Abstract
This paper examines adult age-specific mortality patterns of one of the most devastating epidemics in recorded history, the Black Death of A.D. 1347–351. The goal was to determine whether the epidemic affected all ages equally or if it targeted certain age groups. Analyses were done using a sample of 337 individuals excavated from the East Smithfield cemetery in London, which contains only individuals who died during the Black Death in London in 1349–1350. The age patterns from East Smithfield were compared to a sample of 207 individuals who died from non-epidemic causes of mortality. Ages were estimated using the method of transition analysis, and age-specific mortality was evaluated using a hazards model. The results indicate that the risk of mortality during the Black Death increased with adult age, and therefore that age had an effect on risk of death during the epidemic. The age patterns in the Black Death cemetery were similar to those from the non-epidemic mortality sample. The results from this study are consistent with previous findings suggesting that despite the devastating nature of the Black Death, the 14th-century disease had general patterns of selectivity that were similar to those associated with normal medieval mortality.
Keywords: paleodemography, age-specific mortality, medieval plague, Gompertz-Makeham model
1. Introduction
The Black Death, an outbreak of medieval plague that swept through Europe between A.D. 1347–1351, was one of the most devastating epidemics in human history, and it had wide-ranging and long-lasting demographic, economic, social, and political consequences (Bowsky 1971; Cohn 2002; Gottfried 1983; Ziegler 1969). The Black Death is estimated to have killed between 30–50 percent of the population of Europe, and many of the dramatic changes brought about by the epidemic were the direct result of its exceedingly high mortality (Cohn 2002; Poos 1991; Wood et al. 2003). The Black Death has fascinated people for centuries and thus been studied extensively by physicians, historians, demographers, anthropologists, epidemiologists, molecular biologists, and other researchers. Yet, despite such a long history of study, there is still much to learn about the medieval disease, including how risks of mortality varied within affected populations, the members of which presumably were immunologically naïve with respect to the disease that was apparently new to Europe.
In general, individuals within a population vary in their risks of mortality because of such factors as age, sex, exposure to infectious diseases, and nutritional status. Typically, a consequence of this variability is selective mortality, which occurs when causes of mortality target (and thus select out of the population) those individuals at the highest risks of death in the population rather than killing all individuals at the same rate (Ortner 1991; Wood et al. 1992). Normal causes of morality generally behave selectively and thus target very young children, the elderly, people with compromised immune systems, and other such individuals. Selective mortality has been identified as an important problem for paleodemography and related fields because it renders skeletal samples unrepresentative of the original living populations from which they are derived (Milner et al. 2008). Because of selective mortality, cemeteries formed by normal causes of mortality (often referred to as attritional cemeteries because they are formed by the natural attrition of a population over time) generally contain the individuals who were at the highest risk of death at any particular age rather than a representative sample of all individuals who were once alive at that age (Wood et al. 1992).
The Black Death, however, was not a normal cause of mortality, given its tremendously high mortality rate. It has been suggested previously that because it was so virulent, the Black Death might have been an indiscriminant killer (Chamberlain 2006; Gowland and Chamberlain 2005; Margerison and Knüsel 2002). Examination of samples of individuals who died during the Black Death can clarify whether the epidemic’s mortality patterns differed from those of normal mortality. If the Black Death killed indiscriminately, skeletal samples of Black Death victims are expected to resemble living populations much more closely than do normal, attritional cemeteries. If this was the case, Black Death samples should have similar age distributions, sex ratios, and frequencies of individuals with skeletal pathologies to those in the original living populations. On the other hand, if the Black Death behaved more like normal causes of mortality and was thus selective, skeletal assemblages of Black Death victims are expected to resemble those from attritional cemeteries. Clarification of the patterns of mortality during the Black Death is essential not just for a complete understanding of the medieval disease in particular, but is also relevant to understanding how selective morality - which is of theoretical and methodological importance to the fields of paleodemography and paleoepidemiology - behaves under conditions of epidemic versus normal mortality in general.
I previously investigated patterns of Black Death selectivity by examining how preexisting health condition and sex (of adults) affected risks of dying during the epidemic (DeWitte 2009; DeWitte and Wood 2008). The results of these previous studies revealed that the Black Death was selective with respect to frailty, and thus people in poor health before the epidemic were more likely to die during the Black Death than their comparatively healthy peers, and that men and women faced approximately equal risks of death during the epidemic. The current study further contributes to an understanding of Black Death mortality and to efforts to determine whether the epidemic killed indiscriminately by analyzing selectivity with respect to age during the epidemic. Specifically, this study examines how the risk of mortality during the epidemic was distributed by age to address the question: did people of all ages face similar risks of death during the epidemic, or were certain ages at higher risks than others during the Black Death?
Several chronicles written at the time of the 14th-century epidemic provide a few details about the age patterns of Black Death mortality. For example, the chronicler Matteo Villani described the Black Death as “a pestilence among men of every condition, age and sex” (translation quoted in Cohn 2002: 126), and similarly the chronicler Michele da Piazza wrote that the mortality from the Black Death was “so heavy that sex and age made no difference, but everyone died alike” (translation from Horrox 1994: 41). Both of these descriptions might suggest that age had no effect on risk of death during the Black Death. Such a pattern contrasts sharply with later outbreaks of medieval plague that, according to chroniclers, targeted children (Cohn 2002; Hatcher 1977; Holmes 1971). The references to age that exist in chronicles of the Black Death do not provide a complete picture of the age patterns of mortality during the epidemic. Though the chroniclers generally suggest that the Black Death affected all age groups, this does not necessarily indicate that risk of mortality was uniform across all ages.
Several researchers have attempted to infer the age pattern of Black Death mortality from documentary evidence. Russell (1948) produced life-table estimates of age-specific mortality among high-status individuals during the Black Death in England based on information from the inquisitions post mortem, which provide a nation-wide sample of the highest rank of landholders. Unfortunately, the samples from such records are too small to provide useful life-table estimates, particularly for intervals below the age of twenty (Wood et al. 2002a). Despite the limitations of the available data, Russell concluded that age did have an effect on Black Death mortality; he argued that older men were particularly susceptible (although individuals over the age of 60 apparently fared better than those in their late 50s), and children between the ages of ten and fifteen were at a lower risk of dying from the disease than other age groups.
Using manorial court records from the English village of Halesowen, Razi (1980) estimated ages at death among the peasantry during the epidemic. He then estimated age-specific mortality rates for males above the age of 20 during the Black Death and found, similarly to Russell (1948), that for males between 20 and 59, mortality rates increased with age, and that rates decreased with age after 60. Razi cautioned that his observations are not necessarily valid given the small sample sizes available. In addition, he estimated age at death based on the interval between a person’s first appearance in manorial-court records and that individual’s recorded date of death, assuming that the average age of first appearance in court was 20 years; there is debate regarding whether this assumption is valid (Poos and Smith 1984). In general, the existing historical records do not yield a complete understanding of the age patterns of Black Death mortality, as they do not provide very good age estimates and generally provide little information about women and children. Further, age estimates based on the documentary evidence may not be highly accurate.
Skeletal samples of individuals who died during the Black Death can provide data on age patterns of mortality that are not available from existing historical documents. This study uses a sample from the East Smithfield cemetery in London, an epidemic cemetery that was established for and used only during the outbreak in London in 1349–1350 (Grainger et al. 2008; Hawkins 1990). Most, if not all, individuals interred in East Smithfield were victims of the Black Death, and the cemetery contains individuals from all ages and both sexes and thus provides an ideal sample for examining mortality patterns associated with the epidemic.
East Smithfield has been used in previous investigations of Black Death mortality. Waldron (2001) compared East Smithfield to the normal (i.e. non-epidemic) St. Mary Graces cemetery in London (circa 1350–1538). He used traditional methods of age estimation, and compared both cemetery samples to the living age distribution from a model life table thought to be representative of a medieval urban population. Waldron did not find any systematic differences between the age-at-death distributions of the two cemeteries, but both differed from the model living age distribution, which suggests that the Black Death was not an indiscriminant killer. Using Waldron’s (2001) East Smithfield data, Margerison and Knüsel (2002) compared East Smithfield to a normal mortality sample from the St Helen-on-the-Walls cemetery from York, England (circa late 12th century to the mid-16th century). The age-at-death distributions from both cemeteries were compared to a model life table mortality profile. The East Smithfield mortality profile was significantly different from St Helen-on-the-Walls and the model age-at-death distribution, and in general conformed to expectations of a catastrophic profile, which suggests that the Black Death killed indiscriminately. Similarly, researchers at the Museum of London Centre for Bioarchaeology found that the East Smithfield age-at-death distribution resembles a living population age structure rather than a typical non-epidemic mortality sample (Kausmally 2007). Gowland and Chamberlain (2005) compared East Smithfield to a normal mortality sample from Blackgate cemetery in Newcastle, England. Unlike Waldron (2001) and Margerison and Knüsel (2002), who used traditional age-estimation methods, Gowland and Chamberlain used Bayesian inversion to estimate individual adult ages at death based on age-related changes of the pubic symphysis and iliac auricular surface. These skeletal features were scored according to the protocols established by Brooks and Suchey (1990) and Lovejoy et al. (1985), respectively (the current study used a similar statistical approach but a different scoring protocol; details are provided below in section 2.2.1). The Blackgate age-at-death distribution was similar to the normal mortality profile from a model life table, whereas the East Smithfield distribution resembled a living population age distribution, suggesting that risks of mortality were similar for all age groups during the Black Death.
The current study was done because previous studies of East Smithfield yielded contradictory conclusions about Black Death age patterns. Furthermore, the current study differs from previous investigations of East Smithfield in two important ways: the use of new, rather than traditional, methods of adult age estimation and a hazards model approach. Most of the previous studies, with the exception of the study by Gowland and Chamberlain (2005), were based on adult ages estimated using traditional methods which have been shown to be biased (more details are provided in section 2.2.1) (Bocquet-Appel and Masset 1982). However, this study uses transition analysis, an age estimation method that was developed to avoid such biases in adult ages (Boldsen et al. 2002). Unlike all other studies of East Smithfield age patterns, this study uses the parametric Gompertz-Makeham model of adult mortality, which is well suited to evaluating human mortality risks across all adult ages using the incomplete and otherwise problematic data typically available from skeletal samples (more details are provided in section 2.3) (Gage 1988).
2. Materials and Methods
2.1 Skeletal samples
2.1.1 East Smithfield Black Death Cemetery
The East Smithfield cemetery in London is one of the few exclusively Black Death cemeteries in Europe that has been excavated. The purpose, location, and size of the East Smithfield cemetery were recorded in contemporary records, and there is also archaeological evidence dating it to the time of the Black Death (Grainger and Hawkins 1988; Hawkins 1990). The East Smithfield cemetery London was established right before the epidemic struck London in 1349, and there is no evidence that it was used for burials after the epidemic. So, the individuals interred there died while the Black Death devastated London, and most, if not all, were victims of the epidemic (Grainger and Hawkins 1988; Hawkins 1990). For this study, a sample of 337 individuals was selected from the East Smithfield cemetery and analyzed by the author at the Museum of London Centre for Human Bioarchaeology. This sample comprises all of the excavated adult individuals from East Smithfield who were preserved well enough to provide sufficient data on age using the method described in section 2.2.1.
2.1.2 Medieval Danish Cemeteries: St. Mikkel and Albani Church
For this study, the East Smithfield cemetery was compared to a normal (i.e. non-epidemic) mortality sample from the medieval Danish urban parish cemeteries of St. Albani Church, Odense, and St Mikkel Church, Viborg (circa 1100s- mid 1500s), both of which form part of the current Anthropological Database at Odense University, Denmark (ADBOU) collection. The Danish cemeteries were used because it was possible to obtain a pre-Black Death sample from them using the arm positions of the interred individuals (Jantzen et al. 1994; Kieffer-Olsen 1993). Analysis of medieval and post-medieval burials of known date from Denmark revealed a series of changes in the predominant arm position of buried individuals, and arm position during this time period provides dates with narrower margins of error than those produced by radiocarbon dating. This study includes only individuals from the Danish cemeteries interred with arm positions used exclusively or predominantly before the Black Death arrived in Denmark in 1350. Post-Black Death burials were avoided given that the epidemic caused dramatic demographic changes throughout Europe (Bowsky 1971; Cohn 2002; Gottfried 1983; Hatcher 1977; Herlihy 1997). According to Paine (2000), episodes of catastrophic mortality can have effects on the age-at-death distribution that are evident for decades. The Danish sample contains only individuals who died before the Black Death arrived in Denmark in 1350, and thus provides a baseline of normal, non-epidemic mortality patterns for comparison with East Smithfield.
In addition to providing a pre-Black Death normal mortality sample, there were several advantages to using the Danish sample. It is sufficiently large to allow for the estimation of the parameters of the model used in this study, and it is drawn from a population very similar economically, socially, and demographically to that of southern England (Benedictow 1993; Poulsen 1997; Roesdahl 1999; Sawyer and Sawyer 1993; Widgren 1997). Further, all cemeteries used in this study are from urban areas. Though smaller than London, the Danish cities were major centers for politics, religion, and trade during the Middle Ages. Lastly, there were probably genetic similarities between the two populations, which persist today, as a result of the pre-Conquest Norse settlement of England (e.g. Capelli et al. 2003). This means that observed mortality pattern differences between the East Smithfield and Danish cemeteries could be at least partly attributed to differences between Black Death and normal medieval mortality. However, given that the two samples are not from the same population, if mortality patterns differ between the East Smithfield and Danish cemeteries, potential population differences must also be considered as an explanation.
For this study, a combined sample of 207 individuals was selected from the St. Mikkel and St. Albani Church cemeteries and scored for age by the author. This sample includes all of the individuals from these cemeteries who died before the Black Death and were preserved well enough to yield data on age.
2.2 Age Estimation
This study differs from most previous studies of East Smithfield and Black Death mortality by using a new method of adult age estimation instead of traditional methods, which researchers have found to be biased. Traditional methods of age estimation are associated with the problem of age mimicry whereby estimated ages are biased toward the age distribution of the known-age reference sample that is used as a standard (Bocquet-Appel and Masset 1982; Boldsen et al. 2002). To avoid such bias, for this study, adult ages were estimated using the method of transition analysis described by Boldsen et al. (2002). In transition analysis, data from a known-age reference collection are used to obtain the conditional probability that a skeleton will exhibit a particular age indicator stage or suite of age indicator stages given the individual’s known age. Using Bayes’ theorem, this conditional probability is then combined with either a prior distribution of ages at death based on documentary information or a uniform prior to determine the posterior probability that a skeleton died at a certain age given that it displays particular age indicator stages. For this study, transition analysis was applied to skeletal age indicators on the pubic symphysis and the iliac auricular surface and to cranial suture closure as described by Boldsen et al. (2002), and the ADBOU (Anthropological Database, Odense University) Age Estimation software was used to determine individual ages-at-death. The ADBOU program uses data from 17th-century Danish rural parish records for an informative prior distribution of ages at death (the Gompertz-Makeham parameter estimates for this prior are: α1 = 0.01273, α2 = 0.00002478, and β = 0.01618). The program uses a conditional probability estimated from the Smithsonian Institution’s Terry Collection that an individual at a given age will be in the observed age indicator states; by combining the informative prior and the conditional probability, the program uses Bayes’ theorem to obtain the highest posterior point estimate of age for each skeleton.
2.3 Gompertz-Makeham Model
To determine the age pattern of mortality within the East Smithfield and Danish samples, individual age estimates were used to estimate the parameters of the Gompertz-Makeham model of mortality (Makeham 1860):
where α1 is the constant age-independent risk that everyone within the population faces (i.e. the chance of dying from causes that are unrelated to aging), and α2eβ2a is the exponentially increasing senescent risk where α2 is the risk of death associated with senescence at the moment of birth and β2 is the rate at which this risk increases with age (Gage 1988). The two components of the Gompertz-Makeham model are independent, so surviving one component of mortality does not affect one’s risk of death from the other (Wood et al. 2002b).
There are several advantages to using a hazards model for investigating age patterns of mortality in past population, as compared to the traditional model life table approach. Life tables are considered by many researchers to be an inefficient way to deal with paleodemographic age-at-death data, as they require the estimation of numerous parameters, i.e. the central mortality rate for every age interval in the table (Buikstra 1997; Hoppa and Vaupel 2002; Konigsberg and Frankenberg 2002; Milner et al. 2008; Wood et al. 2002b). Such estimation requires two things that are typically not available from cemeteries: huge sample sizes and information about the original population at risk (Wood et al. 2002b). Rather than construct life tables, some paleodemographers use a model life table, i.e. a theoretical life table that matches the age-at-death distribution in a particular cemetery (Weiss 1973; Wood et al. 2002b), but there is often more than one model life table that fits observed cemetery data and no method to choose among them (Gage 1988; Milner et al. 2008). Many researchers therefore recommend the use of parametric hazards analysis rather than life tables for paleodemographic studies (Buikstra 1997; Gage 1988; Hoppa and Vaupel 2002; Konigsberg and Frankenberg 1992; Konigsberg and Frankenberg 2002; Wood et al. 2002b). The Gompertz-Makeham model used for this study requires the estimation of just three parameters, and thus is a more efficient and less problematic use of paleodemographic data than the life table approach. It can also be applied to small samples, like those typical of paleodemography, as it smoothes the random variation in mortality data that is an artifact of small samples, without imposing any particular age pattern on the data (Gage 1988).
It is possible to assess the risk of mortality across all ages using the five-parameter Siler model (Siler 1979), which adds an exponentially decreasing juvenile risk to the Gompertz-Makeham model. However, I performed preliminary analyses using the Siler model and did not obtain convincing estimates of the parameters of the juvenile component for the East Smithfield sample. All known human patterns of mortality (and, in fact, all known mammalian patterns) include a juvenile risk of mortality that is initially high at birth but declines very rapidly with small changes in age for the first few years of life (Gage 1988), and my preliminary analyses revealed such a pattern for the Danish sample. However, the East Smithfield juvenile mortality pattern differed from what is expected, as the risk was initially low at age 0 and increased slowly with age, with no drop in risk during early childhood. At face value, the estimated parameters from this preliminary analysis might suggest that infants faced relatively low risks of mortality during the Black Death, which is possible, as there are some modern diseases that affect adults more severely than children. For example, in some recent outbreaks of dengue, case-fatality rates were higher among adults than children (Guha-Sapir and Schimmer 2005). However, the apparently low risk for juveniles in East Smithfield might have been the result of the small sample of juveniles available and the general difficulty associated with estimating juvenile risk (Wood et al. 2002b). Information on the juvenile component of mortality is based primarily on individuals in the sample who are between the ages of 0 and 5 years, and it is difficult to accurately estimate the juvenile risk with small sample sizes (Wood et al. 2002b). These ages made up a small percentage of the samples used for this study (only 8% of the East Smithfield sample and 15% of the Danish sample). Given the difficulty associated with estimating the juvenile risk, the current study focuses on adult mortality. Further study using larger sample sizes might resolve the pattern of mortality for children during the Black Death.
The Gompertz-Makeham model parameters were estimated using maximum likelihood analysis with the program mle (Holman 2005), and the model was fit separately to the data from the East Smithfield and Danish samples. For this study, both the full three-parameter Gompertz-Makeham model and a reduced model with the α1 parameter set equal to zero were estimated for both samples (this reduced model without the baseline risk of mortality is the Gompertz model). Previous research has shown that the baseline risk of mortality often cannot be estimated from paleodemographic data, and thus many paleodemographic applications of the Gompertz-Makeham or Siler models do not include the baseline component of mortality (Gage 1988; Herrmann and Konigsberg 2002; Nagaoka et al. 2006). This study uses point estimates of age without their associated errors to estimate model parameters. Because of this, the reported standard errors for the parameter estimates are likely underestimated to an unknown degree; readers should therefore view the standard error estimates with caution. It should also be noted that the use of an informative prior for age estimation, as was done here, might influence the hazard model parameter estimates. An alternative approach, which avoids these problems, is the Rostock protocol, whereby the age-at-death distribution is estimated directly from a cemetery sample (i.e., rather than estimating individual ages first and then constructing the age-at-death distribution or estimating age-specific mortality from the individual age estimates) (Hoppa and Vaupel 2002). With the Rostock approach, a cemetery age-at-death distribution is estimated based on the observed frequencies of skeletal age indicator stages in the cemetery combined with estimates from a reference sample of the probability of observing certain age indicator stages given age. The Rostock approach, though mathematically ideal, requires a large sample size to provide good estimates of the cemetery sample age-at-death distribution, and sufficiently large sample sizes are often not available for paleodemographic studies (Boldsen et al. 2002). Given that the approach of the current study deviates from the Rostock protocol, the parameter estimates and their associated standard errors reported below in section 3 should be viewed as providing a general picture of Black Death and normal medieval adult mortality; i.e., the patterns the parameter estimates reveal are more important than their numerical values.
3. Results
The age-at-death distributions from the East Smithfield and Danish cemeteries are shown in Figure 1. A Kolmogorov-Smirnov test was performed to compare the age-at-death distributions, and the results indicate that the two distributions are not significantly different (Dm,n=0.10, p>0.05) As can be seen in Figure 1, the East Smithfield cemetery contains a higher proportion of young adults (15–25 years) and a lower proportion of adults between the ages 25–45 compared to the Danish cemeteries. There are slight differences between the two cemetery samples at older adult ages. At face value, these results might suggest that compared to normal medieval mortality, the Black Death disproportionately affected young adults and very old adults. However, without reliable information about the living age distributions of the original populations at risk, the observed age-at-death distributions alone do not provide conclusive evidence of Black Death mortality patterns. That is, the populations of London and Denmark might have faced identical age-specific mortality, in which case the differences in the age-at-death distributions would reflect differences in the age structure of the respective living populations, rather than differences in mortality patterns. Alternatively, the different age-at-death distributions from East Smithfield and Denmark might really reflect differences in age-specific mortality associated with normal versus epidemic mortality. Because of such limitations of the existing raw data, the hazard model parameter estimates are necessary for a complete understanding of the age patterns of mortality in these samples.
Figure 1.
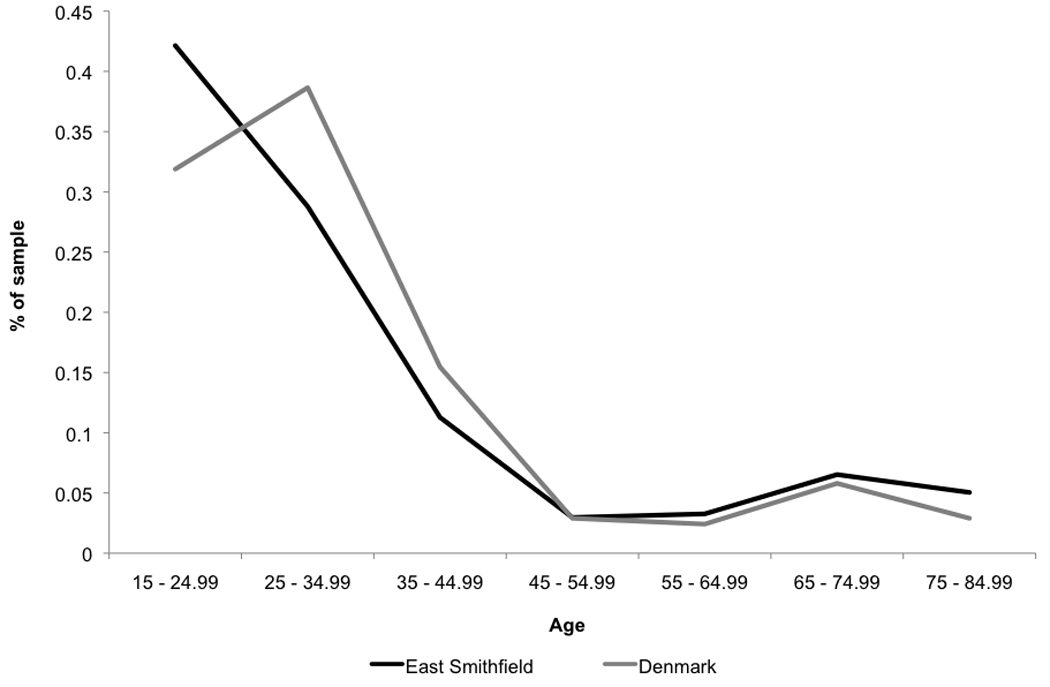
Age-at-death distributions from the East Smithfield Black Death and Danish normal mortality cemeteries.
The Gompertz model parameter estimates for the East Smithfield and Danish samples are shown in Table 1. Only the results of the two-parameter Gompertz model, which excludes the age-independent risk of mortality, are shown here, as estimation of the full three-parameter Gompertz-Makeham model resulted in estimates of the α1 parameter that did not differ significantly from zero (inclusion of the baseline risk had a negligible effect on the estimates of the α2 and β2 parameters). The graphs of the corresponding hazard functions, with their confidence intervals, are shown in Figure 2. These results indicate that risks of mortality increased with adult age in both cemeteries, which is consistent with the typical human mortality pattern (Gage 1989).
Table 1.
Estimates of the hazard model parameters. Standard errors are provided in the parentheses.
| Parameter | East Smithfield | Denmark |
|---|---|---|
| α2 | 0.011 (0.002) | 0.010 (0.003) |
| β2 | 0.032 (0.003) | 0.037 (0.005) |
Figure 2.
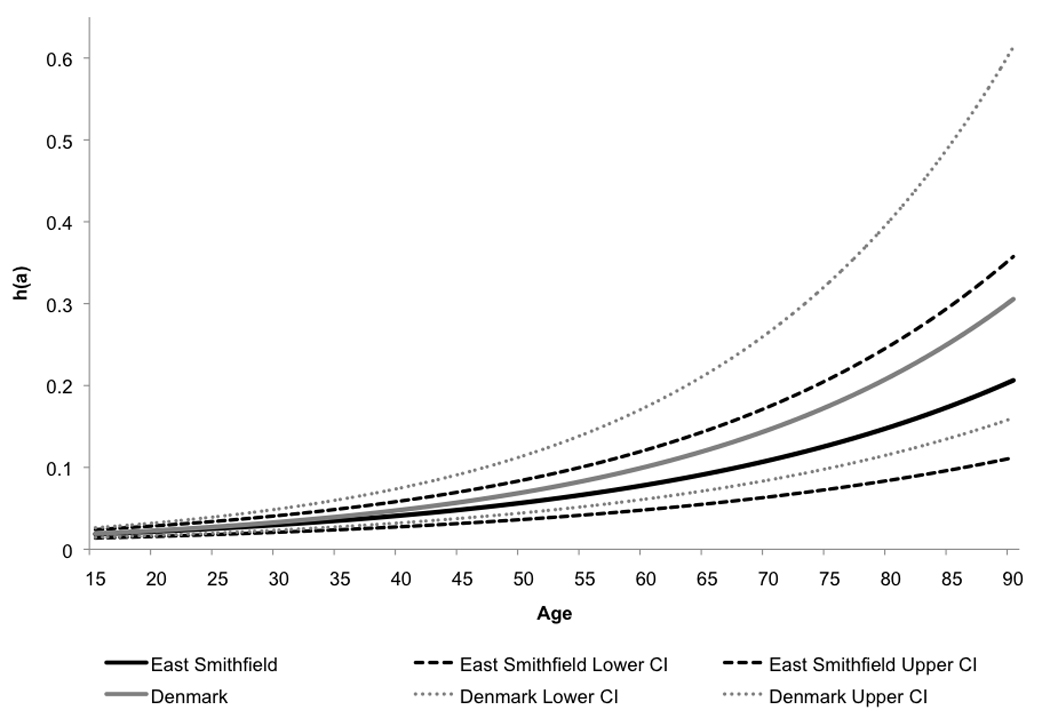
Gompertz hazards estimated from the East Smithfield and Danish samples.
4. Discussion
4.1 Age patterns of Black Death mortality
The results of this study indicate that the risk of mortality was not uniform across age during the Black Death. Age apparently did have an effect on risk of death during the epidemic, such that the risk for older adults was higher than that of younger individuals during the Black Death.
There is an apparent difference between the East Smithfield and Danish samples in the rate at which senescent mortality increases with age. As shown in Figure 2, based on the estimated parameters of the hazards model, the senescent risk increased more rapidly with adult age in the Danish sample than in the East Smithfield cemetery. These results might indicate that the risk of death for older adults was lower during the Black Death than it was during times of normal mortality. A lower estimated risk of death for older adults in East Smithfield compared to the Danish sample might have occurred if the Black Death targeted children and younger adults, as has been observed with some modern diseases such as several recent outbreaks of bubonic plague (Boisier et al. 2002; Kamugisha et al. 2007). However, if this were the case, one would expect to see a higher estimated risk of death for younger adults during the Black Death compared to normal all-cause medieval mortality, but the current study did not reveal such a pattern.
Alternatively, a lower estimated risk of senescent mortality in East Smithfield might have been the result of the epidemic being less strongly selective against frail individuals (and, conversely, more deadly for relatively healthy people) than normal medieval mortality. That is, if the Black Death was so deadly that it killed more otherwise healthy individuals than normal mortality, the difference in risk between younger and older adults in East Smithfield might be lower than that observed in the normal mortality cemetery. However, one would expect such an age pattern of selectivity to result in higher risks across younger adult ages in East Smithfield than in Denmark, but again, this was not revealed by the current analyses.
As described in section 2.1.2, the samples used for this study come from two different geographic regions, and rather than indicating a reduced risk of death for older adults during the Black Death compared to normal mortality, the results might reflect underlying population differences between southern England and Denmark that simply persisted during the Black Death. This is possible given that there are differences among modern populations in the age patterns of mortality (Coale et al. 1988; Gage 1989).
Given that the confidence intervals for the hazards for East Smithfield and Denmark overlap (Figure 2), the risk for older adults might not actually have been lower during the Black Death. In fact, the overlap might be even greater than shown in Figure 2 given that the standard errors associated with the parameter estimates are likely underestimated, as described in section 2.3. The risk of death for older adults might actually have been lower during times of normal mortality than during the Black Death, or the risks might have been identical under the two mortality conditions. However, for the purposes of this study, the general pattern of increasing risk with adult age during the Black Death that is revealed by the analysis is more important than the numerical estimates of the Gompertz model parameters themselves and whether the risk of death for adults truly increased more or less steeply with age in East Smithfield compared to the Danish sample.
4.2 Comparisons with previous research
As described in section 1, Russell (1948) and Razi (1980) estimated age-specific mortality among men in England during the Black Death, based on documentary evidence, and found that the risk of death increased with age among men up to age 60, after which the risk decreased. The results of the current study, which includes both men and women, are generally consistent with these earlier studies, though the drop in risk after age 60 was not observed with the skeletal data.
Comparisons between the age-at-death distributions based on the data used in the current study to those obtained in earlier studies of East Smithfield are already described in detail in DeWitte (2009). In summary, the East Smithfield distribution based on data used in the current study and that obtained by Museum of London researchers differ from the distributions obtained in all other studies of the cemetery, whereas the distributions from Gowland and Chamberlain (Gowland and Chamberlain 2005), Margerison and Knüsel (Margerison and Knüsel 2002), and Waldron (Waldron 2001) do not differ significantly from one another. Variation in age-at-death patterns obtained by different studies of East Smithfield is not surprising given that different age-estimation methods were used by most of the researchers (the exceptions are Margerison and Knüsel (2002) and Waldron (2001), as the former used the latter’s East Smithfield data). The age-at-death distribution from the current study and that from Gowland and Chamberlain (2005) had higher proportions of 15–24 year olds and lower proportions of 25–34 year olds than those from other studies. Traditional methods of adult age estimation tend to overestimate the ages of young adults (Aykroyd et al. 1997; Martrille et al. 2007; Saunders et al. 1992), so the higher proportion of younger adults (15–24) observed in the current study and that of Gowland and Chamberlain compared to others might be the result of our using less biased, Bayesian approaches to age estimation. The peak in adult mortality at ages 25–34 observed in other studies, which in part led some researchers to conclude that the Black Death cemetery more closely resembles a living population age structure than a normal attritional cemetery (Kausmally 2007; Margerison and Knüsel 2002), might have been an artifact of traditional methods rather than truly reflecting mortality patterns of the epidemic.
As mentioned above in section 1, I have avoided drawing any conclusions about the age patterns of Black Death mortality based on the age-at-death distribution alone, given a lack of detailed information about the living age structure of the population of London at the time of the Black Death. Furthermore, for the reasons given in section 2.3, I avoided the model life table approach used by several previous studies of East Smithfield, all of which used different life tables for their comparisons. Waldron (2001) found that the East Smithfield distribution was similar to a normal cemetery mortality profile rather than a living population profile, which suggests that the Black Death did not kill indiscriminately. However, both Margerison and Knüsel (2002) and Gowland and Chamberlain (2005) found that East Smithfield differed from a normal attritional cemetery and more closely resembled a living population age structure, which suggests that the Black Death killed indiscriminately. The estimates of the Gompertz model parameters from this study provide evidence that the Black Death targeted older individuals, and thus that the Black Death did not affect all age groups equally, a conclusion that is consistent with the findings of Waldron (2001).
The current study contributes to existing evidence concerning selectivity of the Black Death with respect to various biological factors. I previously applied hazards analysis to the East Smithfield sample to determine whether the Black Death was selective with respect to preexisting health condition, i.e. whether individuals who were already in poor health before the epidemic arrived in London faced elevated risks of mortality compared to their peers who were relatively healthy before the epidemic (DeWitte and Wood 2008). The results of that analysis indicated that exposure to physiological stress (episodes of infection or malnutrition) before the Black Death (as indicated by macroscopic skeletal pathologies/stress markers) increased risks of dying during the epidemic. The results from East Smithfield were very similar to those from the 21 Danish normal mortality sample, which was used in the current study. I have also used hazards analysis to examine whether sex in adults affected risks of death during the Black Death, as is common with modern infectious diseases, or if men and women faced the same risks of dying (DeWitte 2009). The results of that study indicated that during the Black Death and under conditions of normal medieval mortality, men and women faced similar risks of dying. The results of the current study are consistent with the results from these previous studies, as they reveal similarities between the age specific mortality patterns of the Black Death and normal medieval mortality. Overall these studies indicate that one of the most devastating diseases in human history, which killed millions of people in a very short period of time, behaved in ways comparable to normal causes of mortality and was apparently not an indiscriminant killer. More generally, the results of these studies suggest that even in the case of catastrophic, epidemic mortality, individuals in a population will vary in their risks of dying.
5. Conclusion
The results of this study indicate that older adults faced higher risks of dying during the Black Death compared to younger individuals. Contrary to what has been inferred from contemporary descriptions of the Black Death, the medieval epidemic did not kill indiscriminately with respect to age. When combined with previous paleodemographic analyses of Black Death mortality patterns, these results show that like normal medieval mortality, the epidemic disproportionately affected certain individuals, i.e. those who were already in relatively poor health and older adults. Presumably these individuals faced elevated risks of death during the epidemic because they had immune systems that were compromised by previous exposure to physiological stressors (e.g. past episodes of malnutrition or disease). Together, these results reveal that despite the devastating nature of the disease, the mortality patterns of the 14th-century Black Death were not totally unlike those of normal medieval mortality.
Acknowledgements
I would like to thank Ottar Bjørnstad, Jesper Boldsen, Ken Weiss, Jeffrey Kurland, and James Wood for providing feedback on an earlier version of the analyses presented here. I am also grateful to Richard Klein, Editor, four anonymous reviewers, and Eric Jones for reading and providing comments on an earlier version of this manuscript. Grant Sponsors: National Science Foundation (grant number BCS-0406252), The Wenner-Gren Foundation for Anthropological Research (grant number 7142), The American-Scandinavian Foundation, The Pennsylvania State University’s Research and Graduate Studies Office, University at Albany Center for Social and Demographic Analysis (CSDA), University at Albany Research Foundation.
References
- Aykroyd RG, Lucy D, Pollard AM, Solheim T. Technical note: Regression analysis in adult age estimation. American Journal of Physical Anthropology. 1997;104(2):259–265. doi: 10.1002/(SICI)1096-8644(199710)104:2<259::AID-AJPA11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Benedictow OJ. Plague in the late medieval Nordic countries: epidemiological studies. Oslo: Middelalderforlaget; 1993. p. 329. [Google Scholar]
- Bocquet-Appel JP, Masset C. Farewell to paleodemography. Journal of Human Evolution. 1982;11:321–333. [Google Scholar]
- Boisier P, Rahalison L, Rasolomaharo M, Ratsitorahina M, Mahafaly M, Razafimahefa M, Duplantier JM, Ratsifasoamanana L, Chanteau S. Epidemiologic features of four successive annual outbreaks of bubonic plague in Mahajanga, Madagascar. Emerging infectious diseases. 2002;8(3):311–316. doi: 10.3201/eid0803.010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldsen JL, Milner GR, Konigsberg LW, Wood JW. Transition analysis: A new method for estimating age from skeletons. In: Hoppa RD, Vaupel JW, editors. Paleodemography: Age distributions from skeletal samples. Cambridge: Cambridge University Press; 2002. pp. 73–106. [Google Scholar]
- Bowsky WM. The Black Death: a turning point in history? New York: Holt, Rinehart and Winston; 1971. [Google Scholar]
- Brooks S, Suchey J. Skeletal age determination based on the os pubis: a comparison of the Ascadi-Nemeskéri and Suchey-Brooks methods. Human Evo. 1990;5:227–238a. [Google Scholar]
- Buikstra JE. Paleodemography: Context and promise. In: Paine RR, editor. Integrating archaeological demography : multidisciplinary approaches to prehistoric population. Carbondale, Ill: Center for Archaeological Investigations, Southern Illinois University at Carbondale; 1997. [Google Scholar]
- Capelli C, Redhead N, Abernethy JK, Gratrix F, Wilson JF, Moen T, Hervig T, Richards M, Stumpf MP, Underhill PA, et al. A Y chromosome census of the British Isles. Curr Biol. 2003;13(11):979–984. doi: 10.1016/s0960-9822(03)00373-7. [DOI] [PubMed] [Google Scholar]
- Chamberlain AT. Demography in archaeology. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Coale A, Demeny P, with Vaughan B. Regional Model Life Tables and Stable Populations. New York: Academic Press; 1988. [Google Scholar]
- Cohn SK. The Black Death transformed: disease and culture in early Renaissance Europe. London: Arnold; 2002. [Google Scholar]
- DeWitte SN. The effect of sex on risk of mortality during the Black Death in London, A.D. 1349–1350. American Journal of Physical Anthropology. 2009;139:222–234. doi: 10.1002/ajpa.20974. [DOI] [PubMed] [Google Scholar]
- DeWitte SN, Wood JW. Selectivity of the Black Death with Respect to Preexisting Health. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(5):1436–1441. doi: 10.1073/pnas.0705460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage TB. Mathematical hazard models of mortality: an alternative to model life tables. American Journal of Physical Anthropology. 1988;76(4):429–441. doi: 10.1002/ajpa.1330760403. [DOI] [PubMed] [Google Scholar]
- Gage TB. Bio-mathematical approaches to the study of human variation in mortality. Yearbook of Physical Anthropology. 1989;32:185–214. [Google Scholar]
- Gottfried RS. The Black Death: natural and human disaster in medieval Europe. New York: Free Press; 1983. [Google Scholar]
- Gowland RL, Chamberlain AT. Detecting plague: palaeodemographic characterisation of a catastrophic death assemblage. Antiquity. 2005;79(303):146–157. [Google Scholar]
- Grainger I, Hawkins D. Excavations at the Royal Mint site 1986–1988. The London Archaeologist. 1988;5:429–436. [Google Scholar]
- Grainger I, Hawkins D, Cowal L, Mikulski R. Museum of London Archaeology Service Monograph. Vol. 43. London: Museum of London Archaeology Service; 2008. The Black Death cemetery, East Smithfield, London. [Google Scholar]
- Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerging Themes in Epidemiology. 2005;2(1):1–1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher J. Plague, population, and the English economy, 1348–1530. London: Macmillan; 1977. [Google Scholar]
- Hawkins D. Black Death and the new London cemeteries of 1348. Antiquity. 1990;64:637–642. [Google Scholar]
- Herlihy D. The Black Death and the transformation of the West. Cambridge, Mass.: Harvard University Press; 1997. [Google Scholar]
- Herrmann NP, Konigsberg LW. A re-examination of the age-at-death distribution of Indian Knoll. In: Hoppa RD, Vaupel JW, editors. Paleodemography : age distribution from skeletal samples. Cambridge: Cambridge University Press; 2002. pp. 243–257. [Google Scholar]
- Holman DJ. mle: A programming language for building likelihood models. Seattle, WA: 2005. Version 2.1 ed. [Google Scholar]
- Holmes GA. England: A decisive turning point. In: Bowsky WM, editor. The Black Death: A turning point in history? NY: Holt, Rhinehart, and Winston; 1971. pp. 91–99. [Google Scholar]
- Hoppa RD, Vaupel JW. Paleodemography : age distribution from skeletal samples. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Horrox R. The Black Death. Manchester: Manchester University Press; 1994. [Google Scholar]
- Jantzen C, Kieffer-Olsen J, Madsen PK. De sma brodres hus i Ribe. In: Gelius W, Guldberg M, Stoumann I, editors. Mark og Montre Årbog for kunst- og kulturhistorie. Vol. 30. Ribe: Ribe Amts Museumsråd; 1994. pp. 26–36. [Google Scholar]
- Kamugisha ML, Gesase S, Minja D, Mgema S, Mlwilo TD, Mayala BK, Msingwa S, Massaga JJ, Lemnge MM. Pattern and spatial distribution of plague in Lushoto, northeastern Tanzania. Tanzania Health Research Bulletin. 2007;9(1):12–18. doi: 10.4314/thrb.v9i1.14286. [DOI] [PubMed] [Google Scholar]
- Kausmally T. East Smithfield Black Death Cemetery. London: Museum of London Centre for Human Bioarchaeology; 2007. [Google Scholar]
- Kieffer-Olsen J. Grav og gravskike i det middelalderlige Danmark. Aarhus, Denmark: Aarhus University; 1993. [Google Scholar]
- Konigsberg LW, Frankenberg SR. Estimation of age structure in anthropological demography. Am J Phys Anthropol. 1992;89(2):235–256. doi: 10.1002/ajpa.1330890208. [DOI] [PubMed] [Google Scholar]
- Konigsberg LW, Frankenberg SR. Deconstructing death in paleodemography. Am J Phys Anthropol. 2002;117(4):297–309. doi: 10.1002/ajpa.10039. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO, Meindl RS, Mensforth RP, Barton TJ. Multifactorial determination of skeletal age at death: a method and blind tests of its accuracy. Am J Phys Anthropol. 1985;68(1):1–14. doi: 10.1002/ajpa.1330680102. [DOI] [PubMed] [Google Scholar]
- Makeham W. On the law of mortality. Journal of the Institute of Actuaries. 1860;13:325–358. [Google Scholar]
- Margerison BJ, Knüsel CJ. Paleodemographic comparison of a catastrophic and an attritional death assemblage. Am J Phys Anthropol. 2002;119(2):134–143. doi: 10.1002/ajpa.10082. [DOI] [PubMed] [Google Scholar]
- Martrille L, Ubelaker DH, Cattaneo C, Seguret F, Tremblay M, Baccino E. Comparison of Four Skeletal Methods for the Estimation of Age at Death on White and Black Adults*. Journal of Forensic Sciences. 2007;52(2):302–307. doi: 10.1111/j.1556-4029.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- Milner GR, Wood JW, Boldsen JL. Paleodemography. In: Katzenberg M, Saunders S, editors. Biological Anthropology of the Human Skeleton. NY: Wiley-Liss; 2008. pp. 561–600. [Google Scholar]
- Nagaoka T, Hirata K, Yokota E, Matsu'ura S. Paleodemography of a medieval population in Japan: analysis of human skeletal remains from the Yuigahama-minami site. Am J Phys Anthropol. 2006;131(1):1–14. doi: 10.1002/ajpa.20402. [DOI] [PubMed] [Google Scholar]
- Ortner DJ. Theoretical and methodological issues in paleopathology. In: Ortner DJ, Aufderheide AC, editors. Human paleopathology: current syntheses and future options. Washington, DC: Smithsonian Institution Press; 1991. pp. 5–11. [Google Scholar]
- Paine RR. If a population crashes in prehistory, and there is no paleodemographer there to hear it, does it make a sound? Am J Phys Anthropol. 2000;112(2):181–190. doi: 10.1002/(SICI)1096-8644(2000)112:2<181::AID-AJPA5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Poos LR. A rural society after the Black Death: Essex, 1350–1525. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Poos LR, Smith RM. 'Legal Windows onto Historical Populations'? Recent Research on Demography and the Manor Court in Medieval England. Law and History Review. 1984;2(1):128–152. [Google Scholar]
- Poulsen B. Agricultural technology in medieval Denmark. In: Astill GG, Langdon J, editors. Medieval farming and technology: the impact of agricultural change in northwest Europe. Leiden: Brill; 1997. pp. 115–146. [Google Scholar]
- Razi Z. Life, marriage, and death in a medieval parish : economy, society, and demography in Halesowen, 1270–1400. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- Roesdahl E, editor. Dagligliv i Danmarks middelalder: En arkæologisk kulturhistorie. Copenhagen: Nordisk Forlag; 1999. [Google Scholar]
- Russell JC. British medieval population. Albuquerque: University of New Mexico; 1948. [Google Scholar]
- Saunders S, Fitzgerald C, Rogers T, Dudar C, McKillop H. Test of several methods of skeletal age estimation using a documented archaeological sample. Canadian Society of Forensic Science Journal. 1992;25(2):97–118. [Google Scholar]
- Sawyer B, Sawyer PH. Medieval Scandinavia : from conversion to Reformation, circa 800–1500. Minneapolis: University of Minnesota Press; 1993. [Google Scholar]
- Siler W. A competing-risk model for animal mortality. Ecology. 1979;60(4):750–757. [Google Scholar]
- Waldron HA. Are plague pits of particular use to palaeoepidemiologists? Int J Epidemiol. 2001;30(1):104–108. doi: 10.1093/ije/30.1.104. [DOI] [PubMed] [Google Scholar]
- Weiss KM. Demographic Models for Anthropology. Washington, D.C.: Society for American Archaeology; 1973. [Google Scholar]
- Widgren M. Fields and field systems in Scandinavia during the middle ages. In: Astill GG, Langdon J, editors. Medieval farming and technology : the impact of agricultural change in northwest Europe. Leiden: Brill; 1997. pp. 173–192. [Google Scholar]
- Wood JW, Ferrell RJ, DeWitte-Avina SN. The temporal dynamics of the fourteenth-century Black Death: New evidence from English ecclesiastical records. Human Biology. 2003;75:427–448. doi: 10.1353/hub.2003.0067. [DOI] [PubMed] [Google Scholar]
- Wood JW, Ferrell RJ, DeWitte-avina SN, Holman DJ. Mortality differentials during the Black Death in England, 1349. The 27th Annual meeting of the Human Biology Council; Buffalo, NY. 2002a. [Google Scholar]
- Wood JW, Holman DJ, O’Connor KA, Ferrell RJ. Mortality models for paleodemography. In: Hoppa RD, Vaupel JW, editors. Paleodemography: Age distributions from skeletal samples. Cambridge: Cambridge University Press; 2002b. pp. 129–168. [Google Scholar]
- Wood JW, Milner GR, Harpending HC, Weiss KM. The Osteological Paradox: Problems of Inferring Prehistoric Health from Skeletal Samples. Current Anthropology. 1992;33:343–370. [Google Scholar]
- Ziegler P. The Black Death. London: Harper Collins; 1969. [Google Scholar]


