Abstract
Perinatal exposure to maternal smoke is associated with adverse pulmonary effects, including reduced lung function and increased incidence of asthma. However, the mechanisms underlying these effects are unknown, and there is no effective preventive and/or therapeutic intervention. Recently, we suggested that downregulation of homeostatic mesenchymal peroxisome proliferator-activated receptor-γ (PPARγ) signaling following in utero nicotine exposure might contribute to chronic lung diseases such as asthma. We used an in vivo rat model to determine the effect of perinatal nicotine exposure on 1) offspring pulmonary function, 2) mesenchymal markers of airway contractility in trachea and lung tissue, and 3) whether administration of a PPARγ agonist, rosiglitazone (RGZ), blocks the molecular and functional effects of perinatal nicotine exposure on offspring lung. Pregnant Sprague-Dawley rat dams received placebo, nicotine, or nicotine + RGZ daily from embryonic day 6 until postnatal day 21, when respiratory system resistance, compliance, tracheal contractility, and the expression of markers of pulmonary contractility were determined. A significant increase in resistance and a decrease in compliance under basal conditions, with more pronounced changes following methacholine challenge, were observed with perinatal nicotine exposure compared with control. Tracheal constriction response and expression of mesenchymal markers of airway contractility were also significantly increased following perinatal nicotine exposure. Concomitant treatment with RGZ completely blocked the nicotine-induced alterations in pulmonary function, as well as the markers of airway contractility, at proximal and distal airway levels. These data suggest that perinatal smoke exposure-induced asthma can be effectively blocked by PPARγ agonists.
Keywords: pregnancy, peroxisome proliferator-activated receptor-γ, smoke
there is strong epidemiological and experimental evidence that perinatal exposure to maternal smoking results in detrimental long-term effects on lung growth and function, including significant suppression of alveolarization and increased predisposition to asthma in the offspring (2, 4–6, 9, 13–15, 17, 27, 30, 42, 44, 45, 49). Since the mechanisms underlying these adverse pulmonary effects remain incompletely understood, there are no effective preventive or therapeutic interventions. Recently, on the basis of in vitro and in vivo studies from our laboratory, we suggested that downregulation of homeostatic lung mesenchymal peroxisome proliferator-activated receptor-γ (PPARγ) signaling might be a key contributor to chronic lung diseases such as bronchopulmonary dysplasia and asthma (23, 34, 36, 37, 48). We previously showed that in vitro and in vivo nicotine exposures result in the downregulation of pulmonary mesenchymal PPARγ and the upregulation of myogenic signaling pathways, triggering the transdifferentiation of lipid-rich alveolar interstitial fibroblasts to myofibroblasts (23, 36, 37). Furthermore, by upregulating PPARγ signaling in vitro, nicotine-induced transdifferentiation of alveolar interstitial fibroblasts to myofibroblasts can be blocked. However, the relevance of these findings to perinatal nicotine exposure-induced asthma in offspring and whether it can be prevented by PPARγ agonists are not known. Here, using a rat model, we show that perinatal nicotine exposure alters proximal and distal airway molecular and functional phenotypes, consistent with smoke-induced asthma, and that these changes can be effectively blocked by the concomitant systemic administration of a PPARγ agonist, rosiglitazone (RGZ).
MATERIAL AND METHODS
Animals.
Time-mated first-time-pregnant, pair-fed Sprague-Dawley rat dams (200–250 g body wt) received placebo (diluent, n = 16), nicotine (1 mg/kg sc, n = 16), or nicotine (1 mg/kg sc) + RGZ (3 mg/kg ip, n = 16) in 100-μl volumes daily from embryonic day 6 to postnatal day 21. After spontaneous delivery at full term, the pups were allowed to breast feed ad libitum. At postnatal day 21, the pups were killed, and the lungs and tracheas were collected for determination of relevant protein levels and tracheal tension studies. Experimental animals were maintained in a 12:12-h light-dark cycle, pair-fed in accordance with the previous day's consumption by the nicotine alone-treated group, and allowed free access to water. Animals were killed at postnatal day 21, and their tracheas and lungs were collected for tracheal tension studies, Western blot analysis, and immunofluorescence staining. In a subset of animals at postnatal days 18–21, pulmonary function was evaluated (see below). All animal procedures were performed following National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committees at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and the University of California Davis.
Pulmonary function testing.
Pulmonary function was measured using a plethysmograph for restrained animals (Buxco, Troy, NY), with the rat in the supine position. Dynamic compliance and resistance of the respiratory system were simultaneously measured on the anesthetized, tracheotomized, and ventilated rats at baseline and immediately following serial 3-min nebulizations of saline and methacholine (MCh, 0.5 and 2.0 mg/ml), with 3-min recovery periods allowed after each exposure to MCh, as previously described (22, 54). The pups were deeply anesthetized and sedated with medetomidine (0.5 mg/kg; Domitor, Orion Pharma) and tiletamine-zolazepam (50 mg/kg; Telazol, Fort Dodge Laboratories, Overland Park, KS) and then ventilated at 7–8 ml/kg with a small animal ventilator (MiniVent, Harvard Apparatus, Cambridge, MA) for the duration of the procedure.
Tracheal tension studies.
The whole trachea was excised immediately after death and dissected free of connective tissue in ice-cold modified Krebs-Ringer bicarbonate buffer (in mM: 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 11.1 glucose). Subsequently, a ∼6-mm tracheal ring was resected from the middle of the trachea and used for tracheal tension studies. The tracheal ring was suspended in an organ chamber filled with 10 ml of modified Krebs-Ringer bicarbonate solution maintained at 37 ± 0.5°C and aerated with 95% O2-5% CO2 (pH 7.4). Each ring was suspended via two stirrups that were passed through the lumen: one stirrup was anchored to the bottom of the organ chamber, and the other stirrup was connected to a strain gauge (model FT03C, Grass Instrument, Quincy, MA) for the measurement of isometric force, as described previously (12).
At the beginning of the experiment, each tracheal ring was stretched to its optimal resting tension, which was achieved by step-wise stretching in 0.1-g increments, until the contractile response to 100 mM KCl reached a plateau. The optimal resting tension was measured, and then each tracheal ring was allowed to equilibrate for 1 h after it was brought to its optimal resting tension. The effects of ACh were determined ≥30 min after the administration of nitro-l-arginine (1 × 10−4 M, an inhibitor of nitric oxide synthase). In all experiments, indomethacin (1 × 10−5 M) was added to the bath to prevent the possible interference by prostanoids.
Lung morphometry.
Lung morphometry was determined from radial alveolar counts and septal thickness, as described previously (7, 23).
Immunoblot analysis.
The isolated tracheas and lungs were flash-frozen in liquid nitrogen and then homogenized and sonicated in four volumes of ice-cold lysis buffer containing 50 mM β-glycerophosphate (pH 7.4), 150 mM NaCl, 1.5 mM EGTA, 1 mM EDTA, 1% Triton X-100, 100 mM NaF, 2 mM Na3VO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 μg/ml pepstatin A. After centrifugation at 13,200 g for 15 min at 4°C, the supernatant was used for Western blot analysis for fibronectin, α-smooth muscle actin (α-SMA), calponin, collagen I, collagen III, and cholinergic ACh receptor-α3 and -α7 (AChRα3 and AChRα7). The protein concentration of the supernatant was measured by the Bradford method, with bovine serum albumin as the standard. Aliquots of the supernatant, each containing 30 μg of protein, were separated by SDS-PAGE and electrically transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with Tris-buffered saline (TBS) containing 5% nonfat dry powdered milk (wt/vol) for 1 h at room temperature. After a brief rinse with TBS containing 0.1% Tween 20 (TBST), the protein blots were incubated in 1:250 diluted anti-fibronectin monoclonal antibody (catalog no. 610078, BD Biosciences), 1:10,000 diluted anti-α-SMA monoclonal antibody (catalog no. A2547, Sigma), 1:6,000 diluted anti-calponin monoclonal antibody (catalog no. C-2687, Sigma), 1:500 diluted anti-collagen I polyclonal antibody (catalog no. RDI-MCOII1abr, Fitzgerald Industries), 1:1,000 diluted anti-collagen III monoclonal antibody (catalog no. C7805, Sigma), 1:400 diluted anti-nicotinic AChRα3 (catalog no. sc-5590, Santa Cruz Biotechnology), 1:20,000 diluted anti-nicotinic AChRα7 (catalog no. N8158, Sigma), and 1:4,000 diluted anti-GAPDH monoclonal antibody (catalog no. MAB374, Millipore) overnight at 4°C. After they were washed three times with TBST, the blots were incubated in 1:1,000 (fibronectin), 1:10,000 (α-SMA), 1:6,000 (calponin), 1:2,500 (collagen I), 1:3,000 (AChRα3), 1:20,000 (AChRα7), and 1:4,000 (GAPDH) diluted horseradish peroxidase-conjugated anti-mouse or anti-rabbit or anti-rat secondary antibody for 1 h at room temperature. After three more washes in TBST, the blots were exposed to X-ray film using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL) and developed. The relative densities of the protein bands were determined with UN-SCAN-IT software (Silk Scientific, Orem, UT) and normalized to the density of GAPDH.
Immunofluorescence staining.
Rat lung was inflated in situ with 4% paraformaldehyde in phosphate buffer at a standard inflation pressure of 5 cmH2O and fixed as described previously (37). Fibronectin, α-SMA, calponin, and collagen III protein expression were assessed by immunofluorescence staining. Briefly, 5-μm sections were incubated with mouse monoclonal antibodies against fibronectin (1:500 dilution; catalog no. 610078, BD Biosciences), α-SMA (1:1,000 dilution; catalog no. A2547, Sigma), calponin (1:250 dilution; catalog no. C2687, Sigma), and collagen III (1:250 dilution; catalog no. C7805, Sigma) at 4°C overnight; then Alexa Fluor 594 (1:500 dilution for fibronectin and 1:250 dilution for collagen III) or Alexa Fluor 488 (1:250 dilution for α-SMA and 1:250 dilution for calponin) goat anti-mouse IgG (Invitrogen) was applied to the sections for 1 h at room temperature. The sections were washed with phosphate-buffered saline and then mounted with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) for visualization under a fluorescence microscope.
Statistics.
Values are means ± SE. Comparisons between the different groups were performed using Student's t-test or a mixed-model repeated-measures ANOVA with unstructured covariance. P < 0.05 was considered statistically significant.
RESULTS
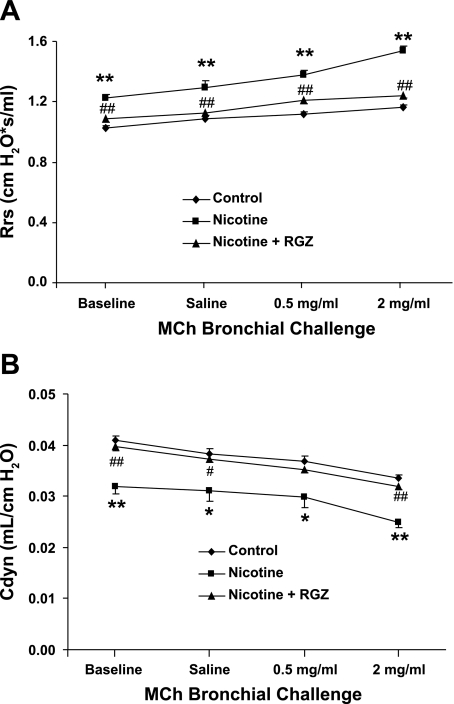
Initially, we examined the effect of the PPARγ agonist RGZ on nicotine-induced alterations in the resistance of the respiratory system at baseline and following MCh challenge. Nicotine administration significantly increased total resistance, at baseline and following MCh challenge, compared with the control group; concomitant RGZ administration blocked total resistance (P < 0.05), such that pulmonary resistance at baseline and following MCh challenge was similar in the control and nicotine + RGZ groups (Fig. 1A). Accompanying the increase in total resistance, there was a significant decrease in total compliance at baseline and following MCh challenge in the nicotine group that was also blocked by RGZ administration (Fig. 1B).
Fig. 1.
Effect of rosiglitazone (RGZ) on perinatal nicotine exposure-induced alterations in respiratory system resistance (Rrs, A) and compliance (Cdyn, B) at baseline and after methacholine (MCh) challenge. Nicotine administration significantly increased total resistance and decreased total compliance of the respiratory system, at baseline and following MCh challenge, compared with control; respiratory system resistance and compliance were blocked by concomitant RGZ administration. Values are means ± SE; n = 6 for each group. *P < 0.05, **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. nicotine.
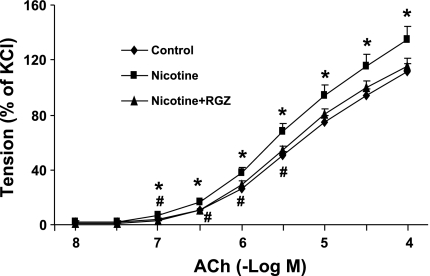
We then determined tracheal morphometry and the tracheal constriction response to an ACh challenge in the various experimental groups. There were no differences in the tracheal ring outside diameter [2.13 ± 0.06 (n = 16), 2.27 ± 0.10 (n = 16), and 2.12 ± 0.10 mm (n = 13)], length [6.34 ± 0.10 (n = 16), 6.54 ± 0.08 (n = 16), and 6.29 ± 0.13 mm (n = 13)], and optimal resting tension [0.54 ± 0.02 (n = 16), 0.53 ± 0.03 (n = 16), and 0.57 ± 0.03 g (n = 13)] for the control, nicotine, and nicotine + RGZ groups, respectively (P > 0.05). The pattern of tracheal constriction to ACh doses was compared among the three groups using a mixed-model repeated-measures ANOVA with unstructured covariance that accounts for the increasing variability in constriction among animals within groups as the dose increases. The overall dose-response pattern differed among the three groups (P = 0.01), with the nicotine group values significantly (P < 0.05) greater than the control group values at all doses except −log 8 and 7.5 and significantly (P < 0.05) greater than nicotine + RGZ group values at −log doses 7, 6.5, 6, and 5.5. The control group values did not differ (P > 0.05) from the nicotine + RGZ group values at any dose. These results demonstrate that the tracheal constriction response to ACh was significantly increased in the nicotine group and was almost completely blocked in the nicotine + RGZ group (Fig. 2).
Fig. 2.
Effect of RGZ on perinatal nicotine exposure-induced alterations in tracheal constriction response to ACh. Nicotine administration significantly increased the tracheal constriction response to ACh compared with control; this response was almost completely blocked by RGZ administration. Values are means ± SE; n = 16 for each group. *P < 0.05 vs. control. #P < 0.05 vs. nicotine.
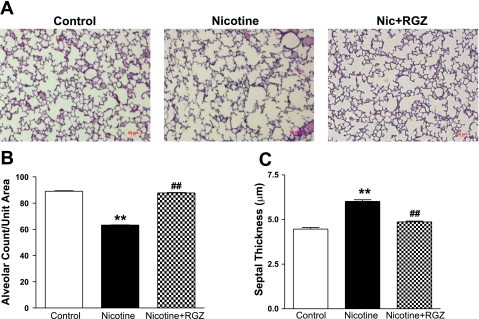
Consistent with previous observations, the radial alveolar count was decreased and the septal thickness increased in the nicotine group, and both of these changes were blocked in the nicotine + RGZ group (Fig. 3): alveolar count was 89.1 ± 0.4, 63.2 ± 0.2, and 87.7 ± 0.3 (SE) alveoli per measured grid (grid size = 0.099 mm2) in control, nicotine, and nicotine + RGZ groups, respectively (Fig. 3B), and septal thickness was 4.46 ± 0.086, 6.01 ± 0.091, and 4.86 ± 0.051 (SE) μm in control, nicotine, and nicotine + RGZ groups, respectively (Fig. 3C).
Fig. 3.
Effect of RGZ on perinatal nicotine (Nic) exposure-induced alterations in radial alveolar count and septal thickness. A: representative hematoxylin-eosin-stained lung sections. Magnification ×100. Nicotine administration decreased radial alveolar count (B) and increased septal thickness (C) compared with control; both changes were blocked in the RGZ-treated group. Values are means ± SE; n = 4 for each group. **P < 0.01 vs. control. ##P < 0.01 vs. nicotine.
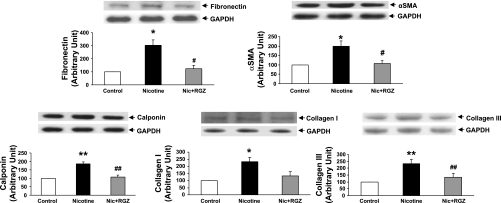
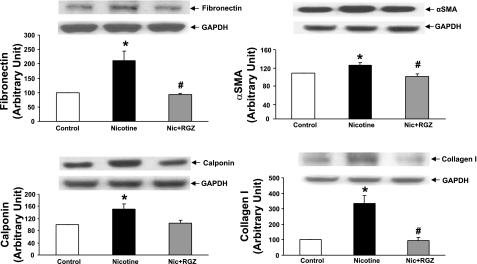
To determine the effect of perinatal nicotine exposure on mesenchymal markers of airway contractility, we next examined the protein levels of fibronectin, α-SMA, calponin, and collagens I and III in the lung and trachea. The levels of all these proteins increased significantly in the lung and trachea (Figs. 4 and 5). In accordance with the pulmonary function and tracheal constriction data, all these changes were largely blocked by concomitant RGZ administration. This finding was corroborated by immunofluorescence staining for the mesenchymal markers of airway reactivity in paraformaldehyde-fixed, paraffin-embedded lung sections (Fig. 6). Staining for fibronectin (Fig. 6A), α-SMA (Fig. 6B), calponin (Fig. 6C), and collagen III (Fig. 6D) was increased significantly in the nicotine group compared with the control group and was blocked in the nicotine + RGZ group.
Fig. 4.
Effect of RGZ on perinatal nicotine exposure-induced alterations in mesenchymal markers of airway reactivity in lung. Nicotine administration significantly increased protein levels of fibronectin, α-smooth muscle actin (α-SMA), calponin, and collagens I and III compared with control; these proteins were blocked in the RGZ-treated group. Representative Western blots for markers and GAPDH are shown above densitometric values of markers normalized to GAPDH. Values are means ± SE; n = 4 for each group. *P < 0.05, **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. nicotine.
Fig. 5.
Effect of RGZ on perinatal nicotine exposure-induced alterations in mesenchymal markers of airway reactivity in trachea. Nicotine administration significantly increased protein levels of fibronectin, α-SMA, calponin, and collagen I compared with control; these proteins were blocked in the RGZ-treated group. Representative Western blots for markers and GAPDH are shown above densitometric values of markers normalized to GAPDH. Values are means ± SE; n = 4 for each group. *P < 0.05 vs. control. #P < 0.05 vs. nicotine.
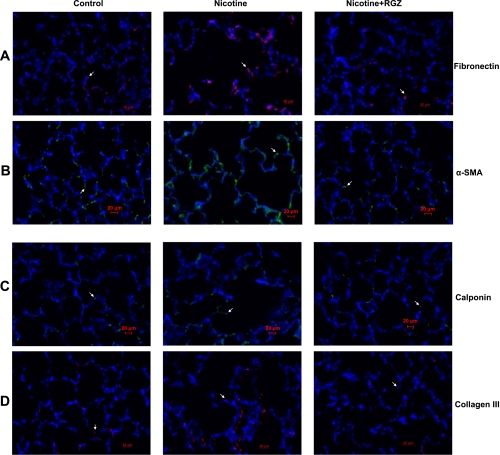
Fig. 6.
Effect of RGZ on perinatal nicotine exposure-induced alterations in mesenchymal markers of airway reactivity in paraformaldehyde-fixed paraffin-embedded lung sections. Nicotine administration significantly increased staining for fibronectin (red, A), α-SMA (green, B), calponin (green, C), and collagen III (red, D) compared with control; these markers were blocked in the RGZ-treated group. Magnification ×400.
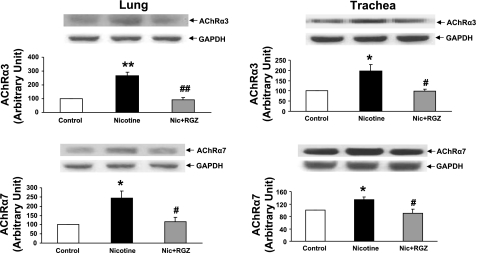
Since we previously determined that, at least under in vitro conditions, the nicotine-induced increase in the alveolar myogenic phenotype is mediated by the nicotinic AChRα3 and -α7 (24, 36), we next determined the levels of these receptors in the lung and trachea. The levels of these receptors increased in the lungs and tracheas of the nicotine-treated animals (Fig. 7). Once again, consistent with the other data in this study, upregulation of the nicotinic AChRα3 and -α7 was blocked by RGZ.
Fig. 7.
Effect of RGZ on perinatal nicotine exposure-induced alterations in nicotinic ACh receptors-α3 and -α7 (AChRα3 and AChRα7) in lung and trachea. Nicotine administration significantly increased levels of both receptors in lung and trachea compared with control; both receptors were blocked in the RGZ-treated group. Representative Western blots for receptors and GAPDH are shown above density histograms. Values are means ± SE; n = 4 for each group. *P < 0.05, **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. nicotine.
DISCUSSION
It is well established that perinatal exposure to tobacco smoke profoundly affects offspring lung development, predisposing them to later asthma and increased hospital visits and admissions due to respiratory problems (6, 9, 14, 15, 17, 18, 21, 30, 45). Consistent with this knowledge, we found evidence of a significant increase in total pulmonary resistance and a decrease in total compliance under basal conditions with perinatal nicotine exposure compared with the control group. With MCh challenge, compliance decreased further significantly, and resistance increased significantly, all changes consistent with airway hyperresponsiveness following perinatal nicotine exposure. Concomitant treatment with RGZ blocked nicotine-induced alterations in pulmonary compliance and resistance under basal conditions and following MCh challenge. Furthermore, perinatal nicotine exposure significantly increased expression of mesenchymal markers of airway reactivity in the trachea and lung parenchyma; expression of these markers was also blocked by the concomitant administration of RGZ. These data lead us to suggest that PPARγ agonists are a promising intervention to prevent tobacco smoke-induced asthma in offspring.
Since prenatal exposure to tobacco smoke has been shown to be more important than postnatal exposure in causing airway hyperresponsiveness in offspring (47), by exposing animals to nicotine throughout lung development, we maximized the likelihood of detecting any differences between the treatment groups. Our results are similar to those of Joad et al. (19), who demonstrated marked increases in airway sensitivity in rats exposed to sidestream smoke during the in utero and postnatal periods, in contrast to animals that had been exposed to sidestream smoke only during the prenatal period or only during the postnatal period. However, in these studies, the molecular markers of airway responsiveness and specific tracheal contractility responses were not determined. Furthermore, no intervention was examined. The present data and our previous studies expand on the findings of Joad et al. and suggest that the underlying molecular pathways involved in linking perinatal nicotine exposure to childhood asthma include enhanced expression of the myogenic pathways, i.e., activation of Wnt signaling and downregulation of PPARγ signaling (23, 34–37, 40, 48). It is important to note that although the mesenchymal proteins examined in this study are not specific for distal airway contractility and may be present in other tissue compartments, such as the vascular tissue, these proteins are hallmarks of a myofibroblast (α-SMA and calponin) and/or have been demonstrated to show increased deposition (fibronectin and collagen III) in the subepithelial space of the airways in all forms of asthma (3, 16, 50). Therefore, we are confident that the increased abundance of these markers in the homogenized lung tissue, along with the evidence of increased airway reactivity on pulmonary function testing, provides a true reflection of nicotine's effect on enhancement of the myogenic phenotype in the affected offspring.
Although some of the effects of maternal smoking on the developing lung have been suggested to be stress-induced, the direct effects of maternal smoke on prenatal lung growth are only due to those components of maternal smoke that are transferred across the placenta. There are many agents in smoke that may be detrimental to the developing lung, but there is plenty of evidence to support direct alteration of fetal lung development by nicotine. Nicotine crosses the human placenta with minimal biotransformation (25), and it accumulates in fetal blood, maternal milk, and amniotic fluid, despite increased nicotine clearance during pregnancy, resulting in exposure of the fetus to even higher levels than the smoking mother (8, 26, 46). Nicotine accumulates in several fetal tissues, including the respiratory tract, suggesting that nicotine is the likely agent that alters lung development in the fetus of the pregnant smoker (46). This is also supported by our in vitro and in vivo studies and studies of others that show direct effects of nicotine on pulmonary epithelial cells, fibroblasts, and the whole lung (23, 36–38, 43, 53). Therefore, although we did not expose animals to cigarette smoke as such, it is likely that our findings, at least to some extent, replicate the circumstances following perinatal tobacco smoke exposure. The dose of nicotine used in this study is approximately equivalent to that used by a moderately heavy human smoker, i.e., ∼1 mg/kg body wt (28, 29). Furthermore, our data probably more directly reflect the consequences of using a nicotine patch, a commonly used nicotine replacement therapy in pregnant smokers (11).
Although a large body of data has demonstrated an association between perinatal smoke exposure and asthma in offspring, the mechanism(s) underlying this association is not fully understood. After in utero tobacco smoke exposure, increases in pulmonary neuroendocrine cells and neuroepithelial bodies (20), lung inflammation (51), allergic sensitization (52), and development of a pulmonary myogenic phenotype (34) have been suggested as possible contributors. The results outlined above reinforce the concept that perinatal nicotine exposure produces a myogenic pulmonary phenotype, as evidenced by the increased levels of myogenic contractility proteins, such as α-SMA, calponin, fibronectin, and collagens I and III, at the proximal and distal airway levels, accompanied by increased contractility responses at both levels.
Our data suggest that increased predisposition to asthma following perinatal smoke exposure is likely due to specific local structural and functional alterations in the developing lung. This is consistent with the findings of Sekhon et al. (41), who reported that maternal nicotine exposure during pregnancy increased collagen deposition around large airways and vessels in rhesus monkey offspring. Consistent with our data, greater inner airway wall thickness in infants who died of sudden infant death syndrome and who were born to mothers who smoked during pregnancy also suggests airway remodeling following in utero smoke exposure (10). The findings of Barrett et al. (1), who report that exposure to sidestream smoke in a transgenic mouse model genetically predisposed to asthma was not associated with the development of allergic sensitization, further reinforce alternative mechanisms, such as pulmonary structural changes, as the possible mechanism for asthma in in utero smoke-exposed offspring. In contrast, some studies have reported higher IgE levels in the children of smoking parents (39). Nevertheless, many studies have reported no differences in IgE levels in children of smoking and nonsmoking parents (31, 32). However, the weight of the evidence seems to suggest that the myogenic pulmonary phenotype induced following perinatal smoke exposure predisposes perinatal smoke-exposed infants to other potential triggers, such as environmental allergens and viral infections, resulting in the asthma phenotype.
It should also be noted that although our study showed effective blockage of pulmonary structural, molecular, and functional changes following in utero nicotine exposure with RGZ, this therapy cannot be recommended for use by pregnant smokers, since there are no safety and efficacy data for such use in human infants. In fact, we have used RGZ only as a proof-of-principle to determine the efficacy of the PPARγ agonist class of drugs to block in utero nicotine-induced damage. Since there are significant cardiovascular risks in adult diabetic paients given RGZ over prolonged periods (33), we strongly recommend detailed safety, pharmacokinetic, and pharmacodynamic studies of this class of drugs before recommending their clinical use in pregnant smokers.
GRANTS
This work was supported by National Institutes of Health Grants HL-75405, HD-51857, HD-058948, HL-76415, and HL-55268 and Tobacco-Related Disease Research Program Grants 14RT-0073, 15IT-0250, and 17RT-0170.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
REFERENCES
- 1. Barrett EG, Wilder JA, March TH, Espindola T, Bice DE. Cigarette smoke-induced airway hyperresponsiveness is not dependent on elevated immunoglobulin and eosinophilic inflammation in a mouse model of allergic airway disease. Am J Respir Crit Care Med 165: 1410–1418, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bassi JA, Rosso P, Moessinger AC, Blanc WA, James LS. Fetal lung growth retardation due to maternal tobacco smoke exposure in the rat. Pediatr Res 18: 127–130, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc 5: 89–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blacquière MJ, Timens W, Melgert BN, Geerlings M, Postma DS, Hylkema MN. Maternal smoking during pregnancy induces airway remodeling in mice offspring. Eur Respir J 33: 1133–1140, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Collins MH, Moessinger AC, Kleinerman J. Fetal lung hypoplasia associated with maternal smoking: a morphometric analysis. Pediatr Res 19: 408–412, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Cunningham J, Dockery DW, Speizer FE. Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol 139: 1139–1152, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-β and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 296: L1031–L1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther 301: 594–598, 2002 [DOI] [PubMed] [Google Scholar]
- 9. DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 113: 1007–1015, 2004 [PubMed] [Google Scholar]
- 10. Elliot J, Vullermin P, Robinson P. Maternal cigarette smoking is associated with increased airway wall thickness in children who die from sudden infant death syndrome. Am J Respir Crit Care Med 158: 802–806, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Forinash AB, Pitlick JM, Clark K, Alstat V. Nicotine replacement therapy effect on pregnancy outcomes. Ann Pharmacother 44: 1817–1821, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Raj JU. Effect of oxygen on cyclic GMP-dependent protein kinase-mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol Lung Cell Mol Physiol 285: L611–L618, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, Avol E, Peters JM. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax 55: 271–276, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haberg SE, Stigum H, Nystad W, Nafstad P. Effects of pre- and postnatal exposure to parental smoking on early childhood respiratory health. Am J Epidemiol 166: 679–686, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Hanrahan JP, Tager IB, Segal MR. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis 145: 1129–1135, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Hocking DC. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest 122: 275S–278S, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Hofhuis W, de Jongste JC, Merkus PJ. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child 88: 1086–1090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hylkema MN, Blacquière MJ. Intrauterine effects of maternal smoking on sensitization, asthma, and chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 660–662, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Joad JP, Bric BM, Peake JL, Pinkerton KE. Perinatal exposure to aged and diluted sidestream cigarette smoke produces airway hyperresponsiveness in older rats. Toxicol Appl Pharmacol 155: 253–260, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Joad JP, Ji C, Kott KS, Bric JM, Pinkerton KE. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol Appl Pharmacol 132: 63–71, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Karaman O, Uguz A, Uzuner N. Risk factors in wheezing infants. Pediatr Int 41: 147–150, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Kenyon NJ, Liu R, O'Roark EM, Huang W, Peng L, Lam KS. An α4β1-integrin antagonist decreases airway inflammation in ovalbumin-exposed mice. Eur J Pharmacol 603: 138–146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krebs M, Sakurai R, Torday JS, Rehan VK. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res 36: 390–398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu B, Yang J, Wen Q, Li Y. Isoliquiritigenin, a flavonoid from licorice, relaxes guinea-pig tracheal smooth muscle in vitro and in vivo: role of cGMP/PKG pathway. Eur J Pharmacol 587: 257–266, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Luck W, Nau H. Nicotine and cotinine concentrations in serum and milk of nursing smokers. Br J Clin Pharmacol 18: 9–15, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther 8: 384–395, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Maritz GS, Dennis H. Maternal nicotine exposure during gestation and lactation interferes with alveolar development in the neonatal lung. Reprod Fertil Dev 10: 255–261, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Maritz GS, Woolward KM. Effect of maternal nicotine exposure on neonatal lung elastic tissue and possible consequences. S Afr Med J 81: 517–519, 1992 [PubMed] [Google Scholar]
- 29. Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190: 269–319, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med 173: 1255–1263, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Oryszczyn MP, Godin J, Annesi I, Hellier G, Kauffmann F. In utero exposure to parental smoking, cotinine measurements, and cord blood IgE. J Allergy Clin Immunol 87: 1169–1174, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Ownby DR, Johnson CC, Peterson EL. Maternal smoking does not influence cord serum IgE or IgD concentrations. J Allergy Clin Immunol 88: 555–560, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Pan GJD. Recommendations for Regulatory Action for Rosiglitazone and Rosiglitazone Containing Products. http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM226240.pdf [12/21/2010]
- 34. Rehan VK, Asotra K, Torday JS. The effects of smoking on the developing lung: insights from a biologic model for lung development, homeostasis, and repair. Lung 187: 281–289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rehan VK, Sakurai R, Wang Y, Santos J, Huynh K, Torday JS. Reversal of nicotine-induced alveolar lipofibroblast-to-myofibroblast transdifferentiation by stimulants of parathyroid hormone-related protein signaling. Lung 185: 151–159, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Rehan VK, Wang Y, Sugano S, Romero S, Chen X, Santos J, Khazanchi A, Torday JS. Mechanism of nicotine-induced pulmonary fibroblast transdifferentiation. Am J Physiol Lung Cell Mol Physiol 289: L667–L676, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Rehan VK, Wang Y, Sugano S, Santos J, Patel S, Sakurai R, Boros LG, Lee WP, Torday JS. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol 292: L323–L333, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Roman J, Ritzenthaler JD, Gil Costa A, Rivera HN, Roser-Page S. Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J 18: 1436–1438, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Ronchetti R, Macri F, Ciofetta G, Indinnimeo L, Cutrera R, Bonci E, Antognoni G, Martinez FD. Increased serum IgE and increased prevalence of eosinophilia in 9-year-old children of smoking parents. J Allergy Clin Immunol 86: 400–407, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Sakurai R, Cerny L, Torday JS, Rehan VK. Mechanism for nicotine-induced up-regulation of Wnt signaling in human alveolar interstitial fibroblasts. Exp Lung Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine exposure increases pulmonary α7-nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 103: 637–647, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med 164: 989–994, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung: association with α7-nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 26: 31–41, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet 348: 1060–1064, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Strachan DP, Cook DG. Health effects of passive smoking. 1. Parental smoking and childhood asthma: longitudinal and case control studies. Thorax 53: 204–212, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szuts T, Olsson S, Lindquist NG, Ullberg S, Pilotti A, Enzell C. Long-term fate of [14C]nicotine in the mouse: retention in the bronchi, melanin-containing tissues and urinary bladder wall. Toxicology 10: 207–220, 1978 [DOI] [PubMed] [Google Scholar]
- 47. Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med 152: 977–983, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Torday JS, Rehan VK. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr Res 62: 2–7, 2007 [DOI] [PubMed] [Google Scholar]
- 49. von Mutius E. Environmental factors influencing the development and progression of pediatric asthma. J Allergy Clin Immunol 109: 5525–5532, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Wicks J, Haitchi HM, Holgate ST, Davies DE, Powell RM. Enhanced upregulation of smooth muscle related transcripts by TGFβ2 in asthmatic (myo) fibroblasts. Thorax 61: 313–319, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu ZX, Lee LY. Airway hyperresponsiveness induced by chronic exposure to cigarette smoke in guinea pigs: role of tachykinins. J Appl Physiol 87: 1621–1628, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Wu ZX, Zhou D, Chen G, Lee LY. Airway hyperresponsiveness to cigarette smoke in ovalbumin-sensitized guinea pigs. Am J Respir Crit Care Med 161: 73–80, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Wuenschell CW, Zhao J, Tefft JD, Warburton D. Nicotine stimulates branching and expression of SP-A and SP-C mRNA in embryonic mouse lung culture. Am J Physiol Lung Cell Mol Physiol 278: L165–L170, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med 180: 731–740, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]