Abstract
The quinones duroquinone (DQ) and coenzyme Q1 (CoQ1) and quinone reductase inhibitors have been used to identify reductases involved in quinone reduction on passage through the pulmonary circulation. In perfused rat lung, NAD(P)H:quinone oxidoreductase 1 (NQO1) was identified as the predominant DQ reductase and NQO1 and mitochondrial complex I as the CoQ1 reductases. Since inhibitors have nonspecific effects, the goal was to use Nqo1-null (NQO1−/−) mice to evaluate DQ as an NQO1 probe in the lung. Lung homogenate cytosol NQO1 activities were 97 ± 11, 54 ± 6, and 5 ± 1 (SE) nmol dichlorophenolindophenol reduced·min−1·mg protein−1 for NQO1+/+, NQO1+/−, and NQO1−/− lungs, respectively. Intact lung quinone reduction was evaluated by infusion of DQ (50 μM) or CoQ1 (60 μM) into the pulmonary arterial inflow of the isolated perfused lung and measurement of pulmonary venous effluent hydroquinone (DQH2 or CoQ1H2). DQH2 efflux rates for NQO1+/+, NQO1+/−, and NQO1−/− lungs were 0.65 ± 0.08, 0.45 ± 0.04, and 0.13 ± 0.05 (SE) μmol·min−1·g dry lung−1, respectively. DQ reduction in NQO1+/+ lungs was inhibited by 90 ± 4% with dicumarol; there was no inhibition in NQO1−/− lungs. There was no significant difference in CoQ1H2 efflux rates for NQO1+/+ and NQO1−/− lungs. Differences in DQ reduction were not due to differences in lung dry weights, wet-to-dry weight ratios, perfusion pressures, perfused surface areas, or total DQ recoveries. The data provide genetic evidence implicating DQ as a specific NQO1 probe in the perfused rodent lung.
Keywords: knockout mice, lung metabolism, duroquinone, isolated perfused mouse lung, coenzyme Q
the pulmonary endothelium and lung tissue participate in altering the redox status and disposition of certain redox-active compounds as they pass through the pulmonary circulation (4–7, 9). This function is carried out by redox enzymes at the pulmonary endothelial surface and within pulmonary endothelial and other lung cells. Since the redox forms of such compounds have different propensities to permeate tissues and carry out pro- and/or antioxidant functions, passage of these compounds through the pulmonary circulation has the potential to influence their dispositions, concentrations, and bioactivities within the pulmonary vessels and lung itself, as well as in downstream organs and vessels.
Quinones comprise a class of redox-active compounds having a wide range of physical and chemical properties, and a number of mammalian enzymes have quinone reductase activity (13). Therefore, quinones are useful for probing lung redox processes that affect their disposition in the blood. We have used the amphipathic quinone duroquinone (DQ) as one model compound for studying quinone metabolism on passage through the pulmonary circulation of the isolated perfused rodent lung (4, 9). DQ is advantageous because the quinone (DQ) and hydroquinone (DQH2) forms are cell membrane-permeable and, thus, freely enter and leave lung tissue from the pulmonary circulation (4). Earlier studies revealed that DQ is reduced to DQH2 on passage through the rat and mouse lung (4, 9). Inhibitor studies indicated that nearly all the DQ reduction in the rat lung was attributable to NAD(P)H:quinone oxidoreductase 1 (NQO1) (4, 9). The contribution of NQO1 to DQ reduction in the mouse lung was not specifically addressed (4).
Since NQO1 is highly expressed in pulmonary endothelium and NQO1 is a two-electron quinone reductase, the impact on DQ may not have been that surprising (17, 40). On the other hand, studies using subcellular fractions, tissue homogenates, or isolated enzymes from various tissues have shown that DQ can also act as an electron acceptor for various other redox enzymes that are also present in lung tissue (19, 35, 37). These observations emphasize the importance of using intact organs in metabolism studies and suggest the intriguing possibility that DQ acts as a specific NQO1 activity probe in the perfused lung. Since there are no means to assess NQO1 activity in vivo, confirmation that DQ can be used for this purpose would represent an advance in studies of NQO1 as a target of drug activation and xenobiotic detoxification and as a factor in susceptibility to oxidative stress, various malignancies, and other pathophysiological conditions in the lung and other organs (1, 10, 11, 14, 15, 21–23, 25, 26, 33, 34, 38, 39, 43–45).
The Nqo1-null (NQO1−/−) mouse has been used extensively to evaluate the contribution of metabolic and nonmetabolic functions of the enzyme, e.g., in susceptibility to carcinogenic and chemotherapeutic compounds and environmental toxins, tissue redox imbalance, and various disease processes (1, 2, 10, 21, 23, 26, 33, 43, 44). Nevertheless, there have been few studies of quinone metabolism in NQO1−/− mouse tissues and none in intact organs (24).
The goal of the present study was to use the NQO1−/− mouse model to evaluate the role of NQO1 in quinone metabolism on passage through the lung. A specific objective was to evaluate previous NQO1 inhibitor studies to determine whether NQO1 is a selective target for DQ reduction in the lung. As a basis for comparison with DQ, we also evaluated the impact of mouse lung NQO1 on the redox status of another amphipathic quinone, coenzyme Q1 (CoQ1). CoQ1 has been shown to act as an electron acceptor for NADH dehydrogenase (complex I of the mitochondrial electron transport chain) and NQO1 in the perfused rat lung (6).
MATERIALS AND METHODS
Materials.
DQ (2,3,5,6-tetramethyl-1,4-benzoquinone), CoQ1 [2,3-dimethoxy-5-methyl-6-(3-methyl-2-butenyl)-1,4-benzoquinone], N-[3-(2-furyl)acryloyl]-Phe-Gly-Gly (FAPGG), FITC-dextran, and other chemicals not otherwise specified were purchased from Sigma-Aldrich Chemical. The goat anti-rabbit polyclonal antibody to NQO1 was purchased from Abcam and the rabbit anti-goat IgG-horseradish peroxidase conjugate from Jackson ImmunoResearch Laboratories. Reagents and gels for immunoblots were purchased from Invitrogen. Primers for genotyping were obtained from MWG Oberon, and the dNTPs and Taq polymerase were from the TaKaRa Ex Taq kit (Clontech Laboratories). Purified human recombinant NQO1 was the generous gift of David Ross, PhD, University of Colorado, Denver.
Animals.
NQO1−/− and NQO1+/− breeder mice were obtained from the colony at the National Cancer Institute. Generation of the NQO1−/− mice is described elsewhere (33). Wild-type (NQO1+/+) mice were obtained from breeding NQO1+/− or NQO1−/− with NQO1+/− mice or were purchased from Jackson Laboratory (129P3/J, strain 000690).
Every animal in the study was genotyped to ensure its placement in the correct study group. Genotyping was carried out by extraction of DNA from snips using the NQO1+/+ primers NQO1-6N (5′-cagatcctggaaggatggaa-3′) and NOQ1-6R (5′-tgtcagctggaatggacttg-3′), product size 196 bp, and the neomycin cassette primers neo57 (5′-ggagaggctattcggctatgac-3′) and neo317R (5′-cgcattcatcagccatgatgg-3′), product size 315 bp, for the NQO1−/− mice. PCR was carried out using a 96-well thermal cycler (Veriti, Applied Biosystems).
Animal breeding, housing, and experimental protocols were approved by the Institutional Animal Care and Use Committee of Zablocki Veterans Affairs Medical Center.
Preparation of isolated perfused mouse lung.
The intact perfused mouse lung preparation is described elsewhere (4). Briefly, the mice were anesthetized with an intraperitoneal injection of 40 mg pentobarbital sodium/kg body wt. The trachea was clamped, the chest was opened, and heparin (0.7 IU/g body wt) was injected into the right ventricle. The pulmonary artery and trachea were cannulated with 0.86-mm-ID, 1.27-mm-OD polyethylene tubing. The heart was cut away, allowing the venous effluent to drain directly from the severed pulmonary vein. The lungs were removed from the chest and attached to a ventilation-perfusion system. The single-pass perfusion system was primed with the perfusate (4.7 mM KCl, 2.51 mM CaCl2, 1.19 mM MgSO4, 2.5 mM KH2PO4, 118 mM NaCl, 25 mM NaHCO3, 5.5 mM glucose, and 5% BSA) maintained at 37°C and equilibrated with a gas mixture of 15% O2 and 6% CO2 in N2, resulting in perfusate Po2, Pco2, and pH of 105 Torr, 40 Torr, and 7.4, respectively. Initially, perfusate was pumped (Masterflex L/S Pump 7523-90, Cole-Parmer) from a reservoir through the lungs at a flow rate of 0.3 ml/min that was gradually increased until the lungs and venous effluent were clear of blood. The flow rate was then set at 2 ml/min, and the lungs were ventilated with 8-Torr end-inspiratory and 3-Torr end-expiratory pressures at 80 breaths/min with the same gas mixture used to gas the perfusate. The pulmonary arterial pressure or perfusion pressure (Pa), referenced to atmospheric pressure at the level of the left atrium, was monitored continuously during the course of the experiments. The venous effluent pressure was atmospheric pressure.
Experimental protocols.
The lung was initially perfused from a reservoir containing control perfusate, as described above, and a venous effluent sample (∼1 ml) was collected as a blank for absorbance measurements. The reservoir was emptied and refilled with 13 ml of perfusate containing FAPGG (145 μM). Between 90 and 150 s from the start of the FAPGG perfusion, two 1-ml venous effluent samples were collected; at 150 s, a reservoir sample was also collected. The reservoir was then emptied and refilled with fresh control perfusate, and the lung and perfusion system were washed free of residual FAPGG by perfusion for 5 min. A single venous effluent sample was collected as a blank, and the reservoir was emptied and refilled with 14 ml of quinone-containing [DQ or CoQ1 (50 or 60 μM, respectively)] perfusate. Between 210 and 240 s from the start of the quinone perfusion, a single 1-ml venous effluent sample was collected; at 240 s, a reservoir sample was also collected. The lung and perfusion system were washed again with the NQO1 inhibitor dicumarol (250 μM) in the perfusate, and the quinone perfusion study was carried out as described above, except 250 μM dicumarol was in the perfusate with the quinone. This dicumarol concentration was selected because of the high degree of dicumarol binding to the perfusate BSA, which substantially lowers its free concentration (9). Then the lung and perfusion system were washed a final time with control perfusate, and the FAPGG infusion was repeated as described above. At the end of each experiment, the lungs were weighed and then dried and reweighed to obtain dry weights and wet-to-dry weight ratios.
Sample processing and concentration calculations.
For determination of venous effluent quinone (DQ or CoQ1) and hydroquinone (DQH2 or CoQ1H2) concentrations, samples were centrifuged at 4°C for 1 min at 10,000 g (AccuSpin Micro R centrifuge, Fisher Scientific), and 100 μl of each sample were added to each of two microcentrifuge tubes: one was prefilled with 10 μl of potassium ferricyanide (1.8 mM) to oxidize any hydroquinone to quinone and the other with 10 μl of 1 mM EDTA in H2O. Ice-cold absolute ethanol (0.8 ml) was added, and the samples were mixed and centrifuged at 10,000 g for 5 min at 10°C.
The concentration of total DQ + DQH2 in each venous effluent sample was calculated from the absorbance at 265 nm of the fully oxidized (with ferricyanide added) sample using the extinction coefficient for DQ (0.02164 μM−1·cm−1). The concentration of DQH2 was calculated from the difference in absorbance (at 265 nm) between the oxidized (with ferricyanide) sample and the unoxidized sample (without ferricyanide) using the extinction coefficient 0.0199 μM−1·cm−1. The DQ concentration was calculated from the difference between the DQ + DQH2 and DQH2 concentrations. CoQ1 and CoQ1H2 concentrations were calculated in a similar manner from absorbance measurements at 275 nm using extinction coefficients of 0.0143 and 0.0121 μM−1·cm−1, respectively (36). Perfusate samples that had passed through the lungs but contained no quinone were treated in the same manner as the rest of the samples and used as the blanks for absorbance measurements. DQH2 and CoQ1H2 autoxidation rates (<1.1%/min) are negligible within the experimental time frame (4, 28).
The permeability-surface area product (PS, ml/min), a measure of the rate constant of angiotensin-converting enzyme (ACE)-catalyzed FAPGG hydrolysis on passage through the lung and an index of perfused capillary surface area, was calculated from the infused arterial FAPGG concentration ([FAPGG]i) and steady-state venous effluent FAPGG concentration ([FAPGG]o) as follows: PS = −F·ln(1 − E), where E = 1 − [FAPGG]o/[FAPGG]i and F is the perfusate flow rate (9). The ACE inhibitor captopril (30 μM) blocked >95% of the FAPGG hydrolysis on passage through the lung, as evidence of the specificity of the substrate for ACE (data not shown, n = 3).
In Fig. 1, where FITC-dextran was used as an intravascular marker, concentrations were quantified from absorbance at 495 nm using the extinction coefficient 0.0935 μM−1·cm−1 (9).
Fig. 1.
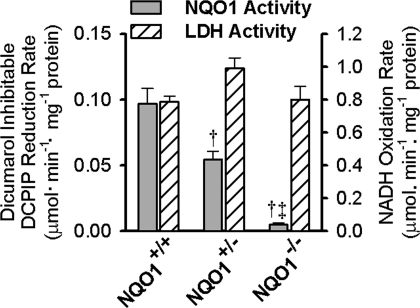
NAD(P)H:quinone oxidoreductase 1 (NQO1) activity in lung cytosol fractions. Values are means ± SE for lung cytosol fractions prepared from homogenates of 5 NQO1+/+, 4 NQO1+/−, and 3 NQO1−/− lungs. Also shown are lactate dehydrogenase (LDH) activities measured in the same lung cytosol fractions. DCPIP, dichlorophenolindophenol. ANOVA followed by Tukey's honestly significant difference test was used to determine statistical significance (P < 0.05): †significantly different from NQO1 activity in NQO1+/+ lungs; ‡significantly different from NQO1 activity in NQO1+/− lungs.
NQO1 immunoblots.
After the lung perfusion protocols, a portion of lung was removed from a subset of lungs and weighed, minced, and homogenized with a Polytron homogenizer in 10 ml of ice-cold homogenization buffer [10 mM HEPES, 250 mM sucrose, 3 mM EDTA, 1 mM PMSF, and 1% protease inhibitor cocktail (catalog no. P8340, Sigma)]. The homogenate was centrifuged for 30 min at 15,000 g. A portion of the resulting supernatant (cytosol fraction, 20 μg of protein) or purified recombinant human NQO1 (0.5 ng protein) was subjected to SDS-PAGE, as previously described (4, 9). The proteins were transferred to a nitrocellulose membrane, which was incubated for 1 h in Tris-buffered saline containing 0.1% Tween 20 and 2% BSA, the latter as a blocking agent. The membrane was then incubated sequentially in a 1:1,000 dilution of primary antibody (polyclonal goat anti-rabbit NQO1), a 1:20,000 dilution of secondary antibody (rabbit anti-goat IgG-horseradish peroxidase conjugate), and the Supersignal West Pico chemiluminescent substrate (Pierce). The signal was captured using a Kodak IS 2000 MMT ImageStation, and the relative intensities of the bands were quantified using the ImageStation software.
NQO1 and lactate dehydrogenase activities in lung homogenates.
A portion of the lung tissue from each mouse used for the lung perfusion protocol was minced and homogenized in ice-cold homogenization buffer (10 ml buffer/g lung tissue, pH 7.4) using a Polytron tissue homogenizer. The homogenate was centrifuged (12,100 g) at 4°C for 30 min, and the supernatant (cytosol fraction) was stored at −70°C. Lung cytosol fraction NQO1 activity was measured as previously described (9). Cytosol fraction protein (∼10 μg) was added to a semimicrocuvette containing 1 ml of reaction buffer consisting of Tris·HCl (25 mM, pH 7.4), BSA (0.23 mg/ml), Tween 20 (0.01% vol/vol), 2,6-dichlorophenolindophenol (DCPIP, 50 μM), and flavin adenine dinucleotide (5 μM) with or without dicumarol (20 μM). The reaction was initiated by the addition of NADPH (200 μM final concentration), and DCPIP reduction was measured spectrophotometrically at 600 nm (25°C). NQO1 activity was calculated as the difference in the initial DCPIP reduction rates in the absence and presence of dicumarol using an extinction coefficient of 0.021 μM−1·cm−1 (9). LDH activity was assayed in the supernatant fraction as NADH oxidation to NAD+ in the presence of pyruvate, measured spectrophotometrically, as previously described (12). The lung cytosol fraction protein content was measured using the Bio-Rad protein assay (9).
Statistics.
Statistical comparisons between hydroquinone efflux rates and other experimental parameters were determined by ANOVA followed by Tukey's honestly significant difference test for multiple comparisons or t-test where noted; P < 0.05 was the criterion for statistical significance. Box-Cox normalizing transforms were used if the data were nonnormal or had heterogenous variability.
RESULTS
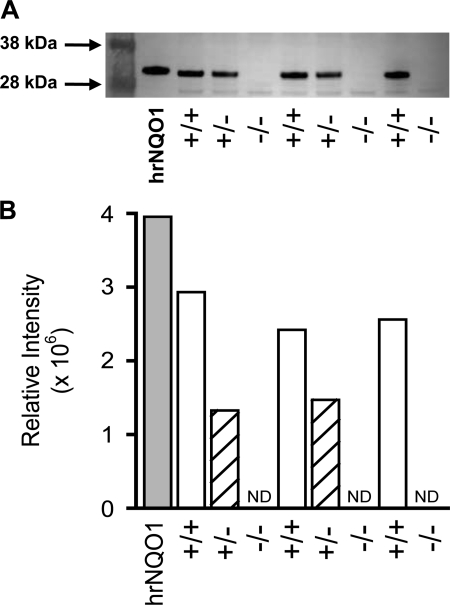
Cytosol fraction NQO1 activities for NQO1+/+, NQO1+/−, and NQO1−/− lungs reveal minimal NQO1 activity in the NQO1−/− compared with NQO1+/+ or NQO1+/− lungs, the latter of which has intermediate activity (Fig. 1). In contrast, the lung cytosol fraction LDH activities are not significantly different between the three groups of lungs, indicating that the impact of genotype on NQO1 activity is not a nonspecific effect on lung enzyme activities (Fig. 1). The immunoblot in Fig. 2 shows that the relative NQO1 protein levels in the lung cytosol fractions from the NQO1+/+, NQO1+/−, and NQO1−/− lungs were qualitatively correlated with the NQO1 activity measurements in Fig. 1.
Fig. 2.
Immunoblots for lung NQO1 protein. A: each lane on the gel was loaded with 20 μg of lung cytosol fraction protein from a different lung. B: relative intensities of corresponding bands on the blot in A. Standard was human recombinant NQO1 (hrNQO1). ND, not determined.
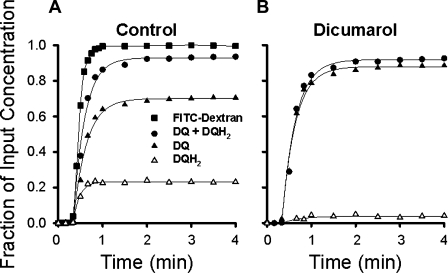
Examples of the general form of the venous effluent concentrations of DQ, DQH2, and DQ + DQH2 vs. time curves for an NQO1+/+ lung are displayed in Fig. 3A, where the concentrations are expressed as fractions of the infused pulmonary arterial DQ concentration (50 μM) at each time point studied. Also shown is the FITC-dextran concentration outflow curve, with concentration normalized to the infused FITC-dextran concentration. FITC-dextran is an intravascular marker; thus its concentration curve represents the DQ curve that would have been observed if the infused DQ had only remained in the perfusate and been convected through the pulmonary circulation without undergoing redox or metabolic reactions within the endothelium or other lung tissue components. Therefore, the FITC-dextran curve emphasizes the impact of passage through the lung on DQ.
Fig. 3.
Duroquinone (DQ) and hydroduroquinone (DQH2) concentration vs. time lung venous outflow curves following DQ infusion in an NQO1+/+ lung in the absence (A) and presence (B) of the NQO1 inhibitor dicumarol. DQ, DQH2, and DQ + DQH2 concentrations are expressed as fractions of infused pulmonary arterial DQ concentration (50 μM), and FITC-dextran concentration is normalized to infused FITC-dextran concentration.
DQH2 first appears in the venous effluent at ∼0.5 min after the start of the DQ infusion; by ∼2 min, the fractional concentrations of DQ and DQH2 reach a quasi-steady state (Fig. 3A). At this time, ∼20% of the DQ infused into the pulmonary arterial inflow appears in the venous effluent as DQH2 and ∼70% as DQ (Fig. 3A). In the presence of the competitive NQO1 inhibitor dicumarol, much less DQH2 emerges from the NQO1+/+ lung, consistent with a greater fraction of infused DQ appearing unchanged in the venous effluent (Fig. 3B). There is no effect of dicumarol on total DQ recovery, measured as DQ + DQH2; i.e., there was no difference in total venous effluent DQ recovery between the conditions used to generate Fig. 3, A and B.
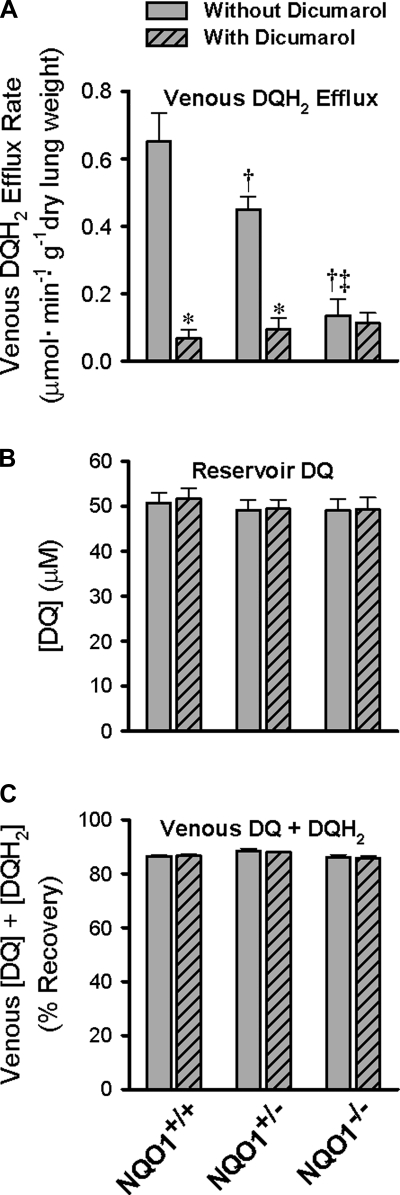
Studies of DQ (50 μM) reduction in the mouse pulmonary circulation revealed the impact of NQO1 genotype. The DQH2 efflux rate is highest in the NQO1+/+ lungs and progressively lower in NQO1+/− and NQO1−/− lungs (Fig. 4A). The dicumarol-sensitive reduction was greater in the NQO1+/+ than NQO1+/− lungs, and there was essentially no effect of dicumarol in the NQO1−/− lungs. In fact, there were no significant differences between the dicumarol-insensitive pulmonary venous DQH2 efflux rates for the three genotypes.
Fig. 4.
Impact of NQO1 genotype on DQ reduction during passage through mouse pulmonary circulation. A: steady-state pulmonary venous DQH2 efflux rate during 50 μM DQ infusion. B: DQ concentration ([DQ]) in the perfusing reservoir. C: total DQ recovery ([DQ] + [DQH2]) in pulmonary venous outflow. DQ infusions were carried out without and with dicumarol. Values are means ± SE; n = 6 NQO1+/+, 10 NQO1+/−, and 8 NQO1−/− lungs. ANOVA followed by Tukey's honestly significant difference test was used to determine statistical significance (P < 0.05): *significantly different from lung without dicumarol; †significantly different from NQO1+/+ lung without dicumarol; ‡significantly different from NQO1+/− lung without dicumarol.
The NQO1 genotype-associated differences in DQ reduction shown in Fig. 4A cannot be attributed to differences in infused DQ concentrations or total recoveries of venous effluent DQ (as DQ + DQH2) shown in Fig. 4, B and C. In addition, neither infused DQ concentration nor total DQ recovery is influenced by dicumarol (Fig. 4, B and C).
DQ recoveries in the perfusion system without a lung in place (reservoir, cannulas, and tubing only) and with a lung in place (mean ± SE, n = 3) are 92.3 ± 0.6% and 87.0 ± 0.3%, respectively (P < 0.05, t-test). The loss to the perfusion system (without a lung) is due to nonspecific interactions between DQ and the perfusion apparatus. The difference between the loss without and with a lung in the system represents DQ and/or DQH2 lost via further metabolism or sequestration (e.g., binding to macromolecular cellular components) in lung tissue. Recovery of DQ in the perfusion system is not affected by the presence of dicumarol (P > 0.05, t-test). Finally, there is no detectable difference between the extent of DQ reduction for two successive infusions of DQ (data not shown, P > 0.05, t-test, n = 3), ruling out a cumulative effect of multiple DQ infusions. These control studies provide evidence that the impact on DQ shown in Fig. 4A required passage through the lung and is not attributable to loss of DQ or DQH2 to the perfusion system or lung tissue and that the effect of dicumarol is not an artifact of the experimental protocol wherein the DQ + dicumarol infusions followed the DQ infusions in each lung studied.
Body weight of the NQO1+/+ mice is ∼10% lower than body weight of the NQO1+/− and NQO1−/− animals, probably as a reflection of age (Table 1). However, the lung dry weights and wet-to-dry weight ratios, reflections of lung size and edema, respectively, are not significantly different between the three NQO1 genotypes. The implication is that there are no gross differences in tissue composition or nonspecific injury in NQO1−/− or NQO1+/− lungs compared with NQO1+/+ lungs. PS and Pa are not significantly different between the genotypes or at the start and end of the experimental protocols (Table 2). Since PS for the ACE substrate provides a reflection of perfused surface area, the implication is that NQO1+/+, NQO1+/−, and NQO1−/− lungs are equivalent with regard to accessibility of quinone to lung tissue reductases via the pulmonary vasculature. That neither PS nor Pa changed as a consequence of the experimental protocol is evidence that the protocol itself did not cause nonspecific lung injury.
Table 1.
Ages and body weights of mice and dry weights and wet-to-dry weight ratios of lungs
| NQO1+/+ | NQO1+/− | NQO1−/− | |
|---|---|---|---|
| Age, days | 77 ± 14 | 116 ± 14 | 108 ± 12 |
| Body wt, g | 24.6 ± 1.3 | 30.7 ± 0.8* | 30.2 ± 1.0* |
| Lung dry wt, mg | 28.1 ± 0.4 | 30.3 ± 0.7 | 28.7 ± 0.9 |
| Lung wet-to-dry wt ratio | 5.85 ± 0.23 | 5.50 ± 0.19 | 5.98 ± 0.26 |
Values are means ± SE for mice and lungs in Fig. 4 study. NQO1, NAD(P)H:quinone oxidoreductase.
Significantly different from NQO1+/+.
Table 2.
Lung perfused surface areas and Pa at start and end of experimental protocols
|
PS, ml/min |
Pa, Torr |
|||
|---|---|---|---|---|
| Start | End | Start | End | |
| NQO1+/+ | 1.80 ± 0.09 | 1.75 ± 0.25 | 6.6 ± 0.5 | 6.2 ± 0.4 |
| NQO1+/− | 1.68 ± 0.08 | 1.50 ± 0.14 | 6.5 ± 0.7 | 6.9 ± 0.8 |
| NQO1−/− | 1.59 ± 0.08 | 1.60 ± 0.09 | 6.7 ± 0.7 | 6.9 ± 0.6 |
Values are means ± SE for lungs in Fig. 4 study before and after duroquinone (DQ) and DQ + dicumarol infusions. There were no significant differences in permeability-surface area product (PS) or perfusion pressure (Pa) between genotypes or at start and end of experimental protocol.
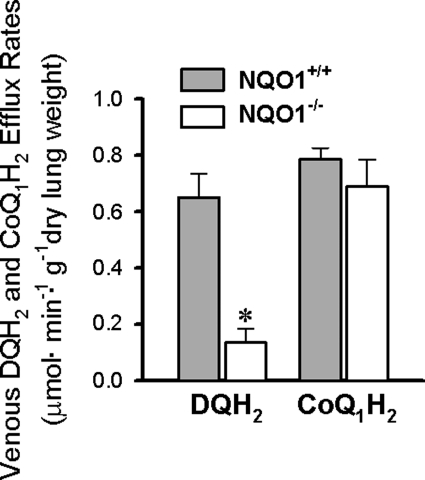
As a basis for comparison with DQ, reduction of another amphipathic quinone, CoQ1, to its hydroquinone, CoQ1H2, was evaluated in NQO1+/+ and NQO1−/− lungs. In contrast to the results obtained for DQ, there is no significant impact of NQO1 genotype on CoQ1H2 efflux rates (Fig. 5).
Fig. 5.
Impact of NQO1 genotype on reduction of DQ vs. coenzyme Q1 (CoQ1) during passage through mouse pulmonary circulation. Bars represent steady-state venous effluent efflux rates for DQH2 or CoQ1H2 during perfusion with DQ (50 μM) or CoQ1 (60 μM), respectively. Values are means ± SE. DQH2 data are from Fig. 3 (n = 6 and 8 for NQO1+/+ and NQO1−/− lungs, respectively). For CoQ1H2, n = 4 each for NQO1+/+ and NQO1−/− lungs. *Significantly different from NQO1+/+, P < 0.05 (t-test).
DISCUSSION
Earlier studies suggested that NQO1 was responsible for the dicumarol-sensitive DQ reduction observed during passage through the rat lung (4, 9). However, the possibility of off-target effects of dicumarol left the specificity of DQ for NQO1 somewhat open to question. The present study provides genetic evidence that reduction of DQ to DQH2 on passage through the mouse lung reflects NQO1 activity in lung tissue accessible via the pulmonary circulation, thereby providing independent confirmation of the utility of DQ as a nondestructive probe of intact rodent lung NQO1 activity.
Earlier studies also revealed that DQ behaves as an NQO1 probe in intact bovine pulmonary endothelial cells in culture (12, 27). In the cell culture studies, DQ was added to the cell culture medium surrounding the cells, and the appearance of DQH2 in the medium was measured. The substantial sensitivity of DQ reduction to two NQO1 inhibitors, the competitive inhibitor dicumarol and the suicide inhibitor ES-936, was used to implicate NQO1 in this redox process. Whereas pulmonary endothelial NQO1 certainly contributes to the fate of DQ on passage through the mouse lung as well, contributions of NQO1 in other lung cells cannot be ruled out, because lung tissue is freely permeable to perfusate DQ and DQH2, even during a single pass through the rodent lung (4). Such behavior is consistent with the amphipathic nature of DQ, which has a reasonably high water solubility and octanol-water partition coefficient (16, 32).
Although we previously studied DQ reduction in the CD-1 mouse as well as the Sprague-Dawley rat lung, the redox processes contributing to DQ fate in the mouse lung had not been as well characterized (4). Thus this is the first report of NQO1-catalyzed DQ reduction on passage through the NQO1+/+ mouse lung. The DQH2 pulmonary venous efflux rate for the NQO1+/+ (129P3/J) lungs (0.65 ± 0.1 μmol·min−1·g dry lung−1) was reasonably close to that for the CD-1 mouse lung at the same DQ inflow concentration (50 μM) (4). The observation that a small fraction of the DQH2 efflux rate in the NQO1+/+ mouse lung was dicumarol-insensitive was also consistent with previous reports of a dicumarol-insensitive “NQO1-like” activity in wild-type mouse lung homogenates (33).
Since NQO1 is predominantly (>90%) cytosolic, our general hypothesis has been that DQ enters the lung tissue from the perfusate in the pulmonary circulation or the endothelial cells in culture from the culture medium and is reduced to DQH2 predominantly via intracellular NQO1, whereupon the DQH2 diffuses back into the perfusate or culture medium (4). However, the rate at which DQH2 emerges in the lung venous effluent is actually the net effect of a combination of redox processes (4, 9). In the rat lung and pulmonary arterial endothelial cells, DQH2 generated by intracellular NQO1 is subject to reoxidation via complex III of the mitochondrial electron transport chain (4, 30). The extent to which DQH2 emerges from the lung will depend on the balance between complex III and NQO1 activities, and we have no reason to suspect differences in lung complex III activity between the three NQO1 genotypes. The key point in this regard is that the venous DQH2 efflux rate, as measured in the present study, provides only a lower threshold on the NQO1-catalyzed DQ reduction rate on passage through the lung.
The differences in DQ reduction in NQO1+/+, NQO1+/−, and NQO1−/− lungs were not attributable to nonspecific effects. Specifically, lung dry weights, wet-to-dry weight ratios, Pa, perfused surface area (i.e., PS), and recoveries of total venous effluent DQ were not detectably different between the genotypes. Since lung dry weights and wet-to-dry weight ratios provide information regarding lung size and nonspecific lung injury in the form of lung edema, respectively, the implication was that neither lung size nor such injury could account for the differences in DQ reduction. The perfused surface area, reflected as PS, was also the same for all three groups of lungs; since we previously demonstrated that DQ and DQH2 are freely permeable from the perfusate into rat and mouse lung tissue, the overall implication was that DQ accessibility to lung tissue NQO1 from the pulmonary blood vessels was the same for all lungs in the study (4). Finally, the total recovery of pulmonary venous effluent DQ was equivalent for all genotypes, indicating that whatever the mechanism(s) by which ∼10% of the DQ and/or DQH2 is lost from the perfusate via, e.g., tissue sequestration, further metabolism, or binding to the tubing in the perfusion system, it cannot account for the differences in DQ reduction in the lung.
NQO1 did not make a dominant contribution to CoQ1 (60 μM) reduction on passage through the mouse lung, as reflected by the observation that CoQ1H2 efflux rates were not significantly different for NQO1+/+ and NQO1−/− lungs. This is in contrast to the rat lung, in which CoQ1 reduction is attributable to both NQO1 and complex I on the basis of inhibitor studies with dicumarol and rotenone (6). It is conceivable that the mouse lung is, instead, analogous to the bovine pulmonary artery endothelial cells, in which CoQ1 appears to act as a selective probe for complex I activity (28). However, it should be noted that the present studies were carried out at a single concentration of DQ or CoQ1 (50 and 60 μM, respectively), selected as advantageous for simultaneous measurement of quinone and hydroquinone (4). Quinone selectivity for any given reductase or other redox process is subject to quinone concentration, its tendency to permeate cell membranes, the propensity for any given quinone to act as an electron donor for a given reductase, and the affinities and kinetics of competing redox processes. In the present study, 60 μM CoQ1 is apparently insufficient to reflect any significant contribution of NQO1 to reduction in the mouse lung. Whether NQO1 contributes to CoQ1 reduction at higher quinone concentrations remains to be seen. For example, it is conceivable that higher concentrations could saturate competing higher-affinity CoQ1 reductases, presumably including complex I, thereby revealing NQO1-catalyzed reduction.
CoQ1 was selected for comparison with DQ, because both were reduced on passage through the rat lung, wherein NQO1 was implicated as the dominant reductase for DQ and a contributing reductase for CoQ1. Their reactivity with NQO1 in the rat lung was not that surprising, since DQ and CoQ1 have nearly the same water and lipid solubility properties and can act as common electron acceptors for NQO1 as the isolated enzyme or within cells (11, 14, 16, 20). For example, NQO1 contributions to CoQ1 reduction have been observed in rat hepatocytes and astrocytes, wherein the protective effect of CoQ1 in hepatocyte complex I dysfunction was attributed to NQO1-mediated CoQ1 reduction followed by CoQ1H2 oxidation at complex III (14). The propensity for any given concentration of CoQ1 to act as an NQO1 electron acceptor in intact rat, but not mouse, lung or intact bovine pulmonary arterial endothelial cells may be attributable to species differences in electron acceptor substrate specificity or activity (18). The preference for DQ over CoQ1 as an NQO1 electron acceptor is suggested by structure-activity studies showing that quinones with van der Waals volumes of <200 Å (162.9 Å for DQ and 243.96 Å for CoQ1) have been observed to act as relatively “fast” NQO1 substrates (high Kcat/Km) (3, 28, 41). In any case, the differential impact of intact mouse and rat lung NQO1 on CoQ1 illustrates the concept that quinone fate in the lung depends on the properties of the substances themselves (e.g., propensity to act as an electron acceptor for any given reductase), as well as the properties of the pulmonary endothelium and lung tissue (e.g., relative activities and complement of available redox enzymes). Accordingly, for a range of redox compounds we have studied, including DQ, CoQ1, coenzyme Q0, and the thiazine polymer (toluidine blue O-polyacrylamide polymer), their different physical and chemical properties influence the subcellular reduction sites, the electron donor utilized, and the dominant reductase(s) involved (4–8, 12, 29, 31).
The present study demonstrates that DQ acts as a probe of lung tissue NQO1 activity on passage through the pulmonary circulation. The need for such a probe is exemplified by the complexity and diversity of roles for NQO1 and polymorphisms resulting in inactive forms of the enzyme in pharmacology, toxicology, and pathophysiology (1, 17, 22, 42). More detailed studies of lung DQ redox metabolism are likely to provide information regarding the therapeutic potential for lung NQO1-targeted activation of anticancer drugs and antioxidants, as well as a better understanding of lung toxicity of various redox-active xenobiotics. The lung may be of particular importance with regard to NQO1 activity because of the high level of NQO1 expression in the endothelium and the very large perfused pulmonary vascular surface area and its position in the circulation. The implication is that NQO1-catalyzed redox metabolism within the pulmonary circulation has the potential to influence quinone bioactivity not only within the lung itself, but also in the systemic circulation and downstream organs and vessels.
GRANTS
This work was supported by National Institutes of Health Grant R01 HL-5065537 and Childrens Environmental Health Core Center Grant ES-004184-23, the National Cancer Institute Intramural Research Program, and the Department of Veterans Affairs (VA Medical Research Funds).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
ACKNOWLEDGMENTS
The authors thank R. Babygirija and T. Takahashi (Dept. of Surgery, Medical College of Wisconsin and Zablocki Veterans Affairs Medical Center) for their generous help in carrying out the genotyping and Philip C. Merker for helpful discussions.
REFERENCES
- 1. Adikesavan AK, Barrios R, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 in metabolic activation of mitomycin C and bone marrow cytotoxicity. Cancer Res 67: 7966–7971, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ahn K, Sethi G, Jain A, Jaiswal A, Aggarwal B. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-κB, IκBα kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem 281: 19798–19808, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Anusevicius Z, Sarlauskas J, Cenas N. Two-electron reduction of quinones by rat liver NAD(P)H:quinone oxidoreductase: quantitative structure-activity relationships. Arch Biochem Biophys 404: 254–262, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Audi SH, Bongard RD, Dawson CA, Siegel D, Roerig DL, Merker MP. Duroquinone reduction during passage through the pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 285: L1116–L1131, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Audi SH, Bongard RD, Okamoto Y, Merker MP, Roerig DL, Dawson CA. Pulmonary reduction of an intravascular redox polymer. Am J Physiol Lung Cell Mol Physiol 280: L1290–L1299, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Audi SH, Merker MP, Krenz GS, Ahuja T, Roerig DL, Bongard RD. Coenzyme Q1 redox metabolism during passage through the rat pulmonary circulation and the effect of hyperoxia. J Appl Physiol 105: 1114–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Audi SH, Olson LE, Bongard RD, Roerig DL, Schulte ML, Dawson CA. Toluidine blue O and methylene blue as endothelial redox probes in the intact lung. Am J Physiol Heart Circ Physiol 278: H137–H150, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Audi SH, Zhao H, Bongard RD, Hogg N, Kettenhofen NJ, Kalyanaraman B, Dawson CA, Merker MP. Pulmonary arterial endothelial cells affect the redox status of coenzyme Q0. Free Radic Biol Med 34: 892–907, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Audi SH, Bongard RD, Krenz GS, Rickaby DA, Haworth ST, Eisenhauer J, Roerig DL, Merker MP. Effect of chronic hyperoxic exposure on duroquinone reduction in adult rat lungs. Am J Physiol Lung Cell Mol Physiol 289: L788–L797, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Bauer AK, Faiola B, Abernethy DJ, Marchan R, Pluta LJ, Wong VA, Roberts K, Jaiswal AK, Gonzalez FJ, Butterworth BE, Borghoff S, Parkinson H, Everitt J, Recio L. Genetic susceptibility to benzene-induced toxicity: role of NADPH:quinone oxidoreductase-1. Cancer Res 63: 929–935, 2003 [PubMed] [Google Scholar]
- 11. Beyer RE, Segura-Aguilar J, di Bernardo S, Cavazzoni M, Fato R, Fiorentini D, Galli MC, Setti M, Landi L, Lenaz G. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc Natl Acad Sci USA 93: 2528–2532, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bongard RD, Lindemer BJ, Krenz GS, Merker MP. Preferential utilization of NADPH as the endogenous electron donor for NAD(P)H:quinone oxidoreductase 1 (NQO1) in intact pulmonary arterial endothelial cells. Free Radic Biol Med 46: 25–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cadenas E, Hochstein P, Ernster L. Pro- and antioxidant functions of quinones and quinone reductases in mammalian cells. Adv Enzymol Relat Areas Mol Biol 65: 97–146, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Chan TS, Teng S, Wilson JX, Galati G, Khan S, O'Brien PJ. Coenzyme Q cytoprotective mechanisms for mitochondrial complex I cytopathies involves NAD(P)H:quinone oxidoreductase 1(NQO1). Free Radic Res 36: 421–427, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Choi EK, Terai K, Ji IM, Kook YH, Park KH, Oh ET, Griffin RJ, Lim BU, Kim JS, Lee DS, Boothman DA, Loren M, Song CW, Park HJ. Upregulation of NAD(P)H:quinone oxidoreductase by radiation potentiates the effect of bioreductive β-lapachone on cancer cells. Neoplasia 9: 634–642, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Virgilio F, Azzone GF. Activation of site I redox-driven H+ pump by exogenous quinones in intact mitochondria. J Biol Chem 257: 4106–4113, 1982 [PubMed] [Google Scholar]
- 17. Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501: 116–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faig M, Bianchet MA, Talalay P, Chen S, Winski S, Ross D, Amzel LM. Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release. Proc Natl Acad Sci USA 97: 3177–3182, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fato R, Bergamini C, Leoni S, Lenaz G. Mitochondrial production of reactive oxygen species: role of complex I and quinone analogues. Biofactors 32: 31–39, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Fato R, Estornell E, di Bernardo S, Pallotti F, Parenti CG, Lenaz G. Steady-state kinetics of the reduction of coenzyme Q analogs by complex I (NADH:ubiquinone oxidoreductase) in bovine heart mitochondria and submitochondrial particles. Biochemistry 35: 2705–2716, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Gaikwad A, Long DJ, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem 276: 22559–22564, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Ginsberg G, Guyton K, Johns D, Schimek J, Angle K, Sonawane B. Genetic polymorphism in metabolism and host defense enzymes: implications for human health risk assessment. Crit Rev Toxicol 40: 575–619, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Iskander K, Gaikwad A, Paquet M, Long DJ, Brayton C, Barrios R, Jaiswal AK. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res 65: 2054–2058, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Joseph P, Jaiswal AK. A unique cytosolic activity related but distinct from NQO1 catalyses metabolic activation of mitomycin C. Br J Cancer 82: 1305–1311, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis AM, Ough M, Hinkhouse MM, Tsao MS, Oberley LW, Cullen JJ. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol Carcinog 43: 215–224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long DJ, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gonzalez FJ, Jaiswal AK. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res 62: 3030–3036, 2002 [PubMed] [Google Scholar]
- 27. Merker MP, Audi SH, Bongard RD, Lindemer BJ, Krenz GS. Influence of pulmonary arterial endothelial cells on quinone redox status: effect of hyperoxia-induced increase in NAD(P)H quinone oxidoreductase 1 (NQO1). Am J Physiol Lung Cell Mol Physiol 289: L788–L797, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Merker MP, Audi SH, Lindemer BJ, Krenz GS, Bongard RD. Role of mitochondrial electron transport complex I in coenzyme Q1 reduction by intact pulmonary arterial endothelial cells and the effect of hyperoxia. Am J Physiol Lung Cell Mol Physiol 293: L809–L819, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Merker MP, Bongard RD, Kettenhofen NJ, Okamoto Y, Dawson CA. Intracellular redox status affects transplasma membrane electron transport in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 282: L36–L43, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Merker MP, Bongard RD, Krenz GS, Zhao H, Fernandes V, Kalyanaraman B, Hogg N, Audi SH. Impact of pulmonary arterial endothelial cells on duroquinone redox status. Free Radic Biol Med 37: 86–103, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Merker MP, Bongard RD, Linehan JH, Okamoto Y, Vyprachticky D, Brantmeier BM, Roerig DL, Dawson CA. Pulmonary endothelial thiazine uptake: separation of cell surface reduction from intracellular reoxidation. Am J Physiol Lung Cell Mol Physiol 272: L673–L680, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Powis G, Appel PL. Relationship of the single-electron reduction potential of quinones to their reduction by flavoproteins. Biochem Pharmacol 29: 2567–2572, 1980 [DOI] [PubMed] [Google Scholar]
- 33. Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, Gonzalez FJ, Jaiswal AK. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem 273: 7382–7389, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Rooseboom M, Commandeur JNM, Vermeulen NPE. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev 56: 53–102, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Ruzicka FJ, Crane FL. Quinone interaction with the respiratory chain-linked NADH dehydrogenase of beef heart mitochondria. II. Duroquinone reductase activity. Biochim Biophys Acta 226: 221–233, 1971 [DOI] [PubMed] [Google Scholar]
- 36. Schatz G, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VII. Oxidative phosphorylation in the diphosphopyridine nucleotide-cytochrome b segment of the respiratory chain: assay and properties in submitochondrial particles. J Biol Chem 241: 1429–1438, 1966 [PubMed] [Google Scholar]
- 37. Schmalix WA, Lang D, Schneider A, Bocker R, Greim H, Doehmer J. Stable expression and coexpression of human cytochrome P450 oxidoreductase and cytochrome P450 1A2 in V79 Chinese hamster cells: sensitivity to quinones and biotransformation of 7-alkoxyresorufins and triazines. Drug Metab Dispos 24: 1314–1319, 1996 [PubMed] [Google Scholar]
- 38. Shen J, Barrios RJ, Jaiswal AK. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res 70: 1006–1014, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D. The reduction of α-tocopherolquinone by human NAD(P)H:quinone oxidoreductase: the role of α-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol 52: 300–305, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med 29: 246–253, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Siraki AG, Chan TS, O'Brien PJ. Application of quantitative structure-toxicity relationships for the comparison of the cytotoxicity of 14 p-benzoquinone congeners in primary cultured rat hepatocytes versus PC12 cells. Toxicol Sci 81: 148–159, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Volpato M, Phillips RM. Tailoring targeted therapy to individual patients: lessons to be learnt from the development of mitomycin C. Cancer Genomics Proteomics 4: 175–186, 2007 [PubMed] [Google Scholar]
- 43. Voynow JA, Fischer BM, Zheng S, Potts EN, Grover AR, Jaiswal AK, Ghio AJ, Foster WM. NAD(P)H quinone oxidoreductase 1 is essential for ozone-induced oxidative stress in mice and humans. Am J Respir Cell Mol Biol 41: 107–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 44. Zhou GD, Randerath K, Donnelly KC, Jaiswal AK. Effects of NQO1 deficiency on levels of cyclopurines and other oxidative DNA lesions in liver and kidney of young mice. Int J Cancer 112: 877–883, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Zhou H, Dehn D, Kepa JK, Siegel D, Scott DE, Tan W, Ross D. NAD(P)H:quinone oxidoreductase 1-compromised human bone marrow endothelial cells exhibit decreased adhesion molecule expression and CD34+ hematopoietic cell adhesion. J Pharmacol Exp Ther 334: 260–268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]