investigator gets exactly what the investigator requests (e.g., fresh uninvolved kidney minced in RPMI media); the investigator must however wait for specimen availability (months) and on clinical outcome (years) if required.
Another approach to obtaining high quality human tissues to support biomedical research is to utilize a “banking model”. In a banking model, standard operating procedures (SOPs) are followed for obtaining, processing and storing human tissues. Tissues maintained in tissue banks are allowed to “mature” so that outcome data can be obtained for the stored samples. For example, a tissue bank may store only frozen tissues and/or paraffin blocks processed by SOPs. “Specially processed” tissues as well as fresh, unfrozen samples usually are not available from a tissue bank; another major disadvantage is that the specimens may not meet the specific needs of the investigator in areas such as aliquot size, % of tumor in the sample, and processing and storage conditions (1-4). Advantages of the “banking model” are that large numbers of specimens are available immediately and clinical and demographic information including clinical outcome are readily available.
A clinical trial model is usually a type of banking model in that the bank stores the remnants of the tissues/bodily fluids collected from one or more clinical trials. These specimens are banked to support future studies. The problems with a general banking model may be magnified in that the original consent form of the clinical trial may not clearly state that the specimens can be used for other research. Additionally, the institution's IRB may prohibit the utilization of specimens for a different type of research. Similarly, the remnants of the clinical study may not meet the needs of a wide range of investigators.
The tissue resource model uses a combination of the approaches of the prospective and the banking models and may incorporate the advantages of each of these models. The main potential problem in the operation of a tissue resource facility is that there are complex and numerous administrative requirements as well as the need for a more complex bioinformatics system.
In each of these models, a strong quality assurance program is critical if high quality tissues are to be provided to support research projects. Because investigators may devote extensive research time and effort to tissues used in biomedical research, tissues of poor quality or incorrect diagnoses can significantly impede the progress of a specific research project or even an area of research.
Bias in use of tissue collections
Without detailed and accurate records, bias can easily be introduced into research projects and incorrect conclusions can be accepted as to the meaning of the research. For example, if cases of a specific disease were to be evaluated using serum that had multiple freeze thaws, a storage time of over 6 years at −80°C, and collection prior to an operation, but the associated controls had only one freeze thaw cycle, a storage time of less than two years at −80°C, and collection in the community using a mobile van, mass spectrometry methods might identify the differences in freeze thaw cycles, collection methods and storage time (5) and researchers might incorrectly conclude that there were proteomic differences between the serum from patients with the specific disease and individuals who did not have the disease; a conclusion based upon the bias of the two sample sets. Such bias might not be identified until attempts are made to validate the initial experimental results. Also, this example emphasizes the importance of using SOPs which are standardized across different collection sites, so that tissues are collected, processed and stored similarly. In this case, careful records might have emphasized the differences, and the conclusions might have been confirmed before reporting on a subset of samples collected, processed and stored more uniformly. Some of the potential causes of bias are listed in Table 1.
Table 1.
Examples of Potential Sources of Bias in Tissue Sets
| 1. | Population (e.g., racial mixture) |
| 2. | Fed or fasting state |
| 3. | Diurnal variations (i.e., time of collection) |
| 4. | Stress |
| 5. | Collection container (red top vs. separator) |
| 6. | Time to processing |
| 7. | Time to freezing |
| 8. | Temperature and length of storage |
| 9. | Freeze thaw cycles |
| 10. | Sites of sample collection |
Staff Responsibilities
Personnel assigned to the QA programs have the responsibility for ensuring compliance with all SOPs and regulatory requirements, and should report directly to high levels of management concerning all QA issues. These personnel should aid in the development of SOPs for specimen collection, handling, processing, storage and distribution of specimens to assure that each of these processes is performed in a consistent fashion. In cases where different groups are collecting specimens for a single repository, SOPs should be standardized across the different collection sites. When problems in these areas are identified and/or if any specimens of poor quality are identified, personnel should initiate and participate in efforts to correct these problems. Personnel assigned to QA should be responsible for designing, overseeing and evaluating audits of overall operations regarding adherence to QA requirements
Aids to Develop Good Operational Practices
There are several documents which can aid repositories in developing their procedures. For example, Good Manufacturing Practices are regulatory guidelines originally developed to aid the pharmaceutical industry; components of these can be adapted by tissue resource organizations to meet the organization's operational goals. Generally, these standards should include or address the following:
The facility is located in a secure, isolated area with limited access.
The facility is locked when tissue resource personnel are not on site.
Policies and procedures are documented in SOPs that are approved by appropriate personnel and changed or updated under strict document control rules.
Personnel are trained in all SOPs, safety and other operational issues and such training is documented.
The facility is subject to internal QA audits by external clients and agencies as appropriate.
An extensive audit trail is maintained for all specimens.
Equipment maintenance and calibration procedures are performed as required by manufacturers or by local SOPs. This maintenance and calibration is documented.
Access to the informatics system is limited and is controlled via a series of codes. These codes permit various levels of access by facility personnel. Per HIPAA requirements, access to informatics systems is documented.
Deviation is documented for all events that fall outside SOPs. Corrective action is identified, if necessary.
The FDA's Good Tissue Practices (21 CFR Part 1271), although focused on human tissues to be used for transplantation, might also be applicable to assuring good tissue procurement practices.
The International Organization for Standardization (ISO), a worldwide federation of national standards organizations, has produced document ISO9001 which has a primary purpose of providing organizations with useful internationally recognized models for operating a quality management system. Tissue facilities may wish to utilize ISO9001 in developing and monitoring a QA/QC program.
Audits
Audits are written periodic evaluations of operating procedures and infrastructure. Tissue resource facilities should conduct regular audits such as those listed subsequently. Audits may be as simple as a weekly check of freezer temperature logs or liquid nitrogen levels or may be more complex such as a review of specimens collected. QA personnel quarterly document problems and report them to upper management and the chief executive officer of the facility.
The QA program at a facility should describe how and when audits are conducted. Examples of specific audits and documentation include the following:
SOPs and adherence to these procedures,
Equipment maintenance and repair,
Equipment monitoring (e.g., determining the cutting thickness of microtomes),
Training records and adherence of staff to required training (e.g.. training in biohazards),
Data management,
Record keeping and monitoring of records,
Specimen tracking (e.g., monitoring of specimen locations in freezer),
Safety plan,
Adherence to standard documents such as ISO9001, as needed,
Shipping problems as reported by investigators.
Reports and Surveys of Users of the Repository
Tissue resource organizations should consider distributing an annual survey to determine the satisfaction of users/investigators who obtained tissue during the preceding year. It would be useful if the survey could be completed “on line”; however, a paper survey could also be sent to users. The results of the survey should be evaluated carefully. Investigators reporting unsatisfactory results should be contacted and their problems discussed, documented, and corrected if practicable.
If the organization provides tissues to extramural investigators, each shipment should be monitored closely. Such monitoring can be accomplished by including a short questionnaire with each shipment that documents receipt of the shipment and provides an opportunity to report immediate feedback if problems were encountered (e.g., not enough dry ice in frozen shipments). These questionnaires could be returned via fax, e-mail, or the recipient could be referred to a website for completion of the questionnaire.
Annotation of the Specimen
One of the most important aspects of a QA program is to insure that all aliquots of tissues supplied for research are identified correctly and that the clinical and demographic information supplied with the specimen are from the patient from whom the tissue specimen was collected.
A labeling method should be used that a) minimizes the chances for the label to separate from the specimen, b) prevents mislabeling due to personnel error and c) avoids problems with reading the specimen identification (e.g., poor handwriting). For some resources, this is best accomplished by the use of bar codes which link the specimen to a database containing pertinent information. It should be understood that a barcode is only a “number” and this number contains no other information; it is only linkage of the barcode number to the database through interface software that actually identifies information regarding the specimen. Thus, unless the same identical software is used at a second site, the second site can only read the specimen number without a link to information in the database. Note that other information may be included on the printed label (e.g., race, age, etc.) because the barcoding software is coded to print additional information on the label.
Quality Control of Tissues
Monitoring the quality and diagnoses of the actual tissues provided for research (i.e., quality control) is a very important component of the QA program. Tissue facilities have used various forms of QC to aid investigators with their studies in order to ensure that the tissues and associated information provided meet the needs of the investigator (1-3). Many tissues, especially tumors, are heterogeneous; thus, specimens from tumors vary as to the extent of neoplastic cells, mucin production, inflammatory cells and/or necrosis. Fibrosis in and adjacent to tumors may be intermixed with or mistaken for tumor and some tumors may diffusely infiltrate normal tissues making areas of tumor difficult to identify grossly. Therefore, in general, just knowing the diagnosis of a patient from whom tissues are obtained is not adequate quality control for tissues provided for research.
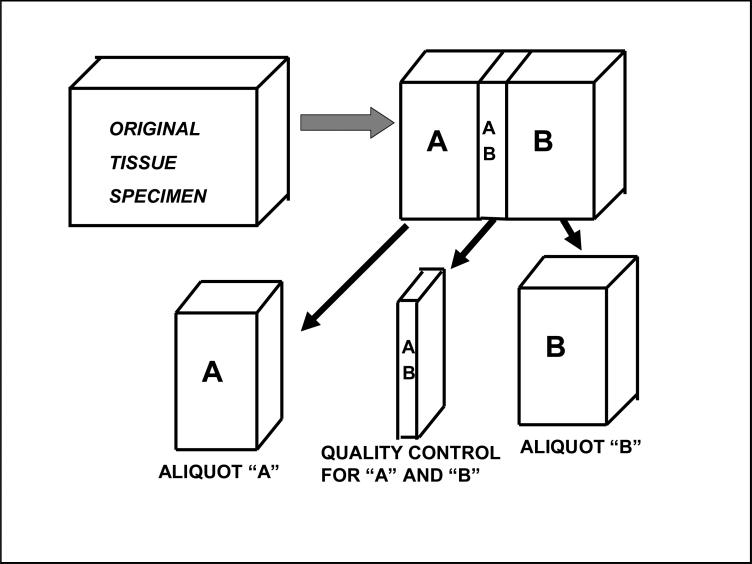
The minimum QC of each tissue resource organization should be the microscopic examination by a pathologist of an aliquot of tissue that is very representative of the specific tissue that is supplied for research. Optimally, unless the tissue is very small, a QC examination (Figure 1) is made on a mirror image piece of tissue to that supplied for research. Note that in Figure 1, AB is processed to a paraffin block and histopathologic examination of AB is the quality control mirror image for both specimen A and specimen B.
Figure 1.
Tissue Specimens Supplied for Research.
Using this or similar methods of QC, the Cooperative Human Tissue Network (CHTN) has found that about 15% of tissues collected for specific research cannot be used for the research for which the specimens originally were collected (1-3). Specifically, areas of tissue that appear grossly to be unaffected by a disease process may be microscopically involved by disease or specimens which appear to be diseased may include such a large component of other processes such as scaring / fibrosis / accumulation of mucin or damage by radiation as to be not useful for specific research. Other reasons for rejection of a specimen of tissue because of the QC examination may include extensive ischemia, inflammation, or necrosis. For example, some focal areas of large tumors (e.g., large renal cell carcinomas or liver metastases of colorectal cancer) may be so necrotic such that only a few recognizable tumor cells remain in the areas of the tumor collected for research. Typically in the QC examination, the proportion (percent) of the specimen that is diseased is specified along with the percent necrosis/fibrosis of the diseased areas as well as the percent of other factors such as mucin formation. For example, a mucinous tumor may be composed of greater than 90% acellular mucin and the lack of cellular representation is likely to impede many specific forms of analysis. Another important quality control index of tissues used in research is the proportion of cells within the tumor that are neoplastic. This is necessary because a tumor may be infiltrated by a large proportion of inflammatory cells. Quality control also can be performed on frozen sections of a specimen embedded in frozen section support medium (e.g., Optimal Cutting Temperature [O.C.T.] Compound). Note that O.C.T. Compound or other similar heavy alcohol based support mediums needed to obtain frozen sections may superficially contaminate the specimen and subsequently interfere with some assays (e.g., biological assays for folate).
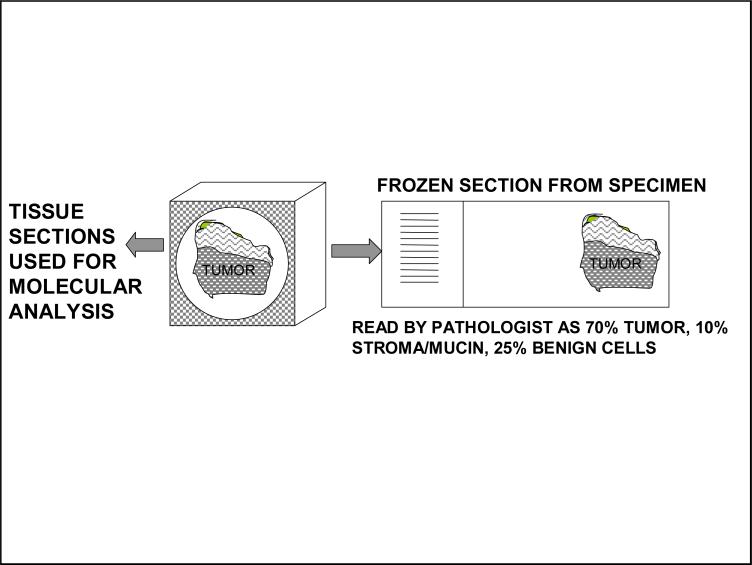
Figure 2 demonstrates QC via a frozen section including the minimum information needed by most investigators as well as molecular information provided if requested.
Figure 2.
Histopathologic and Molecular Quality Control Based on Frozen Sections
The quality control examination also can be in part based on “molecular quality control” in which mRNA, DNA and protein are extracted from small aliquots followed by molecular characterization of the molecules using various analytical methods ranging from mass spectrometry or gene arrays to examination of ribosomal bands of RNA using gels (5) or other systems such as the Agilent® 2100 System (Figure 2 and 3). Molecular quality control is performed when investigators request this level of QC and periodically to verify the quality of specimens in general provided by the facility. It may also be performed when investigators indicate that there is a specific problem with any of the specimens with which they have been supplied, if additional aliquots of those specimens are still available at the facility.
Figure 3.
Platinum Level of Quality Control Based Upon Frozen Sections, Macrodissection and Molecular Assays
Even more extensive QC examinations of tissue are sometimes needed by investigators or specific collaborative research projects; however, as the required QC examinations become more extensive, these more extensive QC measurements have a “price” for their use, specifically, increased time and effort put into the QC examination of the specimen by the tissue resource organization and thus usually an increased “cost” of the specimen to the investigator.
QC can also be tailored based upon the request of individual investigators. Rarely an investigator may request a “platinum level” of QC, which is demonstrated in Figure 3. In this approach to QC, frozen sections of the whole specimen are made followed by macrodissection to enrich the specimen in diseased cells followed by a QC examination of the opposite side of the specimen plus additional macrodissection if necessary. Also, aliquots of the front and back of the specimen after macrodissection would be analyzed molecularly. Thus, frozen sections from both sides are not only used in microscopic examinations for QC, but also are used in molecular quality control. Such an approach to QC increases the costs to the tissue resource of processing specimens and to the investigator. This approach may also reduce the tissue available to support research because the QC examination begins to exhaust the specimen. If the research projects of an investigator do not require extensive QC, such an approach is not cost effective. Also, investigators may wish to perform molecular QC and/or microdissection (e.g., laser capture) or macrodissection at their laboratories or organization, hence reducing the need for extensive efforts in QC at the tissue repository. Most research with today's microtechniques should not require this extent of quality control.
The quality control examination of tissues for research should match the research planned for the tissues and that requested by the specific investigator. For most tissue specimens and investigators, the approach shown in Figure 1 is adequate. If tumor enrichment or molecular analysis is required, this can be requested by the investigator or be performed at the investigator's home institution. If mRNA is to be analyzed using long amplicons greater than 200 base pairs, a section of tissue at the request of an investigator can be examined to determine the quality of RNA using the Agilent 2100 method; obviously, if the specimen is not to be used in RNA analysis, such analysis adds unnecessary costs.
Note that using short amplicons in real time quantitative PCR techniques permits the use of somewhat degraded RNA and even RNA extracted from paraffin blocks can be used to give equivalent results to those using frozen tissues (7). The best and most cost effective approach for QC is to utilize a simple approach (Figure 1) that can be expanded to the platinum level, but only at the request of an investigator and payment by the investigator of the added costs of this approach.
Quality Assurance for the Collection of Bodily Fluids
The QA of bodily fluids primarily involves selecting the parameter of collection, processing and storage, and incorporating these into SOPs. It is also important to avoid bias in the selection of patients and in the collection, processing and storage of the specimens (6). Most studies rely on specimens of bodily fluids that are frozen within 4 hours of collection. For a discussion of other issues concerning QC of a tissue repository, see the ISBER Best Practices (7 and 8).
Acknowledgments
Supported in part by the Cooperative Human Tissue Network (CHTN Grant #5U01 CA44968-18S2 to William E. Grizzle), the Early Detection Research Network (EDRN Grant # CA086359-08 to William E. Grizzle) and the NCI Specialized Program of Research Excellence (SPORE) [P20 CA101955-05 and 5P50 CA89019 to William E. Grizzle] and the Skin Disease Research Center at UAB (P30AR50948 to Craig Elmets). Presented in part at the American College of Veterinary Pathologists and the American Society for Veterinary Clinical Pathology Concurrent Annual Meetings, Savannah Georgia, 2007.
References
- 1.Grizzle WE, Bell W, Sexton KC. Best practices and challenges in collecting and processing human tissues to support biomedical research.. AACR 96th Annual Meeting, Education Book.2005. pp. 305–310. [Google Scholar]
- 2.Grizzle WE, Sexton KC. Development of a Facility to Supply Human Tissues to Aid in Medical Research. In: Srivastava S, Henson DE, Gazdar A, editors. Molecular Pathology of Early Cancer. IOS Press; Van Diemenstratt, Amsterdam, Netherlands: 1999. pp. 371–383. Chapter 24. [Google Scholar]
- 3.Grizzle WE, Aamodt R, Clausen K, et al. Providing human tissues for research: how to establish a program. Arch. Pathol. Lab. Med. 1998;122(12):1065–1076. [PubMed] [Google Scholar]
- 4.Grizzle WE, Semmes OJ, Bigbee W, et al. The need for the review and understanding of SELDI/MALDI mass spectroscopy data prior to analysis. Cancer Informatics. 2005;1(1):86–97. [PMC free article] [PubMed] [Google Scholar]
- 5.Jewell SD, Srinivasan M, McCart LM, et al. Analysis of the molecular quality of human tissues: An experience from the Cooperative Human Tissue Network. Am. J. Clin. Pathol. 2002;118(5):733–741. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 6.Steg A, Wang W, Blanquicett C, et al. Multiple gene expression analyses in paraffin-embedded tissues by Taqman low density array: application to Hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J. Mol. Diagn. 2006;8(1):76–83. doi: 10.2353/jmoldx.2006.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aamodt RL, Anouna A, Baird P, et al. Best practices for repositories I: collection, storage and retrieval of human biological materials for research. Cell Preservation Tech. 2005;3:5–48. [Google Scholar]
- 8.Pitt KE, Campbell LD, Skubitz APN, et al. Best practices for repositories: Collection, storage, distribution and retrieval of biological materials for research. Cell Preservation Tech. 2008 in press. [Google Scholar]