Abstract
Suppressor of cytokine signaling-3 (SOCS3) is thought to be involved in the development of central leptin resistance and obesity by inhibiting STAT3 pathway. Because phosphatidylinositol 3-kinase (PI3K) pathway plays an important role in transducing leptin action in the hypothalamus, we examined whether SOCS3 exerted an inhibition on this pathway. We first determined whether leptin sensitivity in the hypothalamic PI3K pathway was increased in brain-specific Socs3-deficient (NesKO) mice. In NesKO mice, hypothalamic insulin receptor substrate-1 (IRS1)-associated PI3K activity was significantly increased at 30 min and remained elevated up to 2 h after leptin intraperitoneal injection, but in wild-type (WT) littermates, the significant increase was only at 30 min. Hypothalamic p-STAT3 levels were increased up to 5 h in NesKO as opposed to 2 h in WT mice. In food-restricted WT mice with reduced body weight, leptin increased hypothalamic PI3K activity only at 30 min, and p-STAT3 levels at 30–120 min postinjection. These results suggest increased leptin sensitivity in both PI3K and STAT3 pathways in the hypothalamus of NesKO mice, which was not due to a lean phenotype. In the next experiment with a clonal hypothalamic neuronal cell line expressing proopiomelanocortin, we observed that whereas leptin significantly increased IRS1-associated PI3K activity and p-JAK2 levels in cells transfected with control vector, it failed to do so in SOCS3-overexpressed cells. Altogether, these results imply a SOCS3 inhibition of the PI3K pathway of leptin signaling in the hypothalamus, which may be one of the mechanisms behind the development of central leptin resistance and obesity.
Keywords: phosphorylated signal transducer and activator of transcription-3, proopiomelanocortin, leptin resistance
leptin plays an obligatory role in regulation of food intake and body weight by acting primarily at the level of the hypothalamus (16, 36, 37, 41), particularly through the activation of the long form (ObRb) of the leptin receptor, and subsequent activation of the JAK2-STAT3 pathway (14, 30, 43). The JAK2-STAT3 pathway of leptin signaling is also under the negative feedback control of suppressor of cytokine signaling-3 (SOCS3), a negative regulator of cytokine signaling (21). An initial study by Bjorbaek et al. (5) suggested SOCS3 as a potential mediator of central leptin resistance. Subsequent studies in Chinese hamster ovary (CHO) cells suggested SOCS3 as a negative regulator of proximal leptin signaling (4). Further in vitro studies have shown that SOCS3 inhibits leptin-induced signal transduction by binding to the phosphorylated leptin receptor and suppressing JAK2 activity (6, 15, 45).
In line with its role as a negative regulator of leptin signaling in the hypothalamus, peripheral injection of leptin induces SOCS3 mRNA expression in hypothalamic regions expressing ObRb (5). Specifically, leptin activates SOCS3 expression in neuropeptide Y (NPY) and proopiomelanocortin (POMC) neurons (2, 13). Moreover, neuron-specific deletion of Socs3 (27) or haploinsufficiency of Socs3 (18) increases leptin sensitivity through hypothalamic STAT3 activation and confers resistance to diet-induced obesity (DIO). In addition, in DIO mice an increased SOCS3 expression is associated with impaired leptin-induced STAT3 activation in the arcuate nucleus (29). Overall, in vivo and in vitro studies suggest a primary role of SOCS3 in the inhibition of ObRb signaling, particularly through the STAT3 pathway.
It is now established that, besides the JAK2-STAT3 pathway, leptin engages several other pathways in the hypothalamus that have been shown to regulate feeding, including the phosphatidylinositol 3-kinase (PI3K) (32, 47), mammalian target of rapamycin (11), AMPK (25), and phosphodiesterase-3B-cAMP (47) pathways. Moreover, many of these pathways are defective during the development of DIO (10, 23, 24), and the underlying mechanisms are incompletely understood. Importantly, it is still unknown whether SOCS3 inhibits these non-STAT3 pathways of leptin signaling in the hypothalamus. Thus, the present study examined whether SOCS3 inhibited the PI3K pathway, one of the leptin-induced non-STAT3 pathways in the hypothalamus.
Herein, we report that brain-specific deletion of Socs3 results in enhanced leptin sensitivity in the PI3K pathway of leptin signaling in the hypothalamus and that SOCS3 overexpression in a clonal, hypothalamic neuronal cell line expressing POMC reverses the effects of leptin on the PI3K activity. These findings demonstrate that SOCS3 inhibits the PI3K pathway of leptin signaling in the hypothalamus.
MATERIALS AND METHODS
Animals.
Breeding pairs of Socs3flox/flox (Socs3fl/fl) and Socs3fl/fl mice carrying nestin-Cre (Socs3fl/fl-Cre), as described previously (27), were obtained from Dr. Yoshimura. Socs3fl/fl-Cre (NesKO) mice were bred with Socs3fl/fl mice to generate knockout NesKO and Socs3fl/fl mice. Socs3fl/fl mice were used as wild-type (WT) control as described previously (27). These mice were generated as 129/BL6 background and littermates were used in the first study. Mice were housed as 4 males per cage in a light (lights on 0500–1900) and temperature-controlled (22°C) room with food (pelleted rodent chow) and water ad libitum. In the second study, the WT (Socs3fl/fl) mice backcrossed to C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME, USA) for three generations were used. The Institutional Animal Care and Use Committee of the University of Pittsburgh approved all animal experiments.
Experiment 1: effects of brain-specific Socs3 deficiency on leptin sensitivity in the PI3K pathway of signaling in the hypothalamus.
Socs3fl/fl (wild-type) and Socs3fl/fl-Cre. (NesKO) mice were generated as described earlier. Body weight was recorded in some animals for about 5 mo. Daily food intake was measured for 5 days in 6-mo-old mice that were individually caged; the data were expressed as cumulative 5-day food intake per animal. In some mice (5–6 mo old) that were fasted overnight, blood was collected from the retro-orbital sinus under isoflurane anesthesia, and plasma was collected and stored at −20°C. Plasma leptin levels were measured using a RIA kit (Linco Research, St. Louis, MO).
To examine the effects of leptin on PI3K activity, we injected leptin (3.5 mg/kg body wt ip; recombinant murine leptin obtained from Dr. A. F. Parlow, National Hormone and Peptide Program, Torrance, CA) to overnight fasted mice. Animals were killed at different time intervals between 0 and 300 min after injection. The medial basal hypothalamus (MBH) was dissected out and frozen in liquid nitrogen, and kept at −80°C until processing for protein extraction with PI3K extraction buffer (50 mM HEPES, 150 mM NaCl, 20 mM Na-pyrophosphate, 20 mm B-glycerophosphate, 10 mM NaF, 2 mM NaVO, 2 mM EDTA, 1% 1GEPAL, 10% glycerol, 2 mM phenylmethylsulfonyl fluoride, 1 mM MgCl2, 1 mM CaCl2, 10 μg/ml leupeptin, 10 μg/ml aprotinin). The MBH tissue was bounded rostrally by the posterior border of the optic chiasma, laterally by the lateral sulcus, and caudally by the mammillary bodies, and cut to a depth of ∼1.5 mm. We also examined p-STAT3 levels in the same hypothalamic extracts. Epididymal fat (E-fat), retroperitoneal-fat (RP-fat), and brown adipose tissue (BAT) were dissected out and weighed.
Experiment 2: effects of food restriction on leptin sensitivity in the PI3K pathway of signaling in the hypothalamus.
Seventeen- to twenty-two week-old WT (Socs3fl/fl) mice were individually caged, and body weight was recorded daily throughout the experiment. Food intake was measured daily for 5 days, after which, one half of the mice were put on food restriction (FR) regimen with a 10% reduction in the 1st wk and 15% reduction in the 2nd wk. Thirteen days later, all animals were fasted overnight and injected with leptin (3.5 mg/kg body wt ip). Animals were killed at different time intervals between 0 and 300 min after injection. The MBH was dissected out and processed for IRS1-associated PI3K measurement, as described in experiment 1. Fat-pads (E-Fat, RP-Fat, BAT) were dissected out and weighed.
Cell culture and treatments.
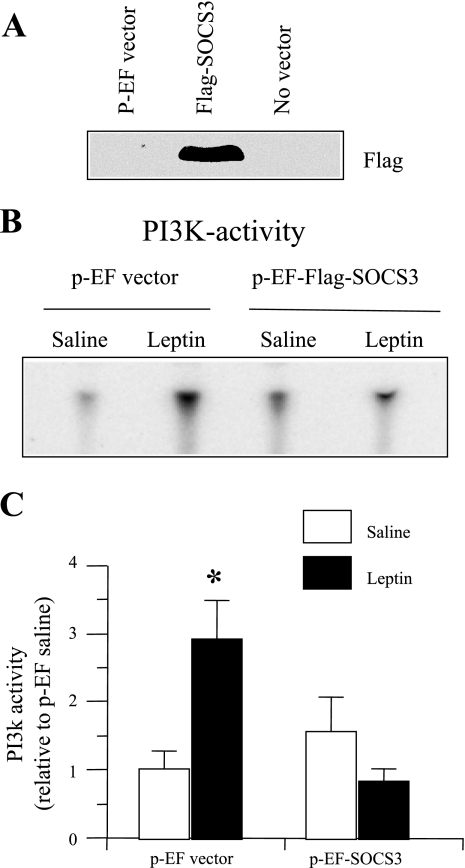
The mHypoE-43/5 cell line expressing POMC has been described previously (9). mHypoE-43/5 cells were grown in DMEM (Gibco, Langley, OK) supplemented with 10% FBS (Invitrogen, Carlsbad, CA), 20 mM glucose (Sigma, St. Louis, MO), and 1% penicillin/streptomycin (Gibco); they were then maintained at 37°C with 5% CO2. For SOCS3 overexpression, we used pEF-Flag1-mSOCS3 expression vector (kindly provided by Drs. D. J. Hilton and Tracy Wilson, Walter and Eliza Hall Institute of Medical Research, Australia). mHypoE-43/5 cells were transfected with pEF-Flag1-mSOCS3 expression vector using Lipofectamine 2000 method, according to the manufacturer's protocol (Invitrogen). For transfection, cells were grown in media without antibiotic. In a preliminary study, cells grown overnight to 80–90% confluency were transfected with 0.25 μg GFP plasmid (pMAX GFP, Amaxa, Cologne, Germany) + 1.5 μg pEF-Flag1-mSOCS3 DNA/35-mm plate. After 4–5 h of transfection, the media were replaced with fresh media without antibiotic for 24 h, and the cells were examined under fluorescence microscope to visualize GFP expression. We observed GFP expression in ∼80% cells, suggesting a high transfection efficiency. We then examined whether transfection with 3 μg of pEF-Flag1-mSOCS3 DNA resulted in SOCS3 overexpression in mHypoE-43/5 cells. In this study, we also had two additional groups, e.g., cells transfected with pEF vector or without any vector. After 24 h of transfection, the cells were lysed in a high-salt buffer (42), and 50 μg of protein was subjected to 15% PAGE followed by Western blot analysis with anti-Flag-M2 antibody (cat. 1804; Sigma). We observed an increased level of SOCS3 protein in the cells transfected with pEF-Flag1-mSOCS3 DNA, as indicated by an increased Flag protein expression (Fig. 5A).
Fig. 5.
Changes in leptin-induced IRS-1-associated PI3K activity in mHypoE-43/5 POMC neuronal cell line. A: Western blot for Flag protein, indicator of SOCS3 overexpression, in mHypoE-43/5 POMC neuronal cells transfected with p-EF vector, p-EF-Flag1-mSOCS3, or no vector. Note that the cells transfected with p-EF vector (control) or without vector did not express any flag protein. B: representative phosphor-images obtained from the TLC plate showing PI(3)P production, an indicator of PI3K activity, are shown. C: results obtained by phosphorimaging showing the changes in PI3K activity. The values are expressed as relative to the p-EF saline group. Values are expressed as means ± SE of three independent incubations. *P < 0.01 vs. saline control.
Experiment 3: effect of overexpression of SOCS3 on leptin-induced IRS1-associated PI3K activity in mHypoE-43/5 hypothalamic neuronal cell line expressing POMC.
Prior to examining the effects of overexpression of SOCS3 on leptin-induced IRS1-associated PI3K activity in mHypoE-43/5 cells [a clonal hypothalamic neuronal cell line expressing POMC (9)], we have observed in a preliminary study that leptin (A. F. Parlow, National Hormone and Peptide Program, Torrance, CA) consistently increased PI3K activity in this cell line at a dose of 0.5 to 4 ng/ml (data not shown). Therefore, we then examined whether SOCS3 overexpression inhibited leptin (0.5 ng/ml)-induced PI3K activity. Cells were transfected with pEF-Flag1-mSOCS3 vector or empty vector (p-EF) at a dose of 3 μg DNA/60 mm plate, as described previously. Eight hours later, the media were discarded and replaced with a fresh media, and the cells were grown for 24 h. Thereafter, the cells were serum deprived for 16 h and then treated with leptin (0.5 ng/ml) or vehicle (saline). Thirty minutes later, the cells were harvested, and protein was extracted with an extraction buffer to measure PI3K activity by enzymatic assay, as described previously (31, 38). We did not examine the effect of SOCS3 overexpression on p-STAT3 levels in this study because leptin at a dose of 0.5–4 ng/ml failed to reliably increase p-STAT3 in mHypoE-43/5 neuronal cells (data not shown). However, SOCS3 overexpression has been shown to prevent leptin-induced p-STAT3 activation in another cell line (12).
Measurement of IRS1-associated PI3K activity.
PI3K activity in the hypothalamus and in the cell line was measured according to a standard protocol, as described elsewhere (31), and which we have used in recent studies (24, 38). Briefly, 150 μg of protein (MBH or cell extract) was immunoprecipitated with 0.9 μg of IRS-1 antibody (Sc-559, polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4°C followed by precipitation of the immunocomplexes with 50 μl of protein A-Sepharose (Amersham Biosciences, Piscataway, NJ) for 1.5 h at 4°C. The immune complex was then incubated at 22°C with phosphatidylinositol (10 μg) (Avanti Polar Lipids, Alabaster, AL) in the presence of 50 μM [γ-32P]ATP (5 μCi) (PerkinElmer Life and Analytical Sciences, Waltham. MA). The reaction was stopped by adding 20 μl 8 N HCl and 160 μl CHCl3: methanol (1:1) followed by centrifugation for 3 min at 14,000 rpm in a Microfuge (Marathon micro A; Fisher Scientific, Pittsburgh, PA). The lower organic phase with 32P-containing PI(3)P was separated on a silica gel TLC plate. 32P-containing PI(3)P was quantitated with phosphorimaging (Bio-Rad Laboratories, Hercules, CA).
Measurement of p-JAK2 levels in SOCS3 overexpressing mHypoE-43/5 cells by Western blot analysis.
mHypoE-43/5 cells were transfected with pEF-Flag1-mSOCS3 or pEF, as described above, and were treated with leptin (0.5 ng/ml) or saline control for 15 min. Cells were then harvested with a 1× lysis buffer (Cell Signaling Technology, Danvers, MA) supplemented with 1 mM PMSF and phosphatase inhibitor cocktail 2 (Sigma-Aldrich Canada, Oakville, ON, Canada). The protein (25 μg) was resolved on an 8% SDS-PAGE and blotted overnight onto Immobilon-P PVDF membrane (Millipore, Billerica, MA). The blots were blocked with 5% BSA in Tris-buffered saline with 0.1% Tween (TBS-T) for 1 h, then incubated overnight at 4°C with p-JAK2 (p-JAK2: monoclonal, #3776; Cell Signaling Technologies, Beverly, MA) primary antibody. Blots were then washed 3 times in TBS-T and then incubated with secondary horseradish peroxidase-labeled goat anti-rabbit at 1:5,000 dilutions for 1–2 h at room temperature. Blots were then washed 3 times in TBS-T and visualized with enhanced chemiluminescence (ECL kit; GE Healthcare, Buckinghamshire, UK). The blots were stripped (Re-Blot; Millipore, Billerica, MA) and then blotted with JAK2 (JAK2: monoclonal, #3230; Cell Signaling Technologies). p-JAK2 levels were normalized to total JAK2 protein and then expressed as relative to saline control.
Measurement of p-STAT3 and SOCS3 levels in the hypothalamus by Western blot analysis.
To measure p-STAT3 levels, aliquots (50 μg protein) of MBH protein extracts used in the PI3K assay were subjected to SDS-PAGE (7% gel), followed by transfer of the resolved polypeptides to polyscreen polyvinylidene difluoride (PVDF) membranes. The membranes were then blotted with p-STAT3 antibody (p-STAT3: B7, monoclonal, Sc-8059; Santa Cruz Biotechnology, Santa Cruz, CA) followed by enhanced chemoluminescence as described by the manufacturer (New England Nuclear life Science Products Life Sciences, Boston, MA). The membranes were stripped and then blotted with STAT3 (K-15, polyclonal, Sc-483; Santa Cruz Biotechnology). p-STAT3 levels were first calculated as a ratio of STAT3 protein and then expressed as relative to saline control.
To compare whether there was any change in SOCS3 protein levels between ad libitum and FR mice, aliquots (50 μg protein) of MBH extracts from the 0 min group were subjected to SDS-PAGE (12% gel), followed by the transfer of the resolved polypeptide to PVDF membranes. The membranes were then blotted with SOCS3 antibody (M-20, polyclonal, Sc-7009; Santa Cruz Biotechnology) followed by enhanced chemoluminescence. The membranes were stripped and then blotted with a monoclonal anti-β-actin antibody (Sigma). SOCS3 levels were first expressed as the ratio of β-actin protein and then expressed as relative to ad libitum group.
Statistical analysis.
All values are expressed as means ± SE. PI3K activity and p-JAK2 levels in the cell line are presented as a ratio to p-EF-vector-vehicle control, and the data were analyzed by one-way ANOVA followed by the multiple-range test. PI3K and p-STAT3 activity in the hypothalamus was presented as relative to 0 min value of the Socs3fl/fl (wild-type) mice; and the data were analyzed by randomized two-way ANOVA with Fisher's least significant difference (LSD) multiple-range tests. The body weight data were analyzed using repeated-measures two-way ANOVA followed by Fisher's LSD multiple-range tests. All statistical analyses were done using GB-Stat software for the Macintosh (Dynamic Microsystems, Silver Spring, MD). Comparisons with P < 0.05 were considered to be significant.
RESULTS
Changes in food intake, body weight, fat pad weight, and plasma leptin levels in brain-specific Socs3-deficient mice.
To examine whether neuronal SOCS3 deficiency results in a lean phenotype, we examined food intake, body weight, and fat pad weight in Socs3flox/flox (Socs3fl/fl, wild-type) and Socs3fl/fl-Cre (NesKO) mice. Cumulative 5-day food intake (at 6 mo) was essentially similar in wild-type and NesKO mice (wild-type: 17.55 ± 0.70 g, NesKO: 16.73 ± 1.13 g, mean ± SE, n = 9 per group). The Socs3-deficient mice maintained their body weight at a slightly, but significantly (P = 0.023) lower level during the 21-wk period of observation (Fig. 1A). To investigate whether the decreased body weight seen in NesKO mice is a consequence of decreased fat mass, we next examined the weight of white adipose tissue (WAT; E-fat, and RP-fat) and BAT in NesKO and wild-type mice. As expected with the decreased body weight, NesKO mice exhibited significantly decreased WAT weight (E-fat, P = 0.0275; RP-fat, P = 0.0187) compared with wild-type mice; but BAT weight remained unchanged between the groups (Fig. 1B). The decreased body weight in NesKO mice was accompanied by significantly reduced plasma leptin levels [wild-type (n = 5): 14.10 ± 3.14 g; NesKO (n = 6): 4.09 ± 1.51 g; P = 0.0254].
Fig. 1.
Changes in body weight (A) and fat-pad weight (B) in brain-specific suppressor of cytokine signaling-3 (SOCS3)-deficient (Socs3-fl/fl-Cre) and wild-type (Socs3-fl/fl) mice. E-fat, epididymal fat; RP-fat, retroperitoneal fat; BAT, brown adipose tissue. Values are expressed as the means ± SE for the number of animals indicated in parentheses. *P < 0.01 vs. Socs3-fl/fl-Cre group, and aP < 0.05 vs. Socs3-fl/fl group.
Changes in leptin-induced PI3K activity and p-STAT3 levels in the hypothalamus of mice with brain-specific Socs3 deficiency.
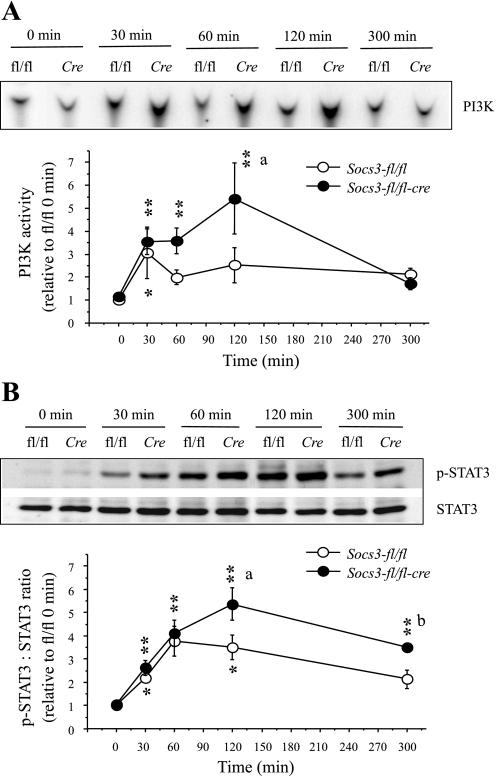
To test the hypothesis that brain-specific Socs3 deficiency increases the sensitivity of the PI3K pathway of leptin signaling in the hypothalamus, we examined IRS1-associated PI3K activity in the MBH of wild-type and NesKO mice at different time points after single peripheral leptin injection. In wild-type mice, leptin increased PI3K activity in the MBH by three-fold (P < 0.05) at 30 min after injection compared with 0 min. Although PI3K activity in response to leptin remained slightly increased beyond 30 min and up to 300 min, it was not significant. In NesKO mice, in contrast, leptin significantly (P < 0.01) increased PI3K activity at 30, 60, and 120 min after injection, and by 300 min, PI3K activity was not significant compared with the 0 min value. Importantly, at 120 min, PI3K activity was significantly (P < 0.01) increased in NesKO mice compared with the wild-type mice (Fig. 2A).
Fig. 2.
Changes in leptin-induced insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase (PI3K) activity (A) and p-STAT3 levels (B) in the hypothalamus of brain-specific Socs3-deficient (Socs3-fl/fl-Cre) and wild-type (Socs3-fl/fl) mice. A, top: representative phosphor-images obtained from the TLC plate showing phosphatidylinositol 3-phosphate [PI(3)P] production, an indicator of PI3K activity, are shown. A, bottom: results obtained by phosphorimaging showing the changes in PI3K activity. B, top: representative p-STAT3 and STAT3 Western blots obtained from the hypothalamic extract. B, bottom: densitometric analysis of the immunoreactive bands for p-STAT3. The values for p-STAT3 were first calculated as the ratio of STAT3. Values represent the means ± SE for 5–8 animals for PI3K and for 4–6 animals for p-STAT3; and presented as relative to 0 min value of the Socs3-fl/fl group. *P < 0.05 and **P < 0.01 vs. corresponding 0 min values. aP < 0.01 and bP < 0.05 vs. corresponding Socs3-fl/fl group.
To ensure that the hypothalamic STAT3 pathway also showed increased leptin sensitivity, as demonstrated previously (27), we examined p-STAT3 levels in the same MBH extracts that were used to measure PI3K activity. In wild-type mice, p-STAT3 levels were significantly increased (P < 0.01) at 30, 60, and 120 min, but not at 300 min postinjection. In NesKO mice, p-STAT3 levels were significantly increased at 30 min and remained increased thereafter, up to 300 min postinjection. In addition, p-STAT3 levels at 120 and 300 min postinjection were significantly increased (P < 0.05) in NesKO compared with the wild-type mice (Fig. 2B), indicating increased leptin sensitivity. These findings confirmed the previous report of increased sensitivity in the STAT3 pathway of leptin signaling in the NesKO mice (27) and further showed that increased sensitivity in this pathway is detectable as early as 2 h after leptin treatment.
Changes in body weight and fat pad weight, and in leptin-induced IRS1-associated PI3K activity, and p-STAT3 and SOCS3 levels in the hypothalamus in food-restricted mice.
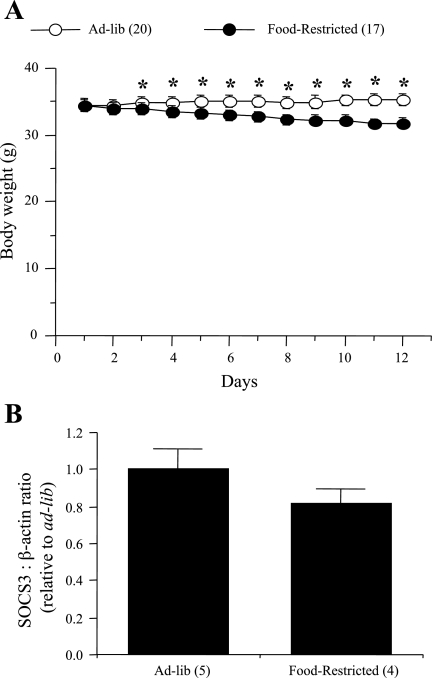
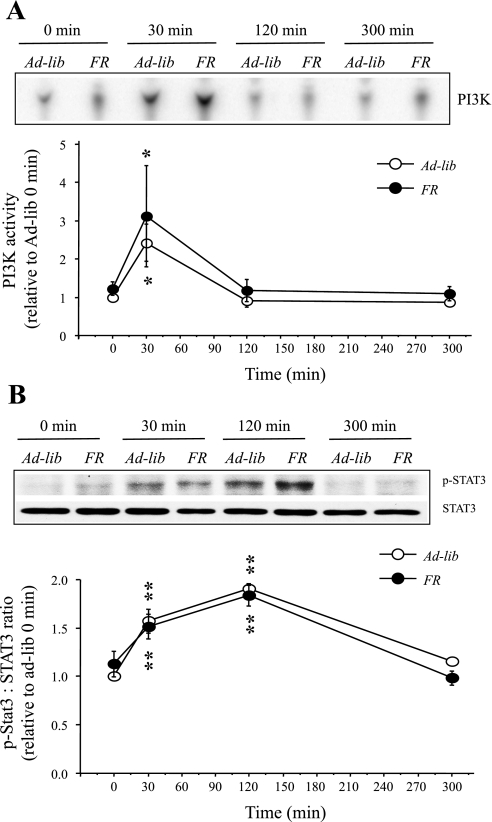
To address whether the increase in leptin sensitivity in the PI3K pathway seen in the hypothalamus of NesKO mice was due to the decrease in body weight, we examined whether food restriction, responsible for decreases in body weight, altered leptin sensitivity in the PI3K pathway in wild-type (Socs3fl/fl) mice. Whereas ad libitum animals showed a small increase (2.8%) in their body weight, FR animals had a 7.75% decrease in body weight over 13 days (Fig. 3A, P < 0.0001). E-fat and RP-fat weight did not differ between the groups (data not shown). However, there was a small but significant increase in BAT in FR animals [ad libitum: 0.0605 ± 0.0047 g (n = 20), FR: 0.0759 ± 0.003 g (n = 17); mean ± SE; P = 0.0115]. IRS1-associated PI3K activity in the MBH was measured at 0, 30, 120, or 300 min after a single peripheral leptin injection. In both ad libitum and FR mice, leptin increased PI3K activity in the MBH by approximately twofold (P < 0.05) at 30 min postinjection compared with 0 min, and there was no increase in PI3K activity at 120 or 300 min postinjection (Fig. 4A). In addition, in both ad libitum and FR mice, leptin significantly increased p-STAT3 levels (P < 0.01) at 30 and 120 min postinjection (Fig. 4B). To investigate whether absence of increased sensitivity in the PI3K pathway of leptin signaling in FR mice is due to abrogated SOCS3 expression, we examined SOCS3 protein levels in the MBH of FR and ad libitum-fed mice. We observed that SOCS3 protein levels in the MBH did not differ between ad libitum and FR groups (Fig. 3B), an important finding that further strengthens the observation of increased PI3K signaling in the NesKO mice.
Fig. 3.
Changes in body weight (A) and hypothalamic SOCS3 protein levels (B) in wild- type (Socs3-fl/fl) mice subjected to food restriction or ad libitum fed. The values for SOCS3 were first calculated as the ratio of β-actin, and they are presented as relative to ad libitum-fed group. Values are expressed as means ± SE for the number of animals indicated in parentheses. *P < 0.01 vs. food-restricted group.
Fig. 4.
Changes in leptin-induced IRS-1-associated PI3K activity (A) and p-STAT3 levels (B) in the hypothalamus of wild-type (Socs3-fl/fl) mice that were fed ad libitum (Ad-lib) or food restricted (FR). A, top: representative phosphor-images obtained from the TLC plate showing PI(3)P production, an indicator of PI3K activity, are shown. A, bottom: results obtained by phosphorimaging showing the changes in PI3K activity. B, top: representative p-STAT3 and STAT3 Western blots obtained from the hypothalamic extract. B, bottom: densitometric analysis of the immunoreactive bands for p-STAT3. The values for p-STAT3 were first calculated as the ratio of STAT3. Values represent the means ± SE for 4 or 5 animals and are presented as relative to 0-min value of the ad-lib group. *P < 0.05 and **P < 0.01 vs. corresponding 0-min values.
Effects of SOCS3 overexpression on leptin-induced IRS1-associated PI3K activity in mHypoE-43/5 neuronal cells.
Because enhanced sensitivity in the leptin-induced PI3K signaling in the hypothalamus of brain-specific Socs3-deficient mice provided indirect evidence in support of negative regulation of the PI3K pathway by SOCS3 in the hypothalamus, we then examined whether overexpression of SOCS3 reversed the stimulatory effect of leptin on PI3K activity in the mHypoE-43/5 cell line—a clonal hypothalamic, neuronal cell line expressing POMC—as well as the active leptin receptor (9). Initial studies established that leptin at a dose of 0.5 to 4.0 ng/ml significantly increased PI3K activity in mHypoE-43/5 cells that were not transfected with an expression vector (data not shown). In the subsequent study, the low dose (0.5 ng/ml) of leptin significantly (P < 0.01) increased PI3K activity in the cells transfected with p-EF vector compared with that in the saline-treated group (Fig. 5). In contrast, leptin failed to increase PI3K activity in the cells that were transfected with p-EF-Flag1-mSOCS3 vector (Fig. 5, B and C) and overexpressing SOCS3, as indicated by Flag protein expression (Fig. 5A).
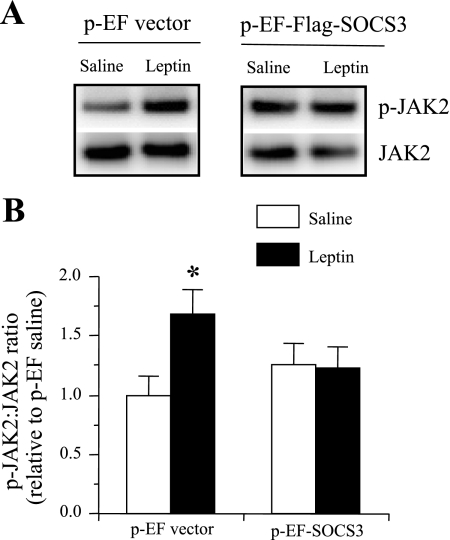
Effects of SOCS3 overexpression on leptin-induced JAK2 phosphorylation in mHypoE-43/5 neuronal cells.
Increased SOCS3 expression that inhibits PI3K activity may occur by inhibiting JAK2 phosphorylation. To test this hypothesis, mHypoE-43/5 cells were transfected with p-EF vector or p-EF-Flag1-mSOCS3 vector and treated with saline or leptin (0.5 ng/ml). Leptin significantly increased JAK2 phosphorylation in the cells transfected with the p-EF vector, compared with saline control after 15 min (Fig. 6). However, leptin failed to induce JAK2 phosphorylation in SOCS3 overexpressing mHypoE-43/5 cells (Fig. 6). These results suggest the inhibition of the PI3K pathway by SOCS3 occurs through the inability of leptin to induce p-JAK2.
Fig. 6.
Changes in leptin-induced p-JAK2 levels in mHypoE-43/5 neuronal cell line. A: representative p-JAK2 and JAK2 Western blot obtained from mHypoE-43/5 neuronal cells transfected with p-EF or p-EF-Flag1-mSOCS3 and treated with saline or leptin (0.5 ng/ml). B: densitometric analysis of the immunoreactive bands of p-JAK2 normalized to total JAK2. The values are expressed as relative to the p-EF saline group and are expressed as means ± SE of 4 independent experiments. *P < 0.05 vs. saline control.
DISCUSSION
The main findings of the present study were that 1) mice with brain-specific Socs3 deficiency demonstrated an increased sensitivity in the PI3K pathway of leptin signaling in the hypothalamus, and 2) SOCS3 overexpression inhibited the leptin-induced increase in IRS1-associated PI3K activity and phosphorylation of JAK2 in a hypothalamic clonal neuronal cell line expressing POMC. In addition, our finding of increased leptin sensitivity in the hypothalamic STAT3 pathway in brain-specific Socs3-deficient mice confirms a previous report using these mice (27) and further shows that increased sensitivity in this pathway is detectable as early as 2 h after leptin exposure.
The existing theory proposes that for normal cytokine signaling, inhibitors keep cytokines such as leptin in check so that signaling is essentially under the control of negative regulators (21). SOCS3 appears to be a major negative regulator of leptin signaling. Whereas studies in cell lines transfected with ObRb showed that an overexpression of SOCS3 inhibited proximal leptin signaling (4), the attention has been mainly on SOCS3 inhibition of leptin-induced STAT3 activation (18, 27, 30). In addition, in vivo studies have shown that SOCS3 is a negative regulator of STAT3 signaling in the hypothalamus, because leptin induces SOCS3 in the hypothalamus (5), and the brain-specific Socs3 deficiency or Socs3 haploinsufficiency increases sensitivity in the STAT3 pathway of leptin signaling (18, 27). As mentioned earlier, the leptin-induced non-STAT3 pathways play a significant role in energy homeostasis, and yet it is unknown whether SOCS3 regulates these pathways in the hypothalamus. To fully understand the mechanisms of leptin signaling in the hypothalamus in normal physiological conditions and during the development of central leptin resistance and obesity, it is quite essential to establish whether SOCS3 regulates the non-STAT3 pathways as well. Because cumulative evidence suggests an important role of the PI3K pathway in leptin signaling in the hypothalamus and energy homeostasis (26, 28, 37, 44), and because it suggests that the PI3K pathway is impaired during the development of DIO in mice (24), here, we sought to examine whether SOCS3 negatively regulated the PI3K pathway of leptin signaling in vivo and in vitro.
To establish the notion that SOCS3 exerts an inhibitory tone on the leptin-induced PI3K pathway in the hypothalamus, we tested in the first study whether the removal of the inhibitory SOCS3 tone in the hypothalamus in brain-specific Socs3-deficient mice would increase leptin sensitivity of PI3K signaling. Our observation that hypothalamic IRS1-associated PI3K activity remained increased for 120 min after leptin injection in NesKO mice, but the increase was restricted to only 30 min postinjection in wild-type mice, which clearly suggests an increased sensitivity in the PI3K pathway of leptin signaling in NesKO mice. In addition, we have confirmed the previous report of increased sensitivity in the STAT3 pathway of leptin signaling in the NesKO mice (27), because the leptin-induced STAT3 activation in the hypothalamus was significantly increased at 120 and 300 min after leptin injection in these mice, compared with the wild-type mice. It is noteworthy that in the wild-type mice, leptin-induced STAT3 activation remained increased up to 120 min postinjection, whereas the increase in PI3K activation was restricted to only 30 min postinjection. Although the underlying mechanisms behind this phenomenon are currently unknown and were not addressed in this study, it is most likely due to the transient nature of leptin-induced JAK2 phosphorylation as opposed to prolonged phosphorylation and/or delayed dephosphorylation of STAT3, as has been shown in a cell line (22).
Additionally, whereas the exact mechanism by which leptin stimulates PI3K activity in the hypothalamus remains unknown in C2C12 muscle cells, leptin has been reported to activate PI3K via JAK2- and IRS-2-dependent pathways (19). In the hypothalamus, leptin-induced activation of PI3K involves both IRS-1 (47) and IRS-2 (32), and further, leptin induces JAK2 phosphorylation (33). Nevertheless, our observation of a prolonged activation of the PI3K pathway by leptin in Socs3-deficient mice suggests SOCS3 as a major candidate for the negative regulation of the PI3K pathway of leptin signaling in the hypothalamus. It however, remains to be seen whether the increased sensitivity in the PI3K pathway occurs in specific leptin-targeted neurons, such as POMC and/or NPY/Agouti-related peptide, or is restricted to specific hypothalamic sites. Nevertheless, previous in vivo studies have shown that leptin increases SOCS3 mRNA expression in the hypothalamus within 1 h of injection with maximum levels at ∼2 h, and by 6 h Socs3 gene expression returns to basal levels (3, 5). Additionally, SOCS3 protein levels in the hypothalamus increased at 3 h after leptin injection (27), and increased SOCS3 protein levels have been reported in CHO cells at 2–3 h after leptin treatment (4). Thus, increased expression of SOCS3 would inhibit PI3K activity, as seen in the mHypoE-43/5 cell line (discussed below), most likely by inhibiting JAK2 phosphorylation (39, 40), whereas removal of SOCS3 would increase JAK2- and IRS-1/2 phosphorylation and associated PI3K activation. Along this line, RNA interference (RNAi)-mediated knockdown of SOCS3 expression reportedly increased tyrosine phosphorylation of JAK2 in a cell line (22).
To further address the leptin-mediated negative regulation of the PI3K pathway by SOCS3, we examined whether overexpression of SOCS3 in a clonal hypothalamic neuronal cell line (mHypoE-43/5) expressing POMC and the active leptin receptor (9) would inhibit the leptin-induced increase in PI3K activity. Here, we demonstrate that exposure of a relatively low dose (0.5 ng/ml) of leptin significantly increased IRS1-associated PI3K activity in the mHypoE-43/5 POMC-expressing cells, compared with control. This result corroborates with an in vivo study, in which leptin has been shown to increase PI3K activity in POMC neurons in the hypothalamus (44). However, when SOCS3 was overexpressed in these cells, leptin was unable to increase PI3K activity. This finding provides evidence for the direct SOCS3 inhibition of the PI3K pathway of leptin signaling in POMC neuronal cells. To address whether inhibition of the PI3K pathway of leptin signaling by SOCS3 was due to the suppression of JAK2 phosphorylation (40), we examined whether SOCS3 overexpression inhibited leptin-induced p-JAK2 levels in mHypoE-43/5 cells. The finding that leptin was unable to induce phosphorylation of Jak2 in SOCS3 overexpressing cells implies that SOCS3 inhibition of the PI3K pathway is due, at least in part, to the inability of leptin to induce p-JAK2. However, whether inhibition of the PI3K pathway by SOCS3 was also due to suppression of leptin receptor phosphorylation (6) and/or due to an alteration in recruitment of PI3K to the receptor, requires further investigation.
Our finding that NesKO mice maintained their body weight at a slightly lower level than the wild-type mice in association with decreased white fat pad weights, but not BAT weights, and with reduced circulating leptin levels, suggests that decreased body weight in NesKO mice is a consequence of decreased white fat mass. However, in a previous study, the body weight in the NesKO mice fed a normal chow diet was comparable with the wild-type mice (27). The reason behind the discrepancy between these two studies is currently unknown. However, differences in genetic backgrounds could play a role, because in the present study the mice were in a 129sv/BL6 mixed background, whereas in the previous report mice were in a BL6 background. Most recently, Briancon et al. (8) reported a decrease in body weight in nestin-cre mice compared with Socs3fl/fl mice. However, the decreased body weight in nestin-cre mice was associated with an increased fat-pad weight and circulating leptin levels (8). On the other hand, a lower body weight in the NesKO (nestin-cre-Socs3fl/fl) mice seen in our study was associated with a decrease in fat-pad weight and reduced circulating leptin levels. In addition, as mentioned above, NesKO mice in the study by Mori et al. (27) had a body weight similar to that in Socs3fl/fl (wild-type) mice. Thus, the contribution of nestin-cre in lower body weight maintenance is not obvious in our NesKO mice, but it warrants further investigation. Further, it is important to consider the source of the nestin-cre mice used while comparing the studies. For example, the nestin-cre mice used in the present study and the study by Mori et al. (27) were developed by Dr. Shigeru Nouguchi (Kanagawa Academy of Science and Technology, Japan), whereas the mice used by Briancon et al. (8) was obtained from the Jackson Laboratory. Nevertheless, it is most likely that increased sensitivity in both the PI3K and STAT3 pathways is responsible for the lower body weight maintenance observed in this study and for the increased sensitivity in the anorectic effects of leptin reported previously in NesKO mice (27). Although previous studies have demonstrated that Tyr985 of the leptin receptor binds SOCS3 and contributes to the attenuation of ObRb signaling (6, 7), it is unknown whether the deficiency of Socs3 with the resultant removal of inhibitory ObRb signaling through Tyr985-SOCS3 contributed to the development of a slightly leaner phenotype in NesKO mice. This is unlikely, however, because Y985F mice expressing the mutant leptin receptor ObRbF985 develop adult-onset obesity (46). Also, the l/l mice (with mutation of Tyr985) fed with normal chow showed no lean phenotype (7).
Because NesKO mice showed a slightly leaner phenotype, it was important to address whether the decreased body weight and/or reduced leptin levels recorded in the NesKO mice played any role in increasing sensitivity in the hypothalamic PI3K pathway. Therefore, we examined leptin-induced PI3K activity in wild-type mice that were entrained to a 10–15% FR diet. While FR caused a decrease in body weight similar to that seen in ad libutum-fed NesKO mice, there was no increase in PI3K activity beyond 30 min and no increase in p-STAT3 levels after 120 min of leptin injection. This finding suggests that neuron-specific removal of inhibitory SOCS3, rather than a decrease in body weight, was responsible for increased leptin sensitivity in the PI3K, as well as the STAT3 pathway in NesKO mice. Also in the previous study (27), increased leptin sensitivity in the STAT3 pathway was observed with a comparable body weight in NesKO and wild-type mice.
Recently, Hill et al. (17) have demonstrated that PI3K knockout in POMC neurons does not alter long-term body weight regulation, but that PI3K signaling in POMC neurons is required for the acute anorectic action of leptin. On the other hand, Plum et al. (34, 35) have shown that whereas selective activation of the PI3K/Akt pathway in ObRb-expressing neurons by ObRb-specific inactivation of the Pten gene results in a lean phenotype; Pten inactivation in POMC neurons, which increases PIP3, leads to leptin-resistant hyperphagia and obesity. In addition, although POMC neuron-specific Socs3-deficient mice show enhanced sensitivity to a low dose of exogenous leptin, body weight and food intake in these animals did not differ from control while on a chow diet (20). Thus, it is most likely that the decrease in body weight seen in NesKO mice is not a result of enhanced leptin sensitivity in the PI3K pathway of leptin signaling in POMC neurons. However, recent evidence that inactivation of p110β PI3K catalytic subunit specifically in POMC neurons exhibits central leptin resistance, increased obesity, and DIO (1), together with attenuation of DIO in mice with Socs3 deficiency selectively in POMC neurons (20), suggests that SOCS3 inhibition of the PI3K pathway in POMC neurons may play a significant role in the development of DIO, although this will require further investigation.
Perspectives and Significance
Central leptin resistance is one of the major causes of DIO, and understanding the molecular mechanism(s) behind this phenomenon is essential toward the development of drugs to treat and/or ameliorate obesity and related disorders. Although SOCS3 is thought to be involved in the development of central leptin resistance, and it is well known that SOCS3 can inhibit the STAT3 arm of the leptin signaling pathway, its effect on PI3K (and other non-STAT3 branches of leptin signaling) remain unknown. Our observation that SOCS3 inhibits the PI3K pathway of leptin signaling in the hypothalamus has important implications in understanding the molecular mechanisms of central leptin resistance and the development of obesity. Our data demonstrate that brain-specific Socs3 deficiency increases leptin sensitivity in both the PI3K and STAT3 pathways, and overexpression of SOCS3 in POMC-expressing hypothalamic neuronal cell line abrogates the leptin-induced PI3K activity, at least in part, through the inhibition of JAK2 phosphorylation. Because PI3K is one of the major non-STAT3 signaling pathways, by which leptin action is mediated in the hypothalamus, SOCS3 regulation of the PI3K pathway is quite significant to a further understanding of the mechanisms controlling leptin signaling in the hypothalamus. We suggest that besides its impact on the STAT3 pathway, regulation of the PI3K pathway is a major mechanism by which SOCS3 inhibits leptin action in the hypothalamus. We speculate that this mechanism could be involved in the development of central leptin resistance and DIO. Future studies should address whether the neuron-specific Socs3-deficient mice on a HFD show enhanced leptin sensitivity through the PI3K, and its downstream second messengers, and other non-STAT3 pathways.
GRANTS
This work was supported by National Institutes of Health Grants RO1 DK61499 and DK78068 (to A. Sahu) and the Canadian Institutes of Health Research (to D. B. Belsham).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. A. F. Parlow and the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone & Pituitary Program (Torrance, CA) for supplying the recombinant murine leptin, and Dr. T. Plant for critical reading of the manuscript. A part of the work was presented at the 36th Annual Meeting of the Society for Neuroscience, Atlanta, GA, October 14–18, 2006.
REFERENCES
- 1. Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJ, Batterham RL, Ashford ML, Vanhaesebroeck B, Withers DJ. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab 10: 343–354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baskin DG, Breininger JF, Schwartz MW. SOCS-3 expression in leptin-sensitive neurons of the hypothalamus of fed and fasted rats. Regul Pept 92: 9–15, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 276: 4747–4755, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 274: 30059–30065, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1: 619–625, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 275: 40649–40657, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 117: 1354–1360, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Briancon N, McNay DE, Maratos-Flier E, Flier JS. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes 59: 3074–3084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai F, Gyulkhandanyan AV, Wheeler MB, Belsham DD. Glucose regulates AMP-activated protein kinase activity and gene expression in clonal, hypothalamic neurons expressing proopiomelanocortin: additive effects of leptin or insulin. J Endocrinol 192: 605–614, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28: 7202–7208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Dunn SL, Bjornholm M, Bates SH, Chen Z, Seifert M, Myers MG., Jr Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol 19: 925–938, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23: 775–786, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138: 839–842, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett 486: 33–37, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118: 1796–1805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10: 734–738, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia 40: 1358–1362, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4: 123–132, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci 113: 2813–2819, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Leinninger GM, Myers MG., Jr LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol (Oxf) 192: 49–59, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 281: 18933–18941, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology 149: 1121–1128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci 5: 54, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289: E1051–E1057, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 59: 287–304, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Myers MG, Jr, Sun XJ, Cheatham B, Jachna BR, Glasheen EM, Backer JM, White MF. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3′-kinase. Endocrinology 132: 1421–1430, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Pal R, Sahu A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology 144: 3789–3798, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116: 1886–1901, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab 6: 431–445, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 24: 225–253, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology 145: 2613–2620, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Sahu A, Metlakunta AS. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J Neuroendocrinol 17: 720–726, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem 275: 29338–29347, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 4: 339–351, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol 20: 2080–2092, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115: 951–958, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol 18: 143–164, 2000 [DOI] [PubMed] [Google Scholar]
- 46. You J, Yu Y, Jiang L, Li W, Yu X, Gonzalez L, Yang G, Ke Z, Li C, Liu Y. Signaling through Tyr985 of leptin receptor as an age/diet-dependent switch in the regulation of energy balance. Mol Cell Biol 30: 1650–1659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci 5: 727–728, 2002 [DOI] [PubMed] [Google Scholar]