Abstract
Our goal was to quantify mitochondrial and plasma potential (Δψm and Δψp) based on the disposition of rhodamine 123 (R123) or tetramethylrhodamine ethyl ester (TMRE) in the medium surrounding pulmonary endothelial cells. Dyes were added to the medium, and their concentrations in extracellular medium ([Re]) were measured over time. R123 [Re] fell from 10 nM to 6.6 ± 0.1 (SE) nM over 120 min. TMRE [Re] fell from 20 nM to a steady state of 4.9 ± 0.4 nM after ∼30 min. Protonophore or high K+ concentration ([K+]), used to manipulate contributions of membrane potentials, attenuated decreases in [Re], and P-glycoprotein (Pgp) inhibition had the opposite effect, demonstrating the qualitative impact of these processes on [Re]. A kinetic model incorporating a modified Goldman-Hodgkin-Katz model was fit to [Re] vs. time data for R123 and TMRE, respectively, under various conditions to obtain (means ± 95% confidence intervals) Δψm (−130 ± 7 and −133 ± 4 mV), Δψp (−36 ± 4 and −49 ± 4 mV), and a Pgp activity parameter (KPgp, 25 ± 5 and 51 ± 11 μl/min). The higher membrane permeability of TMRE also allowed application of steady-state analysis to obtain Δψm (−124 ± 6 mV). The consistency of kinetic parameter values obtained from R123 and TMRE data demonstrates the utility of this experimental and theoretical approach for quantifying intact cell Δψm and Δψp. Finally, steady-state analysis revealed that although room air- and hyperoxia-exposed (95% O2 for 48 h) cells have equivalent resting Δψm, hyperoxic cell Δψm was more sensitive to depolarization with protonophore, consistent with previous observations of pulmonary endothelial hyperoxia-induced mitochondrial dysfunction.
Keywords: mathematical modeling, rhodamine 123, tetramethylrhodamine ethyl ester, multidrug transporter P-glycoprotein, hyperoxia
pulmonary endothelial mitochondrial and plasma membrane potentials (Δψm and Δψp, respectively) are implicated in bioenergetic, metabolic, and signaling processes contributing to normal lung function and in injury (16, 24, 33, 54, 67, 69). In most eukaryotic cells, Δψm is the major component of the mitochondrial electrochemical transmembrane potential and, as such, is involved in pulmonary endothelial mitochondrial ATP generation, regulation of calcium homeostasis, apoptosis, nitric oxide signaling, and other functions (19, 58, 60, 61). Dissipation of Δψm is considered a hallmark of mitochondrial dysfunction in diverse cell types, including pulmonary endothelial cells exposed to oxidative stresses and bleomycin (24, 33, 43, 54, 69). On the other hand, pulmonary endothelial Δψp is implicated in regulating channel-mediated calcium entry as a key signaling response to mechanical stimuli, vasoactive substances, oxidative stress, ischemia, and hypoxia (14, 17, 31, 49, 59, 67, 70). Thus the ability to quantify Δψm and Δψp is important for characterization of mechanisms underlying pulmonary endothelial responses to injury and adaptation and for evaluation of the utility of therapeutics directed at restoration of normal metabolic, signaling, and bioenergetic function.
While various techniques have been reported for evaluating Δψm in different cell types, a typical approach has been to use cationic fluorescent dyes that accumulate in the mitochondrial matrix driven by the voltage gradient across the inner mitochondrial membrane (22, 26, 56, 58, 64). Such studies generally have involved measurements of fluorescence intensity within cells or isolated mitochondria. Because such measurements are easily confounded by the propensity of the dyes to undergo self-aggregation, quenching, and photobleaching and/or to exert phototoxic effects, the outcomes have been predominantly confined to qualitative changes in Δψm (22, 26, 28, 56, 58, 64). In addition, the vast majority of studies have involved mitochondrial isolation, cell permeabilization, or other conditions that have little relevance for intact cells; yet, when intact cells have been studied, the contributions of processes other than Δψm [e.g., the multidrug transporter P-glycoprotein (Pgp) and/or Δψp] to dye disposition have often been overlooked (6, 21, 26, 46, 68). Patch clamping, perhaps considered the gold standard for measuring Δψm, also involves cell disruption (1, 14, 29, 31, 62).
The general focus of our research has been the impact of the pulmonary endothelium on the disposition of redox-active and other compounds within the extracellular medium of cells in culture or in perfusate that has passed through the isolated perfused lung (3–5, 12, 35, 39). An advantage of this approach is that it is applicable to intact cells and organ preparations and, therefore, is generally nondestructive. For pulmonary endothelial cells in culture, we have found it useful to exploit a microcarrier bead preparation consisting of confluent monolayers of pulmonary endothelial cells cultured on polystyrene microcarrier beads (150–300 μm diameter). The cell-coated beads can be suspended in medium containing the substance of interest, and the impact of cellular metabolic and other processes on the disposition of the substance within the medium can be evaluated. The key advantage of this preparation is that a relatively large cell surface area can be studied in a relatively small volume of medium compared with cells grown on culture dishes, without the use of enzymatic or mechanical disruption to remove the cells from the culture surface.
An important and challenging issue is that since such an approach involves intact cells (or perfused lung), it is not an infrequent occurrence that more than process (e.g., enzyme, transporter, and binding site) is implicated in altering the dispositions of compounds under study. In such cases, inhibitors are used to provide discriminating data relevant to these processes, and kinetic modeling is used to interpret such data and obtain values for kinetic parameters descriptive of the individual processes involved. Using this overall strategy, we have studied the impact of pulmonary endothelial cells on a variety of redox-active and other substances (4, 12, 13, 38, 40, 41). The studies have revealed the wide range of pulmonary endothelial redox enzymes and other processes capable of influencing the metabolism, redox status, and disposition of compounds carried in the blood.
Thus the goal of the present study was to apply this general strategy to develop a means to quantify pulmonary endothelial Δψm and Δψp using two common membrane potential-sensitive cationic fluorescent dyes, rhodamine 123 (R123) and tetramethylrhodamine ethyl ester (TMRE). The overall approach was to focus on the impact of the pulmonary arterial endothelial cells in culture on the extracellular concentrations of the dyes under a range of experimental conditions designed to separate the contributions of Δψm, Δψp, and Pgp to the net effect of the cells on the dyes. A kinetic model was developed to interpret the data and to obtain parameter values for Δψm, Δψp, and Pgp for both dyes. R123 and TMRE were selected because, whereas TMRE is more cell membrane-permeable than R123, both have relatively strong fluorescence quantum yields, have sensitivity to changes in the cellular environment, and undergo membrane potential-dependent changes in distribution across the mitochondrial and plasma membranes (21, 22, 32, 58, 64). Thus one concept was that the utility of the proposed approach for quantifying Δψm and Δψp in pulmonary arterial endothelial cells would be revealed by the consistency of the parameter values obtained from the studies with two dyes that have both distinct and common properties.
MATERIALS AND METHODS
Materials.
Fetal bovine serum was purchased from Hyclone Laboratories (Logan, UT), RPMI 1640 tissue culture medium and TMRE from Invitrogen (Carlsbad, CA), Biosilon microcarrier beads from Nunc (Roskilde, Denmark), and protein assay reagent from Bio-Rad Laboratories (Hercules, CA). The multidrug efflux transporter Pgp inhibitor N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF-120918) was generously supplied by GlaxoSmithKline (Research Triangle Park, NC). R123 and other chemicals, unless specifically noted, were purchased from Sigma Chemical (St. Louis, MO).
Endothelial cell culture.
Bovine pulmonary arterial endothelial cells were isolated from segments of calf pulmonary artery obtained from a local slaughterhouse and cultured to confluence on gelatin-coated (2% vol/wt) Biosilon (polystyrene) microcarrier beads (230 μm mean diameter, 160 cm2/ml beads), as previously described (12, 41). The control cells were grown in room air; hyperoxia-exposed cells were subjected to 95% O2-5% CO2 for 48 h, as previously described (36, 37).
General experimental protocols.
For experiments with R123 or TMRE, settled confluent cell-coated beads (∼0.40 ml, ∼59 cm2 cell surface area) or confluent cell-coated beads (∼0.17 ml, ∼28 cm2 cell surface area), respectively (128,000 cells/cm2 surface area), were washed free of the culture medium by resuspension in HBSS containing 5.5 mM glucose and 10 mM HEPES (HBSS-HEPES), pH 7.4, warmed to 37°C, as previously described (41). The cell-coated beads were allowed to settle for ∼15 s, the supernatant was discarded, and the washing procedure was repeated two more times. The washed cell-coated beads were resuspended in 10 × 10 × 48 mm acrylic fluorometric cuvettes containing 2.5 or 3.0 ml of HBSS-HEPES at 37°C (“control medium”) and R123 or TMRE (10, 30, and 100 nM). Immediately, the beads were allowed to settle to the bottom of the cuvette, and the fluorescence intensity in the medium above the settled cell-coated beads was measured (for R123, excitation at 490 nm, emission at 525 nm; for TMRE, excitation at 530 nm, emission at 573 nm) using a luminescence spectrometer (model LS55, Perkin Elmer). The dye concentrations in the extracellular medium ([Re]) were calculated from a standard curve prepared on the same day in the same medium used in cell experiments. After the initial measurements (t = 0 min), the cell-coated bead suspension was placed on a Nutator mixer at 37°C. Periodically, the mixing was stopped, the cell-coated beads were allowed to settle to the bottom of the cuvette, and fluorescence intensity in the medium above the settled beads was measured. The same protocol was also carried out using the protonophore CCCP (0.1, 0.2, 0.3, and 5 μM), high K+ (138 mM KCl/5 mM NaCl), and/or GF-120918 (2.5 μM) and in the absence of cells, the latter to determine the contribution of nonspecific dye interactions with the plasticware.
Additional measurements.
To assess cellular viability, total cellular and medium lactate dehydrogenase (LDH) activities were measured at the end of each experiment to determine percent LDH release into the medium, as previously described (41). Cell protein was measured using the Bio-Rad protein assay, and cell bead weights were obtained by drying and weighing the beads at the end of each experiment (41).
Microcarrier bead surface area, cell protein, cell protein per square centimeter of cell culture area, and percent total cell LDH released into the medium at the end of the experiments, expressed as means ± SE, for all R123 experiments combined were 59.00 ± 1.20 cm2, 1.54 ± 0.06 mg, 26.09 ± 1.04 μg/cm2, and 2.73 ± 0.24%, respectively (n = 55); for all TMRE experiments combined, the values were 28.31 ± 0.77 cm2, 0.74 ± 0.03 mg, 26.35 ± 0.85 μg/cm2, and 2.68 ± 0.20% (n = 68), respectively. There were no detectable differences between values for these parameters in control and experimental groups containing treatments (P > 0.05).
RESULTS
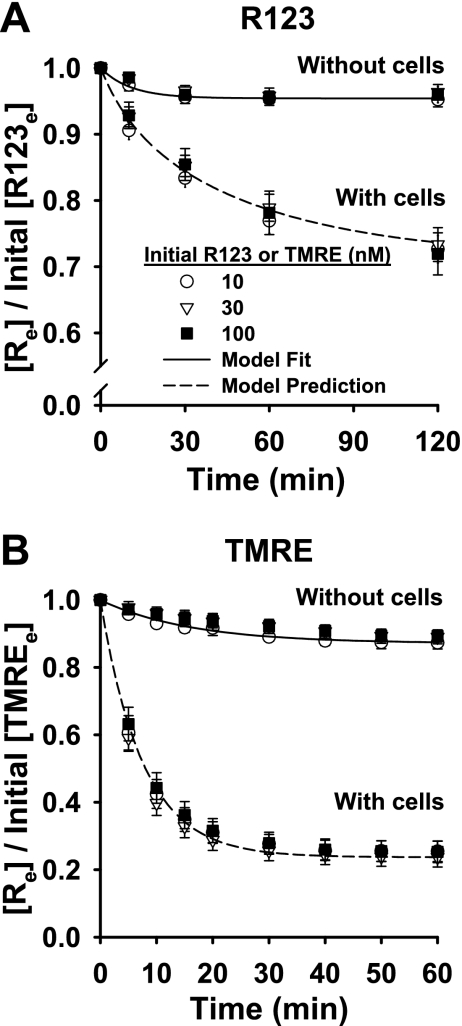
Figure 1 shows R123 and TMRE concentrations in the medium ([Re]) surrounding the pulmonary arterial endothelial cell-coated beads, normalized to the initial dye concentrations. In the presence of the cells, the normalized [Re] for both dyes decreased throughout their respective incubation periods in a manner that was independent of dye concentration. For R123, the normalized [Re] fell continually throughout the 120-min incubation period (Fig. 1A). For TMRE, it reached a steady state within ∼30 min that was maintained throughout the 60-min incubation period (Fig. 1B). The decreases in the normalized [Re] over time were cell-dependent, since there was relatively very little change in the absence of cells (Fig. 1, A and B). Thus the decreases in dye concentration in the extracellular medium were interpreted as dye uptake by the cells.
Fig. 1.
Normalized concentrations of rhodamine 123 (R123) and tetramethylrhodamine ethyl ester (TMRE) in medium surrounding pulmonary arterial endothelial cell-coated beads vs. time. Medium containing R123 (10, 30, and 100 nM) or TMRE (10, 30, and 100 nM) was added to cell-coated beads, and dye concentrations in medium ([Re]) were measured over time. Measured dye concentrations were normalized to initial dye concentrations ([R123e] or [TMREe]). Also shown are normalized R123 and TMRE concentrations in medium over time in the absence of cells. Symbols represent representative data from 3 experiments without cells and 4 experiments with cells for each dye concentration. Solid line, Eq. 8 fit to data; dashed lines, model predictions using Table 1 parameter values.
Uptake of TMRE was more rapid than uptake of R123, because TMRE is more highly cell membrane-permeant (21, 32). Accordingly, [Re] for TMRE reached steady state more rapidly than [Re] for R123, thereby allowing the time course for TMRE studies to be shorter. We also used fewer cells (i.e., a lower cell-coated bead surface area) for TMRE than for R123 studies (28 vs. 59 cm2 confluent cell-coated beads), because if we used the higher number for TMRE also, the cellular uptake of TMRE would be so extensive that [Re] would rapidly approach zero, decreasing the sensitivity (i.e., dynamic range) of the measurement to experimental manipulation. On the other hand, if we used the lower surface area for the R123 studies, the time course required to obtain data sufficient for interpretation would be too long to be consistent with high cell viability. The dye concentrations selected for further study were within the linear dye concentration vs. fluorescence intensity range, i.e., below the aggregation threshold concentration for both dyes (10 nM for R123 and 20 nM for TMRE; data not shown) (21, 26).
The next step was to separate the contributions of Δψm, Δψp, and Pgp to the net effect of the cells on [Re]. The approach was to use the protonophore CCCP, high K+, or GF-120918. CCCP was primarily directed at dissipation of Δψm and high K+ at Δψp (1, 9, 16, 17). GF-120918 was used as the Pgp inhibitor (53, 63).
Previously, 5 μM CCCP was used to target the mitochondrial respiratory chain in cerebral microvascular endothelial cells (9). The high K+ concentration was used to accomplish complete Δψp depolarization, as specified by the Nernst equation and as used for simultaneous determination of cell membrane potentials using microscopic measurements of rhodamine dyes (23, 25). Our use of 2.5 μM GF-120918 was based on our previous studies demonstrating that the maximum effect of GF-120918 on rhodamine 6G accumulation in the perfused rabbit lung was attained at 2 μM (53).
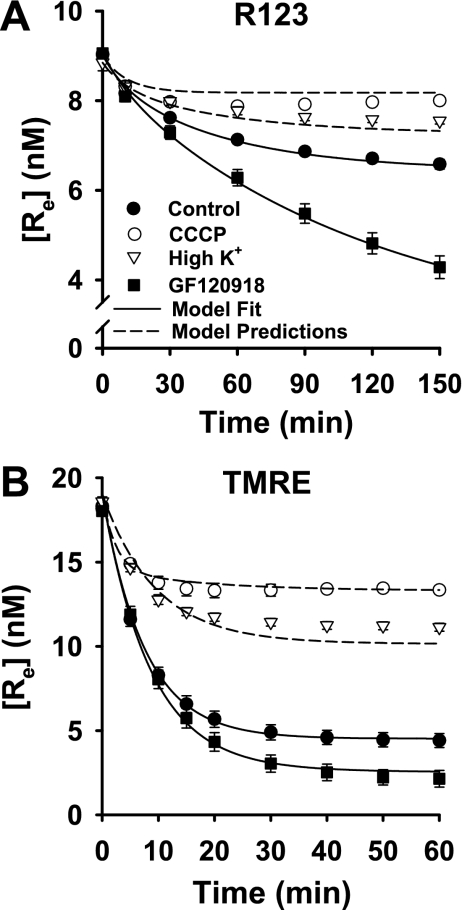
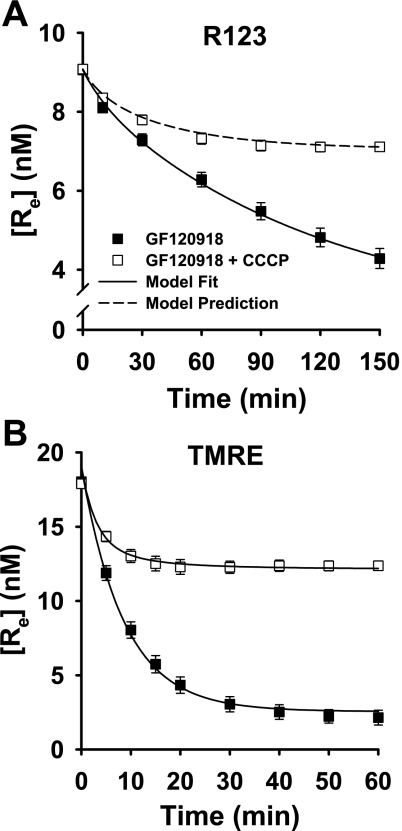
As shown in Fig. 2, the effects of the treatments on the [Re] vs. time progress curves for R123 and TMRE were qualitatively similar but quantitatively different. In general, CCCP and high K+ attenuated the fall in [Re] for both dyes, whereas GF-120918 had the opposite effect (Fig. 2). The overall implication was that dissipation of Δψm or Δψp with CCCP or high K+, respectively, decreased the extent of dye uptake by the cells. On the other hand, Pgp inhibition had the apparent effect of increasing dye uptake, but this was presumably via blockade of dye release from the cells (30, 68).
Fig. 2.
Effects of carbonyl cyanide 3-chlorophenylhydrazone (CCCP), high K+, and GF-120918 on concentrations of R123 and TMRE in medium surrounding cell-coated beads. Symbols represent [Re] (means ± SE) for 11 control experiments, 10 experiments with CCCP, 4 with high K+, and 5 with GF-120918 in A and 8 experiments for each condition in B. Solid lines, model fit to data; dashed lines, model predictions using Table 1 parameter values.
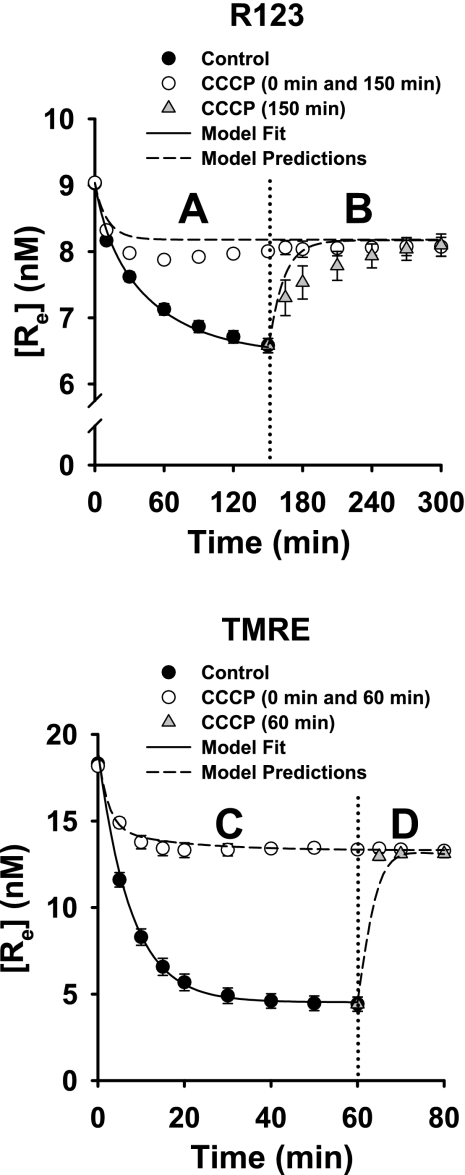
In Fig. 3, A and C, [Re] vs. time for R123 and TMRE was measured for control and CCCP-treated cells. As in Fig. 2, the fall in [Re] for R123 and TMRE was attenuated in the presence of CCCP. In Fig. 3, B and D, CCCP was added to control cells and cells that had been treated with CCCP from the beginning of the experiment. The latter was to control for any effect of experimental time course on the addition of CCCP.
Fig. 3.
Effects of CCCP on concentrations of R123 and TMRE in medium surrounding cell-coated beads. A: [Re] for R123 in samples containing R123 only (control, n = 11) or R123 + CCCP (n = 4). B: at 150 min (dotted vertical line), CCCP was added to 4 R123 samples and to all R123 + CCCP samples. C: [Re] for TMRE (control, n = 4) or TMRE + CCCP (n = 4). D: at 60 min (dotted vertical line), CCCP was added to all 4 TMRE samples and all 4 TMRE + CCCP samples. Symbols are data (means ± SE). Solid lines, model fit to data; dashed lines, model predictions using Table 1 parameter values.
For the control cells that had been allowed to accumulate the dyes in the absence of CCCP, [Re] increased when CCCP was added, approaching that for cells that had been treated with CCCP from the beginning of the experiment (Fig. 3, B and D). That is, CCCP not only blocked dye uptake by the cells if it was present from the beginning of the experiments, it also caused cell-accumulated dye to be released into the medium. TMRE is taken up more rapidly and to a proportionately greater extent by control cells than is R123, as also seen in Fig. 2. In addition, when CCCP was added to cells that had already accumulated dye, efflux into the medium from the cells was more rapid for TMRE than for R123, as reflected in the more rapid increase in [Re] for TMRE than for R123 (Fig. 3, B and D).
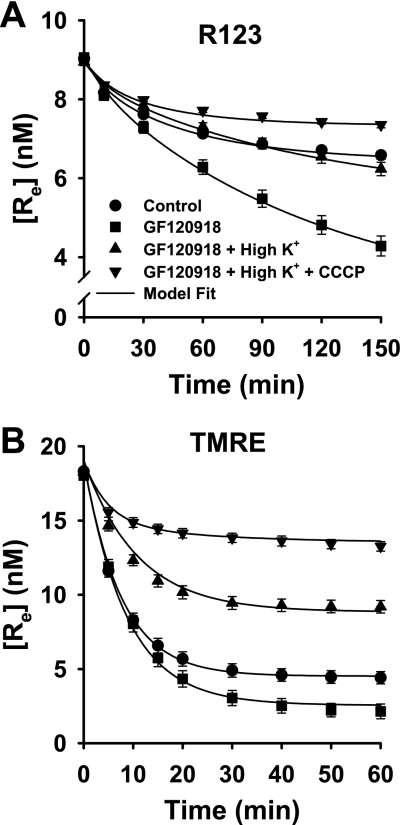
The data in Figs. 2 and 3 imply that Δψm, Δψp, and Pgp contributed to the net effect of the cells on [Re] for R123 and TMRE. Since kinetic model parameters cannot be estimated from a single data set or experimental condition, we designed an experimental data set to contain sufficiently discriminating data to break correlations between effects of Δψm, Δψp, and Pgp on [Re]. Figure 4 shows the effects of progressive, cumulative elimination of Pgp, Δψp, and Δψm on the [Re] vs. time progress curves for R123 and TMRE, using GF-120918 only, GF-120918 + high K+, or GF-120918 + high K+ + CCCP.
Fig. 4.
Cumulative elimination of processes contributing to dye fate on R123 and TMRE concentrations in medium surrounding cell-coated beads. Symbols are data (means ± SE) for 11 control experiments, 5 experiments with GF-120918, 4 with GF-120918 + high K+, and 4 with GF-120918 + high K+ + CCCP in A and 8 experiments for each condition in B. [Re] data for control and GF-120918 are the same as those in Figs. 2 and 3. Solid lines, model fit to data.
To take into account the possibility that CCCP might also depolarize Δψp, an additional study was carried out. Figure 5 shows the impact of CCCP on [Re] for R123 or TMRE in the presence of GF-120918 and the absence of high K+. Under these conditions, which include blocking any effect of Pgp, and without the high K+ that would depolarize Δψp, any impact of CCCP on Δψp should be unmasked.
Fig. 5.
Impact of CCCP on R123 and TMRE concentrations when contribution of P-glycoprotein (Pgp) is minimized with GF-120918. Experimental medium did not contain high K+. Symbols are data (means ± SE) for 5 experiments with GF-120918 and 5 with GF-120918 + CCCP in A and 8 experiments for each condition in B. Symbols are data (means ± SE). Solid lines, model fit to data; dashed line, model prediction.
Data analysis.
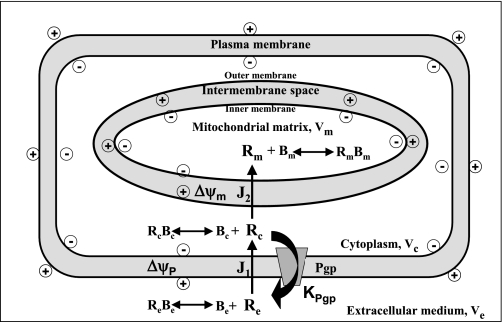
A mathematical model was developed to quantify the contributions of Δψm, Δψp, and Pgp to [Re] for R123 and TMRE. The model includes three regions, the extracellular medium, cytoplasm, and mitochondrial matrix, with volumes Ve, Vc, and Vm, respectively (Fig. 6). The dye flux across plasma (J1) or inner mitochondrial membrane (J2) is represented by a modified one-dimensional Goldman-Hodgkin-Katz equation (26). Because the fractional loss of dye from the extracellular medium in Fig. 1 is dose-independent, Pgp-mediated dye efflux from Vc to Ve is hypothesized to follow linear kinetics (32). Moreover, the model allows for slowly equilibrating nonspecific dye interactions with the cuvette (Be) within Ve and rapidly equilibrating nonspecific dye interactions with binding sites Bc and Bm within Vc and Vm, respectively. Then variations in dye concentrations in Ve, Vc, and Vm with time are described by the ordinary differential equations
| (1) |
| (2) |
| (3) |
| (4) |
where
| (5) |
| (6) |
[Re](t), [Rc](t), and [Rm](t) are dye concentrations in Ve, Vc, and Vm, respectively, at time t; [ReBe](t) is the dye concentration bound to the cuvette at time t; α = ZF/RT = 0.0374 mV−1 at 37°C is a constant dependent on the universal gas constant (R), Faraday constant (F), dye valence (Z), and absolute temperature (T) (26); KPgp (ml/min) is Pgp-mediated dye release from the cells; P1S1 (ml/min) and P2S2 (ml/min) are products of dye permeabilities (P) across plasma and mitochondrial membranes, respectively, and the surface areas (S) of these membranes; and V1 = {1 + (k2[Bc])/k−2}Vc (ml) and V2 = {1 + (k3[Bm])/k−3}Vm (ml) are apparent cytoplasm and mitochondrial matrix volumes, respectively, where ki and k−i are dye association and dissociation rate constants, respectively, with Bc (i = 2) and Bm (i = 3), respectively, k̄1 = k1[Be] (min−1) and k−1 (min−1) are rate constants for dye-cuvette binding and unbinding, respectively, and [Be] is the concentration of cuvette dye binding sites.
Fig. 6.
Schematic representation of kinetic model of disposition of R123 or TMRE in pulmonary arterial endothelial cells and extracellular medium. Ve, Vc, and Vm, volumes of medium, cytoplasm, and mitochondrial matrix, respectively; Re, Rc, and Rm, free R123 or TMRE concentrations in medium, cytoplasm, and mitochondrial matrix, respectively. Within medium, dyes participate in nonspecific binding interactions with fluorometric cuvette (Be). Dyes also participate in nonspecific rapidly equilibrating interactions with binding sites Bc and Bm within Vc and Vm, respectively. RcBc and RmBm, concentrations of bound dye in cytoplasm and mitochondrial matrix, respectively; J1 and J2, dye fluxes across plasma cell membrane and inner mitochondrial membrane, respectively; Δψm and Δψp, mitochondrial and plasma membrane potential, respectively; KPgp, conductance of P-glycoprotein (Pgp)-mediated dye release from cells.
Although typically thought of as a mitochondrial uncoupler, the protonophore carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) depolarizes bovine aortic endothelial and cultured neuronal cell Δψp in a concentration-dependent manner (46, 50). Because CCCP is also an uncoupling protonophore and 5 μM CCCP used in our study is in the range reported for the FCCP-induced plasma membrane depolarization, we used model simulations to evaluate whether CCCP might have such an effect in the present study (not shown). We found an effect of CCCP on [Re] for both dyes that was greater than could be accounted for solely by dissipation of only Δψm, as described by the empirical equation
| (7) |
where (1 − δ) is the Δψp fraction dissipated by CCCP and τ is the associated time constant (min), such that, in the absence of CCCP, δ = 1 and = Δψp.
To break the high correlation between V2 and Δψm in the model, the ratio V2/V1 was set to 0.02, consistent with a lower bound measured for this ratio in rat pulmonary endothelium (48). Then, Δψm, Δψp, V1, KPgp, P1S1, P2S2, δ, τ, k̄1, and k−1 are the unknown model parameters. The model-governing differential equations were solved numerically using the MATLAB (MathWorks) function “ode45,” which is based on an explicit Runge-Kutta formula.
Estimation of model parameters.
In the absence of cells, Eqs. 1–4 become
| (8) |
where [Re]0 is the initial (t = 0) dye concentration and [Re](t) is the concentration at time t. Fitting Eq. 8 to the without-cell data in Fig. 1 gives k̄1 and k−1 of 4.3 × 10−3 and 8.9 × 10−2 min−1, respectively, for R123 and 7.9 × 10−3 and 5.4 × 10−2 min−1, respectively, for TMRE. The fitting procedure was implemented in MATLAB using the function “lsqcurvefit,” which solves a nonlinear curve-fitting problem in the least-squares sense using the Levenberg-Marquardt algorithm.
Estimation of the values of model parameters descriptive of the contributions of membrane potentials and Pgp to changes in [Re] vs. time requires a collection of data sets that provides sufficiently discriminating information about these cellular processes. Thus, for each dye, the values of Δψm, Δψp, V1, KPgp, P1S1, and P2S2 were obtained by fitting the solution of Eqs. 1–4 simultaneously to the mean [Re] vs. time data in Fig. 4. The hypothesis was that Δψm, Δψp, and KPgp could be set to zero in the presence of CCCP, high K+, or GF-120918, respectively. In addition, it was hypothesized that, in the presence of CCCP + high K+, any effect of CCCP on Δψp would be negligible, allowing δ to be set to 1 in Eq. 7. The parameter values and the 95% confidence intervals (CI) are given in Table 1 (10). Data in Fig. 4B do not have sufficient temporal resolution to provide a value for P2S2 for TMRE other than an upper bound set in the nonlinear regression algorithm.
Table 1.
Kinetic model parameter values
| Δψm, mV | Δψp, mV | P1S1, μl•min−1•cm−2 | P2S2, μl•min−1•cm−2 | KPgp, μl•min−1•cm−2 | V1, μl/cm2 | |
|---|---|---|---|---|---|---|
| R123 | −130 ± 7 | −36 ± 4 | 0.17 ± 0.02 | 0.18 ± 0.20 | 0.41 ± 0.07 | 7.4 ± 0.8 |
| TMRE | −133 ± 4 | −49 ± 4* | 5.20 ± 0.79* | 37.67 ± 47.81 | 1.92 ± 0.41* | 30.5 ± 3.8* |
Values are means ± asymptotic 95% confidence intervals. Δψm and Δψp, mitochondrial and plasma membrane potentials, respectively; KPgp, conductance of P-glycoprotein-mediated release of rhodamine 123 (R123) or tetramethylrhodamine ethyl ester (TMRE) from cells; P1S1 and P2S2, permeability-surface area products descriptive of R123 or TMRE conductance across plasma and mitochondrial membranes, respectively; V1, apparent volume of cell cytoplasm. Parameter values were obtained by fitting model solution to mean values of extracellular R123 or TMRE concentration ([Re]) vs. time data in Fig. 4.
Significantly different from the same parameter value obtained using R123 data, P < 0.05 (by t-test).
The means ± 95% CI for the parameters δ and τ were determined to be 0.25 ± 0.02 and 2.3 ± 0.7 min, respectively, by fitting the solution of model Eqs. 1–4 to the GF-120918 + CCCP data for TMRE in Fig. 5B, with KPgp and Δψm set to zero and with Δψm and other parameters set to the values shown in Table 1. Model simulations revealed that the effect of CCCP on Δψp is more apparent in the presence of GF-120918 than for any other experimental condition studied.
Model simulations based on Eqs. 1–4 revealed that increasing or decreasing V2/V1 by 50% changed Δψm by +10 and −18 mV, respectively, consistent with previous estimates of the sensitivity of Δψm to V2/V1 (44). Model simulations further demonstrated that values for Δψp and KPgp are insensitive to changes in V2/V1.
Model predictions (validation).
The kinetic model was evaluated by testing its ability to predict [Re] under experimental conditions that were not used for estimating model parameters in Table 1. The values in Table 1 were used to generate model predictions for data sets in Figs. 2, 3, and 5. The values for δ and τ, which quantify a time-dependent CCCP-mediated Δψp depolarization, obtained from the TMRE data in Fig. 5B, were used to predict the effect of CCCP on the R123 [Re] vs. time curve in the presence of CCCP + GF-120918 (Fig. 5A). That this prediction was a reasonable explanation of the R123 CCCP + GF-120918 data provided support for the hypothesis of a depolarizing effect of CCCP on Δψp under the study conditions.
Steady-state analysis.
Since [Re] for TMRE reaches steady state over the experimental time period (∼30 min; Figs. 1–5), we reasoned that steady-state analysis could be used to estimate Δψm under just two experimental conditions, high K+ + GF-120918 and high K+ + GF-120918 + CCCP, using the following algebraic equation (for derivation see appendix)
| (9) |
where [Re]s0 is the steady-state [Re] in the absence of cells, [Re]s2 is the steady-state [Re] for high K+ + GF-120918, [Re]s1 is the steady-state [Re] for high K+ + GF-120918 + CCCP, and β is V2/V1. The Δψm determined using Eq. 9 is −124 ± 6 (SE) mV.
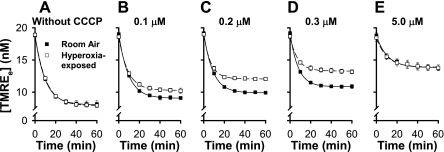
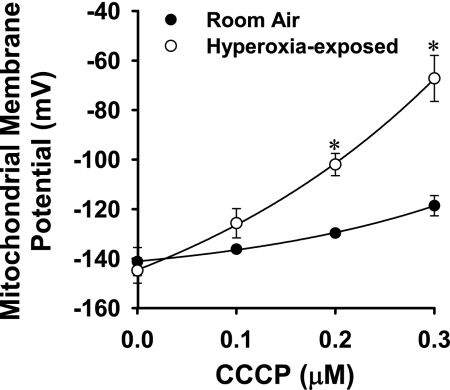
Figures 7 and 8 exemplify the use of steady-state TMRE data and steady-state data analysis to evaluate the effect of a physiological stimulus on the Δψm response to uncoupling with CCCP. The studies included GF-120918 and high K+ to eliminate the contributions of Δψp and Pgp to the fate of TMRE, thereby isolating and emphasizing the impact of Δψm. Figure 7 shows [Re] vs. time data for control and hyperoxia-exposed cells in which the CCCP concentration varies from 0 (Fig. 7A) to 0.1, 0.2, 0.3, and 5.0 μM (Fig. 7, B–E). Figure 7, A and E, shows that the steady-state [Re] is nearly the same for hyperoxia-exposed and control cells in the absence of CCCP and at the highest CCCP concentration (5 μM). However, at intermediate CCCP concentrations, the steady-state [Re] is higher for hyperoxia-exposed than control cells (Fig. 7, B–D). Figure 8 shows the Δψm for control and hyperoxia-exposed cells calculated from Eq. 9 and the Fig. 7 [Re] vs. time data at each CCCP concentration. As anticipated from Fig. 7, the resting Δψm are not detectably different, but the hyperoxia-exposed cell Δψm is more sensitive to the depolarizing effects of uncoupling with CCCP.
Fig. 7.
Impact of 0.1, 0.2, 0.3, or 5.0 μM CCCP on TMRE concentrations in medium surrounding control (room air-exposed) and hyperoxia-exposed pulmonary arterial endothelial cells. Experiments were carried out in the presence of GF-120918 and high K+ without CCCP or with 0.1, 0.2, 0.3, or 5.0 μM CCCP. Symbols are means ± SE for n = 3 determinations at each CCCP concentration.
Fig. 8.
Hyperoxia-exposed cell Δψm is more sensitive to CCCP-induced depolarization than control (room air-exposed) cell Δψm. Symbols represent values for Δψm obtained using steady-state TMRE data from Fig. 7 and Eq. 9. *Significantly different from control values at the same CCCP concentration, P < 0.05 (by t-test).
DISCUSSION
The present study was based on the general hypothesis that changes in the concentrations of rhodamine dyes in the medium surrounding the intact pulmonary arterial endothelial cells ([Re]) would be sensitive to perturbations in Δψm and Δψp. The study reveals that the observed changes in extracellular dye disposition in response to the perturbations were highly predictable on the basis of previous measurements of intracellular rhodamine dye disposition using the same or very similar kinds of perturbations, albeit in different cell types (23). Taken together with the knowledge that such intracellular dye measurements had been used to quantify Δψm and Δψp, our reasoning was that the extracellular dye concentration vs. time kinetic data would also contain this quantitative information.
A key challenge in unlocking this quantitative information was that the net effect of the cells on the rhodamine dye concentrations in the medium involved multiple interacting processes. A general strategy in this situation is to use inhibitors and treatment conditions to provide discriminating data relevant to these processes and kinetic modeling to obtain values for kinetic parameters descriptive of the individual processes involved. We used a variety of treatment conditions and inhibitors to target the processes hypothesized to be involved in dye disposition and a computational model to interpret the data.
Computational modeling provides an integrated framework for quantifying the qualitative features of the data, as well as a means to evaluate mechanistic hypotheses regarding the processes that produce the data (11). Generally speaking, computational models make use of physical laws (e.g., mass balance) together with existing models of subsystems (e.g., the Goldman-Katz equation) to describe the interactions among the components of a more complex system (e.g., intact cells) (11). Our model incorporates mass balance, the Goldman-Katz equation, and linear kinetics to describe the cellular disposition of the rhodamine dyes within the cells and the medium. The model is distinct from, and complementary to, certain existing models for evaluating cell membrane potentials, in that it allows for contributions of additional cellular processes to dye disposition (e.g., Pgp), utilizes extracellular dye concentration data (rather than microscopic measurements of intracellular dye), and takes advantage of an entire intact cell population (instead of individual cells or isolated mitochondria) (64).
In general, model parameters cannot be estimated from a single data set or experimental condition (4, 39). Instead, a diverse set of experimental protocols are needed to provide sufficiently discriminating data to break correlations between the contributing processes. In the present study, the data sets used to obtain the parameter values consist of concentration data for two different dyes under four experimental conditions as a function of time (Fig. 4). The model provided a good fit to the data for the two different dyes, and the values obtained for Δψm and Δψp were consistent for the two dyes. They were also consistent with values reported for pulmonary arterial endothelial cells in culture using an array of other approaches (see below). Since the values representing Pgp activity (KPgp) and dye permeability-surface area product (PS) are determined by physical and chemical properties of the dyes, rather than by intrinsic properties of the cells, they are not expected to be the same for R123 and TMRE. However, as anticipated from the reported higher cell membrane permeability of TMRE, the values of P1S1 and P2S2 (normalized to cell surface area) for TMRE are higher than those for R123 (Table 1) (21, 32).
To our knowledge, the values for Δψm of −130 ± 7 and −133 ± 4 mV (means ± 95% CI) using R123 and TMRE, respectively, obtained in the present study are the first quantification of Δψm for bovine pulmonary artery endothelial cells in culture. Previous studies of bovine aortic endothelial cells using the potential-sensitive triphenylphosphonium ion yielded a Δψm of −220 mV (47). However, it is difficult to make a comparison, because the latter studies involved isolated mitochondria under hyperpolarizing conditions (e.g., in the presence of oligomycin) (47).
The study further showed that steady-state analysis of TMRE data can be used to obtain values for Δψm that were consistent with the values obtained from transient kinetic R123 and TMRE data. An application of the steady-state approach to quantify the impact of hyperoxic exposure on pulmonary endothelial Δψm was demonstrated and, to our knowledge, provides the first quantitative assessment of the impact of an oxidative stress on pulmonary endothelial cell Δψm. Hyperoxic exposure was selected as the stress, because elevated oxygen is the most common treatment for respiratory failure, yet it is injurious to the lung, with endothelial cells being a prime target (18, 42).
For the hyperoxia-exposed cells, resting Δψm was not detectably different from control cells, but hyperoxia-exposed cell Δψm was more sensitive to depolarization via uncoupling. It should be emphasized that the effect of hyperoxic exposure on pulmonary endothelial mitochondria would not have been revealed by measuring only the resting Δψm or using only a single commonly used CCCP concentration (5 μM) to depolarize the mitochondria. Various indications of mitochondrial dysfunction have been reported by us and others in hyperoxia-exposed rat lung, pulmonary endothelial cells in culture, and other cell types (2, 3, 5, 7, 8, 36, 37, 51, 57). In this context, the present observations are consistent with a hyperoxia-induced mitochondrial deficiency.
Previous methods for evaluating stimulation- or stress-induced alterations in pulmonary endothelial cell Δψm using rhodamines or ratiometric probes have, in general, provided only indexes of relative changes, estimated from changes in fluorescence intensity (19, 24, 28, 43, 69). A common pitfall in interpreting such data is that the logarithmic form of the Nernst equation specifies that changes in fluorescence intensity (and, for ratiometric dyes, ratios derived from such changes) are not linearly proportional to changes in Δψm (45). In this context, the ratiometric dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide (JC-1) exists in a monomeric form, with an emission wavelength of 527 nm (green) when excited at 490 nm, but when the dye accumulates in polarized mitochondria, it forms aggregates associated with a large shift in emission wavelength to 590 nm (red) (20). Changes in green-to-red fluorescence ratio have been useful in giving a semiquantitative picture of the impact of various stimuli on Δψm. However, aside from the apparently very few attempts that have been made to quantify the association between these ratios and Δψm in isolated mitochondria, the quantitative relationship between any given ratio change and the corresponding change in Δψm in millivolts in intact cells is not generally considered (20). Our approach could be viewed as complementary to that of JC-1, wherein it would provide a means for quantitative interpretation of changes in green-to-red fluorescence ratios.
The values for Δψp obtained in the present study were −36 ± 4 and −49 ± 4 mV (means ± 95% CI) using R123 and TMRE, respectively (Table 1). Although reasonably close, the difference between the two may be attributable to the longer experimental time course needed to overcome the lower membrane permeability of R123 than TMRE (as represented by PS products in Table 1). Previous estimates of cultured bovine pulmonary arterial endothelial Δψp made using whole cell patch clamp have yielded mean values of −26 to −67 mV (1, 14, 29, 31, 62). The variation may be attributable to differences in culture conditions or error arising from leakage currents (1, 14). The issue of leakage currents has been addressed by rejecting cells displaying resting potentials below a set seal resistance, narrowing the range in values from −56 to −67 mV (1, 14, 29). When comparing these values with those obtained in the present study, we must consider that the “whole cell” patch-clamp studies were carried out at room temperature, used estimated values based on reversal potentials, and involved rupturing the plasma membrane and cell dialysis with pipette solutions. In contrast, our studies were carried out at 37°C using a nondestructive protocol. In addition, patch clamp focuses on relatively small numbers of individual cells, which reveals heterogeneity within the population, while our approach is directed at entire cell populations (14, 15, 21, 31).
Previous measurements of Δψm and Δψp using rhodamine dyes have commonly not taken into account that these dyes are also Pgp substrates (22, 34, 64, 65, 68). For cell types that have few or no multidrug transporters, this may be of minimal importance, but multidrug transporters perform a key function in the pulmonary endothelium, which is in direct contact with blood-borne pharmacological, physiological, and toxicological substances. In the present study, the effect of increasing or decreasing KPgp by 50% on the dye concentration in the mitochondria [Rm] would be a ∼45% increase or ∼40% decrease for R123 and a 12% increase or 8% decrease for TMRE. These effects could be misinterpreted to represent changes in Δψm and/or Δψp. This concept may be of particular importance in studying cell injury, which has been observed to affect Pgp protein levels in brain endothelial cells (52).
Whereas inhibitors (e.g., GF-120918) and other treatments (e.g., CCCP and high K+) are presumed to affect predominantly their intended targets, their unintended effects may vary for different cell types or experimental conditions. For example, one question is whether the extracellular high K+ concentration needed to fully depolarize Δψp might also influence Δψm. There is evidence to suggest that if such an effect of high K+ is present, it is not dominant under the experimental conditions used. 1) The model can explain all the data, with and without high K+, CCCP, and GF-120918 and for R123 and TMRE, without the need to allow for an effect of high K+ on Δψm. According to the concept of parsimony, there is therefore no basis for adding complexity (i.e., additional parameters) to the model. 2) Values of Δψm and Δψp are within the range of values reported for pulmonary endothelial cells, as discussed above. If we allow for a depolarizing effect of high K+ on Δψm in the model, the resulting simulations produce less negative values for Δψp, which are then less, rather than more, consistent with previously reported values. Farkas et al. (23) also asked whether >100 mM extracellular K+ used to distinguish effects of Δψm and Δψp on dye disposition in intact cell imaging studies was affecting Δψm. The relatively small amount of dye leakage from mitochondria observed in these high K+ conditions was attributed to depolarization of Δψp, rather than Δψm (23). Thus interpretations of measurements of dye disposition within cells are associated with the same kinds of complexity as the [Re] measurements of the present study.
Rhodamine dyes and other probes have been used in previous experimental and theoretical studies to simultaneously assess Δψm and Δψp in a variety of nonendothelial mammalian cell types (23, 55, 65, 66). Nicholls (46) and Park et al. (50) used a proprietary Δψp indicator, PMPI (plasma membrane potential indicator), along with TMRE to monitor changes in Δψp and Δψm in cultured neurons. The combination of probes allowed for a means to account for the impact of changes in Δψp on the rhodamine dye disposition. Ward et al. (65, 66) developed an elegant computational model to interpret intracellular rhodamine dye fluorescence measurements in terms of transmembrane potentials for cultured cerebellar neuronal and granular cells. These studies (46, 50, 65, 66) accounted for the impact of Δψm, Δψp, membrane permeability, and mitochondrial volume fraction, but not for effects of Pgp, on dye disposition (46, 50, 65, 66). In contrast to our approach, the latter modeling approaches relied on input of initial values for Δψm and Δψp obtained in separate studies. A MATLAB-based public web service, TOXI-SIM, was developed as a tool for implementing the model of Ward et al., the output of which is information on changes in Δψm and Δψp relative to fixed initial values (27). The approach described in the present study may be viewed as complementary, in that it could provide initial values for the TOXI-SIM tool. This would allow for quantitative assessment of changes in pulmonary endothelial Δψm and Δψp, including those that occur under conditions in which changes are rapid relative to the time scale of the present studies.
In summary, we took several approaches to optimize utility of rhodamine dyes for assessing Δψm and Δψp in intact pulmonary arterial endothelial cells. Dye concentrations were maintained in the linear range of fluorescence intensity vs. concentration, overcoming many of the complexities associated with interpreting contributions of quenching and dequenching to the signals. Solid microcarrier bead cell cultures optimized the cell surface-to-medium volume ratio, maximizing the impact of the cells on substances in the medium. Additionally, since the cell-coated beads rapidly (within ∼15 s) settle out of suspension, medium fluorescence measurements are made without exposing cells to light or requiring dissociation from the culture surface.
The advantages and limitations of the proposed strategy for quantifying membrane potentials in intact pulmonary endothelial cells depend, to some extent, on the question to be asked, and in many respects our approach can be viewed as complementary to other methods. For example, as previously discussed, the quantitative information revealed using our approach would be very useful in interpreting the semiquantitative information obtained using other probes, including ratiometric dyes (e.g., JC-1), or in providing input values for resting membrane potentials for the TOXI-SIM tool. For comparisons between methodologies, our approach involves the entire cell population, whereas microscopic observation of individual cells or fluorescence-activated cell sorting supplies more detailed information on membrane potential heterogeneity within a population. In the present study, mitochondrial volume was hypothesized to be constant, yet, depending on the conditions, it could be important to measure this volume, as has been done for model-based determinations of neuronal cell membrane potentials (46). Finally, the present approach requires the use of inhibitors and high K+, which may have nontargeted effects; however, such perturbations are commonly used in various other methods to quantify cell membrane potentials, including patch-clamp and microscopic studies (1, 20, 21, 23).
In conclusion, we present a unique strategy for quantifying the impact of various stresses and stimuli on membrane potentials in intact pulmonary endothelial cells. The strategy does not rely on prior knowledge of resting Δψm and Δψp to estimate the impact of perturbations on these parameters but, instead, produces actual parameter values in millivolts. Furthermore, this approach allows for quantitative assessment of various injurious conditions on the bioenergetic properties of pulmonary endothelial cells, as was demonstrated by the observations of the impact of chronic hyperoxic exposure on pulmonary endothelial Δψm.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL-24349, HL-65537, and HL-51055 and the Department of Veterans Affairs.
APPENDIX
After addition of the dye along with GF-120918 and CCCP to high-K+ medium, the steady-state dye concentrations in Ve, V1, and V2 should be equal to that in the medium ([Re]s1)
| (A1) |
Using mass balance
| (A2) |
where [Re]s0 is the steady-state value of dye concentration in the medium following the addition of dye in the absence of cells (Fig. 1).
After addition of the dye along with GF-120918 to high-K+ medium, the steady-state dye concentrations in Ve and V1 should be equal and different from that in V2. Thus, using mass balance
| (A3) |
where [Re]s2 and [Rm]s are the respective steady-state dye concentrations within Ve and V2 following the addition of dye + GF-120918 to high-K+ medium. [Re]s2 and [Rm]s can be related to Δψm using the Nernst equation
| (A4) |
Using algebraic manipulations, Eqs. A1–A4 lead to the following equation
| (A5) |
where β is the ratio V2/V1.
- Bc
- Dye binding sites within the cytoplasm
- Be
- Dye-cuvette binding sites
- Bm
- Dye binding sites within mitochondrial matrix
- BPAEC
- Bovine pulmonary arterial endothelial cells
- CCCP
- Carbonyl cyanide 3-chlorophenylhydrazone
- F
- Faraday constant (C/mol)
- FCCP
- Carbonylcyanide p-trifluoromethoxyphenylhydrazone
- GF-120918
- N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide
- J1
- Dye flux across plasma membrane (pmol/cm2)
- J2
- Dye flux across inner mitochondrial membrane (pmol/cm2)
- k1
- Rate constant for dye-cuvette binding (min−1·nM−1)
- k−1
- Rate constant for dye-cuvette unbinding (min−1)
- k̄1 = k1[Be]
- Rate for dye-cuvette binding (min−1)
- k2
- Association rate constant of dye binding with Bc (min−1·nM−1)
- k−2
- Dissociation rate constant of dye binding with Bc (min−1)
- k3
- Association rate constant of dye binding with Bm (min−1·nM−1)
- k−3
- Dissociation rate constant of dye binding with Bm (min−1)
- KPgp
- Pgp-mediated dye efflux rate (ml/min)
- LDH
- Lactate dehydrogenase
- P1
- Dye permeability across plasma membrane (cm/min)
- P2
- Dye permeability across mitochondrial membrane (cm/min)
- P1S1
- Dye permeability-surface area product across plasma membrane (ml/min)
- P2S2
- Dye permeability-surface area product across mitochondrial membrane (ml/min)
- Pgp
- P-glycoprotein multidrug efflux pump
- R
- Universal gas constant (J·K−1·mol−1)
- R123
- Rhodamine 123
- [Rc](t)
- Dye concentration in cytoplasm at time t (nM)
- [Re](t)
- Dye concentration in extracellular medium at time t (nM)
- [Re]s0
- Dye steady-state concentration in extracellular medium in absence of BPAEC (nM)
- [Re]s1
- Dye steady-state concentration in extracellular medium in presence of BPAEC, GF-120918, and high K+ (nM)
- [Re]s2
- Dye steady-state concentration in extracellular medium in presence of BPAEC, GF-120918, high K+, and CCCP (nM)
- [Rm](t)
- Dye concentration in mitochondrial matrix at time t (nM)
- [Rm]s
- Dye steady-state concentration in mitochondrial matrix (nM)
- S1
- Surface area of plasma membrane (cm2)
- S2
- Surface area of mitochondrial membrane (cm2)
- T
- Absolute temperature (Kelvin)
- TMRE
- Tetramethyrhodamine ethyl ester
- V1
- Apparent cytoplasm volume (ml)
- V2
- Apparent mitochondrial matrix volume (ml)
- Vc
- Physical cytoplasm volume (ml)
- Ve
- Extracellular medium volume (ml)
- Vm
- Physical mitochondrial matrix volume (ml)
- Z
- Dye valence
- αc
- ZF/RT (mV−1)
- β
- V2/V1
- δ
- Fraction of Δψp not dissipated by CCCP
- τ
- Time constant of Δψp decay in the presence of CCCP (min)
- Δψp
- Plasma membrane potential (mV)
- Δψm
- Mitochondrial membrane potential (mV)
REFERENCES
- 1. Adams DJ, Hill MA. Potassium channels and membrane potential in the modulation of intracellular calcium in vascular endothelial cells. J Cardiovasc Electrophysiol 15: 598–610, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Ahmad A, Ahmad S, Chang LY, Schaack J, White CW. Endothelial Akt activation by hyperoxia: role in cell survival. Free Radic Biol Med 40: 1108–1118, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Audi SH, Merker MP, Krenz GS, Ahuja T, Roerig DL, Bongard RD. Coenzyme Q1 redox metabolism during passage through the rat pulmonary circulation and the effect of hyperoxia. J Appl Physiol 105: 1114–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Audi SH, Zhao H, Bongard RD, Hogg N, Kettenhofen NJ, Kalyanaraman B, Dawson CA, Merker MP. Pulmonary arterial endothelial cells affect the redox status of coenzyme Q0. Free Radic Biol Med 34: 892–907, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Audi SH, Bongard RD, Krenz GS, Rickaby DA, Haworth ST, Eisenhauer J, Roerig DL, Merker MP. Effect of chronic hyperoxic exposure on duroquinone reduction in adult rat lungs. Am J Physiol Lung Cell Mol Physiol 289: L788–L797, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Baracca A, Sgarbi G, Solaini G, Lenaz G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim Biophys Acta 1606: 137–146, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bassett DJ, Bowen-Kelly E. Pyruvate metabolism of perfused rat lungs after exposure to 100% oxygen. J Appl Physiol 60: 1605–1609, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Bassett DJ, Bowen-Kelly E, Reichenbaugh SS. Rat lung glucose metabolism after 24 h of exposure to 100% oxygen. J Appl Physiol 66: 989–996, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 296: C422–C432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bates DM, Watts DG. Non-Linear Regression Analysis and Its Applications. New York: Wiley, 1983 [Google Scholar]
- 11. Beard DA, Bassingthwaighte JB, Greene AS. Computational modeling of physiological systems. Physiol Genomics 23: 1–3, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Bongard RD, Lindemer BJ, Krenz GS, Merker MP. Preferential utilization of NADPH as the endogenous electron donor for NAD(P)H:quinone oxidoreductase 1 (NQO1) in intact pulmonary arterial endothelial cells. Free Radic Biol Med 46: 25–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bongard RD, Merker MP, Shundo R, Okamoto Y, Roerig DL, Linehan JH, Dawson CA. Reduction of thiazine dyes by bovine pulmonary arterial endothelial cells in culture. Am J Physiol Lung Cell Mol Physiol 269: L78–L84, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Campbell DL, Strauss HC, Whorton AR. Voltage dependence of bovine pulmonary artery endothelial cell function. J Mol Cell Cardiol 23 Suppl 1:133–144, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Cannell MB, Sage SO. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol 419: 555–568, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chatterjee S, Chapman KE, Fisher AB. Lung ischemia: a model for endothelial mechanotransduction. Cell Biochem Biophys 52: 125–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chatterjee S, Levitan I, Wei Z, Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 13: 633–644, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122: 123–143, 1980 [DOI] [PubMed] [Google Scholar]
- 19. Dedkova EN, Blatter LA. Modulation of mitochondrial Ca2+ by nitric oxide in cultured bovine vascular endothelial cells. Am J Physiol Cell Physiol 289: C836–C845, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol 486: 1–13, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol 361: 353–389, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Ehrenberg B, Montana V, Wei MD, Wuskell JP, Loew LM. Membrane potential can be determined in individual cells from the Nernstian distribution of cationic dyes. Biophys J 53: 785–794, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farkas DL, Wei MD, Febbroriello P, Carson JH, Loew LM. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J 56: 1053–1069, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han YH, Moon HJ, You BR, Park WH. Propyl gallate inhibits the growth of calf pulmonary arterial endothelial cells via glutathione depletion. Toxicol In Vitro 24: 1183–1189, 2010 [DOI] [PubMed] [Google Scholar]
- 25. He P, Curry FE. Measurement of membrane potential of endothelial cells in single perfused microvessels. Microvasc Res 50: 183–198, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Huang M, Camara AK, Stowe DF, Qi F, Beard DA. Mitochondrial inner membrane electrophysiology assessed by rhodamine-123 transport and fluorescence. Ann Biomed Eng 35: 1276–1285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huber HJ, Plchut M, Weisova P, Dnssmann H, Wenus J, Rehm M, Ward MW, Prehn JHM. TOXI-SIM—a simulation tool for the analysis of mitochondrial and plasma membrane potentials. J Neurosci Methods 176: 270–275, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J 343: 311–317, 1999 [PMC free article] [PubMed] [Google Scholar]
- 29. Johns A, Lategan TW, Lodge NJ, Ryan US, van BC, Adams DJ. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell 19: 733–745, 1987 [DOI] [PubMed] [Google Scholar]
- 30. Katzir H, Yeheskely-Hayon D, Regev R, Eytan GD. Role of the plasma membrane leaflets in drug uptake and multidrug resistance. FEBS J 277: 1234–1244, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Koliwad SK, Kunze DL, Elliott SJ. Oxidant stress activates a non-selective cation channel responsible for membrane depolarization in calf vascular endothelial cells. J Physiol 491: 1–12, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loetchutinat C, Saengkhae C, Marbeuf-Gueye C, Garnier-Suillerot A. New insights into the P-glycoprotein-mediated effluxes of rhodamines. Eur J Biochem 270: 476–485, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, RamachandraRao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol 170: 1079–1090, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mandala M, Serck-Hanssen G, Martino G, Helle KB. The fluorescent cationic dye rhodamine 6G as a probe for membrane potential in bovine aortic endothelial cells. Anal Biochem 274: 1–6, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Merker MP, Armitage IM, Audi SH, Kakalis LT, Linehan JH, Maehl JR, Roerig DL, Dawson CA. Impact of angiotensin-converting enzyme substrate conformation on fractional hydrolysis in the lung. Am J Physiol Lung Cell Mol Physiol 270: L251–L259, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Merker MP, Audi SH, Bongard RD, Lindemer BJ, Krenz GS. Influence of pulmonary arterial endothelial cells on quinone redox status: effect of hyperoxia-induced NAD(P)H quinone oxidoreductase 1 (NQO1). Am J Physiol Lung Cell Mol Physiol 290: L607–L619, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Merker MP, Audi SH, Lindemer BJ, Krenz GS, Bongard RD. Role of mitochondrial electron transport complex I in coenzyme Q1 reduction by intact pulmonary arterial endothelial cells and the effect of hyperoxia. Am J Physiol Lung Cell Mol Physiol 293: L809–L819, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Merker MP, Bongard RD, Kettenhofen NJ, Okamoto Y, Dawson CA. Intracellular redox status affects transplasma membrane electron transport in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 282: L36–L43, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Merker MP, Bongard RD, Krenz GS, Zhao H, Fernandes V, Kalyanaraman B, Hogg N, Audi SH. Impact of pulmonary arterial endothelial cells on duroquinone redox status. Free Radic Biol Med 37: 86–103, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Merker MP, Bongard RD, Linehan JH, Okamoto Y, Vyprachticky D, Brantmeier BM, Roerig DL, Dawson CA. Pulmonary endothelial thiazine uptake: separation of cell surface reduction from intracellular reoxidation. Am J Physiol Lung Cell Mol Physiol 272: L673–L680, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Merker MP, Olson LE, Bongard RD, Patel MK, Linehan JH, Dawson CA. Ascorbate-mediated transplasma membrane electron transport in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 274: L685–L693, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Moody EJ, Simon BA, Johns RA. Therapeutic gases. In: The Pharmacological Basis of Therapeutics, edited by Hardman JG, Limbird LE, Gilman AG. New York: McGraw-Hill, 2001 [Google Scholar]
- 43. Mungunsukh O, Griffin AJ, Lee YH, Day RM. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 298: L696–L703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nicholls DG. Commentary on: “Old and new data, new issues: the mitochondrial Δψ” by H. Tedeschi. Biochim Biophys Acta 1710: 63–65, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev 80: 315–360, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Nicholls DG. Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J Biol Chem 281: 14864–14874, 2006 [DOI] [PubMed] [Google Scholar]
- 47. O'Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem 281: 39766–39775, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1: 409–417, 1977 [DOI] [PubMed] [Google Scholar]
- 49. Paffett ML, Naik JS, Resta TC, Walker BR. Reduced store-operated Ca2+ entry in pulmonary endothelial cells from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 293: L1135–L1142, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Park KS, Jo I, Pak K, Bae SW, Rhim H, Suh SH, Park J, Zhu H, So I, Kim KW. FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells. Pflügers Arch 443: 344–352, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Pruijn FB, Schoonen WG, Joenje H. Inactivation of mitochondrial metabolism by hyperoxia-induced oxidative stress. Ann NY Acad Sci 663: 453–455, 1992 [DOI] [PubMed] [Google Scholar]
- 52. Robertson SJ, Kania KD, Hladky SB, Barrand MA. P-glycoprotein expression in immortalised rat brain endothelial cells: comparisons following exogenously applied hydrogen peroxide and after hypoxia-reoxygenation. J Neurochem 111: 132–141, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Roerig DL, Audi SH, Ahlf SB. Kinetic characterization of P-glycoprotein mediated efflux of rhodamine 6G in the intact rabbit lung. Drug Metab Dispos 32: 953–958, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi AB, Gillespie MN. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L530–L535, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Rugolo M, Lenaz G. Monitoring of the mitochondrial and plasma membrane potentials in human fibroblasts by tetraphenylphosphonium ion distribution. J Bioenerg Biomembr 19: 705–718, 1987 [DOI] [PubMed] [Google Scholar]
- 56. Scaduto RC, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76: 469–477, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schoonen WG, Wanamarta AH, van der Klei-van Moorsel, Jakobs C, Joenje H. Respiratory failure and stimulation of glycolysis in Chinese hamster ovary cells exposed to normobaric hyperoxia. J Biol Chem 265: 1118–1124, 1990 [PubMed] [Google Scholar]
- 58. Solaini G, Sgarbi G, Lenaz G, Baracca A. Evaluating mitochondrial membrane potential in cells. Biosci Rep 27: 11–21, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Stevens T, Cornfield DN, McMurtry IF, Rodman DM. Acute reductions in Po2 depolarize pulmonary artery endothelial cells and decrease [Ca2+]i. Am J Physiol Heart Circ Physiol 266: H1416–H1421, 1994 [DOI] [PubMed] [Google Scholar]
- 60. Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol 294: C1407–C1418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Terminella C, Tollefson K, Kroczynski J, Pelli J, Cutaia M. Inhibition of apoptosis in pulmonary endothelial cells by altered pH, mitochondrial function, and ATP supply. Am J Physiol Lung Cell Mol Physiol 283: L1291–L1302, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Voets T, Droogmans G, Nilius B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J Physiol 497: 95–107, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang L, Leggas M, Goswami M, Empey PE, McNamara PJ. N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) as a chemical ATP-binding cassette transporter family G member 2 (Abcg2) knockout model to study nitrofurantoin transfer into milk. Drug Metab Dispos 36: 2591–2596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ward MW. Quantitative analysis of membrane potentials. Methods Mol Biol 591: 335–351, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Ward MW, Huber HJ, Weisova P, Dussmann H, Nicholls DG, Prehn JH. Mitochondrial and plasma membrane potential of cultured cerebellar neurons during glutamate-induced necrosis, apoptosis, and tolerance. J Neurosci 27: 8238–8249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ward MW, Rego AC, Frenguelli BG, Nicholls DG. Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 20: 7208–7219, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu S, Jian MY, Xu YC, Zhou C, Al-Mehdi AB, Liedtke W, Shin HS, Townsley MI. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 297: L650–L657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yeheskely-Hayon D, Regev R, Katzir H, Eytan GD. Competition between innate multidrug resistance and intracellular binding of rhodamine dyes. FEBS J 276: 637–648, 2009 [DOI] [PubMed] [Google Scholar]
- 69. You BR, Park WH. The effects of antimycin A on endothelial cells in cell death, reactive oxygen species and GSH levels. Toxicol In Vitro 24: 1111–1118, 2010 [DOI] [PubMed] [Google Scholar]
- 70. Zhang Q, Chatterjee S, Wei Z, Liu WD, Fisher AB. Rac and PI3 kinase mediate endothelial cell reactive oxygen species generation during normoxic lung ischemia. Antioxidants Redox Signaling 10: 679–689, 2008 [DOI] [PubMed] [Google Scholar]